Abstract
Deregulation of long noncoding RNAs (lncRNAs) has been implicated in tumourigenesis. Cancer Susceptibility Candidate 2 (CASC2) is a lncRNA downregulated in multiple cancer types, including endometrial, lung, gastric and colorectal cancers. CASC2 functions as a tumour‐suppressive lncRNA though multiple mechanisms, such as sequestration of oncogenic microRNAs and repression of Wnt/β‐catenin signalling. Pertinent to clinical practice, the use of CASC2 as a prognostic marker has been demonstrated in sporadic studies. These findings suggested that CASC2 might play an important role in human cancers and melanoma. More efforts are warranted to examine the function role of CASC2 in other cancer types. Further validation is also needed to promote its development to be a clinically utilizable prognostic biomarker.
Keywords: lncRNA, melanoma, prognostic marker, tumour suppressor
1. INTRODUCTION
It has been demonstrated that 98% of transcripts from the human genome are noncoding RNAs without any protein‐coding potentials.1, 2 Long noncoding RNAs (lncRNAs) are a class of nonprotein‐coding RNAs with more than 200 nucleotides in length.3 Accumulating studies have shown that lncRNAs (eg, HOTAIR, LINC00152, CCAT1/2, TUG1, BANCR, HULC, NEAT1) are differentially expressed in different cancer types and play significant roles in tumourigenesis through crosstalk with oncogenes or tumour suppressor genes (TSGs).4, 5, 6, 7, 8, 9, 10, 11 Functionally, lncRNAs are involved in regulation of many cancer‐pertinent cellular processes, including cell proliferation, migration and invasion, apoptosis and drug resistance through controlling gene expression at multiple levels, including epigenetic (eg, DNA methylation, histone modification and chromatin remodelling), transcriptional (eg, recruitment of RNA polymerase II, transcriptional factors and/or cofactors) and posttranscriptional (eg, sponging of microRNAs, regulation of mRNA stability, alternative splicing and regulation of translation, protein‐protein interactions and localization) levels.12, 13 Pertinent to clinical practice, lncRNAs have emerged as novel biomarkers for cancer diagnosis/prognostication and as therapeutic targets.14, 15, 16, 17
Cancer Susceptibility Candidate 2 (CASC2) is a human lncRNA‐encoding gene originally identified as a TSG in endometrial cancer.18, 19 CASC2 is a 5‐exon gene located on chromosome 10q26,18, 19 which is a candidate deletion interval based on loss‐of‐heterozygosity studies for human chromosome 10q in endometrial cancer. The second allele of CASC2 is also frequently silenced by epigenetic mechanism. Baldinu and colleagues identified three alternative transcripts of CASC2, namely CASC2a, CASC2b and CASC2c, using a positional candidate approach. CASC2a is a 3285‐bp transcript, previously thought to encode a putative protein of 102 amino acids with no similarity to other proteins whereas CASC2b and CASC2c are predicted not to be protein coding.18, 19 An increasing number of studies have revealed the aberrant expression of CASC2 in human cancers, including lung, colorectal, gastric and bladder cancers as well as glioma.20, 21, 22, 23, 24, 25 The present review summarizes the current knowledge about the deregulation of CASC2 in human malignancies (Table 1 and Figure 1) in relation to its biological functions and molecular mechanisms. The clinical utilities of CASC2 as a prognostic biomarker and therapeutic target are also discussed.
Table 1.
Deregulation and function of CASC2 in human cancers
| Cancer type | Expression | Functional role | Mechanism of action | References |
|---|---|---|---|---|
| Lung cancer | Decreased | Tumour suppressor | Not investigated | 22, 23, 24 |
| Melanoma | Decreased | Tumour suppressor | Sponging miR‐18a‐5p | 19 |
| Gastric cancer | Decreased | Tumour suppressor | Inhibiting ERK1/2 and JNK | 13 |
| Colorectal cancer | Decreased | Tumour suppressor | Sponging miR‐18a | 18 |
| Endometrial cancer | Decreased | Not investigated | Not investigated | 12 |
| Renal cell carcinoma | Decreased | Tumour suppressor | Not investigated | 30 |
| Bladder cancer | Decreased | Tumour suppressor | Inhibiting Wnt/β‐catenin signalling | 32 |
| Glioma | Decreased | Tumour suppressor | Sponging miR‐21 and miR‐181a | 35, 37 |
Figure 1.
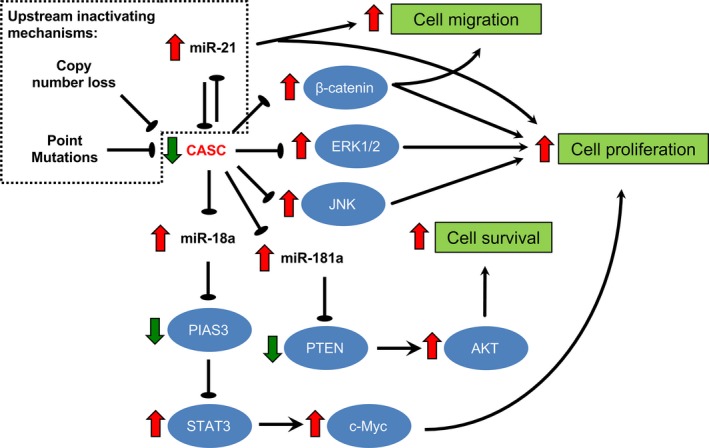
Upstream mechanisms underlying CASC2 downregulation and downstream effector pathways of CASC2 in human cancers. Genetic mechanisms (ie, point mutations and copy number loss) and aberrant upregulation of miR‐21 contribute to loss‐of‐function or downregulation of CASC2 in human cancers. CASC2 exerts its tumour‐suppressive effects through chelation of oncogenic microRNAs (eg, miR‐18a, miR‐21 and miR‐181a) and inhibition of oncogenic pathways (eg, Wnt/β‐catenin, ERK/MAPK and JNK)
2. CASC2 DEREGULATION IN HUMAN MALIGNANCIES
2.1. Melanoma
Zhang et al26 have studied the expression, effect and mechanism of CASC2 in the malignant melanoma. They demonstrated that the expression of CASC2 was downregulated in malignant melanoma tissues and cell lines. Overexpression of CASC2 decreased the malignant melanoma cell proliferation, invasion and migration and colony formation. In silico prediction revealed a binding site for CASC2 on miR‐18a‐5p, which is significantly upregulated in malignant melanoma tissues. The physical interactions between CASC2 and miR‐18a‐5p was confirmed by luciferase assay. This study further identified RUNX1 as a novel target of miR‐18a‐5p. Importantly, miR‐18a‐5p inhibitor phenocopied the effect of CASC2 while upregulation of miR‐18a‐5p reversed the promoting effect of CASC2 on RUNX1. Collectively, this study demonstrated that CASC2 suppressed cell proliferation, migration and invasion partly through acting as a molecular sponge for miR‐18a‐5p to derepress RUNX1 in malignant melanoma.26
2.2. Lung cancer
Lung cancer is the leading cause of cancer‐related death, accounting for more than 20% of cancer mortality worldwide.27 Non‐small cell lung cancer (NSCLC) accounts for approximately 80%‐85% of lung cancer patients.28 Bioinformatic analysis of RNA‐sequencing data from TCGA (The Cancer Genome Atlas) demonstrated that CASC2 expression was significantly downregulated in NSCLC. In addition, the decreased expression of CASC2 was correlated with NSCLC tumour size and advanced tumour‐node‐metastasis (TNM) stage. Multivariate analysis also demonstrated that low CASC2 expression was associated with poorer overall survival of NSCLC patients independent of other clinicopathological parameters,21 indicating that CASC2 is an independent prognosticator. Functionally, enforced expression of CASC2 significantly decreased NSCLC cell proliferation both in vitro and in vivo,21 suggesting that CASC2 might exert a tumour‐suppressing function in NSCLC. Lung squamous cell carcinoma (LSCC) comprises 25%‐30% of NSCLC.29 Huang and colleagues investigated the role of lncRNAs in the regulation process of cancer‐related genes and pathways that are involved in the pathogenesis of LSCC using data from 501 LSCC cancer samples and 51 normal subjects from TCGA.30 Coexpression analysis showed that CASC2 was related to most of the oncogenes and TSGs in LSCC, indicating that CASC2 plays a central role in the pathogenesis of LSCC.
2.3. Gastric cancer
Gastric cancer is the second leading cause of cancer‐related death worldwide.27, 31 The prognosis of gastric cancer is poor with only about one‐fifth of patients survive for 5 years or more after diagnosis.32 Li and colleagues reported that CASC2 expression was significantly lower in human gastric cancer tissues and cell lines.20 Furthermore, restored expression of CASC2 significantly inhibited gastric cancer cell proliferation in vitro and the growth of tumour xenografts in vivo. The authors then examined if CASC2 mediated its biological effects through the mitogen‐activated protein kinase (MAPK) pathway, which contributes to malignant phenotypes in human cancers. Extracellular signal‐regulated kinase (ERK), c‐Jun N‐terminal kinase (JNK) and p38 are three major downstream mediators of MAPK signalling. In this regard, CASC2 inhibited gastric cancer cell proliferation through reducing the phosphorylation levels of ERK1/2 and JNK, in which U0126 (a MAPK/ERK kinase [MEK] inhibitor) or SP600125 (a JNK inhibitor) could by itself inhibit the proliferation of gastric cancer cells.20 Taken together, CASC2 downregulation plays a functional role in the pathogenesis of gastric cancer in which CASC2 functions as a tumour suppressor through inhibiting ERK‐ and JNK‐dependent cell proliferation. Nevertheless, the mechanism by which CASC2 inhibited ERK1/2 and JNK signalling in gastric cancer remains unclear.
2.4. Colorectal cancer
Colorectal cancer is the second most common diagnosed cancer worldwide, with an increasing incidence in Asian countries.33 CASC2 expression was significantly downregulated in colorectal cancer tissues and a panel of colon cancer cell lines (ACO2, SW480, SW620, HCT‐116 and HT‐29) as compared with adjacent noncancer tissues and two nontumourigenic cell lines (CCC‐HIE‐2 and HER293), respectively.25 Decreased expression was remarkably correlated with advanced TNM stage. Functionally, CASC2 suppressed colon cancer cell proliferation in vitro by impeding the G0/G1‐S phase transition and inhibited tumour growth in vivo.25 Furthermore, bioinformatic analysis revealed that three miRNAs (ie, miR‐18a/b, miR‐4735) contain CASC2‐binding sites. The physical interactions between CASC2 and miR‐18a were further confirmed by luciferase assay. Importantly, there was a negative correlation between CASC2 and miR‐18a levels in primary colorectal cancer tissues.25 In this regard, CASC2 derepressed PIAS3 (a repressor of STAT3 and a target of miR‐18a) by sponging miR‐18a, which leads to the suppression of genes downstream of STAT3 (eg, c‐Myc). Clinical sample analysis of colorectal cancer tissues also supported the relationship between CASC2 and PIAS3.25 These findings indicated that CASC2 plays a pivotal role in the development of colorectal cancer and may serve as a prognostic marker.
2.5. Endometrial cancer
Endometrial cancer is the second most frequent gynecologic cancer globally.34 The chromosomal region 10q26, which harbours CASC2, frequently presents allelic deletion or loss of heterozygosity in endometrial cancer.18 In particular, CASC2a transcript was significantly decreased in ~76% of endometrial cancer tissues. Enforced expression of CASC2a remarkably reduced cell proliferation in undifferentiated AN3CA endometrial cancer cells. Aside from copy number loss, CASC2a mutations were found to impair the gene function.18, 19 These findings strongly suggested that CASC2a may act as a tumour suppressor gene, with both epigenetic and genetic alterations resulting in gene inactivation.
2.6. Renal cell carcinoma
Renal cell carcinoma (RCC) is a major pathological type of kidney cancers and is the most lethal genitourinary cancer.35 CASC2 expression was lower in human RCC tissues and 2 RCC cell lines (786‐O and A498) as compared with matched normal tissues and the human embryonic kidney HEK 293 cells, respectively.36 Restoration of CASC2 expression suppressed RCC cell proliferation and migration. Bioinformatics analysis revealed a binding for miR‐21 on CASC2. Subsequent luciferase reporter assays with wild‐type and mutant‐binding sites confirmed that CASC2 was a direct target gene of miR‐21, which is frequently upregulated in RCC. CASC2 RNA levels were also significantly downregulated upon transfection with miR‐21 mimics. Functionally, enforced expression of miR‐21 alleviated CASC2‐mediated inhibition of RCC cell proliferation and migration.36 These results suggest that miR‐21‐mediated downregulation of CASC2 is involved in RCC pathogenesis.
2.7. Bladder cancer
Bladder cancer is the most common type of urogenital malignancy in man.37 CASC2 expression was found to be downregulated in bladder cancer tissues and different bladder cancer cell lines (T24, 5637, SW780, J82 and UMUC3) as compared with adjacent nontumour tissues and normal urothelial cells (SV‐HUC‐1), respectively.24 In addition, decreased CASC2 expression was significantly associated with advanced TNM stage in bladder cancer. Moreover, restored expression of CASC2 expression inhibited bladder cancer cell proliferation, migration and invasion while promoted early apoptosis. Mechanistically, enforced expression of CASC2 reduced the protein levels of β‐catenin, which is an oncogenic transcription factor, and downregulated the downstream target genes c‐Myc and cyclin D1. In human cancers, loss of E‐cadherin, a membranous protein involved in cell‐cell adhesion, often results in nuclear accumulation of β‐catenin. To this end, overexpression of CASC2 strongly upregulated E‐cadherin. These data collectively demonstrated that CASC2 mediates its tumour‐suppressive effect in bladder cancer through E‐cadherin‐dependent inhibition of the Wnt/β‐catenin signalling pathway.24 These findings indicated that CASC2 plays a significant suppressive role in the bladder tumourigenesis and its downregulation contributes to the progression of bladder cancer.
2.8. Glioma
Glioma is the most commonly diagnosed malignancy of the central nervous system, accounting for the majority of brain cancer‐related deaths.38, 39 CASC2 expression was found to be lower in glioma tissues and cell lines (U251, U373, SNB19, U118 and LN229) as compared with peritumoural brain oedema tissues and the normal human astrocyte cell line NHA, respectively.40 Low CASC2 expression was correlated with more aggressive clinicopathologic features (tumour size and WHO stage) and shorter survival time in glioma patients. Restored expression of CACS2 inhibited glioma cell proliferation and amplified the anticancer effect of temozolomide (TMZ),40 a standard and the most widely used treatment for glioma patients.41 In particular, enforced expression of CACS2 resensitized TMZ‐resistant glioma cells to TMZ, while knockdown of CACS2 showed the opposite action. Mechanistically, CASC2 directly inhibited the expression of miR‐181a in TMZ‐resistant glioma cells, in which CASC2 alleviated miR‐181a‐mediated suppression of PTEN and thereby reducing the phosphorylation of AKT. These effects could be partially reversed by the overexpression of miR‐181a. Clinical sample analysis also showed that CASC2 was inversely correlated with miR‐181a, positively correlated with PTEN.40 Similar to above findings, another study showed that CASC2 was downregulated in glioma tissues and cell lines.42 Enforced expression of CASC2 inhibited glioma cell proliferation, migration and invasion and promoted apoptosis. Overexpression of CASC2 significantly decreased miR‐21 expression whereas overexpression of miR‐21 abrogated CASC2‐mediated inhibition of glioma cell proliferation, invasion and migration.42 These two studies collectively demonstrated that CASC2 plays a tumour‐suppressive role in glioma through inhibiting miR‐181a and miR‐21 and may serve as a prognostic marker.
3. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
CASC2, a lncRNA first identified in 2004, has been demonstrated to be downregulated in multiple types of human malignancy, in which it functions as a tumour suppressor. Mechanistically, sponging of oncogenic microRNAs (ie, miR‐21, miR‐18a and miR‐181a) and inhibition of MAPK and Wnt/β‐catenin signalling seem to be important mechanisms underlying the tumour‐suppressive action of CASC2. However, little is known about the detailed mechanism underlying CASC2 downregulation in malignancies. Copy number loss and point mutations at DNA level and targeting by aberrantly upregulated miR‐21 at posttranscriptional level might be key inactivating mechanisms (Figure 1), but the possible contribution of epigenetic and transcriptional mechanisms cannot be neglected. Further investigations into upstream regulation and downstream function of CASC2 by systems biology approaches (eg, proteomics to study DNA‐bound and chromatin‐associated regulatory complexes in CASC2 promoter; RNA pull‐down assay coupled with proteomics or sequencing to identify CASC2‐binding partners) should provide more mechanistic insights.
Pertinent to clinical practice, CASC2 downregulation is associated with aggressive phenotypes and shortened patients’ survival in many cancer types, suggesting CASC2 might be used as a prognostic marker. However, further validation of its prognostic performance in independent cohorts of larger sample size in different ethnic groups is still needed to enhance the clinical utility of CASC2. In this regard, integrative analysis of CASC2 expression in human cancers in relation to patients’ clinicopathological parameters (eg, overall survival) using publicly available data sets (eg, those from the Cancer Genome Atlas [TCGA] Research Network) could readily identify cancer types in which CASC2 might function as a prognostic marker.
Taken together, CASC2 is an important tumour‐suppressive lncRNA with clinical potentials. However, more efforts are warranted to facilitate translation of CASC2 from basic science into clinical utility.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
Yu X, Zheng H, Tse G, Zhang L, Wu WKK. CASC2: An emerging tumour‐suppressing long noncoding RNA in human cancers and melanoma. Cell Prolif. 2018;51:e12506 10.1111/cpr.12506
REFERENCES
- 1. Eddy SR. Non‐coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2:919‐929. [DOI] [PubMed] [Google Scholar]
- 2. Kapranov P, Willingham AT, Gingeras TR. Genome‐wide transcription and the implications for genomic organization. Nat Rev Genet. 2007;8:413‐423. [DOI] [PubMed] [Google Scholar]
- 3. Mercer TR, Dinger ME, Mattick JS. Long non‐coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155‐159. [DOI] [PubMed] [Google Scholar]
- 4. Cai B, Song XQ, Cai JP, Zhang S. HOTAIR: a cancer‐related long non‐coding RNA. Neoplasma. 2014;61:379‐391. [DOI] [PubMed] [Google Scholar]
- 5. Xin Y, Li Z, Zheng H, Chan MTV, Wu WKK. CCAT2: a novel oncogenic long non‐coding RNA in human cancers. Cell Prolif. 2017;50:e12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Z, Shen J, Chan MT, Wu WK. TUG1: a pivotal oncogenic long non‐coding RNA of human cancers. Cell Prolif. 2016;49:471‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu X, Zheng H, Chan MT, Wu WKK. BANCR: a cancer‐related long non‐coding RNA. Am J Cancer Res. 2017;7:1779‐1787. [PMC free article] [PubMed] [Google Scholar]
- 8. Yu X, Zheng H, Chan MT, Wu WK. HULC: an oncogenic long non‐coding RNA in human cancer. J Cell Mol Med. 2017;21:410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer‐related long non‐coding RNA. Cell Prolif. 2017;50:e12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu Y, Yang J, Li Q, Xu B, Lian Y, Miao L. LINC00152: a pivotal oncogenic long non‐coding RNA in human cancers. Cell Prolif. 2017;50:e12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin Y, Li Z, Shen J, Chan M, Wu W. CCAT1: a pivotal oncogenic long non‐coding RNA in human cancers. Cell Prolif. 2016;49:255‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253‐1261. [DOI] [PubMed] [Google Scholar]
- 14. Castellano J, Navarro A, Viñolas N, et al. LincRNA‐p21 impacts prognosis in resected non‐small cell lung cancer patients through angiogenesis regulation. J Thorac Oncol. 2016;11:2173‐2182. [DOI] [PubMed] [Google Scholar]
- 15. Zhang YY, Yang R, Lian JC, Xu HY. LncRNA Sox2ot overexpression serves as a poor prognostic biomarker in gastric cancer. Am J Transl Res. 2016;8:5035‐5043. [PMC free article] [PubMed] [Google Scholar]
- 16. Qi P, Zhou XY, Du X. Circulating long non‐coding RNAs in cancer: current status and future perspectives. Mol Cancer. 2016;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parasramka MA, Maji S, Matsuda A, Yan IK, Patel T. Long non‐coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol Ther. 2016;161:67‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldinu P, Cossu A, Manca A, et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004;23:318‐326. [DOI] [PubMed] [Google Scholar]
- 19. Baldinu P, Cossu A, Manca A, et al. CASC2a gene is down‐regulated in endometrial cancer. Anticancer Res. 2007;27:235‐243. [PubMed] [Google Scholar]
- 20. Li P, Xue WJ, Feng Y, Mao QS. Long non‐coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8:3522‐3529. [PMC free article] [PubMed] [Google Scholar]
- 21. He X, Liu Z, Su J, et al. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non‐small cell lung cancer. Tumour Biol. 2016;37:9503‐9510. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Huang H, Tong S, Huo R. Overexpression of long non‐coding RNA cancer susceptibility 2 inhibits cell invasion and angiogenesis in gastric cancer. Mol Med Rep. 2017;16:5235‐5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang R, Li Y, Zhu G, et al. Long noncoding RNA CASC2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/β‐catenin signaling pathway. Neuropsychiatr Dis Treat. 2017;13:1805‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pei Z, Du X, Song Y, et al. Down‐regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β‐catenin signaling pathway. Oncotarget. 2017;8:18145‐18153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang G, Wu X, Li S, Xu X, Zhu H, Chen X. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR‐18a in colorectal cancer. Sci Rep. 2016;6:26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Qian W, Feng F, et al. Upregulated lncRNA CASC2 may inhibit malignant melanoma development through regulating miR‐18a‐5p/RUNX1. Oncol Res. 2018;. 10.3727/096504018X15178740729367. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 28. Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC Lung Cancer Staging Project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:639‐650. [DOI] [PubMed] [Google Scholar]
- 29. Shen J, Wang S, Zhang YJ, et al. Genome‐wide aberrant DNA methylation of microRNA host genes in hepatocellular carcinoma. Epigenetics. 2012;7:1230‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang G, Ke ZP, Hu HB, Gu B. Co‐expression network analysis of long noncoding RNAs (IncRNAs) and cancer genes reveals SFTA1P and CASC2 abnormalities in lung squamous cell carcinoma. Cancer Biol Ther. 2017;18:115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5(Suppl 1):5‐11. [DOI] [PubMed] [Google Scholar]
- 32. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113‐123. [DOI] [PubMed] [Google Scholar]
- 33. Ait Ouakrim D, Pizot C, Boniol M, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015;351:h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207‐225. [DOI] [PubMed] [Google Scholar]
- 35. Hirsch MS, Signoretti S, Dal Cin P. Adult renal cell carcinoma: a review of established entities from morphology to molecular genetics. Surg Pathol Clin. 2015;8:587‐621. [DOI] [PubMed] [Google Scholar]
- 36. Cao Y, Xu R, Xu X, Zhou Y, Cui L, He X. Downregulation of lncRNA CASC2 by microRNA‐21 increases the proliferation and migration of renal cell carcinoma cells. Mol Med Rep. 2016;14:1019‐1025. [DOI] [PubMed] [Google Scholar]
- 37. Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 2010;60:244‐272. [DOI] [PubMed] [Google Scholar]
- 38. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803‐820. [DOI] [PubMed] [Google Scholar]
- 39. Ludwig K, Kornblum HI. Molecular markers in glioma. J Neurooncol. 2017;134:505‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao Y, Shen L, Zhao H, et al. LncRNA CASC2 interacts with miR‐181a to modulate glioma growth and resistance to TMZ through PTEN pathway. J Cell Biochem. 2017;118:1889‐1899. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Chen X, Zhang Z, et al. Comparison of the clinical efficacy of temozolomide (TMZ) versus nimustine (ACNU)‐based chemotherapy in newly diagnosed glioblastoma. Neurosurg Rev. 2014;37:73‐78. [DOI] [PubMed] [Google Scholar]
- 42. Wang P, Liu YH, Yao YL, et al. Long non‐coding RNA CASC2 suppresses malignancy in human gliomas by miR‐21. Cell Signal. 2015;27:275‐282. [DOI] [PubMed] [Google Scholar]


