Abstract
Objectives
Researches showed that stiffness of the extracellular matrix can affect the differentiation of many stem cells. Dental pulp stem cells (DPSCs) are a promising type of adult stem cell. However, we know little about whether and how the behaviour of DPSCs is influenced by stiffness.
Materials and methods
We carried out a study that cultured DPSCs on tunable elasticity polydimethylsiloxane substrates to investigate the influence on morphology, proliferation, osteogenic/odontogenic differentiation and its possible mechanism.
Results
Soft substrates changed the cell morphology and inhibited the proliferation of DPSCs. Expression of markers related to osteogenic/odontogenic differentiation was significantly increased as the substrate stiffness increased, including ALP (alkaline phosphatase), OCN (osteocalcin), OPN (osteopontin), RUNX‐2 (runt‐related transcription factor‐2), BMP‐2 (bone morphogenetic protein‐2), DSPP (dentin sialophosphoprotein) and DMP‐1 (dentin matrix protein‐1). Mechanical properties promote the function of DPSCs related to the Wnt signalling pathway.
Conclusions
Our results showed that mechanical factors can regulate the proliferation and differentiation of DPSCs via the WNT signalling pathway. This provides theoretical basis to optimize dental or bone tissue regeneration through increasing stiffness of extracelluar matrix.
1. INTRODUCTION
In recent years, tissue engineering has been widely focused and considered an important approach to restore damaged tissues and organs.1, 2, 3, 4 The emergence of tissue engineering technology enables the successful isolation of dental pulp stem cells (DPSCs) and the use of DPSCs for repair of bone tissues or dentin‐pulp tissue and even for the construction of natural teeth.5, 6, 7, 8 DPSCs are tissue‐specific stem cells with a high proliferation rate, self‐renewal capability and high accessibility. Studies have demonstrated that DPSCs can differentiate to multiple cell lineages, including osteoblast,9 chondrocytes,10 odontoblasts11, 12 and neuronal cells.13 DPSCs are thought to be excellent cell sources for regeneration therapy of dental diseases and bone defect based on the characteristics mentioned above.
A substantial amount of literature indicates that the extracellular matrix (ECM) can have an impact on cell behaviour through variable signal pathways.14, 15, 16 Increasing research on the influence of extracellular matrix mechanical factors on cell behaviour in the past decades shows that the physical signals of extracellular matrix stiffness can be converted to biochemical signals and to intracellular signals, so as to influence the regulatory role of cells.17, 18 However, there is limited research on whether and how the behaviour of DPSCs can be affected by the mechanical properties of the extracellular microenvironment.
The osteogenic differentiation of osteoblasts (OBs) has shown to be promoted on stiff substrates.19 Softening substrates promote the chondrocyte phenotype for better functionalization via the RhoA/ROCK pathway.20 Neuronal differentiation of MSCs took place after culturing on a relatively soft polyacrylamide hydrogel with stiffness of 0.1‐1 kPa, whereas mesenchymal stem cells (MSCs) show preference to osteogenic lineage with stiffness of 25‐40 kPa and muscle of 8‐17 kPa.21 Besides, substrate stiffness can regulate proliferation of cells.22 We hypothesized that substrate mechanical properties would contribute to the proliferation and osteogenic/odontogenic differentiation of DPSCs.
In our study, we fabricated tunable elasticity polydimethylsiloxane (PDMS) substrates as a research model, mimicking the extracellular microenvironments of DPSCs. PDMS can meet the requirements of nontoxic effect on the cells cultured on it. Moreover, the variation range of PDMS substrates stiffness is wide.20 After DPSCs were seeded on PDMS substrates of different stiffness, we assessed the expression levels of gene and protein markers associated with odontogenesis and osteogenesis. The Wnt signalling pathway played an important role in lots of physiological processes in cells. Thus, we evaluated whether the Wnt signalling pathway participated in the differentiation of DPSCs on various stiffness PDMS substrates via detecting the expression of GSK‐3β (glycogen synthase kinase ‐3β) and β‐catenin. From the results of this experiment, we can have a more particular knowledge of how DPSCs change their biological behaviour when cultured in extracellular matrix with various biophysical properties. Meanwhile, this study may provide a new way to promote DPSCs function, optimizing the design of scaffolds in tissue engineering.
2. MATERIALS AND METHODS
2.1. Dental pulp stem cells culture
Extracted human third molars and premolars were collected to isolate the human DPSCs used in our study, which were donated by the West China Hospital of Stomatology. The third molars were obtained from donors under 25 years old, and the premolars donors were younger than 18 years old. We notified all tooth donors of our experimental purposes and procedures, which were authorized by the Board of Inspection and Survey, and we asked permission from all donors. As for the minor participants, we obtained the consent of their guardians. Dental pulp was obtained in sterile conditions, after washing twice in PBS and cutting into as small fragments as possible; these were digested in 0.5% type I collagenase for 20 minutes. Then, fresh Dulbecco's modified Eagle's medium (DMEM) (low‐glucose DMEM, 0.1 mmol/l non‐essential amino acids, 4 mmol/L L‐glutamine, 1% penicillin‐streptomycin solution; Hyclone, Logan, UT, USA) containing 10% heat‐activated foetal bovine serum (FBS) was used to terminate digestion. The volume of DMEM was equal to that of 0.5% type l collagenase. Tissues and cells were precipitated from the mixture by centrifuging at 10310 g for 5 minutes, then resuspended with DMEM growth medium and transferred to flasks. DPSCs were maintained in DMEM (low glucose) consisting of 10% FBS (foetal bovine serum) and 1% penicillin/streptomycin (PS) at 37°C in moist atmosphere with 5% CO2 for use.
2.2. Fabrication of various stiffness PDMS substrates
Polydimethylsiloxane substrates (Sylgard 184, Corning, NY, USA) were fabricated on Petri dishes. Different proportions of liquid oligomeric base and curing agent (base/curing agent = 10:1, 20:1, 30:1 and 40:1) which were intensively mixing could be cross‐linked to form PDMS substrates for 24 hours at 60°C in an oven. We have measured the Young modulus of all substrates through Universal Testing Machine and stiffness for each was calculated by an equation in our previous study.
Dopamine solution contained dopamine hydrochloride (BioKem, Chengdu, China) (0.2 mg/ml) and tris (hydroxymethyl) aminomethane (Adamas) (1.2 mg/ml), which were dissolved in double‐distilled water. PDMS was soaked in dopamine solution at room temperature for 24 hours twice in order that self‐polymerization of dopamine could be attached to substrate surface to increase the cell adhesion. After that, PDMS substrates were washed, then dried and sterilized via ultraviolet for 1 hour before use.
2.3. Scanning electron microscope
Dental pulp stem cells were seeded on PDMS substrates with stiffness of about 135 kPa (group of 10:1), 54 kPa (group of 20:1), 16 kPa (group of 30:1) and 6 kPa (group of 40:1) for 2 days to observe cell attachment and morphological transformations. DPSCs were immobilization by 2.5% glutaraldehyde for at least 2 hours at 4°C or overnight. Different concentrations of alcohol (30%, 50%, 75%, 85%, 95% and 100%) were used to make DPSCs gradient dehydration for 15 minutes each on the second day. After being cutting to small pieces, the specimens were covered with a thin layer of gold. Finally, we can observe it by scanning electron microscope (SEM).
2.4. Cell proliferation analysis
To assess cell proliferation, Cell Counting Kit‐8 (Sigma, St. Louis, MO, USA) assay was conducted. We seeded cells on the surface of various elastic PDMS substrates in 96‐well plates at a density of approximately 2000 per well, culturing in DMEM growth media for 7 days, during which the culture media was changed every 3 days. The assay was carried out on the first, third, fifth and seventh days after seeding, which was performed according to the manufacturer's instructions. BioTek ELX800 (Bio‐Tek, Winooski, VT, USA) was taken to determine absorbance of CCK‐8 solutions at 450 nm.
2.5. Odontogenic/osteogenic differentiation of DPSCs
Dental pulp stem cells were induced to differentiate to osteoblasts‐like or odontoblast‐like cells by converting to osteogenic/odontogenic medium, comprising of DMEM (low glucose), 10% FBS, 1% PS, 10 mmol L−1 β‐glycerophosphate (Sigma), 50 μg/mL ascorbic acid 2‐phosphate (Sigma) and 10−7 mol L−1 dexamethasone. In each experiment, DPSCs were seeded onto different ratios of PDMS substrates (10:1, 20:1, 30:1, 40:1) for a specified period of time according to different assays with medium replaced every third day.
2.6. Polymerase chain reaction assays
DPSCs were seeded onto 6‐well plates covered with 4 different elastic substrates at 5 × 104/well, culturing with odontogenic/osteogenic medium. Total RNA of DPSCs was extracted using TRIzol reagent according to the manufacturer's protocol. After purification, TAKARA Reverse Transcriptase kit (TAKARA, Osaka, Japan) was used to perform the reverse‐transcriptional reactions. PCR was then proceeded with PrimeScript™ RT‐PCR kit (Takata, Tokyo, Japan) or ABI 7300 (Applied Biosystems, Shanghai, China). The primers are shown in Table 1. RT‐PCR amplification conditions followed the manufacturer's instructions. Mean data of each group were first quantified relative to GAPDH, and then normalized to that of group 10:1. Experiments were performed in triplicate for each sample.
Table 1.
Sequences of forward and reverse primers of selected genes designed for q‐PCR
| mRNA | Primer pairs |
|---|---|
| GAPDH |
Forward: CAGGGCTGCTTTTAACTCTGG Reverse: TGGGTGGAATCATATTGGAACA |
| ALP |
Forward: ACTGGTACTCAGACAACGAGAT Reverse: ACGTCAATGTCCCTGATGTTATG |
| Runx2 |
Forward: TGGTTACTGTCATGGCGGGTA Reverse: TCTCAGATCGTTGAACCTTGCTA |
| OCN |
Forward: AGCCCATTAGTGCTTGTAAAGG Reverse: CCCTCCTGCTTGGACACAAAG |
| OPN |
Forward: GAA GTT TCG CAG ACC TGA CAT Reverse: GTA TGC ACC ATT CAA CTC CTC G |
| BMP‐2 |
Forward: ACT ACC AGA AAC GAG TGG GAA Reverse: GCA TCT GTT CTC GGA AAA CCT |
| DSPP |
Forward: AAAGTGGTGTCCTGGTGCAT Reverse: CCTGGATGCCATTTGCTGTG |
| DMP‐1 |
Forward: TTCCTCTTTGAGAACATCAACCTG Reverse: ACTCACTGCTCTCCAAGGGT |
2.7. Immunofluorescence
DPSCs on different stiffness substrates were rinsed slightly with PBS for 3 times. Then, freshly prepared 4% paraformaldehyde was added to Petri dish for cell fixation. DPSCs were permeabilized by 0.5% Triton‐100 for 15 minutes, and washed with PBS. After being blocked with 5% sheep serum for 1 hour at 37°, samples were incubated with primary antibody (Abcam, Cambridge, MA, USA) overnight at 4°C. After standing at room temperature for about half an hour, samples were washed by PBS. Alexa Fluor 594 donkey anti‐rabbit, secondary antibody at a concentration of 1:500, was added for cell incubation lasting 1 hour at 37°C. Cell nuclei were stained with the DAPI (Sigma‐Aldrich) for 10 minutes. Images were photographed under fluorescent microscopy.
2.8. Western blot analyses
After culturing on different elastic substrates with odontogenic/osteogenic medium, total cellular protein was collected via lysing in lysis buffer, then collecting the supernatant after centrifuging the lysates at 10310 g for 5 minutes. After being boiled for 5 minutes, the concentration of the protein samples was measured by bicinchoninic acid assay. Proteins were segregated via SDS‐PAGE, then being moved to polyvinylidene fluoride membrane (Bio‐Rad, Munich,Germany) by electrophoresis, and sealed in 5% bovine serum albumin for 45 minutes. Afterwards, membranes were incubated overnight with the individual primary antibody at 4°C. After 3 washes with TBST, membranes were immersed in appropriate secondary antibody at room temperature for 1 hour. The labelled proteins were visualized using the enhanced chemiluminescence reagent (Thermo Scientific, Chelmsford, MA, USA) and exposed to Investigator Proimage (Bio‐Rad).
2.9. Statistical analyses
Results are revealed as mean ± SD from experiments conducted at least 3 times independently and analysed by 1‐way ANOVA with SPSS 21.0. When the 2‐tailed P values were <.05, data were considered statistically.
3. RESULTS
3.1. Cell morphology on elastic PDMS substrates
Stiffness of substrates increased with reducing base to curing proportions. The stiffness of 10:1, 20:1, 30:1 and 40:1 groups was about 135, 54, 16 and 1.4 kPa according to our previous study.20 Using SEM, we observed that DPSCs on matrices with different stiffness had distinct morphologies. DPSCs on stiffer PDMS substrates with stiffness of 135 kPa, 54 kPa (10:1, 20:1) are located on the surface, in a more tridimensional way, compared to those on softer matrices. On the softer matrices (30:1, 40:1), cells sagged into the matrix, especially in the softest substrates with stiffness of 1.4 kPa (Figure 1A). From these SEM images, we discovered that mechanical properties of the surrounding microenvironment influence cell morphology, leading us to explore the microenvironmental influence on cell function.
Figure 1.
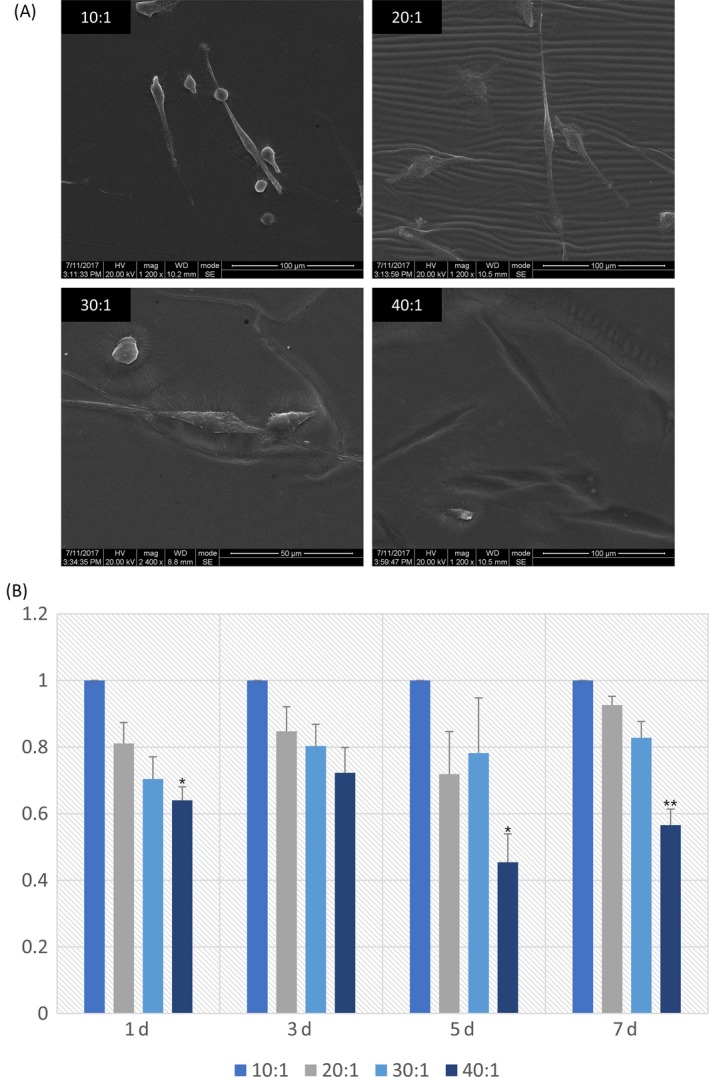
A, SEM images of DPSCs cultured on varying elastic PDMS materials. Scale bars in 10:1, 20:1 are 100 μm, and in 30:1, 40:1 are 50 μm. B, CCK‐8 assay was carried out on the first, third, fifth and seventh day after seeding to detect the proliferation of DPSCs plated on various elasticity substrates. The results were calculated and represented by histogram. Data are normalized to that of group 10:1. The results shown are representative of 3 different samples (n = 5). Data are presented as mean ± SD. Statistical analysis: *P<.05,**P<.01, ***P<.001
3.2. Rigid substrates promoted proliferation of DPSCs
Given tunable elasticity PDMS substrates were fabricated successfully by the mixture of base and curing agent in different proportions, DPSCs were seeded onto 4 different stiffness substrates. The proliferation of DPSCs was quantified by performing CCK‐8 assay. Results showed that the multiplication rate of cells in group 10:1 was significantly faster than that in group 20:1, 30:1 and 40:1 (Figure 1B). Furthermore, the rigidity of the matrix increases with increasing growth rate of DPSCs.
3.3. Rigid substrates induce more DSPP and DMP‐1 expression of DPSCs
QRT‐PCR was used to investigate the odontogenic differentiation of DPSCs at 7 and 14 days post‐seeding. Results of the assay indicated that the expression levels of DSPP and DMP‐1 statistically decreased as PDMS substrates became soft (Figure 2A). We assessed protein expression of DSPP and DMP‐1 in DPSCs induced for 14 days and 21 days by Western blot assays. It showed the same trend that protein expressions distinctively increased on rigid substrates (Figure 2B). In addition, immunofluorescent staining of DSPP and DMP‐1 was conducted after culturing on PDMS substrates for 7 days, showing that stronger expression was observed on rigid substrates (Figure 2C). All tests mentioned above showed that odontogenic differentiation of DPSCs was increased by matrix stiffness.
Figure 2.
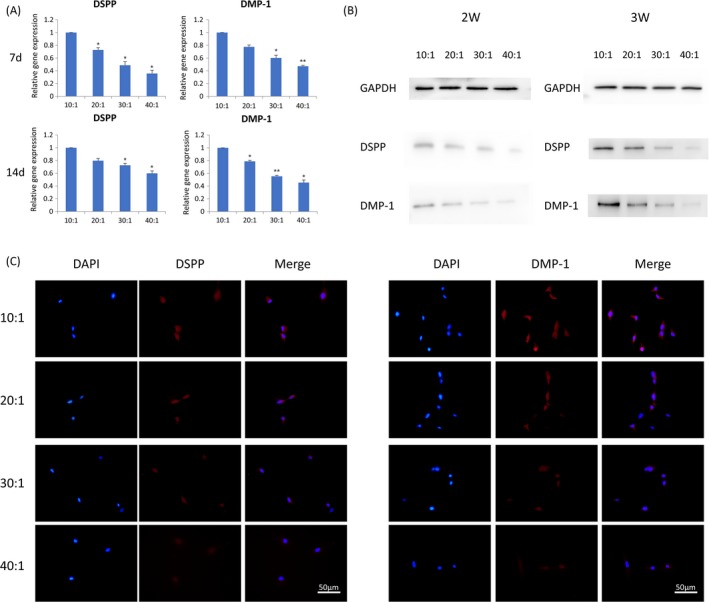
Rigid substrates promote odontogenic differentiation. A, Transcriptional levels of DSPP andDMP‐1 in DPSCs at 7 days and 14 days post‐seeding by q‐PCR. Data have been firstly normalized to GAPDH, and then normalized to that of group 10:1. The results shown are representative of 3 different samples (n = 3). Data are presented as mean ± SD, *P<.05,**P<.01. B, Protein expression of DSPP and DMP‐1 was measured by Western blotting in DPSCs after culturing for 14 days and 21 days. C, Immunofluorescence of DSPP and DMP‐1 expressed in DPSCs cultured on different elastic substrates (from 10:1 to 40:1). Scale bars are 50 μm
3.4. Osteogenic differentiation of DPSCs is regulated by matrix stiffness
The expression levels of ALP (alkaline phosphatase), OPN (osteopontin), RUNX‐2 (runt‐related transcription factor‐2), BMP‐2 (bone morphogenetic protein‐2), OCN (osteocalcin) were highest in group 10:1 comparing those in group 20:1, 30:1 and 40:1 in semi‐quantitative polymerase chain reaction (PCR) induced by osteogenic medium (Figure 3A). Grayscale analysis of semi‐quantitative bands showed a statistically significant difference between group 10:1, 20:1, 30:1 and 40:1 at day 3 (Figure 3B) and day 7 (Figure 3C). The data analysis results of the 5 marker gene transcriptional levels in qRT‐PCR induced for 3 days (Figure 4A) and 7 days (Figure 4B) were similar to that in semi‐quantitative PCR. The protein expression of OPN was evaluated 14 and 21 days after osteogenic induction. There was a trend that when the stiffness of PDMS substrates increased, the protein expression also increased (Figure 4C). After DPSCs were induced for 3 days, the strongest immunofluorescence of OPN and RUNX‐2 in group 10:1 was observed (Figure 5). The strengthened osteogenic differentiation potential of DPSCs on a stiffer matrix can be concluded from the above results.
Figure 3.
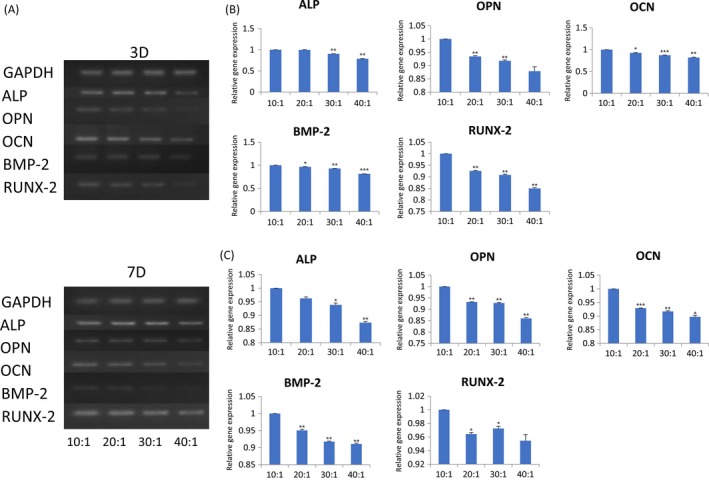
More osteogenic‐related genes expressed on stiffer substrates. A, The gene expression levels of ALP,OPN,OCN,BMP‐2 and RUNX‐2 measured by semi‐quantitative real‐time polymerase chain reaction analysis in DPSCs. B, Transcriptional levels of ALP, OPN,OCN,BMP‐2 and RUNX‐2 of DPSCs culturing on PDMS substrates for 3 days were calculated and represented by histogram. Data have been firstly normalized to GAPDH, and then normalized to that of group 10:1. Data are presented as mean ± SD (n = 3). Statistical analysis:*P<.05,**P<.01,***P<.001. C, The greyscale value of semi‐quantitative bands in DPSCs after seeding 7 days were calculated and represented by histogram
Figure 4.
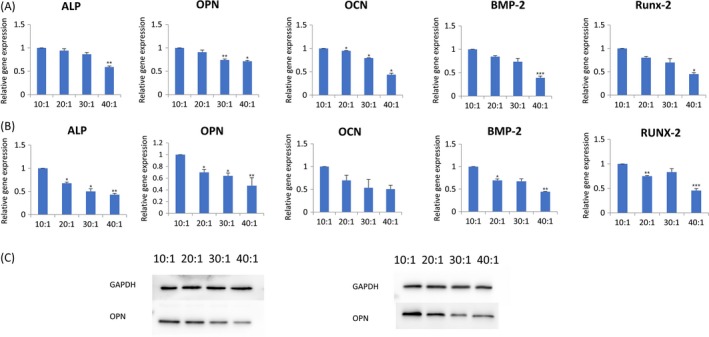
DPSCs preferred osteogenic differentiation on rigid substrates. A, The expression levels of ALP,OPN,OCN,BMP‐2 and RUNX‐2 were analysed by q‐PCR at 3 days. Data have been firstly normalized to GAPDH, and then normalized to that of group 10:1. Data are presented as mean ± SD (n = 3). Statistical analysis:*P<.05,**P<.01,***P<.001. B, The expression levels of ALP,OPN,OCN,BMP‐2 and RUNX‐2 were analysed by q‐PCR at 7 days. C, Protein expression of OPN measured by Western blotting in DPSCs after culturing for 14 days and 21 days
Figure 5.
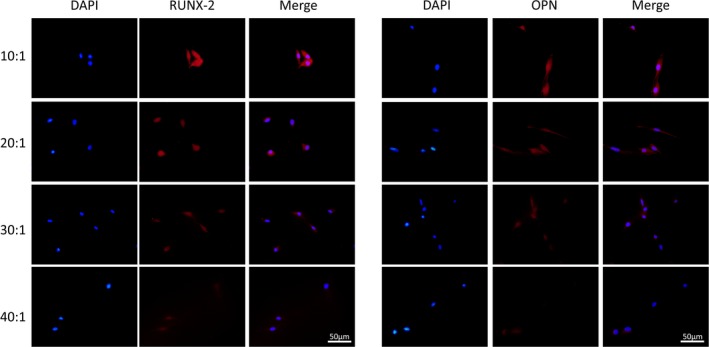
Substrates stiffness modulated the protein expression of RUNX‐2 and OPN. Immunofluorescence of runx‐2 and OPN was observed in DPSCs cultured on different elastic substrates (from 10:1 to 40:1). Scale bars are 50 μm
3.5. Substrate stiffness regulates differentiation of DPSCs through canonical WNT signalling pathway
For the sake of determining whether the Wnt pathway played a role in DPSCs differentiation in response to elastic PDMS substrates, Western blot and immunofluorescence analyses were performed. The protein expressions of β‐catenin were increased in a stiffness‐dependent manner, while the protein GSK‐3β was found to have suppressed expression on rigid substrates after 14‐day (Figure 6A) and 21‐day (Figure 6B) culture of DPSCs. In addition, when paired with the mixture ratios of base to curing agent increasing, enhanced immunofluorescence intensity of β‐catenin was observed in DPSCs at 7 days post‐seeding. The most intense immunofluorescence of GSK‐3β was found in group 40:1, that is the softest substrate in the experiment (Figure 6C). The assays indicate that rigid matrix promotes osteogenic/odontogenic differentiation in DPSCs via the canonical Wnt signalling pathway.
Figure 6.
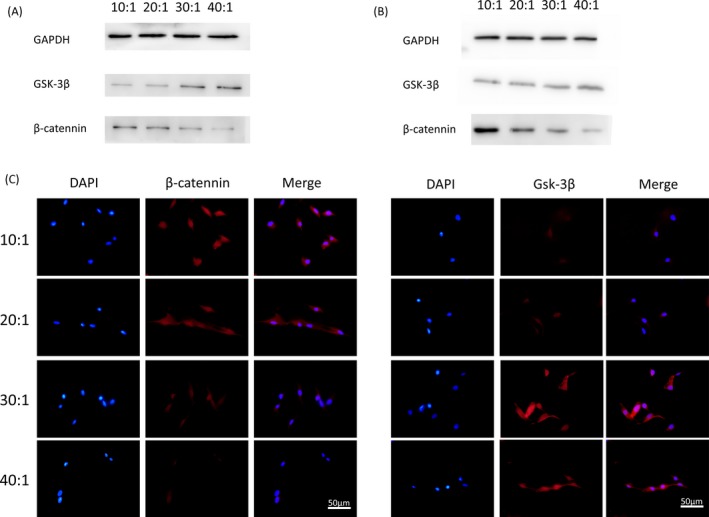
A, Protein expression of β‐catenin in DPSCs plated on different elastic substrates culturing for 14 days. B, Protein expression of Gsk‐3β measured by Western blotting in DPSCs seeded on different elastic substrates culturing for 21 days. C, Immunofluorescence of β‐catenin and Gsk‐3β in DPSCs seeded on substrates with different stiffness for 7 days. β‐catenin (red), cell nuclei (blue). Scale bars are 50 μm
4. DISCUSSION
Our study indicated that DPSCs were more likely to exhibit osteoblastic/odontogenic differentiation and proliferation when seeded on stiff substrates. Based on the favourable cytocompatibility and mechanical properties, like cross‐linking degree and stiffness of 2D PDMS materials, we found out that as substrates stiffness increased, the expression levels of marker genes and proteins related to odontogenic differentiation of DPSCs were upregulated. The potential mechanism of stiffness‐dependent osteogenic/odontogenic differentiation and proliferation was explored, and found to be linked to the canonical Wnt pathway.
Dental pulp stem cells were proved significantly useful in many clinical applications.23 It is important to study the factors—which many researchers have explored24—that affect the biological behaviour of DPSCs. Moreover, there has been an increased focus on the design of scaffolds for dental pulp tissue regeneration or bone tissue engineering.25, 26 However, the design of an overwhelming majority of these scaffolds was focused on the matrix architecture or composition to enhance odontogenic differentiation.27, 28 It remains an unclear subject whether and how the stiffness of the substrates influences the odontogenic differentiation of DPSCs. After the landmark article of Engler et al21 was published, more and more research on the effects of mechanical properties on stem cells was conducted, all certifying that the matrix elasticity impacts the behaviour of different stem cells.19, 20 Hence, the aim of our experiment was to identify the influence of tunable stiffness PDMS substrates on the proliferation and differentiation of DPSCs.
Two‐dimensional (2D) substrates29 with cell seeding on the surface, and three‐dimensional (3D) materials30, 31 with cells encapsulated in them are currently widely used. Although 3D materials have a better spatial construction similar to the growth environment of cells in vivo, it is difficult to achieve a high degree of rigidity. Hence, in this study, we chose the well‐known PDMS substrates, a type of 2D culture, to explore cell‐matrix interactions. Previous literature had shown that the surface roughness of PDMS substrates were 55.67, 53.38, 50.95 and 47.32 nm with the stiffness of 135, 54, 16 and 6 kPa, and statistical differences were found in each 2 groups. But the difference in roughness of PDMS material surface less than 20 nm barely affected cell mechanosensitivity. In another word, it enabled that the effects of stiffness on DPSCs were investigated independently.20 Our results demonstrated obvious variation in DPSCs morphology cultured on elastic substrates. This can be accounted for the dynamic equilibrium between the contraction force of cells and the constrain tension given by matrices in the process of mechanical sensing.
Cell biological function is closely related to cell morphology.32 Remarkably, the proliferation and osteogenic/odontogenic differentiation of DPSCs were regulated. Our study indicated that less proliferation was observed on soft substrates (30:1, 40:1). Interestingly, it was contrary to another study reporting the highest cross‐linked PF‐2.5 hydrogel, namely the stiffest substrate, inhibited cell proliferation.33 This may be due to the fact that the article did not describe a complete quantitative analysis of cell proliferation, but only a qualitative speculation. As to cell differentiation, a trend of increased marker genes and protein expression was noticed with the stiffness increased.
When DPSCs were used as seed cells in tissue engineering, the variation of scaffold materials34, 35 may likewise result in different ECM stiffness. In the present study, different types of cells performed their functions optimally in extracellular matrices with different stiffness.20, 21 Analysis of our experimental data showed it is possible to increase the stiffness of the scaffold material to enhance the osteogenic/odontogenic differentiation of DPSCs, so as to promote the repair of bone defect and the formation of dentin.
The Wnt/β‐catenin signalling pathway involved in biological behaviours of many cells.36 Hunter et al37 announced the Wnt/β‐catenin pathway had an effect on osteoblast and chondrocyte differentiation of MSCs. Tao et al38 found berberine‐induced odontogenic differentiation of MSCs in vitro through the Wnt/β‐catenin signalling pathway. The Wnt pathway is activated in dental epithelium cells and mesenchymal cells, and participates in the formation of dental crown, root and periodontal tissue in the course of tooth morphogenesis.39 It had been demonstrated to influence the biological functions of stem cells from the apical papilla (SCAP).40 Jun activation domain‐binding protein 1 (JAB1) accelerated the odontogenic differentiation of DPSCs via activating the Wnt/β‐catenin pathway.41 In our study, the expression of β‐catenin was significantly upregulated in DPSCs growing on stiffer substrates in the process of differentiation. The expression trend of Gsk‐3β was totally opposite to that of β‐catenin. The results of Western blot analysis of the 2 important members of the Wnt/β‐catenin canonical signalling pathway were consistent with those of immunofluorescence. The above results indicated the possibility that stiffer substrates induced the differentiation through the Wnt/β‐catenin canonical signalling pathway.
Both the influence of stiffness on the behaviour of DPSCs and its possible mechanism were investigated in our study. However, the study has some limitations. Firstly, how the mechanical signal of ECM transformed into cellular biophysical signal influencing the cell behaviour needs to be carefully figured out. Secondly, 2D format material was performed to imitate the ECM experienced by DPSCs in vivo. There still are distinctions between PDMS substrate and the ECM in vivo. More well‐designed materials, including some 3D hydrogels meeting the requirements of high stiffness for cell‐matrix study in dental or bone biology should be evaluated. In summary, physical properties like stiffness of extracellular matrix are an important factor, which should be taken into account in tissue engineering.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (81771125, 81471803) and Sichuan Province Youth Science and Technology Innovation Team (2014TD0001).
Liu N, Zhou M, Zhang Q, et al. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018;51:e12435 10.1111/cpr.12435
REFERENCES
- 1. Shao X, Lin S, Peng Q, et al. DNA nanostructures: tetrahedral DNA nanostructure: a potential promoter for cartilage tissue regeneration via regulating chondrocyte phenotype and proliferation (Small 12/2017). Small. 2017;13:1602770. [DOI] [PubMed] [Google Scholar]
- 2. Zhong J, Guo B, Jing X, et al. Crosstalk between adipose‐derived stem cells and chondrocytes: when growth factors matter. Bone Res. 2015;4:15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi S, Qiang P, Shao X, et al. Self‐assembled tetrahedral DNA nanostructures promote adipose‐derived stem cell migration via lncRNA XLOC 010623 and RHOA/ROCK2 signal pathway. ACS Appl Mater Interfaces. 2016;8:19353. [DOI] [PubMed] [Google Scholar]
- 4. Shi S, Lin S, Shao X, Li Q, Tao Z, Lin Y. Modulation of chondrocyte motility by tetrahedral DNA nanostructures. Cell Prolif. 2017;50:e12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tirino V, Paino F, D'Aquino R, Desiderio V, Rosa AD, Papaccio G. Methods for the identification, characterization and banking of human DPSCs: current strategies and perspectives. Stem Cell Rev. 2011;7:608. [DOI] [PubMed] [Google Scholar]
- 6. Horibe H, Murakami M, Iohara K, et al. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS ONE. 2014;9:e98553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paino F, La NM, Giuliani A, et al. hDPSCs fabricate a vascularised woven bone tissue: a new tool in bone tissue engineering. Clin Sci. 2017;131:CS20170047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phung S, Lee C, Hong C, et al. Effects of bioactive compounds on odontogenic differentiation and mineralization. J Dent Res. 2017;96:107‐115. [DOI] [PubMed] [Google Scholar]
- 9. Alkhalil M. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Medicinski Glasnik. 2015;12:27‐32. [PubMed] [Google Scholar]
- 10. Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813. [DOI] [PubMed] [Google Scholar]
- 11. Bakopoulou A, Leyhausen G, Volk J, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56:709. [DOI] [PubMed] [Google Scholar]
- 12. Yu J, Deng Z, Shi J, et al. Differentiation of dental pulp stem cells into regular‐shaped dentin‐pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006;12:3097. [DOI] [PubMed] [Google Scholar]
- 13. Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435. [DOI] [PubMed] [Google Scholar]
- 14. Maqsood A, Charles FC. Extracellular matrix regulation of stem cell behavior. Curr Stem Cell Rep. 2016;2:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Lin S, Zhang T, et al. Curved microstructures promote osteogenesis of mesenchymal stem cells via the RhoA/ROCK pathway. Cell Prolif. 2017;50 10.1111/cpr.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochem Biophys Acta. 2014;1840:2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye K, Wang X, Cao L, et al. Matrix stiffness and nanoscale spatial organization of cell‐adhesive ligands direct stem cell fate. Nano Lett. 2015;15:4720. [DOI] [PubMed] [Google Scholar]
- 18. Oh SH, Dan BA, Kim TH, Jin HL. Wide‐range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016;35:23. [DOI] [PubMed] [Google Scholar]
- 19. Zhang T, Lin S, Shao X, et al. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017;50:e12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang T, Gong T, Xie J, et al. Softening substrates promote chondrocytes phenotype via RhoA/ROCK pathway. ACS Appl Mater Interfaces. 2016;8:22884. [DOI] [PubMed] [Google Scholar]
- 21. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2016;126:677‐689. [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Sun M, Tan Y, et al. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation. 2017;96:30. [DOI] [PubMed] [Google Scholar]
- 23. Lee SM, Zhang Q, Le AD. Dental Stem Cells: Sources and Potential Applications. Curr Oral Health Rep. 2014;1:34‐42. [Google Scholar]
- 24. Zavatti M, Resca E, Bertoni L, et al. Ferutinin promotes proliferation and osteoblastic differentiation in human amniotic fluid and dental pulp stem cells. Life Sci. 2013;92:993. [DOI] [PubMed] [Google Scholar]
- 25. Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liao J, Tian T, Shi S, et al. The fabrication of biomimetic biphasic CAN‐PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017;5:17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian T, Liao J, Zhou T, et al. The fabrication of calcium phosphate microflowers and their extended application in bone regeneration. ACS Appl Mater Interfaces. 2017;9:30437‐30447. [DOI] [PubMed] [Google Scholar]
- 28. Lambricht L, De BP, Vanacker J, et al. The type and composition of alginate and hyaluronic‐based hydrogels influence the viability of stem cells of the apical papilla. Dent Mater. 2014;30:349‐361. [DOI] [PubMed] [Google Scholar]
- 29. Schellenberg A, Joussen S, Moser K, et al. Matrix elasticity, replicative senescence and DNA methylation patterns of mesenchymal stem cells. Biomaterials. 2014;35:6351‐6358. [DOI] [PubMed] [Google Scholar]
- 30. Xue C, Xie J, Zhao D, et al. The JAK/STAT3 signalling pathway regulated angiogenesis in an endothelial cell/adipose‐derived stromal cell co‐culture, 3D gel model. Cell Prolif. 2017;50:e12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen G, Dong C, Yang L, Lv Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:15790. [DOI] [PubMed] [Google Scholar]
- 32. Treiser MD, Yang EH, Gordonov S, et al. Cytoskeleton‐based forecasting of stem cell lineage fates. Proc Natl Acad Sci USA. 2010;107:610‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu Q, Mirali P, Jalil RA, et al. Modulation of dental pulp stem cell odontogenesis in a tunable PEG‐fibrinogen hydrogel system. Stem Cells Int. 2015;2015:525367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang GT, Yamaza T, Shea LD, et al. Stem/progenitor cell‐mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D'Aquino R, De RA, Lanza V, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75. [DOI] [PubMed] [Google Scholar]
- 36. Cai X, Xie J, Yao Y, et al. The role of WNT Signaling in engineering functional vascular networks for tissue regeneration. Bone Res. 2017;5:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunter DJ, Bardet C, Mouraret S, et al. Wnt Acts as a Prosurvival Signal to Enhance Dentin Regeneration. J Bone Miner Res. 2015;30:1150. [DOI] [PubMed] [Google Scholar]
- 38. Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H. Berberine promotes bone marrow‐derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β‐catenin signaling pathway. Toxicol Lett. 2016;240:68. [DOI] [PubMed] [Google Scholar]
- 39. Liu F, Chu EY, Watt B, et al. Wnt/β‐catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J, Liu B, Gu S, Liang J. Effects of Wnt/β‐catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 2012;45:121‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lian M, Zhang Y, Shen Q, et al. JAB1 accelerates odontogenic differentiation of dental pulp stem cells. J Mol Histol. 2016;47:317. [DOI] [PubMed] [Google Scholar]


