Abstract
Objectives
During long‐term culture, loss of stemness is observed which greatly restricts the application of human periodontal ligament stem cells (hPDLSCs) in tissue regeneration. Oestrogen (E2) was found to significantly enhance the proliferation and osteogenic differentiation capacity in mesenchymal stem cells. Therefore, in this study, we investigated effects of E2 on hPDLSCs stemness in long‐term culture.
Materials and methods
Effects of E2 on hPDLSCs stemness were systematically evaluated. To characterize underlying the mechanisms, its effects on PI3K/AKT signalling pathway were determined.
Results
Our results showed that E2 was able to enhance the proliferation, modify cell cycle, up‐regulate stemness‐related genes expression, promote osteogenic differentiation and elevate the positive rate of CD146 and STRO‐1 over 10 passages in hPDLSCs. Importantly, PI3K/AKT signing pathway might play a role in these effects.
Conclusions
These findings suggest that E2 retains hPDLSCs stemness in long‐term culture, which might enhance its application in tissue engineering.
1. INTRODUCTION
Periodontitis is a highly prevalent inflammatory disease and the common cause of tooth loss worldwide; however, the conventional periodontal therapy is unable to reconstruct the injured or lost periodontal tissue.1, 2 The development of stem cell‐based tissue engineering provides a new and promising approach for the treatment of periodontitis.2 The periodontal ligament is a complex connective tissue located between the alveolar bone and the cementum, playing an important role in maintaining teeth in situ.3 Human periodontal ligament stem cells (hPDLSCs), a unique population of mesenchymal stem cells (MSCs), are found in periodontal ligaments. These stem cells are both pluripotent and capable of self‐renewal.4 Compared with human bone marrow mesenchymal stem cells (hBMSCs), hPDLSCs are readily accessible.5, 6 Therefore, hPDLSCs are considered a favourable candidate for periodontal tissue regeneration.
However, increasing evidence suggests that MSCs lose their stemness gradually during long‐term culture in vitro, which is accompanied by a decline in proliferation and differentiation, a shortening of telomeres and an increase in apoptosis.7, 8 The loss of stemness in hPDLSCs limits the application of hPDLSCs in tissue engineering.9 Thus, retaining hPDLSCs stemness during long‐term culture becomes essential for further application of hPDLSCs in treatment of periodontitis.
Oestrogen (E2) is a hormone that plays a key role in body metabolism, affecting biological processes that include bone formation,10 angiogenesis11 and autoimmunity.12 Recently, studies reported that exogenous E2 enhanced the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), dental pulp stem cells and stem cells from apical papilla.13, 14, 15 It suggests that E2 might have the potential to enhance the proliferation and osteogenic differentiation of hPDLSCs during in vitro culture.
In this study, we investigated the effects of E2 on stemness of hPDLSCs. We found that E2 enhanced the proliferation and the ratio of G2/M + S phase, up‐regulated the expression of stemness‐related genes, promoted osteogenic differentiation and elevated the positive rate of CD146 and STRO‐1 over 10 passages, which caused by stimulation of PI3K/AKT signalling pathway. Taken together, our results showed that E2 treatment efficiently retained the long‐term self‐renewal ability and pluripotency of hPDLSCs, providing a candidate strategy for the application of hPDLSCs in tissue engineering.
2. MATERIALS AND METHODS
2.1. Cell isolation and culture
Healthy premolars were extracted from 12 adults (18‐20 years of age; 6 male and 6 female) for the reason of orthodontics at the Department of Oral and Maxillofacial Surgery, the Affiliated Stomatological Hospital of Sun Yat‐sen University. Informed consent was obtained from all of the participants. Approval was granted by the Sun Yat‐sen University Research Ethics Committee. Human periodontal ligament stem cells were isolated and collected as previously described.4, 16 Briefly, hPDLSCs were isolated from periodontal tissue in the middle one third of the root, digested with 3 mg/mL collagenase type I and 4 mg/mL dispase (Gibco‐BRL, Grand Island, NY, USA) for 1 hour at 37°C. Colony‐forming cells were collected and cultured in phenol red‐free alpha modified Eagle medium (α‐MEM; Gibco‐BRL) supplemented with 10% charcoal‐treated (Gibco‐BRL), 100 μg/mL streptomycin, 100U/mL penicillin (HyClone, Logan, UT, USA), and 5 mmol/L‐glutamine (Gibco‐BRL) at 37°C in 5% CO2. The all hPDLSCs isolated from 12 same individuals were mixed together for all experiments.
2.2. E2 treatment
Passage 3 (P3) hPDLSCs were treated with 10−7 mol/L E2 (Sigma‐Aldrich, ST.Louis, MO, USA) for 48 hours for short‐term stimulation. Human periodontal ligament stem cells cultured with 0.01% (V/V) ethyl alcohol were used as control. ICI 182780 (10−6 mol/L; Sigma‐Aldrich), the E2 receptor (ER) antagonist, was used to abrogate the function of E2.17 The PI3‐kinase inhibitor LY294002 (2.5 μmol/L; Cell Signaling Technology, Danvers, MA, USA) was used to eliminate the activation of the AKT pathway in hPDLSCs.18 P3 hPDLSCs were cultured to passage of 10 hPDLSCs with E2 for long‐term stimulation. For the control, hPDLSCs were cultured in same medium but without E2.
2.3. Cell proliferation assay
Human periodontal ligament stem cells were seeded in 96‐well plates at the density of 2 × 103 cells per well. The cell viability was determined using the colorimetric MTS assay (CellTiter 96 Aqueous One solution, Promega, USA). After rinsed with PBS, cells were incubated with 20 μL MTS reagent in 100 μL serum‐free medium for 3 h at 37°C containing 5% CO2. Absorbance of the obtained dye at 490 nm was recorded by automatic microplate reader (Bio‐TEK, Winooski, VT, USA). All values are analysed as mean of quadruplicate samples from triplicate repeats.
2.4. Surface markers expression of hPDLSCs
Cells were collected and washed with PBS. Cells were then incubated with PE‐coupled antibodies against CD34, CD44, CD90, CD105, CD166 (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA) and FITC‐coupled antibody against CD146 (Becton Dickinson Biosciences). For the examination of STRO‐1 marker, cells were incubated with STRO‐1 antibody (R&D Systems, Minneapolis, MN, USA) for 1 hour on ice. After washing, cells were incubated with PE‐conjugated secondary antibody (R&D Systems) for 30 minutes on ice, and then washed with PBS.19, 20 Fluorescence was detected using a BD Accuri C6 (Becton Dickinson Biosciences). Data were analysed using CF Low Plus Software (Becton Dickinson Biosciences).
2.5. Cell cycle of hPDLSCs
Human periodontal ligament stem cells were first trypsinized and washed with PBS, then fixed with 70% ethanol at 4°C overnight. Cells were then washed with PBS 3 times, and incubated with 100 mg/L propidium iodine (Sigma‐Aldrich) and 100 mg/L RNase (Sigma‐Aldrich) for 30 minutes in the dark. Then, the cell cycles of hPDLSCs were detected and analysed by flow cytometry (Becton Dickinson Biosciences). The rate of proliferation period was measured as a percentage of cells in (G2/M + S) phases.21
2.6. Colony‐forming unit‐fibroblast (CFU‐F) assay
To assess the colony‐forming efficiency of hPDLSCs, cells were seeded at a density of 1000 cells /10 cm dishes in phenol red‐free α‐MEM. After 10 days, the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Aggregates of 50 or more cells were scored as a colony unit. Colony‐forming unit‐fibroblast efficiency was determined by the number of colony units relative to the total number of seeded cells.
2.7. Immunofluorescence staining
For immunofluorescence staining analysis, hPDLSCs were fixed with 4% paraformaldehyde in PBS for 20 minutes, rinsed with PBS 3 times and blocked with 5% bovine serum albumin for 1 hour. Human periodontal ligament stem cells were incubated with the following primary antibodies: mouse anti‐vimentin (1:100; Life Technologies, Carlsbad, CA, USA) and mouse anti‐cytokeratin (1:100; Life Technologies) overnight at 4°C. Mouse IgG was used under the same conditions to serve as negative control. After washes with PBS, the cells were incubated with goat anti‐mouse IgG antibody (1:300; Alexa Fluor 488; Life Technologies) for 45 minutes at room temperature in the dark. After Hoechst 33342 staining (Life Technologies), the cells were photographed with a Zeiss Axio Observer Z1 (Carl Zeiss, Germany).
2.8. Pluripotency of hPDLSCs
Human periodontal ligament stem cells were seeded at a density of 1 × 105 cells/well in 12‐well plates. For osteogenic differentiation, cells were cultured in an osteogenic induction medium (phenol red‐free α‐MEM supplemented with 10% FBS, 10 mmol/L β‐glycerophosphate, 10 nmol/L dexamethasone and 50 μg/mL ascorbic acid).22 The medium was replaced every other day with fresh osteogenic induction medium. Three weeks later, cells were fixed with 4% paraformaldehyde and stained by the Alizarin red. After dissolving with 10% (w/v) cetylpyridinium chloride (Sigma‐Aldrich), quantification of stained nodules was detected by automatic microplate reader at 562 nm.
For adipogenic differentiation, cells were cultured in adipogenic medium (Cyagen Biosciences Inc, Santa Clara, CA, USA). Two weeks later, cells were fixed with 4% paraformaldehyde and stained with oil red O.
2.9. Alkaline phosphatase (ALP) activity
After 7 days of osteogenic induction, hPDLSCs were fixed and stained following the protocol provided by the BCIP/NBT alkaline phosphatase colour development kit (Beyotime Biotechnology, China) for 30 minutes. ALP activity of hPDLSCs was measured according to the manufacturer's instructions using the Alkaline Phosphatase Assay kit (Beyotime Biotechnology).23, 24 Cells were incubated for 30 minutes in the dark. Quantification of ALP activity was detected using an automatic microplate reader at 405 nm.
2.10. Quantitative real‐time reverse transcription polymerase chain reaction (RT‐PCR)
Total RNA was isolated from hPDLSCs using TRIzol reagent (Life Technologies), and first‐strand cDNA was synthesized using a reverse transcriptase M‐MLV Kit (TaKaRa, Japan). Gene expression level was quantified by RT‐PCR using a SYBR Green kit (Roche, Switzerland) with the gene‐specific primers. Cycling parameters were set as follows: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 s, 60°C for 20 s, and 72°C for 20 s. The gene‐specific primers used are reported in Table 1.
Table 1.
Quantitative real‐time reverse transcription polymerase chain reaction (RT‐PCR) primers
| Genes target | Sequence | Predicted size (bp) |
|---|---|---|
| hTERT | Forward: 5′‐CCGATTGTGAACATGGACTACG‐3′ | 99 |
| Reverse: 5′‐CACGCTGAACAGTGCCTTC‐3′ | ||
| Oct4 | Forward: 5′‐GGGCTCTCCCATGCATTCAAAC‐3′ | 197 |
| Reverse: 5′‐CACCTTCCCTCCAACCAGTTGC‐3′ | ||
| Sox2 | Forward: 5′‐GTGAGCGCCCTGCAGTACAA‐3′ | 82 |
| Reverse: 5′‐GCGAGTAGGACATGCTGTAGGTG‐3′ | ||
| c‐Myc | Forward: 5′‐AATGAAAAGGCCCCCAAGGTAGTTATCC‐3′ | 112 |
| Reverse:5′‐GTCGTTTCCGCAACAAGTCCTCTTC‐3′ | ||
| GAPDH | Forward: 5′‐AGCCACATCGCTCAGACAC‐3′ | 67 |
| Reverse: 5′‐GCCCAATACGACCAAATCC‐3′ |
hTERT, human telomerase reverse transcriptase.
2.11. Western blotting
Cell extracts containing 40 μg of total protein were separated by electrophoresis on sodium dodecyl sulphate polyacrylamide gels, and subsequently transferred to nitrocellulose membranes. After transfer, the membranes were blocked with PBS containing 5% non‐fat milk for 1 hour at room temperature, and then incubated at 4°C overnight with primary antibodies against Total AKT (T‐AKT, Boster, Wuhan, China), Phospho‐AKT (P‐AKT; Cell Signaling Technology), T‐GSK‐3β (Cell Signaling Technology), Phospho‐GSK‐3β (Cell Signaling Technology), β‐catenin (Cell Signaling Technology), Cyclin D1 (Abcam, Cambridge, MA, USA), Runt‐related transcription factor 2 (RUNX2; Boster), Bone morphogenic protein‐2 (BMP2; Boster) and Bone sialoprotein (BSP; Boster). Antibody against β‐actin (Sigma‐Aldrich) was used as normalizing control. The membranes were subsequently incubated with the secondary antibodies for 1 hour at room temperature. The results were analysed using an Odyssey 2‐colour infrared laser imaging system (LI‐COR Biosciences, Lincoln, NE, USA). Relative density of labelled protein band was analysed by Image‐ProPlus 5.0 software (Media Cybernetics Inc, Rockville, MD, USA)
2.12. Statistical analysis
All experiments were performed in triplicate. The SPSS 20.0 software package (SPSS, Chicago, IL, USA) was used for the statistical tests. Values are expressed as the mean ± SD. A 1‐way ANOVA was used for the comparison among groups. The comparison between P3 hPDLSCs and P10 hPDLSCs used the Student's t test. In this study, P<.05 is considered statistically significant.
3. RESULTS
3.1. Characterization of hPDLSCs
Human periodontal ligament stem cells formed colonies from a single cell, and exhibited a typical fibroblast‐like morphology (Figure 1A). To assess the pluripotency of hPDLSCs, alizarin Red S staining and oil red O staining were performed. The results showed that hPDLSCs had osteogenic and adipogenic differentiation abilities (Figure 1B,C). Human periodontal ligament stem cells were positive for MSC surface markers, including CD44 (98.5%), CD90 (99.2%), CD105 (99.7%), CD146 (37.9%) and CD166 (97.8%), but they were negative for the hematopoietic cell marker CD34 (0.056%; Figure 1D). Human periodontal ligament stem cells positively expressed the vimentin, a marker of MSC, and negatively expressed the cytokeratin, which is an epithelial marker (Figure 1E). Therefore, the results suggested that the isolated hPDLSCs were mesenchymal origin.
Figure 1.
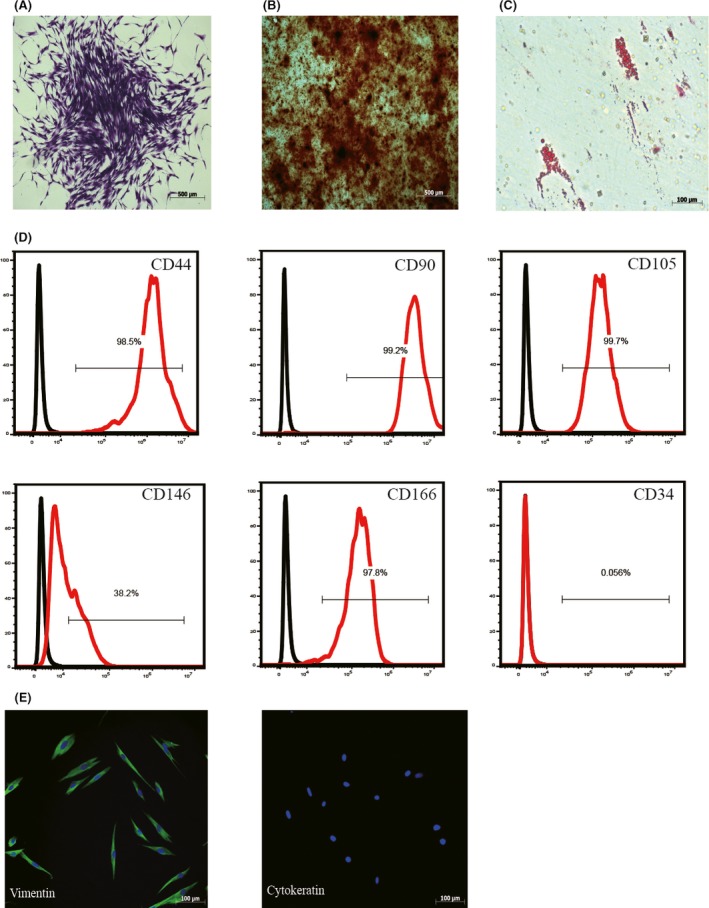
Human periodontal ligament stem cells (hPDLSCs) characterization. hPDLSCs showed the capacity of colony‐forming (A), osteogenic differentiation (B) and adipogenic differentiation (C). Flow cytometric analysis confirmed that hPDLSCs positively expressed CD44, CD90, CD105, CD146 and CD166, while negatively expressed CD34 (D). Immunofluorescence staining demonstrated that hPDLSCs positively expressed vimentin and negatively expressed cytokeratin (E)
3.2. E2 improved the proliferation and the expression of stemness‐related genes of hPDLSCs
The effects of E2 on the proliferation of P3 hPDLSCs were assessed by MTS assay. Results showed that, in the time points of 48 and 72 hours, cells of 10−7 mol/L E2 group had a higher proliferation rate than Control group, 10−8 and 10−6 mol/L E2 groups (Figure 2A). The concentration of 10−7 mol/L was selected as the optimal concentration in the following experiments. To confirm this effect was caused by E2, we added an oestrogen receptor antagonist, ICI 182780 (ICI)which would specifically block E2 signalling.25 The results showed that ICI impaired the promotion of cells proliferation induced by E2 and indicated that this effect was specifically induced by E2. We noticed that E2 affected the cells started at 48 hours (Figure 2B). Thus, the time of 48 hours was used as the appropriate observing time in the following experiments. In cell cycle analysis, we found that the ratio of E2 group during the proliferation period of G2/M + S phase was significantly higher than Control group. However, the rate of proliferation period (G2/M + S) significantly decreased in E2 + ICI group (Figure 2C). It suggested that E2 enhance the proliferation rate of hPDLSCs via prolong the ratio of G2/M + S phase. Human telomerase reverse transcriptase (hTERT), the catalytic component of telomerase, is closely involved in the stem‐like properties in hBMSC.26 E2 treatment significantly increased the levels of hTERT, while the effect could be abrogated by the additional ICI treatment (Figure 2D). Pluripotent transcription factors Sox2, Oct4 and c‐Myc play important roles in regulation of MSCs cycle, which is considered as stemness‐related genes in stemness maintenance.27 We evaluated the effects of E2 on the gene expression of Sox2, Oct4 and c‐Myc by RT‐PCR. Similarly, E2 treatment significantly increased the levels of Sox2, Oct4 and c‐Myc, while the expression decreased in E2 + ICI group (Figure 2D).
Figure 2.
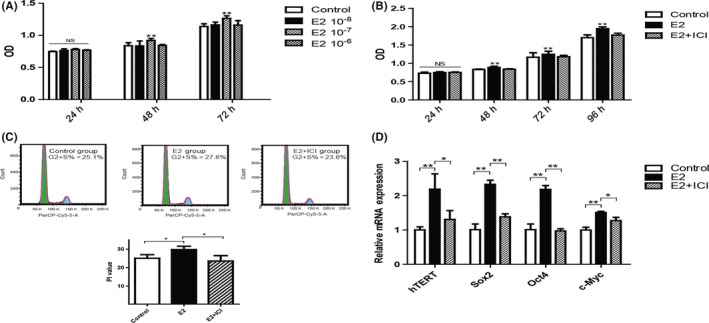
Oestrogen improved the stemness of human periodontal ligament stem cells (hPDLSCs) in short‐term culture. Human periodontal ligament stem cells were treated with different concentrations of oestrogen (10−6, 10−7 and 10−8 mol/L) during various culture times, and the proliferation of cells was subsequently assessed (A). The effect of 10−7 mol/L oestrogen (E2) on the proliferation of hPDLSCs was detected by MTS assay (B). Cell cycle distribution of hPDLSCs under 10−7 mol/L E2 during short‐term stimulation and the percentages of cells residing in the G2 + S phases are shown in the graphs (C). The expression of human telomerase reverse transcriptase (hTERT) and stemness‐related genes (Sox2, Oct4 and c‐Myc) was tested by RT‐PCR during short‐term culture. The mRNA expression levels were normalized to GAPDH mRNA (D). Control group: cells were cultured in 0.01% (V/V) ethyl alcohol. E2 group: cells were cultured with 10−7 mol/L oestrogen. E2 + ICI group: cells were cultured with 10−7 mol/L oestrogen and 10−6 mol/L ICI 182780. Data are shown as the mean ± SDs of 3 separate experiments. *P<.05; **P<.01; NS, no statistical significance
3.3. E2 treatment activated PI3K/AKT signalling pathway in hPDLSCs
We next investigated the potential signalling pathway involved in the function of E2 to P3 hPDLSCs. It has been reported that PI3K/AKT signalling pathway plays a crucial role in stem cells self‐renewal, differentiation and stemness maintenance.28 Therefore, we focused on PI3K/AKT signalling pathway during E2 treatment in 48 hours. Compared with Control group, the expression levels of P‐AKT and P‐GSK‐3β significantly increased in E2 group. Then, we continuously detected the expression of β‐catenin and Cyclin D1, which are the downstream molecules of AKT signalling pathway. We found that the expression of both molecules were increased in E2 group, on the contrary, the expression of these proteins were inhibited in E2 + ICI group (Figure 3A,B). Furthermore, LY294002, a PI3K/AKT inhibitor, was used to elucidate the role of the PI3K/AKT signalling pathway in the observed stemness improvement of E2 treated hPDLSCs. Cells in E2 + LY294002 group (E2 + LY group) expressed relatively lower levels of P‐AKT, P‐GSK‐3β and their downstream proteins compared with E2 group (Figure 3A,B). MTS assay exhibited that the proliferation rate of E2 + LY group lower than E2 group after 48 hours (Figure 3C). RT‐PCR results showed that the expression of hTERT, Sox2, Oct4, and c‐Myc were significantly down‐regulated in E2 + LY group in comparison to E2 group (Figure 3D). These results exhibited that E2 treatment improved the stemness of hPDLSCs via the PI3K/AKT signalling pathway.
Figure 3.
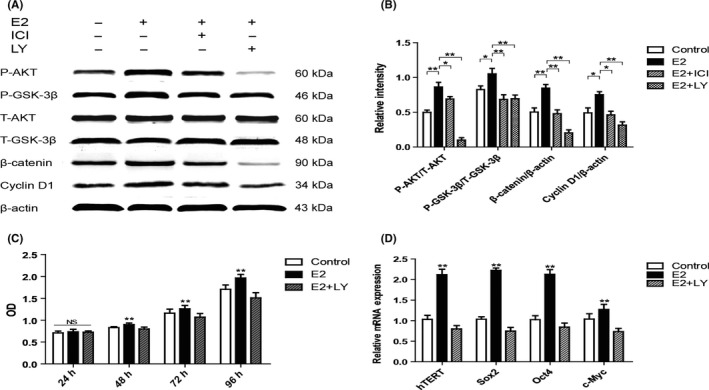
Oestrogen improved the stemness of human periodontal ligament stem cells (hPDLSCs) by the activation of PI3K/AKT signalling pathway. Compared with Control group, PI3K/AKT/GSK‐3β signalling pathway was significantly activated in E2 group, and the activation was inhibited in E2 + ICI and E2 + LY groups (A). The relative expression of proteins is shown in the graph (B). The rate of proliferation of Control group, E2 group and E2 + LY group were assessed by MTS assay (C). The expression of hTERT and stemness‐related genes (Sox2, Oct4 and c‐Myc) was tested by RT‐PCR. The mRNA expression levels were normalized to GAPDH mRNA (D). E2 + LY group: cells were cultured with 10−7 mol/L oestrogen and 2.5 μmol/L LY294002. Data are shown as the mean ± SDs of 3 separate experiments. *P<.05; **P<.01; NS, no statistical significance
3.4. E2 improved the proliferation and stemness‐related genes of hPDLSCs in long‐term culture
To determine the effect of long‐term in vitro passaging on the stemness of hPDLSCs, we compared the ability of proliferation and osteogenic differentiation between P3 hPDLSCs and P10 hPDLSCs. Proliferation assay showed that the growth rate of P10 hPDLSCs is lower than P3 hPDLSCs (Figure 4A). The expression of hTERT, Sox2, Oct4 and c‐Myc was significantly decreased in P10 hPDLSCs in comparison with P3 hPDLSCs (Figure 4B). Alizarin red staining revealed that mineralized nodules formed by P10 hPDLSCs were significantly less than P3 hPDLSCs (Figure 4C). Taken together, these data indicate that the stemness of hPDLSCs is significantly decreased in P10 hPDLSCs in comparison with P3 hPDLSCs.
Figure 4.
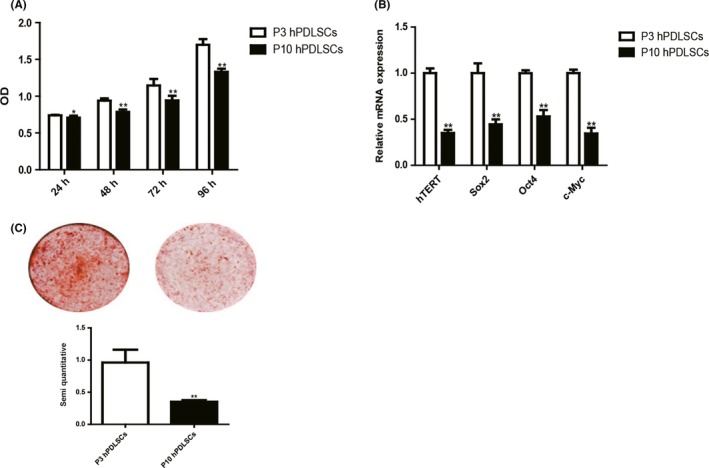
The ability of proliferation and osteogenic differentiation decreased in P10 human periodontal ligament stem cells (hPDLSCs). The rate of proliferation between P3 hPDLSCs and P10 hPDLSCs was compared by MTS assay (A). The expression of human telomerase reverse transcriptase (hTERT) and stemness‐related genes (Sox2, Oct4 and c‐Myc) between P3 hPDLSCs and P10 hPDLSCs was tested by RT‐PCR. The mRNA expression levels were normalized to GAPDH mRNA (B). The ability of osteogenic differentiation between P3 hPDLSCs and P10 hPDLSCs was compared by Alizarin red staining and quantification (C). Data are shown as the mean ± SDs of 3 separate experiments. *P<.05; **P<.01
To evaluate the function of E2 during long‐term culture, we examined the proliferation rate of P10 hPDLSCs. By MTS analysis, we found that the proliferation rate of E2 group was significantly higher than Control group, while the proliferation rate was decreased in E2 + ICI group at 72 and 96 hours (Figure 5A). Colony‐forming unit‐fibroblast assay results showed that E2 group had higher CFU‐F efficiency (25.5% ± 2.587%) than Control group (21.1% ± 0.755%), but ICI treatment reversed the elevated CFU‐F efficiency (20.0% ± 1.716%; Figure 5B). Next, we explored the influence of E2 on hTERT and stemness‐related genes expression in long‐term culture by RT‐PCR. Similar to short‐term culture, the expression of hTERT, Sox2, Oct4 and c‐Myc increased in E2 group, while the expression of genes decreased in E2 + ICI group (Figure 5C). These results revealed that E2 was able to elevate proliferation and the expression of stemness‐related genes of P10 hPDLSCs.
Figure 5.
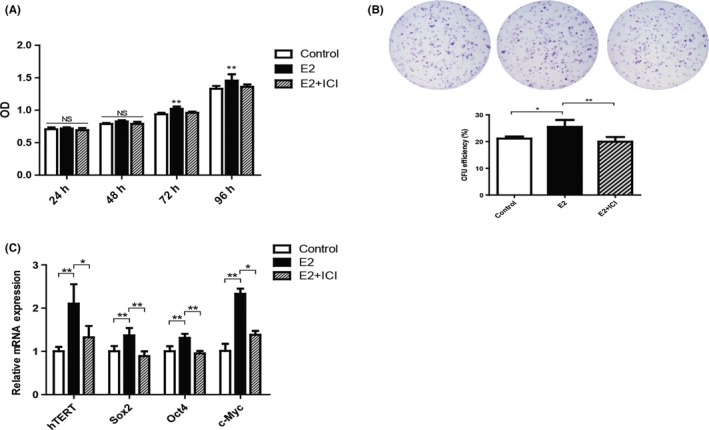
Oestrogen retained the loss of hPDLSCs stemness in long‐term culture. E2 promoted the proliferation rate and colony‐forming capability of hPDLSCs during long‐term culture (A, B). The expression of human telomerase reverse transcriptase (hTERT) and stemness‐related genes was tested by RT‐PCR (C). Data are shown as the mean ± SDs of 3 separate experiments. *P<.05; **P<.01; NS, no statistical significance
3.5. E2 enhanced osteogenic and adipogenic differentiation of hPDLSCs in long‐term culture
To investigate the effects of E2 treatment on the osteogenic differentiation of P10 hPDLSCs, the analyses of alizarin red staining, ALP activity assay and Western blotting were used.
Alizarin red staining and quantification results showed that E2 group formed more noticeable mineral nodules than Control group after osteogenic induction (Figure 6A). From the results of ALP staining and activity assay, the ALP level in E2 group was significantly higher than Control group (Figure 6B). Next we detected the expression of RUNX2, BMP2 and BSP that are considered closely related with osteogenic differentiation.29 Western blotting results showed that the expression of RUNX2, BMP2 and BSP in E2 group was significantly increased (Figure 6C). Nevertheless, the ability of osteogenic differentiation was inhibited by ICI additional treatment. Oil red O staining results showed that E2 elevated the adipogenic differentiation of P10 hPDLSCs, comparing with Control and E2 + ICI group (Figure 6D). These data suggested that E2 promoted osteogenic and adipogenic differentiation of P10 hPDLSCs, compared to Control and E2 + ICI groups.
Figure 6.
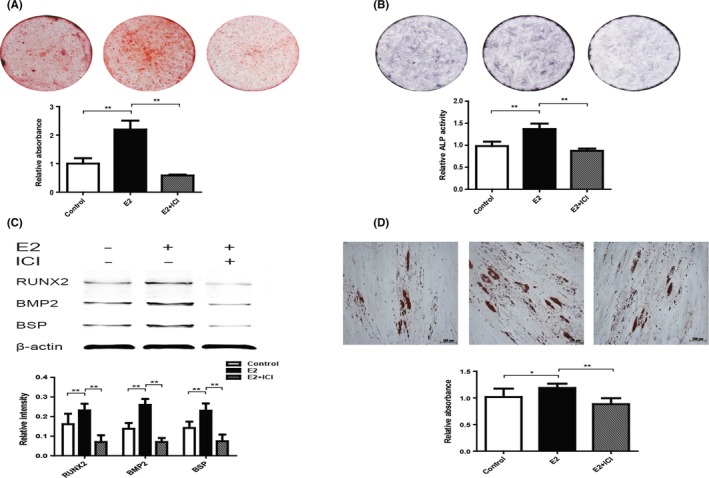
Oestrogen enhanced osteogenic and adipogenic differentiation of hPDLSCs in long‐term culture. The osteogenic differentiation capability of hPDLSCs was tested by Alizarin red staining and quantification (A). Alkaline phosphatase (ALP) staining and ALP activity results were shown in the graph (B). The expression of proteins (RUNX2, BMP2 and BSP) was detected by Western blotting, and the relative protein expression level was normalized to β‐actin (C). The adipogenic differentiation capability of hPDLSCs was tested by oil red O staining and quantification (D). RUNX2: Runt‐related transcription factor 2; BMP2: Bone morphogenic protein‐2; BSP: Bone sialoprotein. Data are shown as the mean ± SDs of 3 separate experiments. *P<.05; **P<.01
3.6. E2 elevated the positive rate of CD146 and STRO‐1 of hPDLSCs in long‐term culture
We examined the effects of E2 on the expression of surface molecules (CD34, CD44, CD90, CD105, CD146, CD166 and STRO‐1) in P10 hPDLSCs. We found that the positive rate of CD146 is 27.87 ± 1.65 in E2 group, which is significantly higher than Control and E2 + ICI group, which are 22.23 ± 2.51 and 21.13 ± 2.36 respectively (Figure 7). The positive rate of STRO‐1 is 3.16 ± 0.28 in E2 group, which is significantly higher than Control and E2 + ICI group, which are 2.57 ± 0.15 and 2.60 ± 0.36 respectively (Figure 7). In addition, there was no significant difference for other mesenchymal markers (CD34, CD44, CD90, CD105 and CD166) among control, E2 and E2 + ICI groups (Figure 7). As the positive rate of CD146 and STRO‐1 is related with the multipotency of MSCs,30, 31 the results indicate that the cells have higher multipotency in E2 group.
Figure 7.
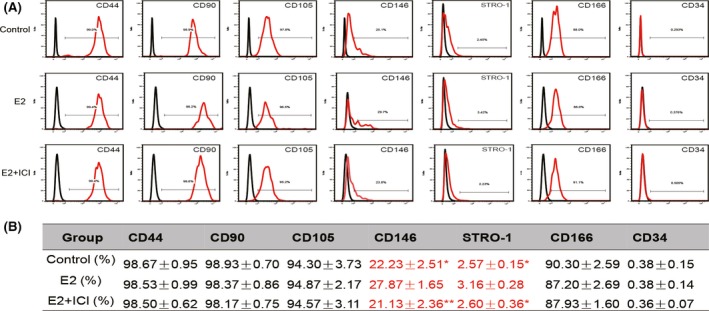
The effect of oestrogen on the surface markers of hPDLSCs during long‐term culture. The effect of oestrogen on the surface markers of hPDLSCs was tested by flow cytometry. Representative figures of cytometric flow tests (A). Percentage of positive expression (B). Data are shown as the mean ± SDs of 3 separate experiments. *P<.05 vs E2 group, **P<.01 vs E2 group
4. DISCUSSION
Tissue engineering provides a promising prospective method for the periodontal tissue regeneration, and stem cell plays a crucial role in the periodontal tissue engineering.32 It has been previously reported that MSCs stemness gradually declines during long‐term culture in vitro, including the decrease in proliferation and differentiation, which strongly limits MSC function in tissue engineering.33, 34 Stemness maintenance of hPDLSCs during in vitro culturing becomes one of the major challenges in tissue engineering.
Oestrogen (E2) plays an essential role in regulating physiological functions of mesenchymal stem cell types. E2 promotes hematopoietic stem cells (HSCs) self‐renewal and erythropoiesis in the spleen, inhibits BMSCs apoptotic rate, and increase colony‐forming via modulating Bcl‐2 and Bcl‐xL proteins.35, 36 Furthermore, E2 can enhance proliferation and odonto/osteogenic differentiation of human dental pulp stem cells and stem cells from apical papilla.14, 15 We found that short‐term E2 treatment significantly improved the ratio of (G2/M+S) phases and the expression of cyclin D1 (Figures 2 and 3). This may be due to E2 regulation of cyclin D1 and CDK4/6, thus promoting cell transformation from the G2 phase to the S phase.37, 38 We also found that the E2‐induced proliferation was reversed by the oestrogen receptor antagonist ICI 182780.
In this study, we mixed the male and female hPDLSCs together. However, previous studies demonstrated that there is a gender difference regarding response of male or female donors when MSCs treated with E2 or its antagonist,13, 39 which probably caused by the gender difference of ER and oestrogen‐related receptor in male and female MSCs. Due to the gender difference, the concentration of E2 to male individual needs to be further explored in the application of tissue engineering, avoiding the occurrence of side effects, such as prostate cancer, heart disease and Alzheimer's disease.40, 41 Therefore, we will focus on the gender difference of the effect of E2 on hPDLSCs in the future study.
During MSC proliferation in vitro, a decrease in telomere length correlates with the loss of cell viability, suggesting that telomere length and telomerase activity are indispensable for the stemness maintenance.42, 43 Human telomerase reverse transcriptase elongates and stabilizes telomeres, alleviating replicative senescence and apoptosis.44 In our study, E2 treatment increased the expression of hTERT in hPDLSCs and prevented cellular senescence (Figures 2 and 5), which suggests that E2 modulate hTERT to maintain hPDLSCs stemness. Sox2, Oct4 and c‐Myc are critical stemness‐related genes that maintain pluripotency and self‐renewal of embryonic stem cells.9 Previous works show that hPDLSCs growth and proliferation can be promoted by bone morphogenetic protein‐4 (BMP‐4) via prompting the expression of Sox2, Oct4 and c‐Myc.9, 45, 46 In our trial, both short‐ and long‐term E2 treatment significantly enhanced the expression of Sox2, Oct4 and c‐Myc. This suggests that E2 treatment retains the loss of hPDLSCs stemness via Sox2‐, Oct4‐ and c‐Myc‐related signalling pathways (Figures 2 and 5).
Previous studies show that activation of PI3K/AKT pathway stimulates MSC proliferation and differentiation and promotes survival rate and stemness during long‐term culture in vitro.46, 47 It has been reported that E2 promotes the proliferation of MSCs and improves the activity of hTERT via the PI3K/AKT pathway.48 We further investigated the mechanism of E2 treatment on hPDLSCs stemness. Our results showed that PI3K/AKT/GSK‐3β signalling was activated by E2 treatment, while the function was inhibited by LY294002 (Figure 3).
Current evidence indicates that during long‐term culture, the proliferation and differentiation capabilities of MSCs decline and the expression of stemness‐related genes decrease.49 In this study, we also found that the stemness of P10 hPDLSCs decreased after long‐term culture, which was accompanied by the down‐regulation of stemness‐related genes (Figure 4). Comparing with short‐term stimulation, long‐term stimulation has the advantages of high efficiency, specificity and safely.50 In long‐term stimulation of E2, we observed that the loss of proliferation and differentiation capacities of hPDLSCs was retained in passages of 10 (Figures 5 and 6). We found that P10 hPDLSCs showed higher positive rate of CD146 and STRO‐1 in E2 group (Figure 7). It has been shown that the high expression of CD146 and STRO‐1 in MSCs is correlated with proliferative potential and osteogenic differentiation capacity.31, 51 Therefore, the result suggests that E2 promotes the stemness of hPDLSCs by enhancing the positive rate of CD146 and STRO‐1.
In summary, our data indicate that short‐term and long‐term E2 treatment are able to promote the stemness of hPDLSCs in vitro cultures, which caused by stimulation of PI3K/AKT signalling pathway. These findings provide a new insight into the stemness modulation of hPDLSCs and may facilitate the application of hPDLSCs in tissue engineering.
COMPETING INTERESTS
The authors have no competing interests to declare.
Ou Q, Wang X, Wang Y, Wang Y, Lin X. Oestrogen retains human periodontal ligament stem cells stemness in long‐term culture. Cell Prolif. 2018;51:e12396 10.1111/cpr.12396
This work was supported by the Natural Science Foundation of China (nos. 81530069) and the Guangdong Innovative Research Team program (no. 2009010058).
Contributor Information
Yan Wang, Email: wang93@mail.sysu.edu.cn.
Xuefeng Lin, Email: linxfeng@mail.sysu.edu.cn.
REFERENCES
- 1. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990‐2010: a systematic review and meta‐regression. J Dent Res. 2014;93:1045‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen FM, Gao LN, Tian BM, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu T, Volponi AA, Babb R, An Z, Sharpe PT. Stem cells in tooth development, growth, repair, and regeneration. Curr Top Dev Biol. 2015;115:187‐212. [DOI] [PubMed] [Google Scholar]
- 4. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149‐155. [DOI] [PubMed] [Google Scholar]
- 5. Zhu W, Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int. 2015;2015:972313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20:271‐285. [DOI] [PubMed] [Google Scholar]
- 7. Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell‐based therapy. Int J Mol Sci. 2016;17:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeong SG, Cho GW. Accumulation of apoptosis‐insensitive human bone marrow‐mesenchymal stromal cells after long‐term expansion. Cell Biochem Funct. 2016;34:310‐316. [DOI] [PubMed] [Google Scholar]
- 9. Liu L, Wei X, Huang R, Ling J, Wu L, Xiao Y. Effect of bone morphogenetic protein‐4 on the expression of Sox2, Oct‐4, and c‐Myc in human periodontal ligament cells during long‐term culture. Stem Cells Dev. 2013;22:1670‐1677. [DOI] [PubMed] [Google Scholar]
- 10. Yao W, Guan M, Jia J, et al. Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013;31:2003‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol. 2013;13:576‐581. [DOI] [PubMed] [Google Scholar]
- 13. Hong L, Zhang G, Sultana H, Yu Y, Wei Z. The effects of 17‐beta estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev. 2011;20:925‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Yan M, Wang Z, et al. 17beta‐estradiol promotes the odonto/osteogenic differentiation of stem cells from apical papilla via mitogen‐activated protein kinase pathway. Stem Cell Res Ther. 2014;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Zheng Y, Wang Z, et al. 10(‐7) m 17beta‐oestradiol enhances odonto/osteogenic potency of human dental pulp stem cells by activation of the NF‐kappaB pathway. Cell Prolif. 2013;46:677‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Wang Y, Dai X, et al. Effects of intermittent administration of parathyroid hormone (1‐34) on bone differentiation in stromal precursor antigen‐1 positive human periodontal ligament stem cells. Stem Cells Int. 2016;2016:4027542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao M, Shu L, Li J, et al. The expression of estrogen receptors and the effects of estrogen on human periodontal ligament cells. Methods Find Exp Clin Pharmacol. 2007;29:329‐335. [DOI] [PubMed] [Google Scholar]
- 18. Xiang Q, Hong D, Liao Y, et al. Overexpression of gremlin1 in mesenchymal stem cells improves hindlimb ischemia in mice by enhancing cell survival. J Cell Physiol. 2017;232:996‐1007. [DOI] [PubMed] [Google Scholar]
- 19. Wang WJ, Zhao YM, Lin BC, Yang J, Ge LH. Identification of multipotent stem cells from adult dog periodontal ligament. Eur J Oral Sci. 2012;120:303‐310. [DOI] [PubMed] [Google Scholar]
- 20. Li Q, Ma Y, Zhu Y, Zhang T, Zhou Y. Declined expression of histone deacetylase 6 contributes to periodontal ligament stem cell aging. J Periodontol. 2017;88:e12‐e23. [DOI] [PubMed] [Google Scholar]
- 21. Liu LY, Hou YS, Chai JK, et al. Basic fibroblast growth factor/vascular endothelial growth factor in the serum from severe burn patients stimulates the proliferation of cultured human umbilical cord mesenchymal stem cells via activation of Notch signaling pathways. J Trauma Acute Care Surg. 2013;75:789‐797. [DOI] [PubMed] [Google Scholar]
- 22. Cai C, Yuan GJ, Huang Y, et al. Estrogen‐related receptor alpha is involved in the osteogenic differentiation of mesenchymal stem cells isolated from human periodontal ligaments. Int J Mol Cell Med. 2013;31:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 23. Wu Y, Yang M, Fan J, et al. Deficiency of osteoblastic Arl6ip5 impaired osteoblast differentiation and enhanced osteoclastogenesis via disturbance of ER calcium homeostasis and induction of ER stress‐mediated apoptosis. Cell Death Dis. 2014;5:e1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie Q, Wang Z, Bi X, et al. Effects of miR‐31 on the osteogenesis of human mesenchymal stem cells. Biochem Biophys Res Commun. 2014;446:98‐104. [DOI] [PubMed] [Google Scholar]
- 25. Zhang W, Schmull S, Du M, et al. Estrogen receptor alpha and beta in mouse: adipose‐derived stem cell proliferation, migration, and brown adipogenesis in vitro. Cell Physiol Biochem. 2016;38:2285‐2299. [DOI] [PubMed] [Google Scholar]
- 26. Huang G, Zheng Q, Sun J, et al. Stabilization of cellular properties and differentiation mutilpotential of human mesenchymal stem cells transduced with hTERT gene in a long‐term culture. J Cell Biochem. 2008;103:1256‐1269. [DOI] [PubMed] [Google Scholar]
- 27. Karamzadeh R, Baghaban EM, Sharifi‐Zarchi A. Comparative in vitro evaluation of human dental pulp and follicle stem cell commitment. Cell J. 2017;18:609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh S, Moirangthem RD, Vaidya A, Jalnapurkar S, Limaye L, Kale V. AKT signaling prevailing in mesenchymal stromal cells modulates the functionality of hematopoietic stem cells via intercellular communication. Stem Cells. 2016;34:2354‐2367. [DOI] [PubMed] [Google Scholar]
- 29. Chatakun P, Nunez‐Toldra R, Diaz LE, et al. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature. Cell Mol Life Sci. 2014;71:113‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O'Connor KC. In vitro high‐capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788‐798. [DOI] [PubMed] [Google Scholar]
- 31. Frank V, Kaufmann S, Wright R, et al. Frequent mechanical stress suppresses proliferation of mesenchymal stem cells from human bone marrow without loss of multipotency. Sci Rep. 2016;6:24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Yu F, Sun Y, et al. Concise reviews: characteristics and potential applications of human dental tissue‐derived mesenchymal stem cells. Stem Cells. 2015;33:627‐638. [DOI] [PubMed] [Google Scholar]
- 33. Ng CP, Sharif AR, Heath DE, et al. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomater. 2014;35:4046‐4057. [DOI] [PubMed] [Google Scholar]
- 34. Farrell MJ, Fisher MB, Huang AH, Shin JI, Farrell KM, Mauck RL. Functional properties of bone marrow‐derived MSC‐based engineered cartilage are unstable with very long‐term in vitro culture. J Biomech. 2014;47:2173‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ayaloglu‐Butun F, Terzioglu‐Kara E, Tokcaer‐Keskin Z, Akcali KC. The effect of estrogen on bone marrow‐derived rat mesenchymal stem cell maintenance: inhibiting apoptosis through the expression of Bcl‐xL and Bcl‐2. Stem Cell Rev. 2012;8:393‐401. [DOI] [PubMed] [Google Scholar]
- 36. Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem‐cell self‐renewal in females and during pregnancy. Nature. 2014;505:555‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor‐dependent divergent functions in breast cancer migration and stem cell‐like activity. Cell Cycle. 2013;12:2384‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han HJ, Heo JS, Lee YJ. Estradiol‐17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006;290:C1067‐C1075. [DOI] [PubMed] [Google Scholar]
- 39. Hong L, Sultana H, Paulius K, Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol. 2009;114:180‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams GP. The role of oestrogen in the pathogenesis of obesity, type 2 diabetes, breast cancer and prostate disease. Eur J Cancer Prev. 2010;19:256‐271. [DOI] [PubMed] [Google Scholar]
- 41. Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185:17‐23. [DOI] [PubMed] [Google Scholar]
- 42. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675‐682. [DOI] [PubMed] [Google Scholar]
- 43. Samsonraj RM, Raghunath M, Hui JH, Ling L, Nurcombe V, Cool SM. Telomere length analysis of human mesenchymal stem cells by quantitative PCR. Gene. 2013;519:348‐355. [DOI] [PubMed] [Google Scholar]
- 44. Zhao Q, Wang XY, Yu XX, et al. Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int J Mol Biol Med. 2015;36:857‐864. [DOI] [PubMed] [Google Scholar]
- 45. Chen X, Xu H, Yuan P, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106‐1117. [DOI] [PubMed] [Google Scholar]
- 46. Palumbo S, Tsai TL, Li WJ. Macrophage migration inhibitory factor regulates AKT signaling in hypoxic culture to modulate senescence of human mesenchymal stem cells. Stem Cells Dev. 2014;23:852‐865. [DOI] [PubMed] [Google Scholar]
- 47. Zhou H, Li D, Shi C, et al. Effects of Exendin‐4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep. 2015;5:12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cha Y, Kwon SJ, Seol W, Park KS. Estrogen receptor‐alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Mol Cells. 2008;26:454‐458. [PubMed] [Google Scholar]
- 49. Gu Y, Li T, Ding Y, et al. Changes in mesenchymal stem cells following long‐term culture in vitro. Mol Med Rep. 2016;13:5207‐5215. [DOI] [PubMed] [Google Scholar]
- 50. Shuai Y, Liao L, Su X, et al. Melatonin treatment improves mesenchymal stem cells therapy by preserving stemness during long‐term in vitro expansion. Theranostics. 2016;6:1899‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu W, Tan Y, Qiu Q, et al. Comparison of the properties of human CD146+ and CD146− periodontal ligament cells in response to stimulation with tumour necrosis factor alpha. Arch Oral Biol. 2013;58:1791‐1803. [DOI] [PubMed] [Google Scholar]


