Abstract
Objectives
Lung cancer is still a disease with high morbidity and mortality in the world. MicroRNAs have been proven to act as an indispensable role in the reuse of multiple solid tumours. Although miR‐1258 plays a vital role in suppressing metastasis in breast cancer and gastric cancer, the specific biological function of miR‐1258 in non‐small‐cell lung cancer remains unclear.
Methods
The differential expression of miR‐1258 in NSCLC tissues and corresponding paracancerous tissues was detected by qRT‐PCR and ISH. Flow cytometry and CCK‐8, EdU, tubule formation, and senescence assays were performed, and xenograft models were studied to explore the function of miR‐1258. Potential targets of miR‐1258 were verified by dual luciferase reporter assay, qRT‐PCR, IHC and Western blotting.
Results
In vitro and in vivo gain‐ and loss‐of‐function assays suggested that miR‐1258 inhibits NSCLC cell proliferation and induces senescence and apoptosis. The luciferase reporter assay, IHC and Western blotting analysis showed that GRB2 is one of the direct targets of miR‐1258. The GRB2 overexpression plasmid can reverse the functional changes after overexpression of miR‐1258. In contrast, miR‐1258 inhibitor significantly reversed si‐GRB2‐induced GRB2 down‐regulation. Mechanistically, overexpression of miR‐1258 inhibits GRB2 expression and then leads to inactivation of the Ras/Erk oncogenic pathway.
Conclusions
Our results indicate that miR‐1258 can suppress NSCLC progression by targeting the GRB2/Ras/Erk pathway, which may lead to different insights into potential biomarkers and novel therapeutic strategies for NSCLC patients.
1. INTRODUCTION
Currently, lung cancer is still the leading cause of cancer death in men, and it is the second leading cause of death after breast cancer in women.1 According to the WHO classification, lung cancer is predominantly non‐small‐cell lung cancer (NSCLC) (85%), which is subdivided into adenocarcinoma, squamous cell carcinoma and large cell carcinoma.2 Although much progress has been made in targeted therapy and novel immunotherapy, the 5‐year survival rate of patients is only 17%.3 Hence, it is necessary to further elucidate the molecular mechanisms involved in the development of NSCLC to provide evidence for the treatment of patients with NSCLC.
MicroRNAs (miRNAs) are small RNAs of 21‐25 nucleotides that bind to partially complementary sequences in the 3′‐untranslated region of mRNA (3′‐UTR) and negatively affect post‐transcriptional regulation.4, 5 miRNAs have been found to be dysregulated in a variety of tumours and to regulate cell proliferation, apoptosis, tumorigenesis and multiple signalling pathways, thereby acting as a tumour promoter or tumour suppressor.6, 7 Recent studies have shown that aberrant miRNA expression may play an important role in the development of non‐small‐cell lung cancer.8, 9, 10 Previous studies have found that miR‐1258 can play its role in the regulation of invasion and metastasis by targeting heparanase in gastric cancer; while in breast cancer, miR‐1258 can also inhibit brain metastasis by degrading heparanase.11, 12 Furthermore, in NSCLC, a negative correlation between the expression of miR‐1258 and heparanase was found.13 Despite this, the mechanism of miR‐1258 in the development of NSCLC remains unknown, especially in terms of proliferation, senescence and apoptosis.
In the present study, we found that miR‐1258 is generally expressed at low levels in the cancerous tissues of patients with non‐small‐cell lung cancer. In vitro experiments revealed that miR‐1258 inhibits cell proliferation and promotes cell senescence and apoptosis. The bioinformatics prediction, dual luciferase reporter assay and Western blotting showed that GRB2 was the downstream gene target of miR‐1258 and verified the relationship between GRB2 and the Ras/Erk pathway. Finally, it was demonstrated that miR‐1258 exerts its anti‐tumour effect through the GRB2/Ras/Erk signalling pathway.
2. MATERIALS AND METHODS
2.1. Non‐small‐cell lung cancer tissues and cancer cell lines
Tumour tissue samples and adjacent non‐cancerous tissue samples from a total of 50 patients were tested in this study. All samples were from February 2015 from patients undergoing thoracic surgery at the First Affiliated Hospital of Nanjing Medical University. Patients sampled had no preoperative radiotherapy or chemotherapy, and patients with previous histories of cancer were excluded from the study. All tissue samples are taken intraoperatively, and the samples are quickly frozen in liquid nitrogen until needed. We recorded the clinical characteristics of the patients, including TNM staging based on the AJCC staging system (the 7th edition). The study was approved by the Ethics Committee of Nanjing Medical University, and informed consent was obtained from all patients. Human non‐small‐cell lung cancer cell lines including A549, SPCA1, H1299, H358, PC9, 95D, 16HBE (human bronchial epithelial cells), HUVEC (human umbilical vein endothelial cells) and HEK293 cells were provided by the Shanghai Academy of Sciences. All cells were cultured in RPMI‐1640 medium (Gibco, NY, USA) supplemented with 10% foetal bovine serum (Gibco, NY, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, NY, USA) at 37°C in a humidified cell incubator in a 5% CO2 atmosphere.
2.2. In situ hybridization (ISH) staining
We detected the expression level of miR‐1258 in tissues using digoxigenin‐labelled sense and antisense miR‐1258 probes. The slides were dewaxed and rehydrated before being incubated with proteinase K at 37°C for 15 minutes. Then, the slides were washed 3 times for 15 minutes with 0.1 m TBS/diethyl pyrocarbonate. After incubation with 5x SSC solution for 15 minutes at room temperature, the miR‐1258 probe was added and hybridized overnight at 50°C. The sections were then washed with a gradient diluted SSC solution at 50°C for 30 minutes followed by incubation with anti‐digoxigenin (1:1000) (Roche, Mannheim, Germany) for 2 hours at room temperature. Finally, hybridization signals were visualized by NBT/BCIP (Sigma). The reaction was stopped by washing with water for 5 minutes. The slide was counterstained with haematoxylin, fixed with an aqueous solution, and photographed.
2.3. RNA extraction and quantitative reverse transcription PCR
According to the manufacturer's instructions, TRIzol reagent (Invitrogen, CA, USA) was used to isolate the total RNA from tissues and cells. cDNA was generated using total RNA and the PrimeScript RT reagent (Takara, Kusatsu, Japan), and qRT‐PCR was performed with SYBR Green Master Mix II (Takara) on a ABI 7900 fast real‐time PCR system (ABI, CA, USA). mRNA and miRNA were normalized using GAPDH and small RNA RNU6B (U6) as endogenous controls. The 2−ΔΔCT method was used to quantify the relative levels of miR‐1258 and GRB2 mRNA. Each sample was run in triplicate. The oligonucleotides used in this study are shown in Table 1.
Table 1.
The oligonucleotides used in this study
| Namea | Sequence (5′→3′) |
|---|---|
| miR‐1258 mimics (Sense) | AGUUAGGAUUAGGUCGUGGAA |
| NC (Sense) | ACUACUGAGUGACAGUAGA |
| miR‐1258 F | CTGCGAGTCCCTGGAGTTAG |
| miR‐1258 R | CGGTCCCCTA‐ACTACCCATT |
| U6 F | CTCGCTTCGGCAGCACATATACT |
| U6 R | ACGCTTCACGAATTTGCGTGTC |
| GRB2 F | CCATCGCCAAATATGACTTCAAA |
| GRB2 R | CTTCGTTCAAAACCTTGAGGATGT |
| GAPDH F | AAGGTGAAGGTCGGAGTCA |
| GAPDH R | GGAAGATGGTGATGGGATTT |
F, forward primer; R, reverse primer.
2.4. Transfection of lentivirus, plasmid and small interfering RNA
The lentiviral (LV‐hsa‐miR‐1258‐mimics, LV‐hsa‐miR‐1258‐inhibitor, LV‐NC), plasmid GRB2, empty vector, siRNA‐GRB2 and siRNA‐NC were purchased from Gene Pharma (Shanghai, China). The lipofectamine 3000 reagent (Invitrogen) was used for transient transfection following the manufacturer's instructions.
2.5. Cell proliferation assay
The Cell Counting Kit‐8 (CCK8) assay (Dojindo, Tokyo, Japan) was used to monitor cell proliferation. Transfected cells were seeded into 96‐well plates at 1000 cells/well. Each group was seeded into 3 replicate wells. Prior to observation, each well was treated with 10 μL/well of CCK8 during the last 2 hours of incubation. Finally, the absorbance at 450 nm was used to determine cell viability. The 5‐ethynyl‐2′‐deoxyuridine (EdU) assay (Ribobio, Guangzhou, China) was also used to determine cell capacity. Cells in the logarithmic growth phase were inoculated into a 96‐well plate at 4000‐10 000 cells per well and cultured in the normal growth stage. EdU labelling, cell immobilization, Apollo staining and DNA staining were used according to the instructions, and finally, images were acquired and analysed by fluorescence microscopy. All experiments were performed independently in triplicate.
2.6. Flow cytometric analysis
In the apoptotic assay, transfected cells were collected in flow tubes, washed with pre‐cooled PBS and resuspended 3 times. After staining with the Annexin V‐FITC Apoptosis Detection Kit (Vazyme, Nanjing, China), cells were analysed using flow cytometry (FACScan, BD Biosciences, USA). In the cell cycle experiment, the cells were harvested, and 70% pre‐cooled ethanol was added for times ranging from 2 hours to overnight, and then the cells were stained with propidium iodide (PI) (Vazyme, Nanjing, China) by FACScan flow cytometry for 30 minutes. The DNA content analysis and light scattering analysis were performed using the analysis software.
2.7. Tube formation
Approximately 1 × 104 cells were resuspended in each well of a 48‐well plate containing Matrigel (BD Bioscience, USA). Tube formation was observed with a microscope after 12 hours of incubation. The branch point of the tube structure was quantified according to the manufacturer's instructions. The total tube length in each well was measured and calculated using Image J. Experiments were performed in triplicate.
2.8. Recruitment assay
Recruitment assay was performed using a 6.5 mm chamber with 8 μm pores (Corning, NY, USA). A cell suspension containing 2.5 × 104 HUVECs per mL was prepared in serum‐free medium, and 400 μL of cell suspension was added to the upper chamber. Subsequently, 600 μL of different conditioned media was added to the lower chamber. After 36 hours of incubation at 37°C in a 5% CO2 humidified incubator, cells that did not migrate through the well and remained in the upper chamber were removed by scraping the membrane with a cotton swab. After immobilization with methanol, the migrated cells on the underside of the membrane were stained with 0.1% crystal violet (Beyotime, Shanghai, China) for 30 minutes at 37°C. Three independent experiments were performed.
2.9. Wound‐healing assay
HUVEC cells were evenly plated into 6‐well plates (Corning, NY, USA), and the cells were completely fused after 48 hours. The 6‐well plate was washed twice with PBS and then evenly scratched in the centre by using a sterile 200 μL pipette tip. After 0 and 48 hours, the distance between wounds was measured on the side. Experiments were performed in triplicate.
2.10. SA‐β‐gal staining
SA‐β‐gal staining was performed using the Senescence β‐Galactosidase Staining Kit (Beyotime, Shanghai, China) according to the manufacturer's instruction. Transfected cells were seeded in 6‐well plates. The next day, the cell culture solution was aspirated and washed once with HBSS (Gibco, NY, USA). Next, 1 mL of β‐galactosidase staining fixative was added, and the solution was fixed at room temperature for 15 minutes. After aspirating the cell‐fixing solution, the cells were washed 3 times for 3 minutes each with HBSS. One millilitre of premixed staining fluid was added to each well. The 6‐well plate was incubated overnight at 37°C and observed under a normal light microscope (Nikon, Japan). Ten fields were photographed randomly at 10× magnification. The number of stained cells were counted and expressed as the average number of cells/field of view. The experiments were performed in triplicate.
2.11. Luciferase reporter assay
The 3′‐UTR sequence of GRB2 or the mutated sequence and the predicted target site were inserted into the pmir‐GLO‐promoter vector (Promega, Madison, USA). They were named pmirGLO‐GRB2 WT and pmirGLO‐GRB2‐MUT. HEK293T cells were seeded in 24‐well plates and transfected with 100 ng of pmirGLO‐GRB2 or pmirGLO‐GRB2‐MUT, miR‐1258 mimics and NC with Lipofectamine 3000 (Invitrogen). After 24 hours, the transfected cells were obtained. The relative luciferase activity was measured using a Dual Luciferase Assay Kit (Promega).
2.12. Orthotropic tumour model and xenograft tumour model in Nod/Scid mice
A group of Nod/Scid mice (4‐5 weeks old) were randomly divided into 2 groups. One group was injected on both sides of the armpit with H1299 cells stably transfected with miR‐1258 and NC. The other group was injected with stably transfected SPCA1 cells. Tumour size was measured every 4 days, and tumour size was calculated as length × width × 0.5 (mm3). After 24 days of injection, mice were killed, and tumour nodules were weighed. All animal experiments were conducted under the supervision of the Institutional Animal Protection and Use Committee of Nanjing Medical University.
2.13. Immunohistochemical (IHC) analysis of specimens and xenograft model
All patient samples and mouse tumours were fixed in 4% formalin and then embedded in paraffin. All paraffin sections were then dewaxed and rehydrated. After the blocking of endogenous peroxides and proteins, the slides were incubated with anti‐p‐Erk1/2 and anti‐CD34 (Cell Signalling Technology, MA, USA) or anti‐GRB2 (Abcam, Cambridge, England) overnight at 4°C. After being washed with PBS the next day, the sections were incubated with HRP‐polymer‐conjugated secondary antibodies for 1 hour at 37°C. The sections were stained with DAB solution for 3 minutes and counterstained with haematoxylin. Then, the percentage of positive tumours and cell staining intensity were determined.
2.14. Western blotting analysis
The total protein was extracted from the cells using the RIPA reagent (Beyotime, Shanghai, China) containing 100 μg/mL PMSF (Beyotime, Shanghai, China) and 2 μg/mL aprotinin (Beyotime, Shanghai, China). Proteins extracted from NSCLC cells and tissues were separated by 10% SDS‐polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% skim milk powder for 2 hours, the membranes were incubated overnight at 4°C with the following specific primary antibodies: GRB2 (Abcam, ab32037), Ras (Cell Signalling Technology, 3339), c‐Raf (Abcam, ab173539), P‐c‐Raf (CST, 53745), Mek1/2 (CST, 8727), P‐Mek1/2 (CST, 9154), Erk1/2 (CST, 4695), P‐Erk1/2 (CST, 4370) and GAPDH (CST, 2118). The membrane was then incubated with HRP‐conjugated anti‐rabbit IgG (1: 2000) for 2 hours at room temperature and then washed 3 times with TBST buffer. Bound secondary antibodies were detected by an enhanced chemiluminescence (ECL) system (Pierce Biotechnology, Rockford, USA). GAPDH was used as an internal control.
2.15. Statistical analysis
All experimental data were analysed using GraphPad software 7.0 and spss 19.0 for statistical analysis. The P‐values were analysed using Student's t test, one‐way ANOVA and Spearman's test. P < .05 for the difference was considered statistically significant.
3. RESULTS
3.1. miR‐1258 was down‐expressed in tumour samples and cell lines of non‐small‐cell lung cancer
To clarify the relationship between miR‐1258 and lung cancer, we performed quantitative real‐time PCR (qRT‐PCR) and in situ hybridization staining (ISH) on tumour tissues and corresponding paracancerous tissues from 50 patients with non‐small‐cell lung cancer. As shown in Figure 1A, B, and D, the expression level of miR‐1258 in cancer tissues was lower than that in the adjacent normal tissues. We further compared the expression level in 16HBE cells with those in 5 NSCLC cell lines (A549, SPCA1, H1299, H358, 95D, PC9). Similarly, the expression level of miR‐1258 in non‐small‐cell lung cancer cells was significantly reduced compared to that in 16HBE cells (Figure 1C). In addition, we analysed the correlation between miR‐1258 expression and clinicopathological features (age, sex, smoking history, histological type, tumour size, lymph node metastasis and TMN staging) in NSCLC patients. As shown in Table 2, the expression level of miR‐1258 was negatively correlated with TNM staging, lymph node metastasis and tumour size.
Figure 1.
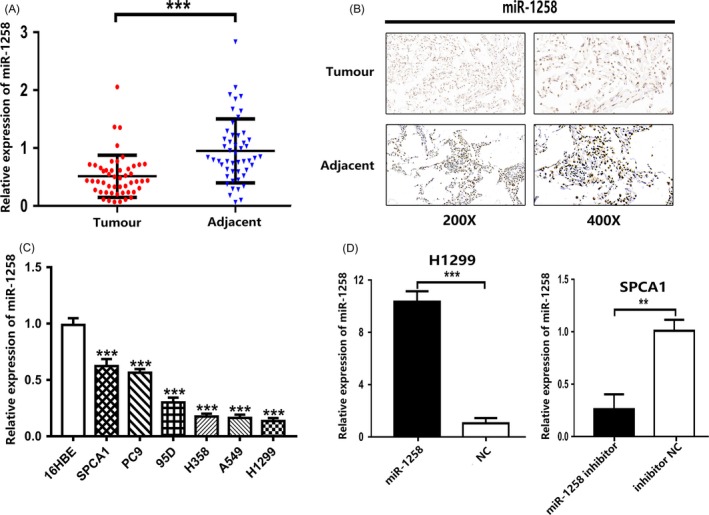
miR‐1258 is down‐regulated in nom‐small‐cell lung cancer tissues and cells. A, The miR‐1258 expression level in 50 NSCLC tissues and paired adjacent tissues was detected by qRT‐PCR. B, ISH of tumour tissue and corresponding paracancerous tissues. C, The expression levels of miR‐1258 in NSCLC cells and 16HBE were detected by qRT‐PCR. D, H1299 cells were transfected with miR‐1258 or NC, SPCA1 cells were transfected with miR‐1258 inhibitor or inhibitor NC, and the expression level of miR‐1258 was detected by qRT‐PCR. The data are represented as mean ± SD. (*P < .05; **P < .01; ***P < .001)
Table 2.
Expression of miRNA‐1258 expression and GRB2 in human NSCLC according to patients’ clinicopathological characteristics
| Factors | miR‐1258 expression | P value | GRB2 expression | P value | ||
|---|---|---|---|---|---|---|
| High | Low | High | Low | |||
| Gender | ||||||
| Male | 16 | 17 | .848 | 18 | 15 | .103 |
| Female | 7 | 10 | 14 | 3 | ||
| Age (y) | ||||||
| ≧60 | 11 | 7 | .189 | 13 | 5 | .547 |
| <60 | 12 | 20 | 19 | 13 | ||
| Smoker | ||||||
| Yes | 8 | 16 | .149 | 17 | 7 | .501 |
| No | 15 | 11 | 15 | 11 | ||
| Histology | ||||||
| LSC | 9 | 6 | .321 | 10 | 5 | .948 |
| LAC | 14 | 21 | 22 | 13 | ||
| Tumour size (cm) | ||||||
| >5 | 3 | 16 | .002a | 16 | 3 | .042a |
| ≦5 | 20 | 11 | 16 | 15 | ||
| Lymph node metastasis | ||||||
| Yes | 9 | 3 | .047a | 6 | 6 | .415 |
| No | 14 | 24 | 26 | 12 | ||
| TNM stage | ||||||
| a‐IIb | 10 | 21 | .028a | 15 | 16 | .008a |
| IIIa | 13 | 6 | 17 | 2 | ||
P < .05 (Chi‐square test).
3.2. miR‐1258 inhibits NSCLC cell proliferation in vitro
To explore the function of miR‐1258 in vitro, we selected H1299 cells for high expression and SPCA1 cells for suppression. LV‐hsa‐miR‐1258‐mimics (miR‐1258) and LV‐NC (NC) were transfected into H1299 cells, while LV‐hsa‐miR‐1258‐inhibitor (miR‐1258 inhibitor) and LV‐NC (inhibitor NC) were transfected into SPCA1 cells. The expression efficiency of miR‐1258 was detected by qRT‐PCR (Figure 1D). CCK‐8 and EdU incorporation assays were used to determine the effect of miR‐1258 on cell proliferation. The results suggested that the overexpression of miR‐1258 significantly inhibited the proliferation of H1299 cells. However, low expression of miR‐1258 significantly promoted the proliferation of SPCA1 cells (Figure 2A,B). Angiogenesis experiments revealed that the overexpression of miR‐1258 inhibited the formation of extravascular blood vessels, while the low expression of miR‐1258 promoted angiogenesis (Figure 2C). To further explore the process of angiogenesis, we conducted recruitment assay and wound‐healing assay and found that the overexpression of miR‐1258 inhibited the migration of HUVECs, whereas low expression promoted the migration (Figure 2D and Figure S1). The correlation of cell cycle arrest induced by proliferation with miR‐1258 overexpression was analysed by flow cytometry. As shown in Figure 2E, H1299 cells transfected with miR‐1258 showed a significant increase in the percentage of cells at the G0/G1 phase, whereas the opposite trend was observed in SPCA1 cells transfected with miR‐1258 inhibitor. Taken together, these results indicate that miR‐1258 inhibits the proliferation of NSCLC cells and increases G0/G1 arrest.
Figure 2.
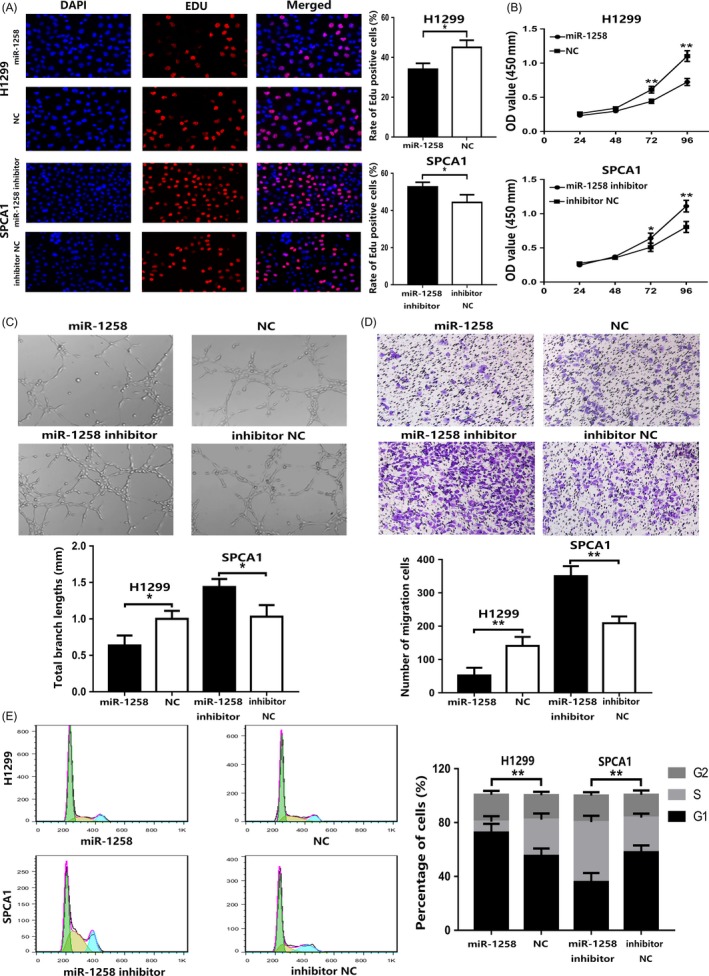
miR‐1258 inhibits NSCLC cell proliferation, angiogenesis and cell cycle progression. A, Compared with the control, Edu cell growth profiles in H1299 cells and SPCA1 cells after transfection with miR‐1258 and miR‐1258 inhibitor, respectively. B, CCK‐8 was used to determine the proliferation of transfected NSCLC cells. C, Effect of miR‐1258 expression on angiogenesis. D, The role of miR‐1258 in the recruitment of HUVECs. E, Effects of miR‐1258 alteration on cell cycle distribution of NSCLC cells. The data are represented as mean ± SD. (*P < .05; **P < .01; ***P < .001)
3.3. miR‐1258 induces senescence and apoptosis in NSCLC cells
Cellular senescence and apoptosis are important causes of tumour suppression. Cellular senescence was revealed by the detection of SA‐β‐gal staining, which is considered a specific marker of senescence. As shown in Figure 3A, H1299 cells transfected with miR‐1258 showed a strong blue SA‐β‐gal staining, while there were only scattered SA‐β‐gal positive cells in miR‐1258 inhibitor group. Flow cytometry analysis of apoptosis was performed to confirm the role of miR‐1258 in apoptosis. H1299 cells transfected with miR‐1258 showed a higher rate of apoptosis than the control group, whereas the opposite tendency was observed in SPCA1 cells transfected with miR‐1258 inhibitor (Figure 3B). On the basis of these findings, we believe that miR‐1258 accelerates the progression of senescence and apoptosis in NSCLC cells.
Figure 3.
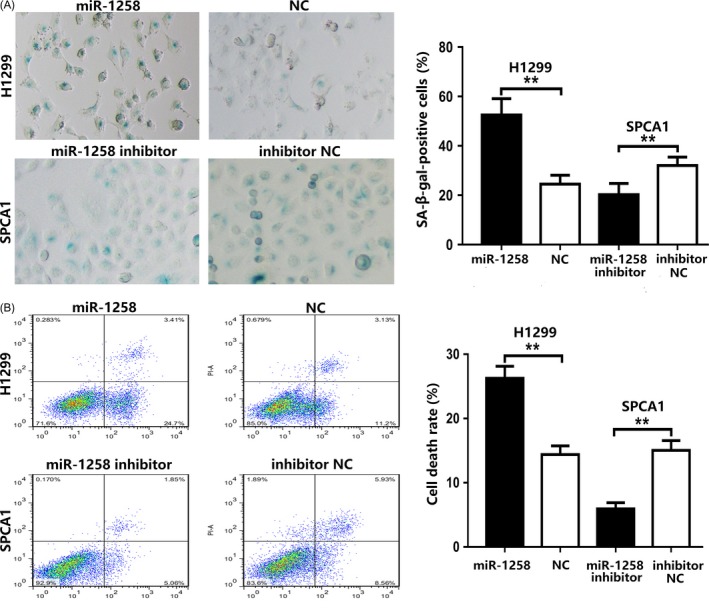
miR‐1258 affects senescence and apoptosis in NSCLC cells. A, Up‐regulation of miR‐1258 promotes cellular senescence by detecting SA‐β‐gal activity, whereas down‐regulation of miR‐1258 suppresses senescence. B, FACS analysis of the effect of miR‐1258 expression alteration on cell apoptosis. The data expressed as the mean ± SD. (*P < .05; **P < .01; ***P < .001)
3.4. miR‐1258 regulates GRB2 by directly targeting the 3′‐UTR
To further explore the downstream regulatory mechanisms of miR‐1258, we identified GRB2 (growth factor receptor binding protein 2) as a potential target for miR‐1258 via an online bioinformatics database (microRNA.org, http://www.microrna.org/). In this study, we found that GRB2 is overexpressed in the cancerous tissues of patients with non‐small‐cell lung cancer and in NSCLC cell lines (Figure 4A,B,C). As shown in Table 2, the expression of GRB2 is related to tumour size and TNM stage. More importantly, GRB2 mRNA levels were negatively correlated with miR‐1258 in 50 NSCLC tissues, with a R 2 of .4119 (Figure 4D). To verify the GRB2 3′‐UTR as a direct target for miR‐1258, the wild‐type (WT) GRB2 3′‐UTR fragment or the MUT was inserted into the pmir‐GLO luciferase miRNA target expression vector (Figure 4E). Pmir‐GLO‐miR‐1258 and GRB2 WT or MUT 3′‐UTR vectors were co‐transfected into HEK293 cells. The relative luciferase activity of the GRB2 WT pmirGLO‐3′‐UTR vector was significantly reduced in HEK293 cells overexpressing miR‐1258, while the luciferase activities of miR‐1258 mimics and mutants were not affected (Figure 4F). The results support that miR‐1258 directly inhibits GRB2 expression by binding the mRNA 3′‐UTR.
Figure 4.
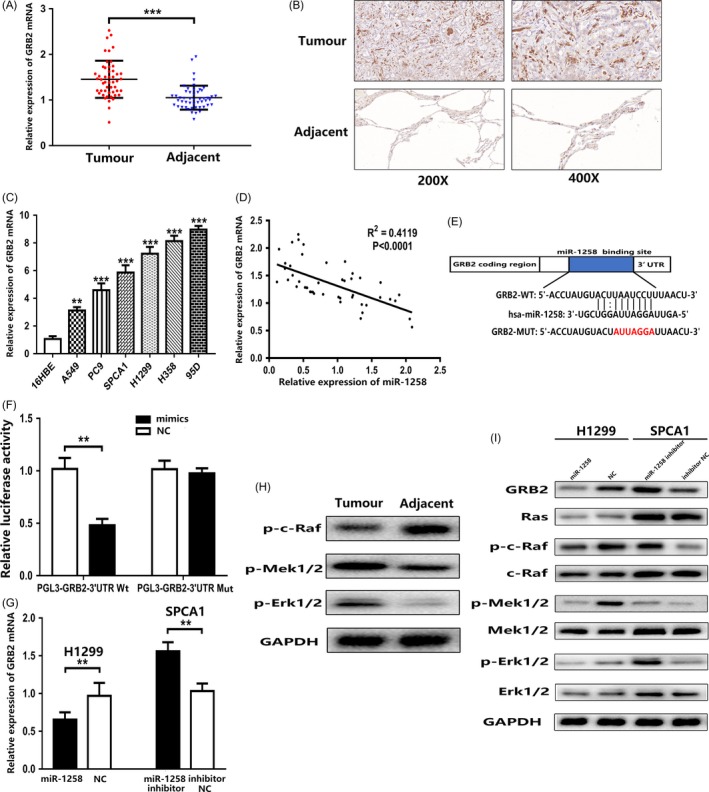
miR‐1258 suppresses GRB2 expression by directly binding its 3′‐UTR and regulating Ras/Erk pathway. A, The GRB2 expression level in 50 NSCLC tissues and adjacent tissues was detected by qRT‐PCR. B, The expression level of GRB2 protein in NSCLC tissues and adjacent tissues was determined by immunohistochemistry staining. C, The expression levels of GRB2 in NSCLC cells and 16HBE were detected using qRT‐PCR. D, Negative correlation between the expression levels of miR‐1258 and GRB2 in NSCLC specimens. E, Luciferase reporter assay was conducted to verify that miR‐1258 is directly bounded to the 3′‐UTR region of GRB2. F, Luciferase activity was analysed in cells co‐transfected with miR‐1258‐mimics or negative control with pmirGLO‐GRB2‐Wt or pmirGLO‐GRB2‐Mut. G, GRB2 mRNA expression levels in transfected H1299 and SPCA1 cells were analysed by qRT‐PCR. H, Ras/Erk pathway‐related phosphorylated protein expression levels were analysed by Western blotting in tissues. I, GRB2 protein and Ras/Erk pathway‐related protein expression levels were analysed by Western blotting in transfected H1299 and SPCA1 cells. GAPDH was used as a control. The data expressed as the mean ± SD. (*P < .05; **P < .01; ***P < .001)
3.5. miR‐1258 suppresses GRB2 expression and regulates the Ras/Erk pathway
Further study found that when miR‐1258 was overexpressed, GRB2 expression decreased; conversely, with low expression levels of miR‐1258, GRB2 expression increased (Figure 4G,I). Previous studies have indicated that GRB2 is a key adapter protein that activates the Ras/Erk pathway.14 At the same time, the expression levels of p‐c‐Raf, p‐Mek1/2 and p‐Erk1/2 were increased in tumour tissues compared with adjacent tissues (Figure 4H). Western blotting results showed that the expression levels of p‐c‐Raf, p‐Mek1/2 and p‐Erk1/2 were significantly decreased when miR‐1258 was overexpressed. However, the expression levels of p‐c‐Raf, p‐Mek1/2 and p‐Erk1/2 were significantly increased in cells with low expression levels of miR‐1258. There was no significant difference in Ras, c‐Raf, Mek1/2 and Erk1/2 expression in either case (Figure 4I). These results indicate that miR‐1258 may be involved in the regulation of the GRB2/Ras/Erk pathway.
3.6. miR‐1258 inhibits proliferation and induces senescence and apoptosis in NSCLC cells via the GRB2/Ras/Erk pathway
To further confirm the role of GRB2 in the miR‐1258‐induced growth inhibition and promotion of senescence and apoptosis, we conducted rescue assays. To up‐regulate GRB2 expression, we stably transfected H1299 cells with the GRB2 plasmid. The mRNA and protein expression levels of GRB2 in the transfected cells were detected by qRT‐PCR and Western blotting. Compared with the control group, the transfection group showed a significantly higher GRB2 expression level. Consistently, GRB2 relative expression levels were down‐regulated with miR‐1258‐down‐regulated expression of si‐GRB2 (Figure 5A,G). Subsequently, EdU incorporation and tubule formation assays and flow cytometry were employed in co‐transfected cells. As shown in Figure 5B, C, and D, up‐regulation of GRB2 effectively counteracted the inhibitory effect of miR‐1258 up‐regulation on H1299 cell proliferation. Similarly, GRB2 down‐regulation in SPCA1 cells reversed the proliferation‐promoting effect of low expression levels of miR‐1258. Moreover, it was found that up‐regulation of GRB2 suppressed cellular senescence and apoptosis, whereas the down‐regulation of GRB2 had an opposite effect (Figure 5E,F). In the meantime, we found that plasmid GRB2‐transfected cells showed an up‐regulation of p‐c‐Raf, p‐Mek1/2 and p‐Erk1/2 compared to the levels in the control group. However, p‐c‐Raf, p‐Mek1/2 and p‐Erk1/2 were down‐regulated in si‐GRB2 transfected cells. Similarly, no significant changes were observed in the expression levels of Ras, c‐Raf, Mek1/2 and Erk1/2 in both groups (Figure 5G). Taken together, our results indicate that the effect of miR‐1258 is mediated through GRB2 down‐regulation and the subsequent inhibition of the Ras/Erk signalling pathway.
Figure 5.
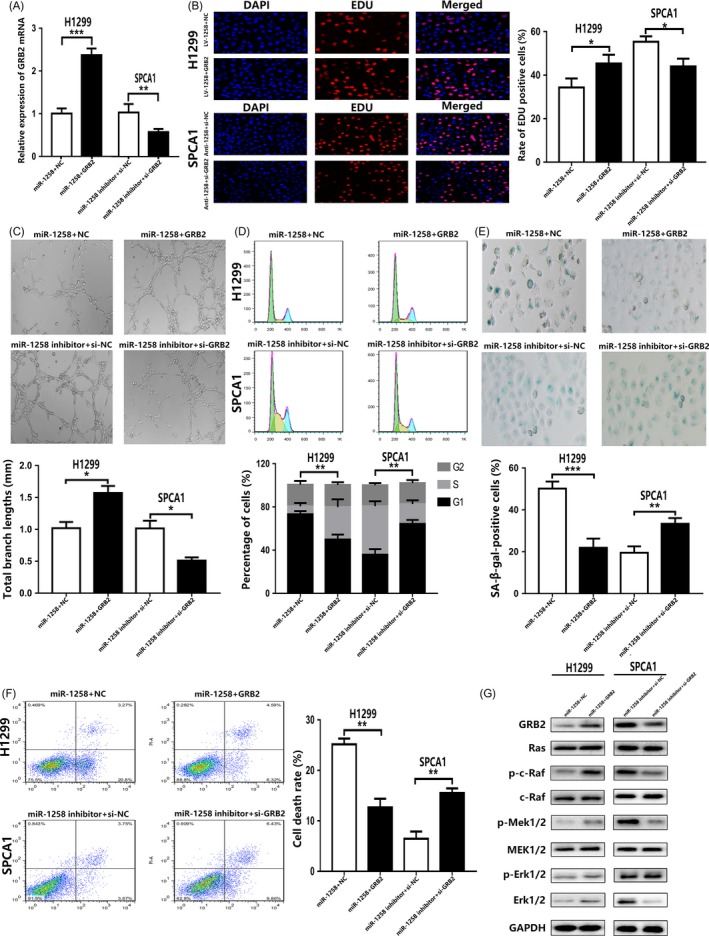
miR‐1258 inhibits proliferation and induces senescence and apoptosis in NSCLC cells by targeting GRB2/Ras/Erk pathway. A, The expression level of GRB2 was verified by qRT‐PCR in co‐transfected cell lines. B, C, D, The effect of inhibiting proliferation of miR‐1258 was reversed by high expression of GRB2, while knockdown of GRB2 enhanced the role of miR‐1258. The roles of miR‐1258 and GRB2 in the regulation of NSCLC cell cycle were verified by flow cytometry. E, F, miR‐1258 up‐regulation or si‐GRB2 induces senescence and apoptosis. G, The expression levels of GRB2 protein and Ras/Erk pathway‐related protein after GRB2 plasmid and si‐GRB2 transfection were analysed by Western blotting in co‐transfected cell lines. The data expressed as the mean ± SD. (*P < .05; **P < .01; ***P < .001)
3.7. Overexpression of miR‐1258 inhibits proliferation of NSCLC cells in vivo
After subcutaneous transfection of lentivirus‐transfected H1299 and SPCA1 cells into Nod/Scid mice, we further evaluated the effect of miR‐1258 overexpression and low expression on the growth rate of NSCLC cells. The results showed that overexpression of miR‐1258 can significantly reduce the growth of tumours in vivo, while the low expression of miR‐1258 has the opposite effect (Figure 6A,B,C). Meanwhile, GRB2 protein expression decreased in the up‐regulated miR‐1258 group and increased in the down‐regulated miR‐1258 group (Figure 6D). The results of the immunohistochemical staining of GRB2, p‐Erk1/2 and CD34 were consistent with miR‐1258 promoting the proliferation of NSCLC (Figure 6E). These observations reveal that miR‐1258 inhibits tumour formation in vivo.
Figure 6.
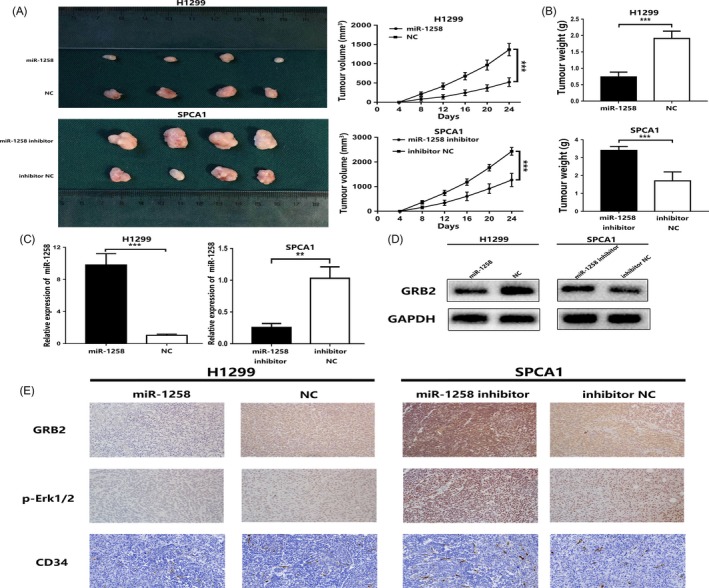
miR‐1258 promotes the tumorigenicity xenograft by targeting GRB2/Ras/Erk pathway in vivo. A, B, Photographs of tumours were obtained from different groups of Nod/Scid mice transfected with miR‐1258, NC, miR‐1258 inhibitor and inhibitor NC, respectively. Growth curve of tumour volumes was calculated. The weight of tumours was measured and analysed. C, The relative expression of miR‐1258 in xenografts was detected by qRT‐PCR. D, The expression levels of GRB2 protein in the implanted tumours were explored by Western blotting. E, Immunohistochemical staining against GRB2, p‐Erk and CD34 assay were used to determine the effects of miR‐1258 expression alteration and GRB2 expression alteration on cell proliferation and angiogenesis in the samples collected from Nod/Scid mice. The data expressed as the mean ± SD. (*P < .05; **P < .01; ***P < .001)
4. DISCUSSION
Lung cancer is still the leading cause of cancer deaths worldwide due to a lack of understanding of the molecular mechanisms of lung cancer development.3 Recently, increasing numbers of studies have revealed that microRNAs function as important diagnostic or prognostic biomarkers for malignancies and have potential as therapeutic targets.5, 15 Meanwhile, the abnormal regulation of miRNAs in NSCLC has also been widely observed, but the specific biological function of most abnormally expressed miRNAs in the proliferation and metastasis of NSCLC remains unclear.8, 9, 10 Recent studies have found that miR‐1258 plays a role in the metastasis of non‐small‐cell lung cancer, but the role of miR‐1258 in cell proliferation, senescence, and apoptosis in NSCLC is unknown.
It has been reported that miR‐1258 can inhibit the migration and metastasis of gastric cancer cells by targeting heparanase.11 In addition, miR‐1258 can inhibit brain metastases in breast cancer.12 In our study, we also found that miR‐1258 levels were significantly reduced in NSCLC tissues and cell lines. The in vitro experiments showed that miR‐1258 overexpression inhibits NSCLC cell proliferation and promotes senescence and apoptosis, but knockdown of miR‐1258 has the opposite effect. Therefore, we hypothesize that miR‐1258 functions as a tumour suppressor in the development of lung cancer. By directly targeting the 3′‐UTR of a gene, miRNAs can inhibit the expression of genes necessary for cellular signalling pathways.16 For example, miR‐6869‐5p inhibits the proliferation of CRC cells by targeting TLR4, and miR‐215‐5p exerts a tumour suppressor effect by targeting EGFR and HOXB9 in colorectal cancer.17, 18 We predicted GRB2 as a downstream target of miR‐1258 using microRNA.org. Studies have found that GRB2 and its signal transduction enhance cell proliferation and cell motility, contributing to the loss control of cell cycle.19 What's more, elevated GRB2 expression is associated with cancer progression and poor prognosis.20 Simultaneously, miR‐411‐5p inhibits the proliferation and metastasis of breast cancer cells by targeting GRB2, whereas miR‐329 limits tumour growth by targeting GRB2 in pancreatic cancer.21, 22 Through the luciferase reporter assay and Western blotting, we confirmed that GRB2 is the target of miR‐1258. We also suspect that GRB2 may regulate a signalling pathway to achieve its function. Earlier studies have found that GRB2, which consists of 2 SH3 domains and one Src homology 2 (SH2) domain, is a receptor tyrosine kinase‐interacting protein that is the key to defining RTK signalling.23 The interaction between GRB2 and SOS and the recruitment of the GRB2‐SOS complex to the plasma membrane to activate the MAPK protein kinase cascade is an important function of GRB2 in cells.24 Consequently, GRB2 is able to link EGFR and downstream Ras/Erk signalling molecules.23 GRB2 promotes tumour cell proliferation and inhibits apoptosis by activating the Ras/Erk pathway, while inhibiting the GRB2/Ras/Erk pathway can limit tumour development by promoting apoptosis.25, 26, 27 In the meantime, there is literature showing that inhibition of the GRB2/Ras/Erk pathway can induce cellular senescence.28, 29 Angiogenesis has long been recognized as a hallmark of cancer and a prerequisite for tumour growth beyond 1‐2 mm in diameter.30, 31 The GRB2/Ras/Erk pathway also plays an important role in angiogenesis.32, 33 At the same time, abnormalities in the GRB2/Ras/Erk pathway promote the development of NSCLC.34, 35 All of this evidence suggests that the GRB2/Ras/Erk pathway acts as a cancer‐promoting agent in the carcinogenesis of a variety of cancers. Thus, we hypothesized that miR‐1258 could inhibit the Ras/Erk pathway downstream by targeting GRB2. Through Western blotting and IHC, we further confirmed the tumour suppressor effect of the miR‐1258/GRB2/Erk axis in NSCLC (Figure 7).
Figure 7.
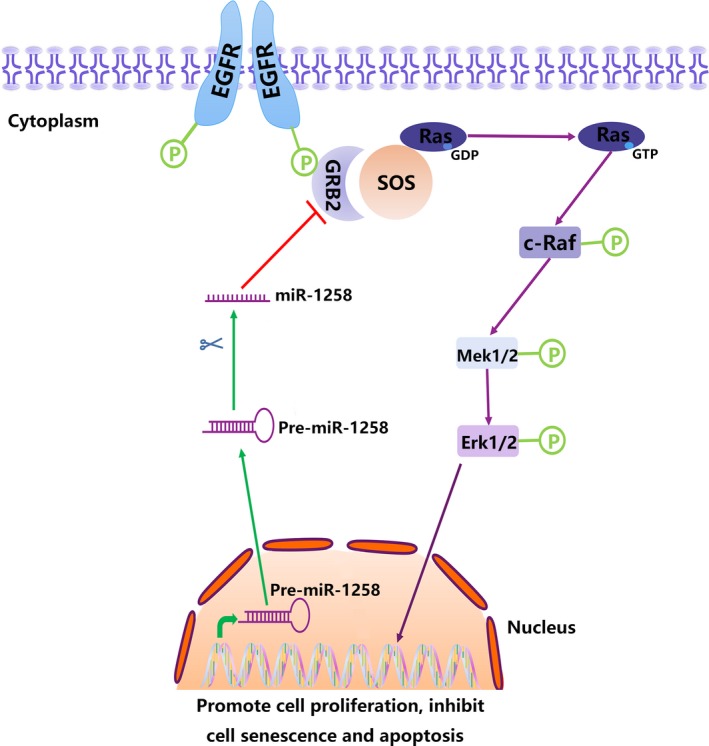
Schematic diagram of miR‐1258/GRB2/Erk pathway
To demonstrate the inhibitory effect of miR‐1258 on the GRB2/Ras/Erk pathway, we transferred plasmids carrying GRB2 into cells that were stably highly expressing miR‐1258. We found that the originally suppressive effect on proliferation was restored, with reduced senescence and apoptosis. In contrast, the group transfected with si‐GRB2 had reduced proliferation and increased senescence and apoptosis. This further confirms the tumour suppressor role of the miR‐1258/GRB2/Erk axis in NSCLC. To explore the function of miR‐1258, we also conducted in vivo experiments. The results suggested that miR‐1258 significantly slowed the growth of the tumour. The effect of miR‐1258 on the GRB2/Ras/Erk pathway was demonstrated by qRT‐PCR, Western blotting and IHC, further supporting the role of the miR‐1258/GRB2/Erk axis in vivo.
Taken together, miR‐1258 inhibits the proliferation and tumorigenesis of NSCLC cells in vitro and in vivo by directly targeting the 3′‐UTR of GRB2 mRNA and regulating Ras/Erk signalling downstream of GRB2. However, the results of this study do not rule out the possibility that other signalling pathways may also be affected by miR‐1258. In addition to Ras/Erk pathway, there may be other mechanisms regulating cell proliferation, senescence and apoptosis. Moreover, whether miR‐1258 can predict the prognosis of patients with cancer still needs to be further studied.
5. CONCLUSION
Our study demonstrated that miR‐1258 plays as a tumour suppressor role in NSCLC. By targeting the 3′‐UTR of GRB2, miR‐1258 can inhibit the proliferation of NSCLC cells and promote their senescence and apoptosis. Although no further studies have identified additional direct targets of miR‐1258 or other signalling pathways in which GRB2 is involved, this preliminary study suggests that recovery of miR‐1258 may be a promising therapeutic option for NSCLC.
AUTHORS’ CONTRIBUTIONS
YJC, WJ, KW, CFP and HL conceived the study. WJ, KW, CFP and HL performed the experiments. JC, XH, SCZ, YZH, YX, YT and LC analysed the data. WJ and WWY wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Jiang W, Wei K, Pan C, et al. MicroRNA‐1258 suppresses tumour progression via GRB2/Ras/Erk pathway in non‐small‐cell lung cancer. Cell Prolif. 2018;51:e12502 10.1111/cpr.12502
Funding information
This work was supported by The National Natural Science Foundation of China (grant no. 81572263), The National Natural Science Foundation of China (grant no. 81702262).
Wei Jiang, Ke Wei, Chunfeng Pan, and Hong Li are the authors contributed equally to this work.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165‐e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 4. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522‐531. [DOI] [PubMed] [Google Scholar]
- 5. Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non‐small cell lung cancer–Recent advances and future perspectives. Int J Cancer. 2016;138:2549‐2561. [DOI] [PubMed] [Google Scholar]
- 6. Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang C, Zhang X, Wang HM, et al. MicroRNA‐18a‐5p functions as an oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis. 2017;8:e2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Y, Ding L, Hu Q, et al. MicroRNA‐218 functions as a tumor suppressor in lung cancer by targeting IL‐6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhuang L, Shou T, Li K, et al. MicroRNA‐30e‐5p promotes cell growth by targeting PTPN13 and indicates poor survival and recurrence in lung adenocarcinoma. J Cell Mol Med. 2017;21:2852‐2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi J, Chen P, Sun J, et al. MicroRNA‐1258: an invasion and metastasis regulator that targets heparanase in gastric cancer. Oncology letters. 2017;13:3739‐3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA‐1258 suppresses breast cancer brain metastasis by targeting heparanase. Can Res. 2011;71:645‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu H, Chen X, Gao W, Jiang G. The expression of heparanase and microRNA‐1258 in human non‐small cell lung cancer. Tumour Biol. 2012;33:1327‐1334. [DOI] [PubMed] [Google Scholar]
- 14. Qu Y, Chen Q, Lai X, et al. SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Mol Cancer. 2014;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levy B, Hu ZI, Cordova KN, Close S, Lee K, Becker D. Clinical utility of liquid diagnostic platforms in non‐small cell lung cancer. Oncologist. 2016;21:1121‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berindan‐Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64:311‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan S, Liu G, Jin C, et al. MicroRNA‐6869‐5p acts as a tumor suppressor via targeting TLR4/NF‐kappaB signaling pathway in colorectal cancer. J Cell Physiol. 2017;233:6660‐6668. [DOI] [PubMed] [Google Scholar]
- 18. Vychytilova‐Faltejskova P, Merhautova J, Machackova T, et al. MiR‐215‐5p is a tumor suppressor in colorectal cancer targeting EGFR ligand epiregulin and its transcriptional inducer HOXB9. Oncogenesis. 2017;6:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giubellino A, Burke TR Jr, Bottaro DP. Grb2 signaling in cell motility and cancer. Expert Opin Ther Targets. 2008;12:1021‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timsah Z, Ahmed Z, Ivan C, et al. Grb2 depletion under non‐stimulated conditions inhibits PTEN, promotes Akt‐induced tumor formation and contributes to poor prognosis in ovarian cancer. Oncogene. 2016;35:2186‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Xu G, Liu G, et al. miR‐411‐5p inhibits proliferation and metastasis of breast cancer cell via targeting GRB2. Biochem Biophys Res Comm. 2016;476:607‐613. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Lu X, Zhang T, et al. mir‐329 restricts tumor growth by targeting grb2 in pancreatic cancer. Oncotarget. 2016;7:21441‐21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowenstein EJ, Daly RJ, Batzer AG, et al. The SH2 and SH3 domain‐containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431‐442. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed Z, Timsah Z, Suen KM, et al. Grb2 monomer‐dimer equilibrium determines normal versus oncogenic function. Nat Commun. 2015;6:7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gril B, Vidal M, Assayag F, Poupon MF, Liu WQ, Garbay C. Grb2‐SH3 ligand inhibits the growth of HER2+ cancer cells and has antitumor effects in human cancer xenografts alone and in combination with docetaxel. Int J Cancer. 2007;121:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li D, Wu LJ, Tashiro SI, Onodera S, Ikejima T. Oridonin‐induced A431 cell apoptosis partially through blockage of the Ras/Raf/ERK signal pathway. J Pharmacol Sci. 2007;103:56‐66. [DOI] [PubMed] [Google Scholar]
- 27. Shan X, Miao Y, Fan R, et al. Suppression of Grb2 expression improved hepatic steatosis, oxidative stress, and apoptosis induced by palmitic acid in vitro partly through insulin signaling alteration. Vitro Cell Dev Biol Anim. 2013;49:576‐582. [DOI] [PubMed] [Google Scholar]
- 28. Liu Q, Xu X, Zhao M, et al. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR‐MEK‐ERK signaling pathway. Mol Cancer Ther. 2015;14:355‐363. [DOI] [PubMed] [Google Scholar]
- 29. Zhang M, Cai S, Zuo B, et al. Arctigenin induced gallbladder cancer senescence through modulating epidermal growth factor receptor pathway. Tumour Biol. 2017;39:1010428317698359. [DOI] [PubMed] [Google Scholar]
- 30. Folkman J. What Is the Evidence That. J Natl Cancer Inst. 1990;82:4‐6. [DOI] [PubMed] [Google Scholar]
- 31. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57‐70. [DOI] [PubMed] [Google Scholar]
- 32. Meadows KN, Bryant P, Pumiglia K. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J Biol Chem. 2001;276:49289‐49298. [DOI] [PubMed] [Google Scholar]
- 33. Simons M, Gordon E, Claesson‐Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611‐625. [DOI] [PubMed] [Google Scholar]
- 34. Swanton C, Govindan R. Clinical implications of genomic discoveries in lung cancer. N Engl J Med. 2016;374:1864‐1873. [DOI] [PubMed] [Google Scholar]
- 35. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


