Abstract
Objectives
Long noncoding RNAs (lncRNAs) play important roles in cancer development and progression. The deregulated expression of LINC00978 has been reported in human cancers. However, the expression pattern and biological roles of LINC00978 in gastric cancer (GC) remain unclear. In this study, we investigated the potential roles and clinical value of LINC00978 in gastric cancer.
Materials and methods
QRT‐PCR was performed to investigate the expression of LINC00978 in gastric cancer cell lines, tissues and serum samples. Cell counting, colony formation, transwell migration and matrigel invasion assays were performed to determine the effects of shRNA‐mediated knockdown of LINC00978 on gastric cancer cell functions. In vivo tumour growth assay was also conducted. Flow cytometry, immunohistochemistry, western blot and qRT‐PCR were used for potential mechanism study.
Results
LINC00978 expression level was elevated in GC tumour tissues, serum samples and cell lines. The expression level of LINC00978 was significantly correlated with tumour size (P = 0.02), lymphatic metastasis (P = 0.009) and TNM stage (P = 0.009). LINC00978 knockdown inhibited the proliferation of GC cells by suppressing cell cycle progression and inducing apoptosis. LINC00978 knockdown also inhibited the migration and invasion of GC cells. In addition, LINC00978 knockdown inhibited the activation of TGF‐β/SMAD signalling pathway and the process of epithelial‐mesenchymal transition (EMT) in GC cells. Moreover, the in vivo tumorigenicity of LINC00978 knockdown GC cells in mice was significantly decreased.
Conclusions
LINC00978 promotes gastric cancer progression and may serve as a potential biomarker for GC.
1. INTRODUCTION
Gastric cancer is one of the most common malignancies and a leading cause of cancer death worldwide, especially in Eastern Asia.1, 2 The prognosis of gastric cancer remains poor since it is mostly diagnosed at advanced stage, usually accompanied by malignant proliferation, extensive invasion and distant metastasis. Therefore, the exploration of novel biomarkers is imperative for early diagnosis and effective therapies of gastric cancer.
Long noncoding RNAs (lncRNAs), defined as transcripts of more than 200 nt in length that lack protein‐coding capacity, are widely transcribed in the genome.3, 4 Compared with miRNAs that have been extensively studied, the roles of lncRNAs in human health and diseases remain to be elucidated.5 Growing evidence suggests that lncRNAs are key players in cancer.6, 7 Up to now, lncRNAs have been demonstrated to play vital roles in tumour initiation, progression and metastasis through modulating oncogenic and tumour‐suppressing pathways.8, 9 In addition, recent studies have indicated that a number of lncRNAs are dysregulated in gastric cancer.10, 11, 12, 13 The aberrant expression pattern of lncRNAs can serve as biomarkers for cancer diagnosis and prognosis as well as targets for potential therapy.14, 15, 16 However, the contributions of lncRNAs to gastric cancer remain largely unknown and suitable biomarkers still need to be explored.
LINC00978, also known as MIR4435‐2HG and AK001796, is a lncRNA located in the 2q13 region of human genome. Yang et al17 first reported that LINC00978 was overexpressed in lung cancer tissues and cell lines. Deng et al18 found that high level of LINC00978 predicted poor prognosis in breast cancer patients. Recently, Ke et al19 demonstrated that a combination of circulating lncRNAs including MIR4435‐2HG in plasma can serve as diagnostic markers for gastric cancer. However, the precise biological function and potential underlying mechanism of LINC00978 in gastric cancer have not been well characterized.
In this study, we determined the expression pattern of LINC00978 in gastric cancer and conducted in vitro and in vivo studies to elucidate the roles of LINC00978 in gastric cancer cell activities. Our results showed that LINC00978 expression is upregulated in gastric cancer tissues and its expression level was associated with tumour size, tumour‐node‐metastasis (TNM) stage and lymph node metastasis. LINC00978 promoted gastric cancer growth in vitro and in vivo and enhanced the metastatic potential of gastric cancer cells in vitro. LINC00978 exerted its oncogenic activities through the activation of TGF‐β/SMAD pathway and the induction of EMT. Our results suggest that LINC00978 is critically involved in gastric cancer progression and may serve as a potential diagnostic biomarker for gastric cancer.
2. MATERIALS AND METHODS
2.1. Microarray data analysis
Human microarray datasets GSE13911 and GSE79973 were obtained from the Gene Expression Omnibus (GEO database, http://www.ncbi.nlm.nih.gov/geo/). The GSE13911 dataset contained the transcriptome data of 69 stomach tissues studied on an Affymetrix Human Genome U133 Plus 2.0 Array with 47 000 transcripts (Affymetrix, Santa Clara, CA, US), with data normalized by the Robust Multichip Average (RMA) algorithm. The GSE79973 dataset contained 20 samples of paired normal gastric mucosa and gastric adenocarcinoma tissues and was analysed with the Affymetrix U133 Array and data normalized by the MicroArray Suite (MAS) 5.0 algorithm. The z‐score of log2 format of normalized data was used for further analysis.
2.2. Ethics statement
This study was performed with the approval of Ethics Review Committee of the Affiliated People's Hospital of Jiangsu University. Informed consents were obtained from each participants included in the study prior to sample collection. All experiments were performed in accordance with relevant regulations and guidelines.
2.3. Clinical samples and data collection
Clinical samples including tissue and serum samples were collected from the Affiliated People's Hospital of Jiangsu University between April 2015 and December 2016. All selected gastric cancer patients met the following inclusion criteria: (i) Patients were newly diagnosed to have gastric cancer with definite pathological evidence or radiological evidence; (ii) No chemoradiotherapies were given before surgery. Preoperative blood samples of gastric cancer patients and healthy controls were collected, separated for serum within 2 hours and stored at −80°C. Tissue samples including tumour and paired non‐tumour tissues were collected within 1 hour after gastric excision. Tissue samples were put into liquid nitrogen immediately and then transferred to −80°C for storage. The characteristics of patients including gender, age, tumour size, differentiation, lymphatic metastasis, venous or perineural invasion, invasion depth, TNM stage and tumour location were recorded.
2.4. Cell culture
The human gastric cancer lines MGC‐803, SGC‐7901, BGC‐823 and HGC‐27 were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The human normal gastric epithelial cell line GES‐1 was purchased from Gefan Biological Technology (Shanghai, China). MGC‐803 was cultured with high glucose‐Dulbecco's modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA), while SGC‐7901, BGC‐823, HGC‐27 and GES‐1 cells were cultured with Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA, USA). All media were supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco). All the cells were cultured at 37°C in a humidified incubator with 5% CO2.
2.5. RNA isolation, reverse transcription and quantitative real‐time polymerase chain reaction (qRT‐PCR)
Total RNA was isolated from tumour and non‐tumour tissues by using Ultrapure RNA Kit following the manufacturer's procedures (CWBIO, Beijing, China). Total RNA from serum samples was extracted by using miRNeasy Serum/Plasma kit according to the manufacturer's instructions (QIAGEN, Hilden, Germany). TRIzol reagent (Invitrogen) was used for the extraction of total RNA from the cultured cells. The cDNAs were generated from 1 μg total RNA by using the HiScript 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). qRT‐PCR was performed using UltraSYBR Mixture (CWBIO) on a CFX96 Real‐time PCR Detection System (Bio‐Rad, Hercules, CA, USA). The qRT‐PCR reactions were performed in triplicate. Changes in gene expression were determined by the −ΔCt or 2−ΔΔCt method. Results were normalized to the expression of small molecular RNA U6. The primer sequences used for qRT‐PCR were presented in Table S1.
2.6. RNA interference by shRNA
The shRNA targeting LINC00978 and shRNA control were synthesized by Hanbio Biotechnology (Shanghai, China). The sequences of shRNAs were as follows: pGPU6/GFP/Neo‐shRNA (sh‐LINC00978): 5′‐CACCGCCCAGATTTAAGGGCTATTTCAAGAGAATAGCCCTTAAATCTGGGCCTTTTTTG‐3′; pGPU6/GFP/Neo‐shNC (sh‐control): 5′‐CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG‐3′. MGC‐803 and SGC‐7901 cells cultured in 6‐well plate were transfected with the sh‐LINC00978 and sh‐control by using LipoFiter (Hanbio, Shanghai, China) according to the manufacturer's instructions. Cells were harvested at 36 hours after transfection. The specific silencing of LINC00978 expression was assessed by using qRT‐PCR.
2.7. Cell counting and colony formation assay
The transfected cells were seeded into 24‐well plates (1 × 104 cells per well). The cells were trypsinized and counted every day for 6 days. The results were plotted as cell growth curves. For colony formation assay, the transfected cells were plated into 6‐well plates (1 × 103 cells per well) and cultured for 10 days, during which the medium was replaced every 3 days. At the end of the experiment, the colonies were washed with phosphate buffered saline (PBS), fixed with 4% paraformaldehyde for 10 minutes and stained with 0.5% crystal violet for 5 minutes. The number of visible colonies containing ≥50 cells was counted. All these experiments were performed in triplicate.
2.8. Flow cytometric analyses of cell cycle and apoptosis
For cell cycle analysis, cells were harvested and fixed with 75% ethanol at 4°C overnight, then treated with RNase A and stained with propidium iodide (PI) for 30 minutes at 37°C. Cell cycle profiles were determined using FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA). For the analysis of cell apoptosis, cells were collected and stained with Annexin V‐FITC and PI for 15 minutes in darkness at room temperature. Cells undergoing early and late apoptosis were then quantified using flow cytometry analysis (BD Biosciences). The experiments were performed in triplicate.
2.9. Cell migration and invasion assay
The transfected cells resuspended in 200 μL serum‐free media were seeded into the upper chamber of transwell with 8 μm pore (Corning, NY, USA), which was pre‐coated with (for invasion assay) or without (for migration assay) 50 μL matrigel. A total of 600 μL DMEM medium containing 10% FBS was added into the lower chamber. After incubation at 37°C with 5% CO2 for 24 hours, cells on the upper surface were removed with a cotton swab and cells on the lower surface were fixed in paraformaldehyde for 15 minutes followed by staining with 0.2% crystal violet for 5 minutes. Cells were photographed and counted under an inverted microscope (Olympus, Tokyo, Japan). The results were mean values of 3 independent experiments.
2.10. Protein extraction and western blot
Cells were washed and lysed in Radio‐Immunoprecipitation Assay (RIPA) extraction reagent (Beyotime, Beijing, China) supplemented with a protease inhibitor cocktail solution (Roche, Indianapolis, IN, USA). The protein samples were separated on 10% SDS‐polyacrylamide gel electrophoresis and transferred to 0.22 μm PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% non‐fat milk, the membranes were incubated with specific primary antibodies against p21, poly(ADP‐ribose) polymerase (PARP), caspase‐3, Bcl‐2, Slug, Twist, E‐cadherin, N‐cadherin, Vimentin, TGF‐β, p‐SMAD2, SMAD2 and MMP9 antibodies (Cell Signaling Technology, Shanghai, China) at 4°C overnight and washed extensively. After incubation with the goat anti‐rabbit or anti‐mouse secondary antibodies (CST), the protein bands were visualized by using chemiluminescence (Millipore). GAPDH (Sigma‐Aldrich, St. Louis, MO, USA) was used as loading control.
2.11. In vivo tumour growth assay
All procedures for animal experiments were approved by the Animal Use and Care Committee of Jiangsu University. Ten BALB/c nude mice (4 weeks old, male) were randomly divided into 2 groups. MGC‐803 cells transfected with sh‐LINC00978 or sh‐control were harvested at 36 hours after transfection. Approximately 2 × 106 MGC‐803 cells were resuspended with 0.2 mL PBS and injected into each mice subcutaneously. Xenograft tumours were observed periodically after cell inoculation. The mice were killed at 6 weeks after injection and the tumours were removed and photographed. Tumour diameters were examined with a vernier caliper. Tumour volumes (V) were calculated using the formula:
2.12. Immunohistochemical staining
Tumour tissue sections were incubated with primary monoclonal antibody against Ki‐67 (Cell Signaling Technology) followed by incubation with the secondary antibody for 30 minutes at room temperature. After being incubated with 3, 3′‐Diaminobenzidine (3, 3′‐DAB, Maxim, Fuzhou, China) for 5 minutes, the sections were counterstain with haematoxylin for 30 seconds. Finally, the sections were photographed under a TE2000 microscope (Nikon, Tokyo, Japan).
2.13. Statistical analysis
All the data were analysed by using SPSS 22.0 software (SPSS, Chicago, IL, USA) or GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). Differences between measured groups were assessed using Student's t test. The associations between LINC00978 expression and clinicopathological features were studied using chi‐square test and Fisher's exact test. The area under the ROC curve (AUC) was analysed to estimate the effectiveness of LINC00978 for prediction. All P values were 2‐sided. Differences were considered as statistically significant for P values less than 0.05. Data were presented as mean with the standard deviation (SD).
3. RESULTS
3.1. LINC00978 is upregulated in gastric cancer tissues and cell lines
The expression levels of LINC00978 in human gastric cancer tissues were analysed by using the microarray data downloaded from GEO (GSE13911 and GSE79973). The results showed that LINC00978 expression level was consistently upregulated in GSE13911 and GSE79973 datasets from either unpaired or paired samples (Figure 1A,B). To validate the findings of GEO data analysis, we examined LINC00978 expression in a cohort of 72 paired gastric cancer and adjacent non‐cancerous tissues by using qRT‐PCR. The expression level of LINC00978 was significantly upregulated in gastric cancer tissues compared with the paired non‐cancerous tissues (P < 0.001, Figure 1C,D). We further analysed the relationship between LINC00978 expression level and the clinicopathological features of gastric cancer patients. As shown in Table 1, the expression level of LINC00978 was associated with tumour size (P < 0.05), lymphatic metastasis (P < 0.01) and TNM stage (P < 0.01). The area under ROC curve (AUC) was 0.746 (95% confidence interval (CI)= 0.663‐0.828, P < 0.001; Figure 1E). The sensitivity and specificity were 0.53 and 0.92, respectively. Moreover, 5 cell lines including one normal gastric epithelial cell line GES‐1 and 4 gastric cancer cell lines (MGC‐803, BGC‐823, SGC‐7901, HGC‐27) were detected for LINC00978 expression. The expression levels of LINC00978 in MGC‐803, BGC‐823, SGC‐7901 and HGC‐27 cells were significantly higher than that in GES‐1 cells (Figure 1F). Taken together, these findings suggest that LINC00978 is upregulated in gastric cancer tissues and cell lines.
Figure 1.
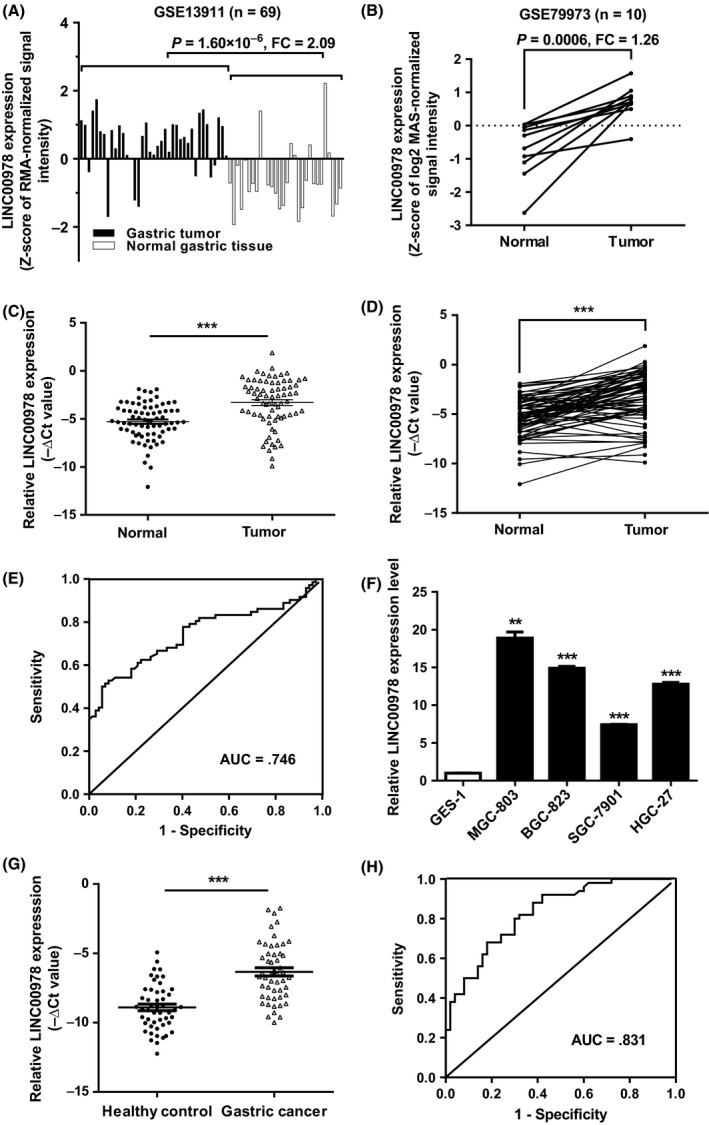
LINC00978 is upregulated in gastric cancer tissues, serums and cell lines. (A) Analysis of LINC00978 expression level in GEO dataset GSE13911 (n = 38 for tumour group; n = 31 for non‐tumour group). (B) Relative LINC00978 expression levels in paired tumour and non‐tumour tissues (n = 10) from GEO dataset GSE79973. (C,D) Relative expression of LINC00978 in paired tumour and non‐tumour tissues (n = 72). (E) ROC curve analysis of the diagnostic potential of LINC00978 in gastric cancer tissues. (F) The expression profiles of LINC00978 in MGC‐803, BGC‐823, SGC‐7901, HGC‐27 and GES‐1 cells. (G) Serum LINC00978 expression levels in gastric cancer patients and healthy controls. (H) ROC curve analysis of the diagnostic performance of serum LINC00978. **P < 0.01, ***P < 0.001
Table 1.
The association between LINC00978 expression levels (−ΔCt) in tumour tissues and the clinicopathological features of gastric cancer patients
| Features | Number | LINC00978 expression | Mean ± SD | P value | |
|---|---|---|---|---|---|
| High | Low | ||||
| Gender | |||||
| Male | 52 | 39 | 13 | 3.31 ± 2.50 | 0.210 |
| Female | 20 | 12 | 8 | 3.29 ± 2.65 | |
| Age, years | |||||
| 60 | 13 | 9 | 4 | 3.55 ± 2.56 | 1.000 |
| ≥60 | 59 | 42 | 17 | 3.25 ± 2.54 | |
| Tumour size (cm) | |||||
| <5 | 36 | 21 | 15 | 3.78 ± 2.75 | 0.020 |
| ≥5 | 36 | 30 | 6 | 2.83 ± 2.12 | |
| Differentiationa | |||||
| Moderate | 32 | 25 | 7 | 3.01 ± 2.53 | 0.173 |
| Poor | 38 | 24 | 14 | 3.54 ± 2.58 | |
| Lymphatic metastasis | |||||
| N0 | 19 | 9 | 10 | 3.54 ± 2.57 | 0.009 |
| N1‐3 | 53 | 42 | 11 | 3.22 ± 2.53 | |
| Venous or perineural invasion | |||||
| Absent | 47 | 32 | 15 | 3.51 ± 2.43 | 0.482 |
| Present | 25 | 19 | 6 | 2.91 ± 2.70 | |
| Invasion depth | |||||
| T1 and T2 | 3 | 1 | 2 | 4.27 ± 3.96 | 0.202 |
| T3 and T4 | 69 | 50 | 19 | 3.26 ± 2.48 | |
| TNM stage | |||||
| I and II | 19 | 9 | 10 | 3.86 ± 2.70 | 0.009 |
| III and IV | 53 | 42 | 11 | 3.10 ± 2.46 | |
| Tumour location | |||||
| Antrum | 16 | 8 | 8 | 4.11 ± 2.42 | 0.195 |
| Body | 4 | 4 | 0 | 2.08 ± 1.91 | |
| Angulus | 7 | 5 | 2 | 3.39 ± 2.72 | |
| Cardia | 24 | 17 | 7 | 3.33 ± 2.69 | |
| Others | 21 | 17 | 4 | 2.85 ± 2.48 | |
Missing 2 cases.
TNM, tumour‐node‐metastasis.
3.2. LINC00978 expression level is elevated in the serum of gastric cancer patients
We further tested the expression of LINC00978 in serum samples from gastric cancer patients and healthy controls. The results showed that the expression levels of serum LINC00978 were significantly higher in gastric cancer patients than those in healthy controls (Figure 1G). Furthermore, the AUC of ROC curve was 0.831 (95% CI 0.754‐0.908, sensitivity 0.80, specificity 0.70), as shown in Figure 1H. In summary, these data suggest that LINC00978 is highly expressed in the circulation of gastric cancer patients and may serve as a potential biomarker for gastric cancer.
3.3. LINC00978 silencing inhibits gastric cancer cell growth in vitro and in vivo
To investigate the roles of LINC00978 in gastric cancer, we inhibited LINC00978 expression in gastric cancer cells by using shRNA‐mediated interference (Figure 2A). The proliferation abilities of gastric cancer cells were determined by using cell counting and colony formation assays. As shown in Figure 2B, the silencing of LINC00978 significantly retarded the growth of gastric cancer cells. The results of colony formation assay showed that gastric cancer cells with LINC00978 knockdown formed significantly less colonies than control cells (P < 0.001, Figure 2C). The effect of LINC00978 on gastric cancer growth in vivo was determined by using a subcutaneous xenograft tumour model in mice. The results showed that the mice injected with sh‐LINC00978 gastric cancer cells developed smaller tumours than that injected with sh‐control cells (P < 0.001, Figure 2D). The results of immunohistochemical staining further revealed that there were less Ki‐67 positive proliferating cells in the tumour tissues from mice in sh‐LINC00978 group than that in sh‐control group (Figure 2E). Thus, these findings indicate that LINC00978 could promote gastric cancer growth in vitro and in vivo.
Figure 2.
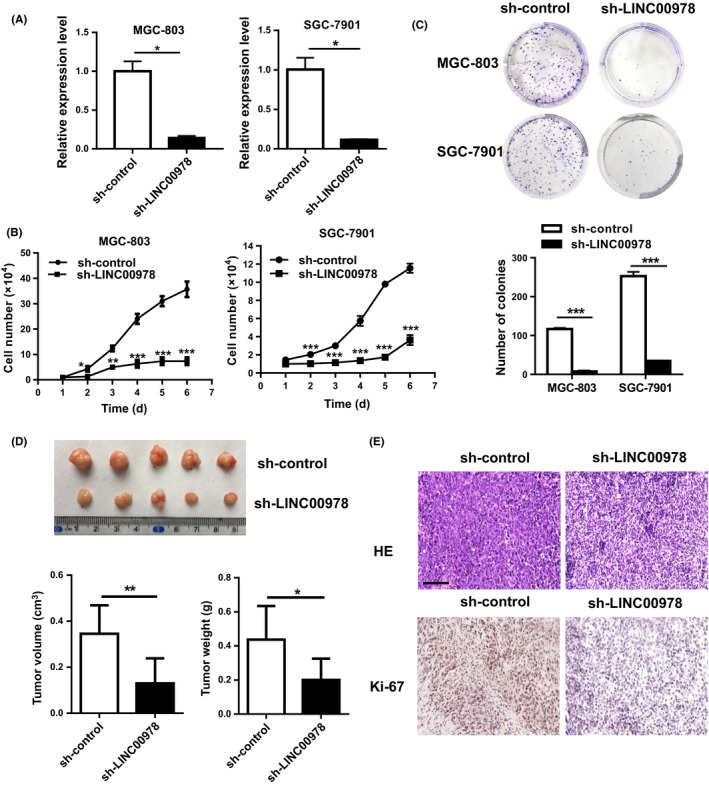
LINC00978 knockdown inhibits the proliferation of gastric cancer cells. (A) Relative expression levels of LINC00978 in shRNA‐transfected cells. (B) Growth curves of gastric cancer cells transfected with sh‐LINC00978. (C) Colony formation assays for gastric cancer cells transfected with sh‐LINC00978. (D) Tumour volumes and weights of mice in sh‐LINC00978 and sh‐control groups. (E) Representative images of HE staining and immunohistochemical staining of Ki‐67 in mouse tumour tissues. *P < 0.05, **P < 0.01, ***P < 0.001
3.4. LINC00978 knockdown induces cell cycle arrest and apoptosis in gastric cancer cells
To further explore the potential mechanisms by which LINC00978 could affect gastric cancer cell proliferation, we performed flow cytometric analyses to determine the cell cycle distribution and apoptosis of gastric cancer cells with or without LINC00978 knockdown. The results of cell cycle analysis revealed that LINC00978 knockdown led to a significant increase in the proportion of cells in G1 phase (P < 0.05) while a significant decrease in the percentage of cells in S phase (P < 0.01), both in MGC‐803 and SGC‐7901 cells (Figure 3A). We further performed Annexin‐V/PI double staining combined with flow cytometric analysis to evaluate the effect of LINC00978 knockdown on cell apoptosis. As shown in Figure 3B, LINC00978 knockdown induced a significant increase in the percentage of apoptotic cells in both MGC‐803 and SGC‐7901 cells. Moreover, we determined the expression of cell cycle and apoptosis‐related proteins by using western blot. As shown in Figure 3C, LINC00978 knockdown promoted the expression of p21 and the cleavage of PARP and caspase‐3 in both MGC‐803 and SGC‐7901 cells. The results of western blot and qRT‐PCR showed that LINC00978 knockdown inhibited the expression of Bcl‐2 in gastric cancer cells (Figure 3C,D). Taken together, these results indicate that LINC00978 knockdown could inhibit gastric cancer cell proliferation by inducing cell cycle arrest and apoptosis.
Figure 3.
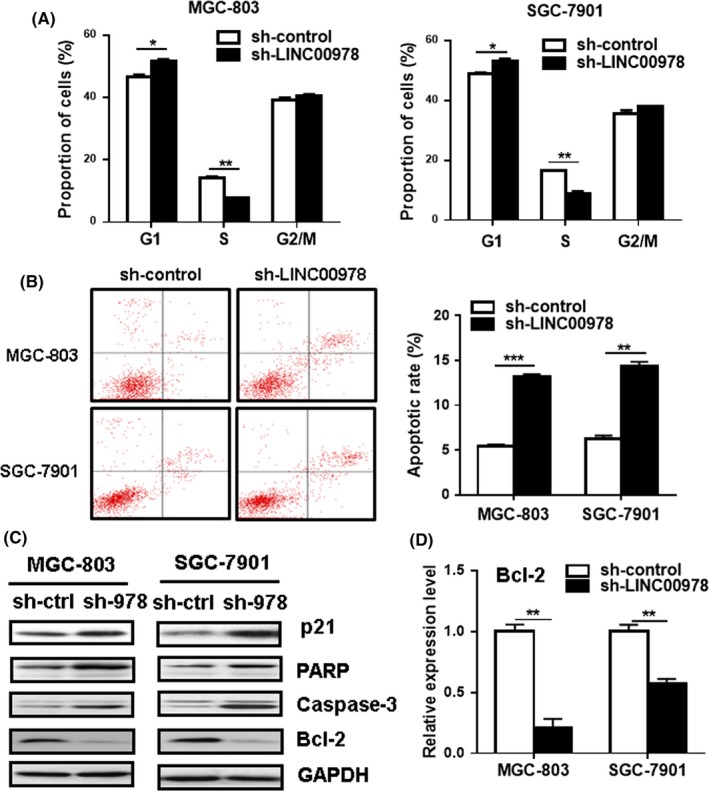
LINC00978 knockdown induces cell cycle arrest and apoptosis in gastric cancer cells in vitro. (A) The cell cycles of MGC‐803 and SGC‐7901 cells transfected with sh‐LINC00978 were analysed by using flow cytometry. (B) Cell apoptosis in MGC‐803 and SGC‐7901 cells transfected with sh‐LINC00978 were determined by using flow cytometry. (C) Western blot analyses of p21, poly(ADP‐ribose) polymerase (PARP), Bcl‐2 and caspase‐3 in LINC00978 knockdown MGC‐803 and SGC‐7901 cells. (D) QRT‐PCR analyses of BCL‐2 gene expression in LINC00978 knockdown GC cells. *P < 0.05, **P < 0.01, ***P < 0.001
3.5. LINC00978 knockdown inhibits the migration and invasion of gastric cancer cells
We next wanted to know whether LINC00978 knockdown affected the metastatic potential of gastric cancer cells. The results of transwell migration assay showed that LINC00978 knockdown inhibited the migration abilities of both MGC‐803 and SGC‐7901 cells (Figure 4A). In addition, the depletion of LINC00978 also suppressed the invasion abilities of gastric cancer cells as shown by matrigel invasion assays (Figure 4B). These findings suggest that LINC00978 may enhance gastric cancer metastasis through the promotion of cell migration and invasion.
Figure 4.
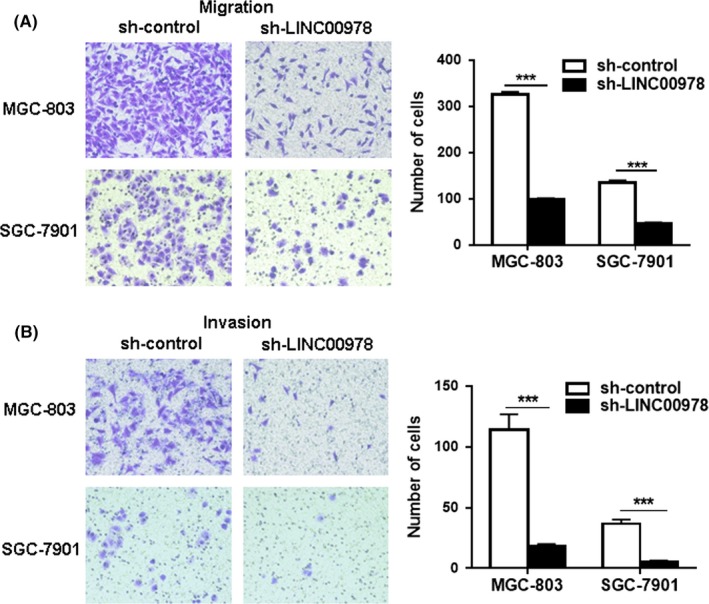
Silencing of LINC00978 inhibits the migration and invasion of gastric cancer cell lines in vitro. (A) Transwell migration assays were performed to investigate the migratory abilities of MGC‐803 and SGC‐7901 cells with LINC00978 knockdown. (B) Matrigel invasion assays for the invasive abilities of sh‐LINC00978 MGC‐803 and SGC‐7901 cells. ***P < 0.001
3.6. LINC00978 knockdown inhibits the activation of TGF‐β/SMAD pathway and suppresses EMT in gastric cancer cells
To elucidate the mechanisms that are responsible for the inhibitory role of LINC00978 in gastric cancer cell migration and invasion, we determined the activation of metastasis‐related pathways in gastric cancer cells with LINC00978 knockdown. As shown in Figure 5A, LINC00978 knockdown downregulated the expression of N‐cadherin, slug and twist genes, while upregulated that of E‐cadherin gene in MGC‐803 and SGC‐7901 cells. The results of western blot also confirmed that LINC00978 knockdown decreased the expression of N‐cadherin, slug, twist and vimentin, while increased that of E‐cadherin in both cells (Figure 5B). In addition, we found that LINC00978 knockdown inhibited the expression of TGF‐β and inactivated SMAD2 pathway in gastric cancer cells (Figure 5C). The expression level of MMP9, an important downstream molecule of TGF‐β/SMAD pathway, was also suppressed by LINC00978 knockdown. Moreover, MGC‐803 and SGC‐7901 cells with LINC00978 knockdown presented an epithelial‐to‐mesenchymal transition in cell morphology (Figure 5D). These data indicate that LINC00978 may activate the TGF‐β/SMAD pathway in gastric cancer cells to induce EMT.
Figure 5.
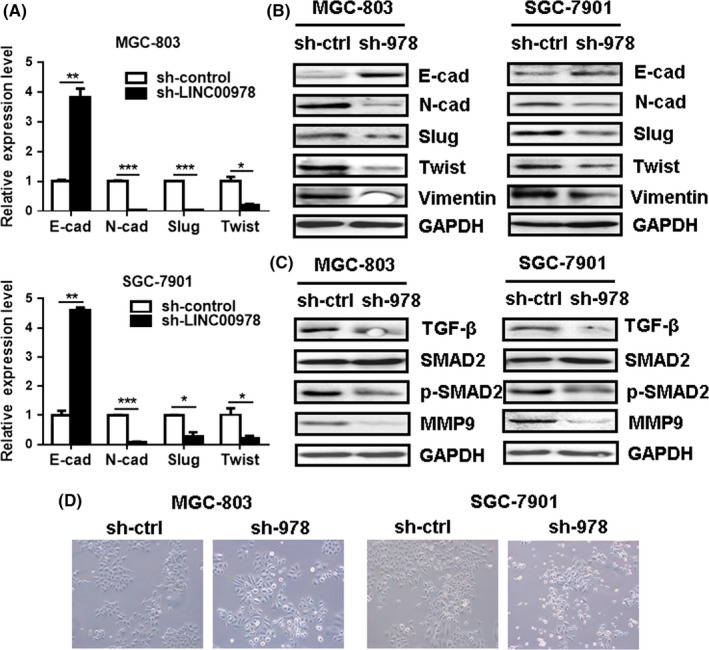
LINC00978 knockdown inhibits the activation of TGF‐β/SMAD pathway and suppresses epithelial‐mesenchymal transition (EMT) in gastric cancer cells. (A) QRT‐PCR and (B) Western blot analyses of EMT‐specific markers in MGC‐803 and SGC‐7901 cells with LINC00978 knockdown. (C) Western blot analysis of TGF‐β, SMAD2, p‐SMAD2 and MMP9 in MGC‐803 and SGC‐7901 cells with LINC00978 knockdown. (D) The morphology of MGC‐803 and SGC‐7901 cells with LINC00978 knockdown
4. DISCUSSION
LINC00978 was first identified in a microarray analysis as one of the mostly altered lncRNAs by resveratrol in lung cancer cells.17 Further study revealed that it may act as an oncogene in lung cancer cells. Deng et al. suggest that LINC00978 can be used as a potential biomarker to predict prognosis in breast cancer patients.18 Recently, LINC00978 was reported as one of the 4 serum lncRNAs that might serve as a panel for gastric cancer diagnosis.19 However, the biological functions and potential underlying mechanisms of LINC00978 in gastric cancer progression remain unclear.
In this study, we first identified LINC00978 as an overexpressed lncRNA in gastric cancer by analysing GEO microarray datasets. We further validated this finding in a cohort of 72 paired tumour and non‐tumour tissues and 4 gastric cancer cell lines. We demonstrated in this study that the expression level of LINC00978 in gastric cancer tissue was associated with tumour size, lymphatic metastasis and TNM stage. Moreover, LINC00978 expression in the serum enabled the discrimination of gastric cancer patients from healthy controls with an AUC of 0.831. Our results are in consistent with that reported in lung cancer and breast cancer, suggesting that LINC00978 may serve as a potential diagnostic and prognostic biomarker for gastric cancer.
We further explored the biological functions of LINC00978 in gastric cancer cells by using shRNA‐mediated gene silencing. The results of cell counting and colony formation assays revealed that LINC00978 knockdown led to a significant inhibition in the proliferation of MGC‐803 and SGC‐7901 cells. Yang et al. reported that LINC0097 knockdown in lung cancer cells inhibited the formation of colonies in soft agar, which might be associated with the induction of cell cycle arrest at G1 phase.17 We found that LINC00978 knockdown also resulted in G1 phase arrest in gastric cancer cells, leading to the inhibition of proliferation. LINC00978 is suggested to regulate multiple cell cycle‐associated genes in lung cancer cells.17 We showed that LINC00978 knockdown upregulated the expression of p21, an important cell cycle regulator, indicating that LINC0097 is critically involved in the regulation of cell cycle progression. We also showed that LINC00978 knockdown induced apoptosis in gastric cancer cells, suggesting that LINC00978 may also promote gastric cancer cell proliferation by protecting them from apoptosis. In support of the in vitro findings, our in vivo animal study data also showed that the tumours generated with sh‐LINC00978 transfected cells had smaller volumes and weights, indicating that the knockdown of LINC00978 inhibits gastric tumour growth in vivo.
About 90% of cancer deaths are caused by metastases in distant organs, making metastasis the major cause of mortality in patients with cancer.20 Deng et al. compared the expression levels of LINC00978 between low‐metastatic and high‐metastatic breast cancer cell lines and found that LINC00978 expression levels were significantly higher in the high‐metastatic cell lines than in the low‐metastasis cell lines.18 However, there was no correlation between LINC00978 expression level and lymph node status in breast cancer patients.18 In this study, we demonstrated that LINC00978 expression level was closely associated with lymphatic metastasis in gastric cancer patients. We further performed functional studies to confirm the potential of LINC00978 in cancer metastasis. Our results revealed that the migration and invasion abilities of gastric cancer cells were significantly inhibited by LINC00978 knockdown. EMT plays a central role in driving cancer metastasis. Many transcription factors can activate EMT, including ZEB, Twist and Snail families.21, 22, 23 We found that LINC00978 knockdown inhibited the expression of Twist1 and Slug (Snail2), leading to the downregulation of N‐cadherin and vimentin but the upregulation of E‐cadherin, indicating that LINC00978 may promote the metastatic potential of gastric cancer cell by inducing EMT. Oncogenic signals, growth factors and inflammatory and hypoxic microenvironment can trigger EMT.24, 25, 26 TGF‐β is one of the best‐studied drivers of EMT.27 We showed that LINC00978 knockdown inhibited the expression of TGF‐β and suppressed the activation of SMAD2 and the expression of MMP9 in gastric cancer cells, suggesting that LINC00978 may promote EMT through the regulation of TGF‐β/SMAD pathway. However, the detailed mechanism by which LINC00978 affects TGF‐β/SMAD pathway warrants further investigation.
In conclusion, we demonstrated in this study the expression pattern, biological function and potential mechanism of LINC00978 in gastric cancer. Our findings partially unveil the roles of LINC00978 in gastric cancer and provide a potential biomarker for the diagnosis and prognosis of gastric cancer.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81572075, 81672416, 81702078, 81702080), the Natural Science Foundation of the Jiangsu Province (BK20141303), Jiangsu Province for Outstanding Sci‐tech Innovation Team in Colleges and Universities (SJK2013‐10), Jiangsu Province's Major Project in Research and Development (BE2015667), the Key Research and Development Project of Zhenjiang (SH2015034, SH2016044), the Medical Science and Technology Development Foundation of Jiangsu University (JLY20160010).
Fu M, Huang Z, Zang X, et al. Long noncoding RNA LINC00978 promotes cancer growth and acts as a diagnostic biomarker in gastric cancer. Cell Prolif. 2018;51:e12425 10.1111/cpr.12425
Contributor Information
Pengcheng Jiang, Email: jpc18906106623@126.com.
Xu Zhang, Email: xuzhang@ujs.edu.cn.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654‐2664. [DOI] [PubMed] [Google Scholar]
- 3. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629‐641. [DOI] [PubMed] [Google Scholar]
- 4. Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435‐1439. [DOI] [PubMed] [Google Scholar]
- 5. Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127:761‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169‐176. [DOI] [PubMed] [Google Scholar]
- 7. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Werven FJ, Neuert G, Hendrick N, et al. Transcription of two long noncoding RNAs mediates mating‐type control of gametogenesis in budding yeast. Cell. 2012;150:1170‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126:2775‐2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Liang G, Yang S, et al. Dysregulated lncRNA‐UCA1 contributes to the progression of gastric cancer through regulation of the PI3K‐Akt‐mTOR signaling pathway. Oncotarget. 2017;8:93476‐93491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan L, Liang W, Fu M, et al. Exosomes‐mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991‐1004. [DOI] [PubMed] [Google Scholar]
- 12. Song YX, Sun JX, Zhao JH, et al. Non‐coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun. 2017;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang K, Shi H, Xi H, et al. Genome‐wide lncRNA microarray profiling identifies novel circulating lncRNAs for detection of gastric cancer. Theranostics. 2017;7:213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qi P, Du X. The long non‐coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155‐165. [DOI] [PubMed] [Google Scholar]
- 15. Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;215:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chandra Gupta S, Nandan Tripathi Y. Potential of long non‐coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int J Cancer. 2017;140:1955‐1967. [DOI] [PubMed] [Google Scholar]
- 17. Yang Q, Xu E, Dai J, et al. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol. 2015;285:79‐88. [DOI] [PubMed] [Google Scholar]
- 18. Deng LL, Chi YY, Liu L, Huang NS, Wang L, Wu J. LINC00978 predicts poor prognosis in breast cancer patients. Sci Rep. 2016;6:37936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ke D, Li H, Zhang Y, et al. The combination of circulating long noncoding RNAs AK001058, INHBA‐AS1, MIR4435‐2HG, and CEBPA‐AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget. 2017;8:21516‐21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 21. Huang R, Zong X. Aberrant cancer metabolism in epithelial‐mesenchymal transition and cancer metastasis: mechanisms in cancer progression. Crit Rev Oncol Hematol. 2017;115:13‐22. [DOI] [PubMed] [Google Scholar]
- 22. Kim NH, Cha YH, Lee J, et al. Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat Commun. 2017;8:14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsen JE, Nathan V, Osborne JK, et al. ZEB1 drives epithelial‐to‐mesenchymal transition in lung cancer. J Clin Invest. 2016;126:3219‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia R, Liang Y, Chen R, et al. Osteopontin facilitates tumor metastasis by regulating epithelial‐mesenchymal plasticity. Cell Death Dis. 2016;7:e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Li W. Epithelial‐mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33‐46. [DOI] [PubMed] [Google Scholar]
- 26. Hwang W, Chiu YF, Kuo MH, et al. Expression of neuroendocrine factor VGF in lung cancer cells confers resistance to EGFR kinase inhibitors and triggers epithelial‐to‐mesenchymal transition. Cancer Res. 2017;77:3013‐3026. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi M, Kizuka Y, Ohtsubo K, Gu J, Taniguchi N. Disease‐associated glycans on cell surface proteins. Mol Aspects Med. 2016;51:56‐70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


