Abstract
Objectives
Skeletal mandibular hypoplasia (SMH), a common type of developmental deformities, results in impaired aesthetics of facial profile, occlusal dysfunction and poor life quality. In this study, BMAL1 deficiency leads to SMH formation, and we aim to investigate the mechanism by which BMAL1 deficiency induces SMH.
Materials and methods
Circadian rhythm‐disordered mouse models were constructed by placing animals in a jet lag schedule of 6‐h light advance every 7 days for 4 or 8 weeks. The OPG expression was evaluated by histomorphometry, immunohistochemistry and western blot analysis. The mechanism by which BMAL1 affects OPG expression was investigated by chromatin immunoprecipitation and luciferase reporter assays. The phenotypes caused by BMAL1 knockout can be rescued by exogenous supplementation with OPG.
Results
We demonstrate that the expressions of BMAL1 and OPG decreased in SMH patients. Circadian rhythm‐disordered mice and Bmal1 −/− mice exhibited decreased expression of OPG, reduced bone mass and bone size of mandibles. Our results revealed that BMAL1 bound directly to the Opg promoter and upregulated its expression, thus inhibiting osteoclast differentiation. BMAL1 deficiency increased osteoclast differentiation by downregulating OPG expression. In vitro, the enhancement effect of osteoclast differentiation caused by BMAL1 knockdown was significantly reversed by exogenous supplementation with OPG. Importantly, bone loss caused by BMAL1 knockout can be partially reversed by injecting OPG Intraperitoneally.
Conclusions
These results indicate that the circadian clock plays a critical role in the growth and development of mandible by regulating OPG expression, and present a potential therapeutic strategy to prevent SMH.
1. INTRODUCTION
Skeletal mandibular hypoplasia (SMH), a common type of craniofacial deformities, can cause serious complications by causing airway obstructions, gastric disturbance, immune deficiencies and delayed developmental growth.1, 2, 3 The development of facial morphology is influenced by both congenital factors and epigenetic factors.4 The clarification of SMH etiology is the key to effective prevention and treatment. Previous studies have provided some insights into the significance of congenital factors,5 but the effects of epigenetic factors on mandibular development have not yet been elucidated.
The circadian rhythm allows life to adapt to periodic changes in the environment and participates in many physiological and behavioural processes, such as cell cycle regulation, energy metabolism, the immune response6 and bone remodeling.7 The circadian clock is involved in the regulation of bone development and bone homeostasis. Molecules associated with osteoblast differentiation are under circadian control, and genes involved in mineral deposition occur in a circadian pattern.7, 8, 9, 10 Brain and muscle ARNT‐like1 (BMAL1) is the core and irreplaceable component of the circadian molecular oscillator, playing critical roles in the regulation of bone resorption11 and bone formation.12 William et al found that femoral bone mass was significantly reduced in BMAL1‐knockout mice and that osteogenic differentiation was decreased in bone marrow stromal cells (BMSCs).12 BMAL1 also affected nucleus pulposus cells and regulated the bone mass of intervertebral discs, which is closely linked with disc height.13 However, whether BMAL1 disturbance could lead to mandibular deformity remains unknown.
Bone development and bone homeostasis are tightly regulated by hormones either systemically or by factors locally secreted by either osteoblasts or osteoclasts, such as leptin, bone morphogenetic proteins (BMPs), osteoprotegerin (OPG) and RANK ligand (RANKL). Systemic leptin causes a shift in mesenchymal stem cell (MSC) lineage and results in decreased osteogenesis.14 BMPs promote osteogenesis as growth factors.15 OPG is a member of the tumour necrosis factor receptor superfamily that inhibits the formation and maturation of osteoclasts by preventing RANKL interaction with its receptor RANK, which plays a critical role in osteoclastogenesis.16 OPG‐deficient mice develop early‐onset osteoporosis.17, 18 In contrast, OPG overexpression suppresses bone resorption and increases bone mass in transgenic mice.19 Additionally, OPG‐deficient male mice can serve as a model for studying severe alveolar bone loss.18 We previously deployed a protein chip analysis and found that OPG expression in the mandible was decreased significantly in Bmal1 −/− mice compared with wild‐type mice.20
In the current study, we found that BMAL1 and OPG expressions decrease concomitantly in clinical cases with SMH. To explore the correlations among circadian dysrhythmia, OPG downregulation and SMH, circadian rhythm‐disordered mouse models were builded by a jet lag schedule. Reduced bone mass and bone size are observed in circadian rhythm‐disordered mice, OPG expression decreasing concomitantly. Reduced bone mass and OPG expression of mandibles are also observed in Bmal1 −/− mice. We further revealed that BMAL1 can bind to the promoter of Opg and consequently modulates osteoclast differentiation. Importantly, bone loss caused by BMAL1 knockout can be partially reversed by injecting OPG intraperitoneally. Taken together, our findings provide new insights into the pathogenesis of SMH and present a potential therapeutic strategy.
2. MATERIALS AND METHODS
2.1. Patient tissue specimens
All the twenty human mandible tissue specimens were collected from the patients at Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Ten specimens were obtained from patients with skeletal mandibular hypoplasia and the other ten specimens were obtained from controlled sex‐matched normal peers. All specimens were critically obtained in the period between 9:00 am and 11:00 am. All subjects’ age was ranged from 10 to 12 years old and their skeletal maturation had not reached at the growth and development peak according to the quantitative cervical vertebral maturation (QCVM) measurement method. Inclusion criteria of the experimental group are listed in Table S1. Any possible impact factors, including whether treated with orthodontics or suffered from maxillofacial trauma and temporomandibular joint disorder, were excluded.
2.2. Animals
C57BL/6J mice were purchased from the Beijing HFK Bioscience Co., Ltd. (Beijing, China). Mice were placed under strict 12‐h light/12‐h dark cycles, with lights on at 8 am and off at 8 pm. For induction of the jet lag group, mice were placed under alternating light‐cycle conditions with 6‐h light advanced every week for 4 or 8 weeks. Five mice per group were sacrificed to collect the mandibles at the indicated time point (ZT0, ZT4, ZT8, ZT12, ZT16, ZT20 and ZT24, 8 am was set as the zeitgeber time 0: ZT0). Homozygous Bmal1‐deficient (Bmal1 −/−) mice were produced by breeding heterozygous BMAL1‐deficiency mating pairs (Bmal1 +/–) and Bmal1 deficiency was confirmed by PCR as described by Bunger et al.21
2.3. Study approval
Human mandible specimens were acquired from patients at Department of Stomatology, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, which was approved by the Institutional Research Ethics Committee of Tongji Medical College (Wuhan, China). This study was conducted in accordance with the Declaration of Helsinki. Get written informed consent before data collection. All procedures involved with animals were committed to abide by the ethical guidelines and get approved of the Institutional Animal Care and Use Committee of Tongji Medical College.
2.4. Other methods
For other Material and methods, please see Data S1.
2.5. Statistical analysis
All statistical analyses were performed using spss 17.0 (spss, Chicago, IL, USA). The two‐tailed, unpaired Student's t test was used to analyse differences between groups. P values were considered significant at <.05.
3. RESULTS
3.1. Circadian dysrhythmia may be involved in the pathogenesis of skeletal mandibular hypoplasia
The circadian clock plays a key role in the regulation of bone development and bone homeostasis.12 To determine the correlation between circadian clock and mandible development, we measured the expression levels of clock genes in human mandibular tissues and found that, in general, the protein levels of BMAL1, CLOCK, PER1, PER2 and CRY2 were changed obviously in SMH patients.20 The core circadian molecular oscillator BMAL1 is essential for circadian pace making, which was lower in the mandibular tissues of SMH patients than in normal controls. OPG is a member of the tumour necrosis factor receptor superfamily that inhibits the formation and maturation of osteoclasts. We also found that OPG protein expression was concomitantly decreased in SMH patients (Figure 1A,B). These data suggest that the expression disruption of BMAL1 and OPG may be involved in SMH formation.
Figure 1.
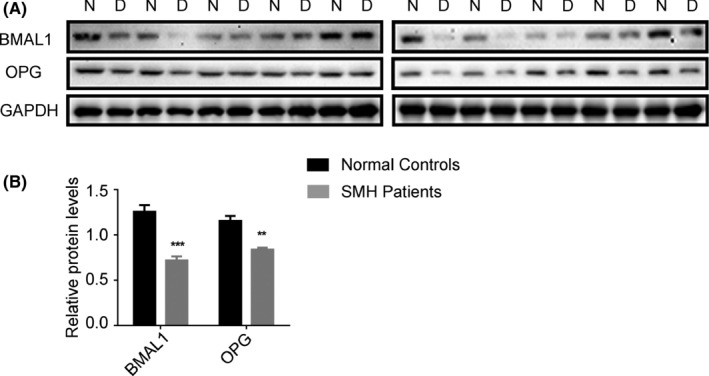
Circadian dysrhythmia may be involved in the pathogenesis of skeletal mandibular hypoplasia. (A‐B) Western blot analysis of BMAL1 and OPG expression in mandibles from skeletal mandibular hypoplasia patients and normal peers. Data represent the mean ± SD (n = 10 individuals per group). **P < .01 and ***P < .001 (compared with control), from Student's t tests
3.2. Circadian dysrhythmia contributes to skeletal mandibular hypoplasia and OPG expression decreased correspondingly
Our data showed that the expression levels of clock genes were changed significantly in the mandibles of SMH patients, indicating that circadian dysrhythmia could be involved in the pathogenesis of SMH. To determine the role of circadian dysrhythmia in SMH formation, we constructed a jet lag mouse model in which mice were under shifting light‐dark cycles of 6‐h light advance every week for 4 or 8 weeks (Figure 2A). We examined the expressions of clock genes in mandibular specimens from jet lag group at several time points, and we confirmed that the circadian rhythm of jet lag mice was indeed disrupted (Figure S1). The mandibles of jet lag mice were significantly smaller than those of the normal group visually (Figure 2B). The heights of the mandibular ramus and the coronoid process were also reduced. Micro‐computed tomography (CT) and three‐dimensional (3D) reconstruction revealed that the bone volume/total volume (BV/TV) and trabecular thickness (Tb.Th) in the condylar regions of the jet lag mice were much lower compared with the control group (Figure 2C,D). Serial sections of mandible tissues were stained with haematoxylin and eosin (H&E). Staining revealed that the number of osteoblasts was decreased in the jet lag group. Tartrate‐resistant acid phosphatase (TRAP) staining was performed to quantify osteoclast‐like cells, which revealed that the osteoclasts were distributed more abundantly in jet lag group than control group (Figure 2E,F). Our findings showed that OPG protein expression was concomitantly reduced in the mandibles of SMH patients, suggesting that OPG may play a role in regulating mandible development. The expression of OPG was further assessed by immunohistochemistry and western blot, which revealed that OPG expression was significantly decreased in the mandibles of jet lag group compared to the control group (Figure 2E, G, H), implying a critical role for OPG in the growth and development of the mandible by responding to circadian clock outputs.
Figure 2.
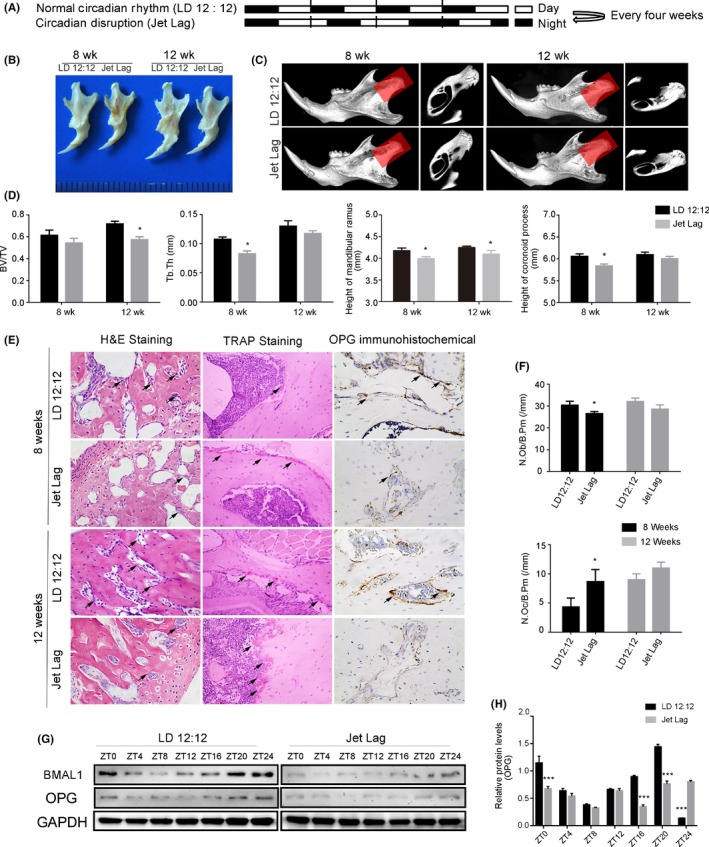
Circadian dysrhythmia contributes to skeletal mandibular hypoplasia and OPG expression decreased correspondingly. (A) A schematic depiction of C57 mice subjected to light/dark 12:12‐h (upper panel) and 6‐h light advanced every week for 4 or 8 wk (lower panel). (B) Photograph of mandibles dissected from 8‐ and 12‐wk‐old jet lag C57BL/6J or control mice. (C) Representative images of micro‐CT reconstructing the mandibles of 8‐ and 12‐week‐old mice in the normal or disrupted status. Shadow areas show cross‐sections of the condylar regions. (D) Micro‐CT reconstruction analysis of mandibles harvested from 8‐ to 12‐wk‐old mice in the normal or disrupted status (BV/TV, Tb.Th, height of the mandibular ramus, and height of the coronoid process) (n = 5 per group). (E) Representative images of H&E and TRAP staining and OPG immunohistochemistry from normal or jet lag mouse mandibles. The arrows indicate osteoblasts in the H&E staining images, and arrows indicate osteoclasts in the TRAP staining images. (magnification, 400x). (F) Osteoblast number (N.Ob/B.Pm), osteoclast number (N.Oc/B.Pm) were measured (n = 5 per group). (G) Western blot analysis of BMAL1 and OPG expression in mandibles from 12‐wk‐old jet lag C57BL/6J or control mice. N = 3 independent experiments. (H) Relative protein level of OPG in mandibles from 12‐wk‐old jet lag C57BL/6J or control mice. N = 3 independent experiments. BV/TV, bone volume/total volume; Tb.Th, trabecular thickness; N.Ob/B.Pm, number of osteoblasts/bone perimeter; N.Oc/B.Pm, number of osteoclasts/bone perimeter. Data represent the mean ± SD. *P < .05, **P < .01, ***P < .001. Two‐tailed Student's t test was used
3.3. Skeletal mandibular hypoplasia in Bmal1 −/− mice with decreasing OPG expression
Since Bmal1 is the core and irreplaceable component of the circadian molecular oscillator, we constructed BMAL1‐knockout mice to assess the effects of the circadian rhythm on mandible development. The BMAL1 knockout genotype was identified by polymerase chain reaction (PCR) (Figure 3A). The mRNA levels of Clock, Per1, Per2, Cry1 and Cry2 were significantly decreased in the mandibles of Bmal1 −/− mice, confirming the circadian rhythm disorder (Figure 3B).
Figure 3.
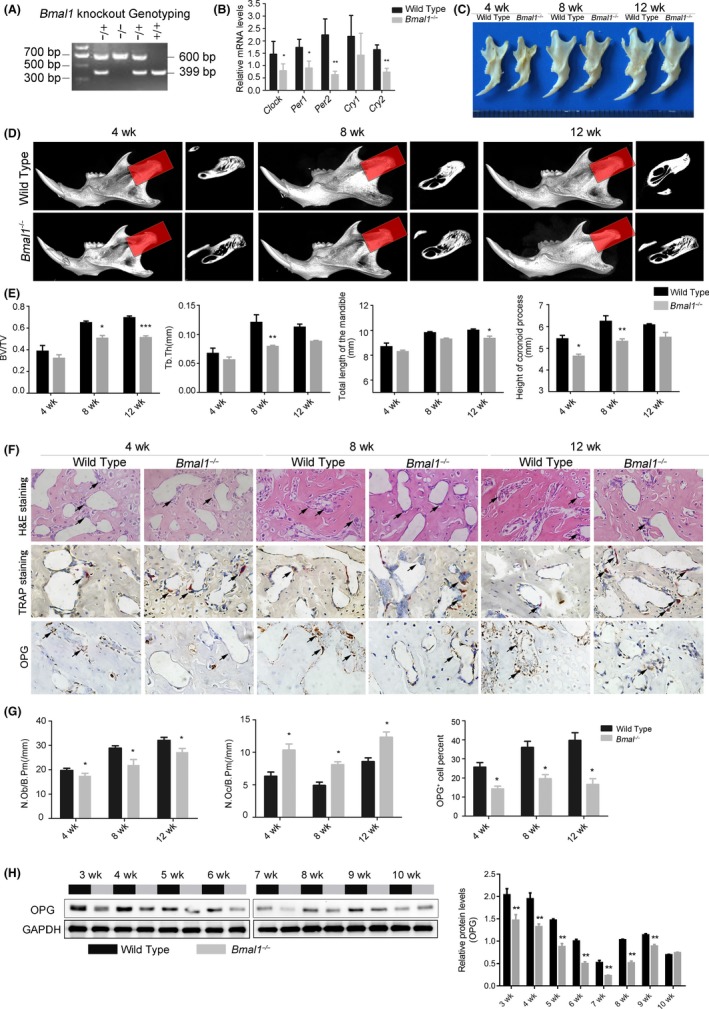
Skeletal mandibular hypoplasia in Bmal1 −/− mice with decreasing OPG expression. (A) Genotype determined by PCR amplification in Bmal1 −/−, Bmal1 +/− , and Bmal1 +/+ mice (n = 5 per genotype). (B) mRNA levels of circadian clock genes Clock, Per1, Per2, Cry1, and Cry2 in wild‐type and Bmal1 −/− mice, as quantified by qRT‐PCR. (C) Photograph of mandibles dissected from 4‐, 8‐, and 12‐week‐old Bmal1 −/− or wild‐type mice. (D) Representative images of micro‐CT reconstructing the mandibles of 4‐, 8‐, and 12‐week‐old Bmal1 −/− or wild‐type mice. Shadow areas show cross‐sections of the condylar region. (E) Micro‐CT reconstruction analysis of mandibles harvested from 4‐, 8‐, and 12‐wk‐old Bmal1 −/− or wild‐type mice (BV/TV, Tb.Th, total length of the mandible, and height of the coronoid process) (n = 5 per group). (F) Representative H&E and TRAP staining and OPG immunohistochemistry images from wild‐type or Bmal1 −/−mice mandibles. The arrows indicate osteoblasts in the H&E staining images, and arrows indicate osteoclasts in the TRAP staining images. (magnification, 400x). (G) Osteoblast number (N.Ob/B.Pm), osteoclast number (N.Oc/B.Pm), and OPG expression level were measured (n = 5 per group). (H) Western blot analysis of OPG expression in the mandibles of 3‐, 4‐, 5‐, 6‐, 7‐, 8‐, 9‐, and 10‐week‐old Bmal1 −/− and wild‐type mice. Data represent the mean ± SD, *P < .05, **P < .01, ***P < .001, from Student's t test
Overall, we observed that the mandibles of Bmal1 −/− mice were significantly smaller than those of the wild‐type mice (Figure 3C). The height of the coronoid process and the total length of mandible were consistently decreased in Bmal1 −/− mice (Figure 3E). Micro‐CT and 3D reconstruction revealed that the BV/TV, Tb.Th and bone mineral density (BMD) were decreased in the condylar regions of Bmal1 −/− mice compared with the age‐matched wild‐type mice (Figure 3D,E; Figure S2). H&E staining revealed that the number of osteoblasts was decreased in mandible sections of Bmal1 −/− mice compared with wild‐type mice. TRAP staining revealed an increased number of TRAP+ cells in Bmal1 −/− mice comparatively. OPG immunohistochemistry revealed that the OPG protein level was decreased in the mandibles of Bmal1 −/− mice relative to wild‐type mice (Figure 3F,G). Next, we examined OPG expression in the mandibles of 3‐, 4‐, 5‐, 6‐, 7‐, 8‐, 9‐, and 10‐week‐old wild‐type and Bmal1 −/− mice. The decreased OPG protein level was consistent in the mandibular tissues of Bmal1 −/− mice throughout the entire growth period (Figure 3H).
3.4. BMAL1 knockout leads to decreased OPG expression
To determine whether Bmal1 regulates OPG expression, we knocked down BMAL1 in murine BMSCs (mBMSCs) and MC3T3‐E1 cells, and found that OPG proteins were highly downregulated. In BMAL1‐overexpressing mBMSCs and MC3T3‐E1 cells, OPG proteins were clearly upregulated (Figure 4A,B). Immunofluorescence revealed that OPG expression was decreased in BMAL1‐knockdown MC3T3‐E1 cells (Figure 4C). Similarly, the levels of secreted OPG were reduced in the supernatant of BMAL1‐knockdown mBMSCs and MC3T3‐E1 cells (Figure 4D).
Figure 4.
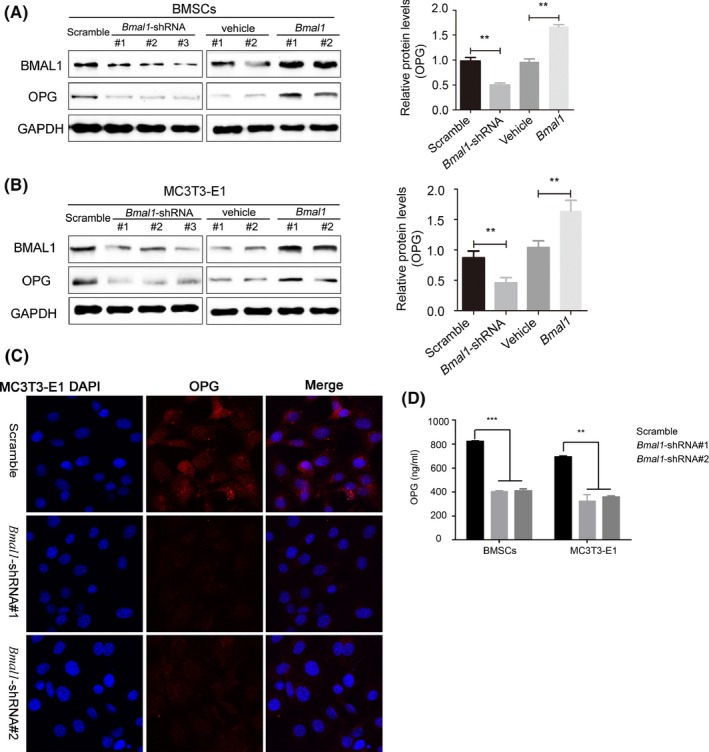
BMAL1 knockout leads to decreased OPG expression. (A‐B) Western blot analysis of BMAL1 and OPG expression in BMAL1‐overexpressing or BMAL1‐knockdown mBMSCs and MC3T3‐E1 cells. (C) Confocal microscope images of OPG expression in BMAL1‐knockdown or scrambled shRNA‐transfected MC3T3‐E1 cells. (magnification, 600x). (D) Enzyme‐linked immunosorbent assay shows the OPG concentration in the supernatant of BMAL1‐knockdown mBMSCs and MC3T3‐E1 cells. N = 3 independent experiments, data represent the mean ± SD, **P < .01, ***P < .001. Two‐tailed Student's t test was used
3.5. OPG is involved in the pathway cascade of BMAL1 regulation on osteoclast differentiation
To investigate the effects of reduced OPG expression on osteoclast differentiation, we co‐cultured RAW 264.7 cells with BMAL1‐knockdown mBMSCs (or BMAL1‐knockdown MC3T3‐E1 cells), and found that osteoclast differentiation was enhanced compared to cells co‐cultured with control mBMSCs (or control MC3T3‐E1 cells) (Figure 5A,B). Additionally, the levels of OPG in the supernatant were lower in RAW 264.7 cells co‐cultured with BMAL1‐knockdown mBMSCs (or BMAL1‐knockdown MC3T3‐E1 cells) (Figure 5C). Next, we determined that the enhancement effect of osteoclast differentiation caused by BMAL1 knockdown was significantly reversed by exogenous supplementation with OPG (Figure 5A,B).
Figure 5.
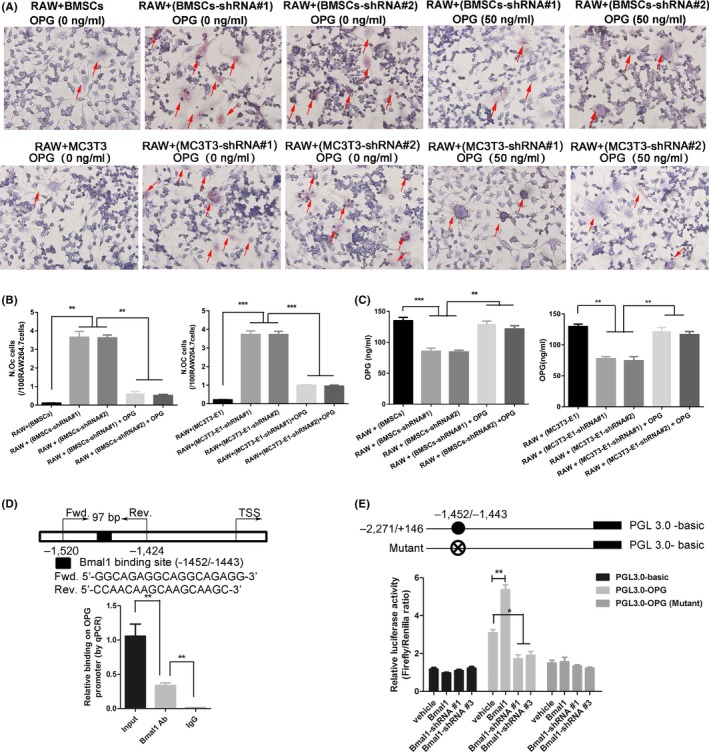
BMAL1 upregulates OPG expression by directly binding to the Opg promoter. (A‐B) Representative images of TRAP staining of RAW 264.7 cells co‐cultured with BMAL1‐knockdown mBMSCs or MC3T3‐E1 cells in the absence or presence of recombinant OPG. (magnification, 400x). (C) OPG concentration in the supernatants of co‐cultured RAW 264.7 cells quantified by enzyme‐linked immunosorbent assay. (D) The transcription factor BMAL1 bound to the Opg promoter in MC3T3‐E1 cells. Chromatin immunoprecipitation assays were performed using anti‐IgG as a negative control. (E) Luciferase reporter assays were performed to measure the activities of the wild‐type or mutated BMAL1‐binding site at the Opg promoter in BMAL1‐overexpressing or BMAL1‐knockdown MC3T3‐E1 cells. N = 3 independent experiments, data represent the mean ± SD, *P < .05, **P < .01, ***P < .001. Analysis of variance with Tukey's post‐hoc test was used
3.6. BMAL1 upregulates OPG expression by directly binding to the Opg promoter
As a widely acknowledged transcription factor, BMAL1 exerts its function by activating the transcription of its downstream target genes.22 To explore how BMAL1 regulates OPG expression, we predicted the BMAL1 binding site in the promoter of Opg, and chromatin immunoprecipitation (ChIP) was performed in MC3T3‐E1 cells. The ChIP assay revealed that BMAL1 selectively bound to the promoter of Opg at putative E‐box (CACGTG) site (Figure 5D). We then used luciferase assays to test whether BMAL1 could alter Opg transcriptional activity. The luciferase assay revealed that BMAL1 directly activated the Opg promoter, whereas the activation effect was abolished when the BMAL1‐binding site was mutated or when BMAL1 was knocked down (Figure 5E).
3.7. Supplemental OPG mitigates skeletal mandibular hypoplasia caused by BMAL1 knockout
Based on these observations, we further explored the prevention potential of OPG for SMH. 4‐week‐old male mice were given intraperitoneal injections of OPG (30 mg/kg, twice a week, 6 weeks). Micro‐CT analyses of the mandibles showed that treatment with the OPG significantly increased BV/TV, Tb.Th and BMD (Figure 6A,B; Figure S3). In addition, the osteoclast number (N.Oc/B.Pm) was significantly suppressed by OPG administration (Figure 6C,D). Taken together, our results suggest that OPG may be a potential prevention approach for SMH caused by Bmal1 knockout.
Figure 6.
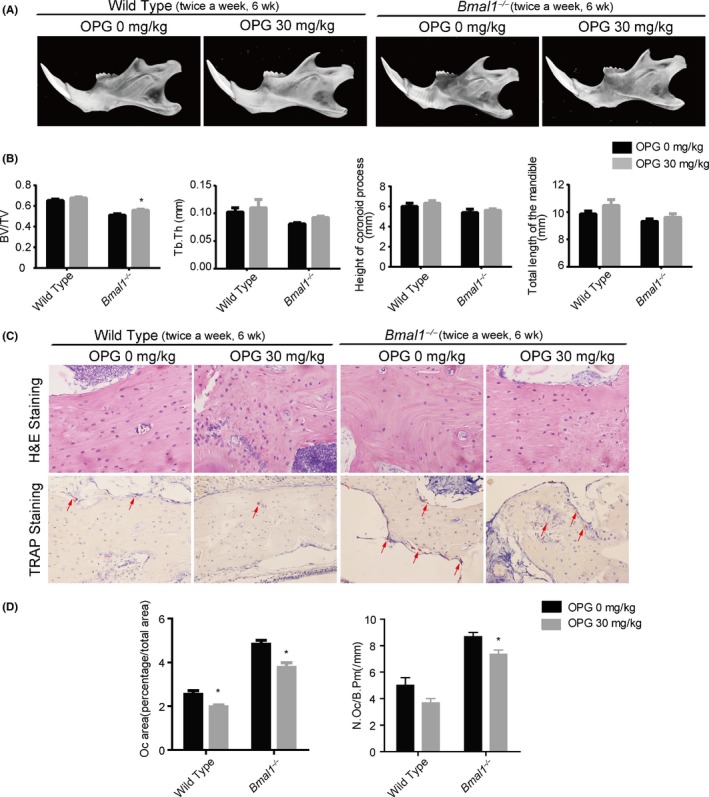
Supplemental OPG mitigates skeletal mandibular hypoplasia caused by BMAL1 knockout. (A) Representative images of micro‐CT reconstructing the mandibles of wild type and Bmal1 −/− mice treated with or without intraperitoneal injections of OPG (30 mg/kg, twice a week) for 6 weeks. (B) Micro‐CT reconstruction analysis of mandibles of wild type and Bmal1 −/− mice treated with or without intraperitoneal injections of OPG (30 mg/kg, twice a week) for 6 weeks(BV/TV, Tb.Th, total length of the mandible, and height of the coronoid process) (n = 5 per group). (C) Representative images of H&E staining (magnification, 400x) and TRAP staining (magnification, 320x) from wild‐type or Bmal1 −/− mice mandibles treated with or without intraperitoneal injections of OPG (30 mg/kg, twice a week) for 6 wk. (D) Oc area (percentage/total area) and osteoclast number (N.Oc/B.Pm) were measured (n = 5 per group). Oc area (percentage/total area) = area of osteoclast/total area, data represent the mean ± SD. *P < .05, from Student's t test
4. DISCUSSION
Skeletal mandibular hypoplasia (SMH) is a common type of developmental deformities, results in impaired aesthetics of facial profile, occlusal dysfunction and poor life quality. In addition to congenital factors such as genetic mutations and maternal pregnant abnormalities,23 epigenetic factors such as chronic malnutrition24, 25 and vitamin D deficiency26, 27 can affect the normal and coordinated development of bone, causing jaw deformity. Currently, the human circadian rhythm has been greatly affected due to active or passive staying up at night, night light pollution, night shifts, etc.28 Circadian dysfunction is closely associated with bone destruction. Indeed, previous studies have described the roles of circadian clock in developing femurs.12, 29 BMAL1 deficiency results in a low bone mass phenotype of femurs. An earlier closure and reduced thickness of growth plates in BMAL1‐deficient mice compared with wild‐type mice suggest alterations in endochondral bone formation.12 Furthermore, the current study demonstrated that the circadian rhythm can affect the growth and development of the mandible. Circadian dysrhythmia significantly inhibits bone growth of the mandible. Based on mandibular morphological measurements, micro‐CT analysis and histomorphometric examination, we found that circadian dysrhythmia can promote bone resorption, suggesting that circadian dysrhythmia is an important acquired factor that may cause jaw deformity. The mandibles of Bmal1 −/− mice were significantly smaller than those of the wild‐type mice, further confirming the effect of the circadian rhythm on mandible development.
In bone tissue, OPG competitively binds to RANKL, thereby blocking RANKL binding to RANK on osteoclast precursor cells, the only known receptor for RANKL, thus inhibiting osteoclast differentiation and maturation.16 OPG‐overexpressing mice exhibit osteopetrosis via decreased osteoclast differentiation, while OPG‐knockout mice are osteoporotic with decreased total bone density and a high incidence of bone deformities and fractures.17 In our study, BMAL1 and OPG expression was lower in SMH patients than in normal controls, and mandibular OPG expression was significantly decreased in jet lag mice. Furthermore, concomitant decreased OPG expression was present in the mandibular tissues of Bmal1 −/− mice throughout the entire growth period, and osteoclast differentiation was significantly promoted in the mandibles of Bmal1 −/− mice. These findings suggest that OPG is a key factor in BMAL1 deficiency‐mediated SMH.
BMAL1 is the core component of the circadian molecular oscillator because it is essential for circadian behavior,21 and the absence of BMAL1 will result in expression disorders of other clock genes in the circadian clock system. BMAL1 is a basic helix‐loop‐helix PAS domain transcription factor that exerts its function by binding to the E‐box elements of CACGTG‐type (or CACGTT‐type‐like) in the promoters of its downstream target genes.30, 31 For example, BMAL1 is a direct regulator of insulin‐mediated osteoblast differentiation by increasing the promoter activity of Bmp2 in MC3T3‐E1 cells.32 OPG is secreted by a variety of MSC‐derived cells, such as osteoblasts and BMSCs,33 which are regulated by various cytokines and hormones.34 Several potential mechanisms have been identified: PGE1 stimulates OPG synthesis via the p38 MAP kinase and SAPK/JNK signalling pathways in osteoblast‐like MC3T3‐E1 cells35; HIF‐1α directly interacts with the Opg‐binding site to stimulate transcription36; and glucocorticoids lower Opg mRNA levels by binding to the Opg promoter,37 as well as indirectly by modulating Wnt/β‐catenin signalling.38 In this study, we searched potential E‐box enhancer elements in Opg. Next, ChIP‐qPCR confirmed the binding of BMAL1 to this E‐box region (‐1520/‐1424, 97 bp) of Opg. Functional luciferase assays validated the BMAL1‐mediated activation of this E‐box sequence in cultured cells. To our knowledge, this is the first study to report that BMAL1 directly upregulates Opg transcription to inhibit osteoclast differentiation, highlighting the importance of the circadian rhythm in the bone‐volume balance. Previous studies have reported that a change in sleep patterns can increase the risk of hip fractures in southern European men,39 and that the risk of hip and wrist fractures is increased in postmenopausal women who experience at least three work shift changes per month.40 Our results support that bone metabolism is directly connected to changes in the circadian rhythm. Thus, circadian rhythm dysfunction is a direct environmental factor that can cause SMH.
Due to its complex aetiology, most current studies mainly focus on the therapeutic approaches of orthodontic treatment or orthognathic surgery for SMH. However, few studies have examined the prevention of SMH. OPG is reportedly a potent inhibitor of bone resorption in human subjects. Supplemental OPG prevented the development of osteoporosis.19 Treatment with OPG‐Fc reduced the formation of evident osteolytic lesions radiographically in tumour‐bearing animals.41 In our in vivo experiment, exposure to recombinant OPG led to increased bone density in normal mice, due to a decrease in osteoclast numbers. Intraperitoneal injection of OPG can partially rescue bone loss in Bmal1 −/− mice. Our results suggest that supplemental OPG may mitigate jaw deformity formation caused by circadian rhythm dysfunction to a certain extent.
Collectively, our data demonstrate that the circadian rhythm is closely associated with mandible development, and that circadian dysfunction can induce SMH formation by decreasing OPG expression. BMAL1 directly interacts with the Opg‐binding site to stimulate Opg transcription and suppresses osteoclast differentiation. The results of our study will enhance our understanding of the aetiology of SMH, thereby providing us with a better understanding of the molecular targets for prevention and therapeutic approaches. OPG may serve as an important target molecule for the prevention of SMH caused by circadian dysrhythmia.
AUTHOR CONTRIBUTIONS
L. L. Chen, contributed to conception, design, study supervision, data analysis, and interpretation, drafted and critically revised the manuscript; X. Zhou, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; R. Yu, Y. L. Long, contributed to data acquisition and analysis, critically revised the manuscript; J. J. Zhao, S. L. Yu, contributed to data acquisition, critically revised the manuscript; Q. M. Tang, contributed to data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work.
CONFLICT OF INTEREST
The authors have no competing interests.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by National Science Fund for Excellent Young Scholars (31725011, to L.L. Chen), National Science Fund for Outstanding Young Scholars (31422022, to L.L. Chen), and Clinical Research Physician Program of Tongji Medical College, HUST (to L.L. Chen). The authors thank Prof. Ying Xu of Soochow University for the heterozygous BMAL1‐deficient mating pairs (Bmal1 +/−).
Zhou X, Yu R, Long Y, et al. BMAL1 deficiency promotes skeletal mandibular hypoplasia via OPG downregulation. Cell Prolif. 2018;51:e12470 10.1111/cpr.12470
Xin Zhou, Ran Yu, Yanlin Long are the authors who contributed equally to this article.
REFERENCES
- 1. Paladini D. Fetal micrognathia: almost always an ominous finding. Ultrasound Obstet Gynecol. 2010;35:377‐384. [DOI] [PubMed] [Google Scholar]
- 2. Bollhalder J, Hanggi MP, Schatzle M, Markic G, Roos M, Peltomaki TA. Dentofacial and upper airway characteristics of mild and severe class II division 1 subjects. Eur J Orthod. 2013;35:447‐453. [DOI] [PubMed] [Google Scholar]
- 3. Masood Y, Masood M, Zainul NN, Araby NB, Hussain SF, Newton T. Impact of malocclusion on oral health related quality of life in young people. Health Qual Life Outcomes. 2013;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanco C, Kasai K, Sergi R, Richards LC, Townsend GC. Genetic and environmental influences on facial profile. Aust Dent J. 1995;40:104‐109. [DOI] [PubMed] [Google Scholar]
- 5. da Fontoura CS, Miller SF, Wehby GL, et al. Candidate gene analyses of skeletal variation in malocclusion. J Dent Res. 2015;94:913‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spengler ML, Kuropatwinski KK, Comas M, et al. Core circadian protein CLOCK is a positive regulator of NF‐kappaB‐mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457‐E2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zvonic S, Ptitsyn AA, Kilroy G, et al. Circadian oscillation of gene expression in murine calvarial bone. J Bone Miner Res. 2007;22:357‐365. [DOI] [PubMed] [Google Scholar]
- 8. He Y, Chen Y, Zhao Q, Tan Z. Roles of brain and muscle ARNT‐like 1 and Wnt antagonist Dkk1 during osteogenesis of bone marrow stromal cells. Cell Prolif. 2013;46:644‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guntur AR, Kawai M, Le P, et al. An essential role for the circadian‐regulated gene nocturnin in osteogenesis: the importance of local timekeeping in skeletal homeostasis. Ann N Y Acad Sci. 2011;1237:58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElderry JD, Zhao G, Khmaladze A, Wilson CG, Franceschi RT, Morris MD. Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J Bone Miner Res. 2013;28:1846‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu C, Ochi H, Fukuda T, et al. Circadian clock regulates bone resorption in mice. J Bone Miner Res. 2016;31:1344‐1355. [DOI] [PubMed] [Google Scholar]
- 12. Samsa WE, Vasanji A, Midura RJ, Kondratov RV. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone. 2016;84:194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suyama K, Silagi ES, Choi H, et al. Circadian factors BMAL1 and RORalpha control HIF‐1alpha transcriptional activity in nucleus pulposus cells: implications in maintenance of intervertebral disc health. Oncotarget. 2016;7:23056‐23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18:782‐796. [DOI] [PubMed] [Google Scholar]
- 15. Liu Z, Yuan X, Liu M, et al. Antimicrobial peptide combined with BMP2‐modified mesenchymal stem cells promotes calvarial repair in an osteolytic model. Mol Ther. 2017;26:199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mizuno A, Amizuka N, Irie K, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610‐615. [DOI] [PubMed] [Google Scholar]
- 17. Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin‐deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koide M, Kobayashi Y, Ninomiya T, et al. Osteoprotegerin‐deficient male mice as a model for severe alveolar bone loss: comparison with RANKL‐overexpressing transgenic male mice. Endocrinology. 2013;154:773‐782. [DOI] [PubMed] [Google Scholar]
- 19. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309‐319. [DOI] [PubMed] [Google Scholar]
- 20. Zhao J, Zhou X, Tang Q, et al. BMAL1 deficiency contributes to mandibular dysplasia by upregulating MMP3. Stem Cell Reports. 2018;10:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271‐290. [DOI] [PubMed] [Google Scholar]
- 23. De Mori R, Romani M, D'Arrigo S, et al. Hypomorphic recessive variants in SUFU impair the sonic hedgehog pathway and cause joubert syndrome with cranio‐facial and skeletal defects. Am J Hum Genet. 2017;101:552‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565‐1575. [DOI] [PubMed] [Google Scholar]
- 25. Sugatani T, Agapova OA, Fang Y, et al. Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int. 2017;91:86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Handel MN, Frederiksen P, Cohen A, Cooper C, Heitmann BL, Abrahamsen B. Neonatal vitamin D status from archived dried blood spots and future risk of fractures in childhood: results from the D‐tect study, a population‐based case‐cohort study. Am J Clin Nutr. 2017;106:155‐161. [DOI] [PubMed] [Google Scholar]
- 27. McCarty DE, Chesson AL Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18:311‐319. [DOI] [PubMed] [Google Scholar]
- 28. Copinschi G, Caufriez A. Sleep and hormonal changes in aging. Endocrinol Metab Clin North Am. 2013;42:371‐389. [DOI] [PubMed] [Google Scholar]
- 29. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin‐regulated bone formation. Cell. 2005;122:803‐815. [DOI] [PubMed] [Google Scholar]
- 30. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935‐941. [DOI] [PubMed] [Google Scholar]
- 31. Yoo SH, Ko CH, Lowrey PL, et al. A noncanonical E‐box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci USA. 2005;102:2608‐2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Min HY, Kim KM, Wee G, Kim EJ, Jang WG. Bmal1 induces osteoblast differentiation via regulation of BMP2 expression in MC3T3‐E1 cells. Life Sci. 2016;162:41‐46. [DOI] [PubMed] [Google Scholar]
- 33. Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal‐osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768‐4776. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, He H, Cao Z, Fang Y, Du M, Liu Z. Regulatory effects of bone morphogenetic protein‐4 on tumour necrosis factor‐alpha‐suppressed Runx2 and osteoprotegerin expression in cementoblasts. Cell Prolif. 2017;50:e12344.doi: 10.1111/cpr.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto N, Otsuka T, Kuroyanagi G, et al. Resveratrol reduces prostaglandin E1‐stimulated osteoprotegerin synthesis in osteoblasts: suppression of stress‐activated protein kinase/c‐Jun N‐terminal kinase. Prostaglandins Other Lipid Mediat. 2015;116–117:57‐63. [DOI] [PubMed] [Google Scholar]
- 36. Shao J, Zhang Y, Yang T, Qi J, Zhang L, Deng L. HIF‐1alpha disturbs osteoblasts and osteoclasts coupling in bone remodeling by up‐regulating OPG expression. Vitro Cell Dev Biol Anim. 2015;51:808‐814. [DOI] [PubMed] [Google Scholar]
- 37. Le Phuc P, Friedman JR, Schug J, et al. Glucocorticoid receptor‐dependent gene regulatory networks. PLoS Genet. 2005;1:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005;329:177‐181. [DOI] [PubMed] [Google Scholar]
- 39. Kanis J, Johnell O, Gullberg B, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9:45‐54. [DOI] [PubMed] [Google Scholar]
- 40. Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int. 2009;20:537‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Canon JR, Roudier M, Bryant R, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119‐129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


