Abstract
The oxygenation of polyunsaturated fatty acids such as arachidonic and linoleic acid through lipoxygenases (LOXs) and cyclooxygenases (COXs) leads to the production of bioactive lipids that are important both in the induction of acute inflammation and its resolution. Amongst the several isoforms of LOX that are expressed in mammals, 15‐LOX‐1 was shown to be important both in the context of inflammation, being expressed in cells of the immune system, and in epithelial cells where the enzyme has been shown to crosstalk with a number of important signalling pathways. This review looks into the latest developments in understanding the role of 15‐LOX‐1 in different disease states with emphasis on the emerging role of the enzyme in the tumour microenvironment as well as a newly re‐discovered form of cell death called ferroptosis. We also discuss future perspectives on the feasibility of use of this protein as a target for therapeutic interventions.
Keywords: 15‐LOX‐1, cancer, ferroptosis, inflammation, tumour microenvironment
1. INTRODUCTION
The polyunsaturated fatty acids (PUFAs) arachidonic acid (AA) and linoleic acid (LA) and the enzymes that metabolize these PUFAs have emerged to be essential regulators of crucial cellular processes in the context of cancer and inflammation. AA is a 20‐carbon fatty acid that is released from phospholipids of the nuclear membrane with the activity of phospholipase A2 (PLA2), which is further metabolized by cyclooxygenases (COXs) or lipoxygenases (LOXs).1 Alternatively, the essential fatty acid LA can be processed in most mammalian cells to AA.2 When AA is metabolized by COX‐1 or COX‐2, it gives rise to eicosanoids such as prostaglandins. COX‐1 is expressed constitutively for many essential functions, while COX‐2 expression is inducible and the produced prostaglandins are essential for pain and inflammation. On the other hand, metabolism of AA through the LOX pathway leads to the production of leukotrienes (LTs), which can act as inflammatory mediators, or in a temporal manner be further metabolized into precursors such as lipoxins (Lxs) that are essential for the resolution of inflammation.1 Metabolism of other PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) through LOXs result in the production of a series of compounds known as resolvins (Rvs) and protectins that have roles in the resolution of inflammation.3, 4
Lipoxygenases can be classified into several categories based on the position of the carbon on AA that is oxygenated. Human LOX enzymes include 5‐LOX, 12‐LOX and 15‐LOX. Human ALOX15 was initially named arachidonate 15‐lipoxygenase (ALOX15) or 15‐lipoxygenase (15‐LOX). Subsequent studies uncovered a second human enzyme with 15‐lipoxygenase activity. Therefore, the product of human ALOX15 gene is now referred to as 15‐LOX‐1, and the second discovered human 15‐lipoxygenase, a product of the ALOX15B gene, is called 15‐LOX‐2.5, 6 15‐LOX‐1 catalyses its preferred substrate LA to 13‐hydroxyoctadecadienoic acid (13‐HODE); 15‐LOX‐2 preferentially acts on AA to produce 15‐hydroxy‐5Z,8Z,11Z,13E‐eicosatetraenoic acid (15‐HETE).7 The production of 13‐HODE or 15‐HETE may have very different outcomes in a cell in terms of neoplastic transformation8 (Figure 1; described in detail later).
Figure 1.
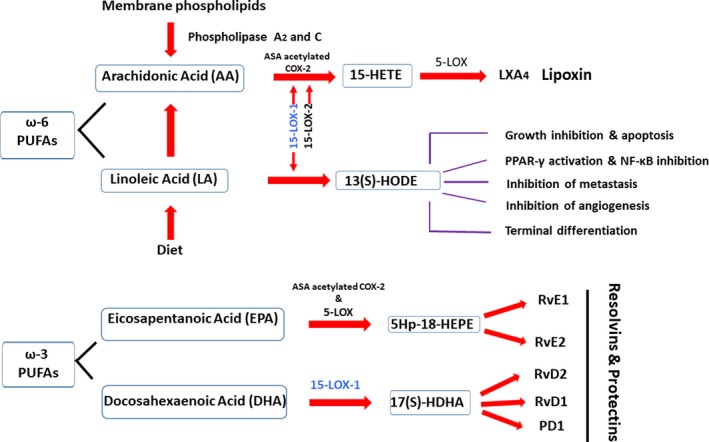
Metabolism of ω‐3 & ω‐6 PUFAs by 15‐LOX‐1 results in the production of bioactive lipids with varying functions in inflammation and cancer. The ω‐6 polyunsaturated fatty acid (PUFAs) arachidonic acid (AA) is obtained from membrane phospholipids and converted to 15‐HETE as a minor product of 15‐LOX‐1 and a major product of 15‐LOX‐2. 15‐HETE can be further converted to Lipoxins in the presence of 5‐LOX. The PUFA LA, an essential fatty acid, is oxygenated to 13(S)‐HODE by 15‐LOX‐1. Both of the bioactive lipids have important roles in cancer. The ω‐3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), also known as fish oils, are oxygenated by aspirin acetylated COX‐2, 15‐LOX‐1 and 5‐LOX in a transcellular and temporal manner producing the E‐ and D‐series of Resolvins and Protectins. These autacoids, along with Lipoxins, are generated during the resolution phase of inflammation
15‐LOX‐1 is primarily expressed in reticulocytes and macrophages.9 The ALOX15 gene is located on chromosome 17, p13.3 locus, and has 14 exons (GenBank: NC_000017). It shares 40% identity with 15‐LOX‐2.10 The 15‐LOX‐1 protein has a C‐terminal domain that has been shown to be important for catalytic activity and an N‐terminal β‐barrel domain that resembles the C‐terminal β‐barrel domain of the human pancreatic lipase.11 The enzyme is located in the cytosol, but may be associated with organelles such as the endoplasmic reticulum and mitochondrial membranes.12 Herein, we summarize available data on the activity, transcriptional regulation and inflammatory functions of 15‐LOX‐1 and discuss the newly described roles of 15‐LOX‐1 and its related pathways in the context of inflammation and cancer, with particular emphasis on the tumour microenvironment (TME).
2. ENZYMATIC ACTIVITY OF 15‐LOX‐1
In addition to free fatty acids such as AA and LA, 15‐LOX‐1 can oxygenate a broad range of substrates that include esterified forms of naturally occurring polyenoic fatty acids, even when they are bound to biomembranes or lipoproteins.12 The enzyme has been shown to bind to biomembranes through its N‐terminal domain12 and is therefore thought to be in close proximity for direct oxygenation of complex lipids. Alternatively, it may oxygenate free fatty acids; the products, namely 15‐HETE and 13‐HODE, are more polar and may be incorporated into the membrane phospholipids.13 Additionally, 15‐LOX‐1 metabolites have been shown to activate NADPH oxidases leading to the production of reactive oxygen species (ROS),14 which in turn may also oxygenate membrane lipids. It is therefore plausible that 15‐LOX‐1 itself, or its oxygenation products may cause alterations in the structure of biomembranes and thereby alterations in cellular functions.
3. REGULATION OF 15‐LOX‐1 EXPRESSION
Regulation of ALOX15 expression is complex and involves multiple mechanisms including transcriptional and epigenetic regulation.
3.1. Transcriptional regulation
The expression of 15‐LOX‐1 in various cell types (both epithelial and stromal) was shown to be up‐regulated when the cells were treated with the anti‐inflammatory T‐helper subset 2 (TH‐2) cytokines IL‐4 or IL‐13.15, 16 An up‐regulation of 15‐LOX‐1 expression was also observed during differentiation of CD34+ hematopoietic progenitor cells to dendritic cells in the presence IL‐4 and GM‐CSF.17 In human macrophages, which appear to constitutively express 15‐LOX‐1 at low levels, IL‐13 induced 15‐LOX‐1 mRNA and protein synthesis leading to enhanced production of 15‐HETE.18
Stimulation of 15‐LOX‐1 expression by IL‐4 and IL‐13 involves the transcription factor STAT‐615 (Figure 2A). In human monocytes, induction of JAK2 and TYK2 tyrosine kinases by IL‐13 induced 15‐LOX expression, through STAT‐6 dimerization and nuclear import.19 However, IFN‐γ, a product of TH‐1 lymphocytes, was observed to inhibit ALOX15 gene expression induced by IL‐13 in monocytes.20 IL‐4 mediated induction of 15‐LOX‐1 in A549 cells21, 22 was shown to be through the up‐regulation of the histone acetyltransferase activity of the Creb‐binding protein/p300 (CBP/p300), which can acetylate both nuclear histones and STAT‐6 in lung carcinoma cells (Figure 2B). In the same study it was suggested that IL‐4 stimulation caused STAT‐6 phosphorylation; and ALOX15 expression was enhanced provided the relevant histones were acetylated and the STAT‐6 binding sites became unmasked.23 Ku autoantigen (DNA helicase) was also shown to induce 15‐LOX‐1 gene expression in A549 cells after treatment with IL‐4 and IL‐13.24 Additionally, STAT‐1 and STAT‐3 phosphorylation through p38 MAPK activation was shown to be important for 15‐LOX‐1 expression in human primary monocytes after IL‐13 treatment.25
Figure 2.
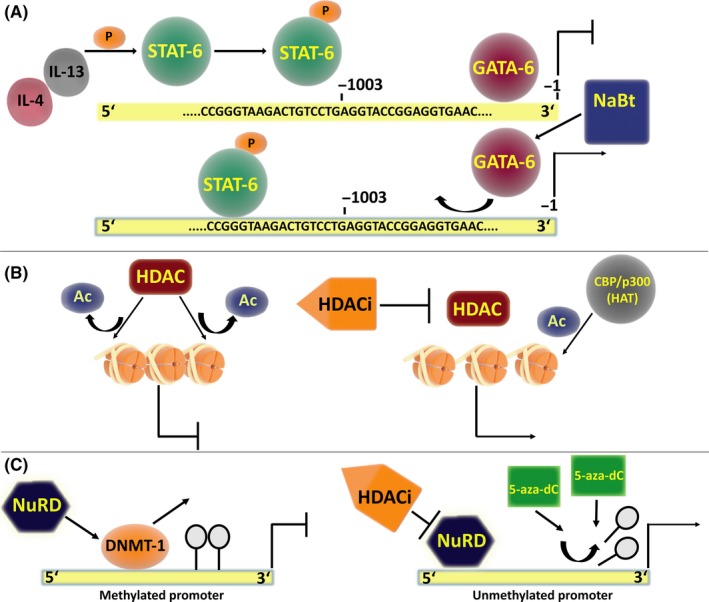
Transcriptional regulation of ALOX15 promoter in cancer. A, IL‐4 and IL‐13, mediated phosphorylation of STAT‐6 protein allows its entry to the nucleus to act as a transcription factor for 15‐LOX‐ 1. GATA‐6 acts as a repressor of ALOX15 expression; treatment with the HDACi sodium butyrate has been shown to inhibit the binding of GATA‐6 to the promoter, thus enhancing the expression of 15‐LOX‐1. B, Continued acetylation of histones by histone deacetylase inhibitors (HDACi) or acetylation of histones with histone acetyl transferases (HATs) such as CBP/p300 prevents chromatin condensation and induce the expression of 15‐LOX‐1. C, Methylation of the ALOX15 promoter is a commonly observed in colon cancer samples and cell lines where the gene is silenced. The DNA methyl transferase DNMT‐1 and the chromatin remodelling complex NuRD compete for binding and repress the ALOX15 promoter. HDACi may deactivate the NuRD complex for the re‐expression of 15‐LOX‐1. Treatment with the DNA methyltransferase agent 5‐Aza‐2′‐deoxycytidine(5‐aza‐dC) can de‐methylate the promoter and enhance expression in colon cancer cells.
Wittwer et al screened the entire coding region and 2 kb from 3′UTR of ALOX15 from 44 healthy Caucasians and reported that a single nucleotide polymorphism ‐292C‐to‐T base exchange, resulted in higher transcription of 15‐LOX‐1 in human macrophages by generating a novel SPI1 transcription factor binding site.26 On the other hand, GATA‐6 was shown to contribute to transcriptional suppression of 15‐LOX‐1 probably by preventing the binding of activator proteins. GATA‐6 expression was reported to be higher in transformed colon epithelial cells than the normal epithelia.27 Shureiqi et al have shown that in colon cancer cell lines, GATA‐6 knockdown was insufficient by itself but contributed significantly to restoring 15‐LOX‐1 expression.27
3.2. Epigenetic regulation
Chromatin structure can influence gene transcription, through post‐translational modifications of histones (eg, acetylation, methylation etc.).28 Histone remodelling appears to be an important regulatory mechanism for the near universal loss of 15‐LOX‐1 expression in cancer cells, particularly in colorectal cancer (CRC). Methylation of histones was also implicated in the regulation of 15‐LOX‐1 expression. Liu et al showed that independent of STAT‐6 activation, 15‐LOX‐1 transcriptional up‐regulation in colon cancer cells required H3K9me2 demethylation by Lysine‐specific Demethylase 3A (KDM3A) and inhibition of histone H3 and H4 acetylation by depsipeptide, a selective Histone Deacetylase 1 and 2 (HDAC1 and HDAC2) inhibitor (HDACi).29 Similarly, other nonspecific HDACi's, suberoylanilide hydroxamic acid (SAHA), sodium butyrate (NaBt) and HC toxin, were also shown to induce 15‐LOX‐1 transcription and protein expression in colon cancer cells.30, 31, 32 In line with this, the nucleosome remodelling and deacetylase (NuRD) complex, consisting of core proteins HDAC1, HDAC2, Mi2, MTA1/2/3 and others, was shown to suppress 15‐LOX‐1 expression in CRC.33 HDACi can inhibit the core HDAC components of this complex, which in turn may contribute to the re‐activation of 15‐LOX‐1 transcription33 (Figure 2C).
Methylation status of ALOX15 promoter was also studied in a broad range of cell types. ALOX15 was shown to be methylated in healthy human primary monocytes and T lymphocytes, lymphoma, lung, epidermoid and cervical cancer cell lines in vitro, and the methylation was associated with the transcriptional repression of 15‐LOX‐1. Methylation of the 15‐LOX‐1 promoter was reported in CRC cell lines as well as in 18 of 50 CRC patients, while promoter methylation was not observed in the colonic mucosa of the control group with no history of CRC or polyps. This process was proposed to also be responsible for the loss of expression of 15‐LOX‐1 in CRC.34 In L‐428 lymphoma cells treated with the DNA methyltransferase (DNMT) inhibitor 5‐aza‐dC, IL‐4 enhanced mRNA expression of 15‐LOX‐1, providing direct evidence that demethylation of the 15‐LOX‐1 promoter was required for IL4‐induced 15‐LOX‐1 expression. Similar results were obtained with TSA treated L‐428 cells that were pre‐incubated with 5‐aza‐dC. Although a robust increase in the mRNA transcript of 15‐LOX‐1 was obtained in these cells, 15‐LOX‐1 protein expression was not detected, indicating that together with transcriptional suppression mechanisms, post‐transcriptional and/or post‐translational mechanisms can contribute to the regulation of 15‐LOX‐1 expression.35
Interestingly, the binding (rather than activity) of the enzyme DNA methyltransferase‐1 (DNMT‐1) to the promoter of 15‐LOX‐1 was found to directly suppress its transcription. Dissociation of DNMT‐1 from the promoter was necessary for the reactivation of transcription in the presence of the HDACi SAHA. Interestingly, Kamitani et al did not find any effect of 5‐aza‐dC treatment on 15‐LOX‐1 expression,31 which is compatible with the findings of Zuo et al who showed that 15‐LOX‐1 promoter DNA methylation was not correlated with 15‐LOX‐1 mRNA levels in colon cancer, and that promoter demethylation failed to reactivate 15‐LOX‐1 expression in vitro.34 It was thus proposed that DNA methylation was not a primary event in the suppression of the gene. DNMT1, on the other hand, appeared to play an important role, as it could bind to the same region on the promoter where HDAC1 and HDAC2 of the NuRD complex can bind to; therefore, the occupation of this region by either DNMT1 or the members of the NuRD complex was reported to be crucial in the transcriptional fate of 15‐LOX‐1 in colon cancer cells.34, 35
Expression of the mutant form of the tumour suppressor protein p53 was shown to up‐regulate the human 15‐LOX promoter activity in a mouse embryo fibroblast cell line, whereas it markedly inhibited mouse 12/15‐LOX expression.36 Although these results are important to indicate the divergence of these the human and mouse enzymes at the level of transcriptional regulation, further data are needed to understand whether a similar regulation may occur in human cancer cells, in tumours harbouring different types of p53 mutations, or in tumours where p53 is lost. Additionally, since human ALOX15 is present on chromosome 17p13.3 in close proximity to TP53,36 the co‐regulation of these two genes at the level of transcription needs to be analysed in terms of the dynamics of the chromatin structure.
4. 15‐LOX‐1 IN CARCINOGENESIS
The expression of 15‐LOX‐1 is lower in diverse cancer types compared to the normal cells. In a screen of 128 randomly collected cancer cell lines representing more than 20 types of human cancers, Moussalli et al showed that 15‐LOX‐1 mRNA expression was considerably lower in cancer cells compared to terminally differentiated cells.37 In nonsmall cell lung cancer (NSCLC) patients, decreased expressions of 15‐LOX‐1 and 15‐LOX‐2 were shown to reduce the production of PUFA oxygenation products, which in turn led to decreased activity of peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ),38 resulting in loss of apoptosis and enhanced cell proliferation.39 In breast carcinoma, 15‐LOX‐1 and 15‐LOX‐2 staining was shown to be low in metastatic patients specimens40 and in pancreatic cancer, low 15‐LOX‐1 expression or complete loss of expression was observed.41 Examination of normal human bladder and bladder tumour specimens indicated a statistically significant decrease in 15‐LOX‐1 expression in stage T3/T4 bladder tumours compared with normal tissues.42 Adenoviral transfection of 15‐LOX‐1 in a syngeneic rat model of malignant glioma led to the induction of apoptosis through caspase‐3 activation, reduction in tumour volume and enhanced survival, significantly.43
The effect of 15‐LOX‐1 on CRC has been investigated extensively with a consistent loss in expression and activity of the protein reported. In a cohort of primary colorectal carcinoma, decreased 15‐LOX‐1 expression as well as 13‐HODE levels were reported in human colorectal adenomas and carcinomas compared to the normal mucosa.44, 45 Treatment of colon carcinoma cell line Caco‐2 with 13‐HODE resulted in decreased cell proliferation.46 In the same study, it was shown that in adenoma tissues 15‐LOX‐1 expression was spread throughout the sample with a lower intensity of staining in the neoplastic epithelium and a higher intensity of staining in the inflammatory areas while in normal controls the expression was mostly restricted to the colonic mucosal epithelium.46 Based on this finding, it might be possible to argue that 15‐LOX‐1 expression must be maintained at a high concentration in a confined area for its biological effects, and that in the case of neoplastic transformation, colon cells evade 15‐LOX‐1‐mediated apoptosis through the dispersion of the expression pattern. Furthermore, tumours derived from the colon cancer cell line HCT‐116 that ectopically expressed 15‐LOX‐1 were smaller than the tumours derived from empty vector‐expressing HCT‐116 cells in athymic nude mice.46 Re‐expression of 15‐LOX‐1 by adenoviral transfection to HT‐29 and LoVo CRC cells inhibited growth of cancer cells in xenografts and down‐regulated important anti‐apoptotic markers like XIAP and Bcl‐xL, suggesting pro‐apoptotic properties of 15‐LOX‐1.47 Treatment of RKO and HT‐29 cells with sulindac and NS‐39 led to increased 15‐LOX‐1 expression and subsequent 13‐HODE production, cell growth inhibition and increased apoptosis.48 In the same study, the effects of the NSAID treatments became unobservable when 15‐LOX‐1 was inhibited with caffeic acid.48 In addition, exogenous addition of 13‐HODE to these cells led to growth inhibition and apoptosis.48 Treatment with celecoxib, another NSAID that selectively inhibits COX‐2, resulted in increased 15‐LOX‐1 expression and induction of apoptosis, suggesting a therapeutic role of celecoxib in CRC.44 Interestingly, a negative correlation between the expression and activity of 15‐LOX‐1 and COX‐2 has been reported in colonic neoplasia indicating an alteration in the species of bioactive lipids being produced in the malignant tissues from the pro‐apoptoic molecules to the more mitogenic prostaglandins.49
On the other hand, some studies suggest a pro‐carcinogenic role of 15‐LOX‐1 signalling. 15‐LOX‐1 expression was shown to be dramatically high in prostate cancer tissues, primarily in high‐grade tumours, as compared to normal tissue samples.2 Transgenic mouse models (human fifteen lipoxygenase‐1 in mouse prostate, FliMP), as well as cell line studies suggested enhanced proliferation in the presence of 15‐LOX‐1.50, 51 These studies, however, have been refuted recently. Bioactive lipids produced from the metabolism of DHA by 15‐LOX‐1 in prostate cancer cells were shown to reduce cell proliferation by activating PPAR‐γ signalling and induction of apoptosis.52 Moreover, the same lipid products were shown to be important for the activation of syndecan‐1 (SDC‐1), a protein that is important in cell to matrix interactions, cellular proliferation and migration as well as caspase‐3 activity.52 Zhong et al have shown that 15‐LOX‐1 expression in prostate cancer cells led to the ubiquitin mediated degradation of Hypoxia Inducible Factor 1α (HIF‐1α), a transcription factor that is essential for neoangiogenesis.53 15‐LOX‐1 was shown to be expressed more in hepatocellular cancer cell lines compared to normal hepatic cells with a further exacerbation of expression under hypoxic conditions in cancer cells. Again, in this case the composition of the bioactive lipids produced was of importance since the production of 15‐HETE, but not 13‐HODE, led to the activation of phosphoinositide‐3 kinase (PI3K)/Akt/Heat Shock Protein 90 pathway resulting in several pro‐carcinogenic changes in the cells.54
Ferroptosis is a newly described form of cell death that differs from traditional apoptosis and necrosis. Ferroptosis results from the accumulation iron‐dependent lipid peroxides and is characterized by cell volume shrinkage and increased mitochondrial membrane density.55 Recently, SAT1 (spermidine/spermine N1‐acetyltransferase 1), a transcriptional target of p53, was shown to induce ferroptosis via 15‐LOX‐1 in human nonsmall cell lung carcinoma cell line H1299. The authors claimed that SAT1 increased the expression of 15‐LOX‐1, and the cells treated with the 15‐LOX‐1 inhibitor PD146176 rescued SAT1‐induced ferroptosis.56 These results support earlier findings which state that 12/15‐lipoxygenase dependent lipid peroxide signalling could activate apoptosis‐inducing factor (AIF)‐mediated cell death in a neuronal degeneration model.57 Inhibition of ferroptosis in neurodegenerative diseases, characterized by accumulation of lipid hydroperoxides, offer a therapeutic alternative.58 Similarly, the induction of ferroptosis has the potential to provide a new strategy for killing cancer cells. Future studies will establish the precise mechanisms by which 15‐LOX‐1 is involved in ferroptosis in different cancer models.
5. ROLE OF 15‐LOX‐1 IN INFLAMMATION
Inflammation is the sum of biological processes such as swelling, redness, pain and heat. While essential during pathogenic infections, acute inflammation should be resolved when the infection is cleared out through the process of resolution. A highly regulated spatial and temporal class switching of eicosanoids occurs in inflamed tissues, starting with the production of pro‐inflammatory mediators through the COX and LOX pathways followed by the production of resolution mediators such as resolvins and lipoxins.59, 60 However, overproduction of the pro‐inflammatory mediators and a deficiency of resolution may lead to chronic low grade inflammation and associated diseases such as cancer.61 15‐LOX‐1 plays a dual role in inflammation as its activity generates mediators that function both in the progression and in the resolution of inflammation.
Role of 15‐LOX‐1 expression in colon cancer in the context of inflammation has been addressed in recent years. Since 12‐HETE and 15‐HETE produced by 12/15LOX (an ortholog of human 15‐LOX‐1) in mice may have different biological effects in cells, Shureiqi et al have generated a mouse model where human 15‐LOX‐1 (which oxygenates AA exclusively to 15‐HETE) is overexpressed solely in the intestinal epithelium through the use of the promoter of villin, a brush border protein that is highly expressed in the colon.62 Expression of 15‐LOX‐1 was shown to decrease tumour size in mice administered with the carcinogen azoxymethane (AOM). Data emerging from this model indicated a crosstalk between 15‐LOX‐1 expression and the inflammatory transcription factor Nuclear Factor‐kappa B (NF‐κB). Expression of human 15‐LOX‐1 resulted in a significant inhibition of the activity of NF‐κB, along with lower levels of tumour necrosis factor‐α (TNF‐α) and its target inducible nitric oxide synthase (iNOS).62 Mechanistically, our group has shown that the inhibition of NF‐κB activity was through the phosphorylation and activation of PPAR‐γ by its ligand 13‐HODE in 15‐LOX‐1 overexpressing CRC cell lines.38 In a colitis‐associated colon cancer (CAC) model, 15‐LOX‐1 inhibited IL‐6/STAT‐3 signalling and tumourigenesis by down‐regulating PPAR‐δ.63 Similarly, in a trinitrobenzenesulfonic acid‐induced IBD mouse model, the administration of milk fermented by a Lactococcus lactis strain producing 15‐LOX‐1 alleviated symptoms of colitis.64 These data collectively illustrate that chronic inflammation, a cornerstone of colon cancer may be ameliorated by the expression and activity of 15‐LOX‐1.
Inflammatory resolution is an active process aimed to prevent the progression from acute‐resolving to persistent‐chronic inflammation to inhibit further tissue damage.65 Resolvins, protectins, lipoxins and maresins are endogenous lipid mediators that are synthesized during the resolution phase of acute inflammation.66 The products of 15‐LOX‐1 pathway can induce a number of anti‐inflammatory processes through the production of pro‐resolving mediators. 15‐LOX‐1 can metabolize DHA to form D‐series resolvins (RvDs) and protectins (PDs) that act as pro‐resolution agents.66 Lipoxins (LXs) are pro‐resolution mediators produced by combined action of various LOXs as well as aspirin‐acetylated COX‐2 in a transcellular manner.60, 67 The inhibition of COX‐2 with aspirin leads to the cessation of prostaglandin synthesis, but allows for the production of 15‐R‐HETE from AA and subsequently LXs.66 15‐LOX‐1 is also known to produce LXA4 during resolution of inflammation.60 Production of LXA4 or 15‐epi‐LXA4 from 15‐HETE through the action of 15‐LOX‐1 or aspirin acetylated COX‐2 entails an eicosanoid class switching where the state of tissue goes from active inflammation to resolution.68 LXA4 was shown to inhibit inflammation caused by lipopolysaccharide (LPS)‐induction in keratinocytes through the upregulation of suppressor of cytokine signalling 2 (SOCS2) and the down‐regulation of TRAF6, a TNF receptor associated factor family member.69 LXA4 and 15‐epi‐LXA4 were shown to diminish migration of neutrophils to the site of infection.67 Moreover, it was demonstrated that PUFAs such as AA, LA and EPA could inhibit the growth of the CRC cell lines RKO and LoVo in vitro by decreasing the synthesis of leukotrienes and prostaglandins, and the expression of COX‐2 while increasing the formation of LXA4.70 Similarly, transgenic rabbits overexpressing 15‐LOX‐1 in macrophages showed reduction in inflammation and associated tissue damage as well as up‐regulation of LXA4.71 In addition, IL‐13, an anti‐inflammatory cytokine, was shown to up‐regulate 15‐LOX‐1 expression in human monocytes72 and to induce LXA4 receptor expression in human enterocytes.73 Among other TH‐2 lymphokines, induction of 15‐LOX in human peripheral blood monocytes was achieved by IL‐4 stimulation, but not by IL‐10. However, as a pro‐inflammatory TH‐1 cytokine, IFN‐γ blocked IL‐13‐mediated induction of 15‐LOX, indicating TH‐lymphocyte subpopulations can modulate 15‐LOX expression and inflammatory responses related to 15‐LOX signalling.20 An anti‐inflammatory effect of 15‐LOX‐1 was also demonstrated by Munger et al by taking the advantage of in vivo gene delivery to distinguish between human and rodent 15‐LOX‐1 expression in rat kidney.74 In an experimental glomerulonephritis model, glomerular leukotriene B4 (LTB4) production was reduced while LXA4 concentration in the urine from unilaterally 15‐LOX‐1 transfected animals was shown to increase, further indicating the anti‐inflammatory actions of 15‐LOX‐1 derived eicosanoids during inflammation.74
On the other hand, 15‐LOX‐1 has also been shown to exhibit pro‐inflammatory activities in various models. In dermatitis, inhibition of 15‐LOX‐1 resulted in impaired podosome formation in dendritic cells (DCs), immune system cells important for pro‐inflammatory responses and antigen presentation, which resulted in the inhibition of their antigen uptake and migration abilities, showing the importance of 15‐LOX‐1 for trafficking of DCs to inflamed tissues.75 15‐LOX‐1 expression and activity was also associated to alcoholic liver disease (ALD). The initial step of ALD is the accumulation of lipid droplets in the liver. 15‐LOX was found to be overexpressed in the liver of patients with ALD cirrhosis. Since lipids accumulated in the liver can stimulate inflammation, 15‐LOX‐1 mediated fatty acid derivatives were reported to play an important role in ALD. Furthermore, free radical peroxidation, which is elevated in ALD patients, was suggested to contribute to liver necroinflammatory injury in ALD.76
A number of studies have demonstrated increased expression and activity of 15‐LOX‐1 in lung epithelial cells as a pro‐inflammatory event in the pathogenesis of asthma and other inflammatory airway diseases.77, 78, 79, 80 Zhao et al showed that epithelial 15‐LOX‐1 expression increased with increasing severity of asthma.81 To clarify the mechanism, Liu et al overexpressed 15‐LOX‐1 in A549 human lung epithelial cell line and showed that 15‐LOX‐1 overexpression led to enhanced release of the inflammatory chemokines, and thereby increased recruitment of immature dendritic cells, mast cells and activated T cells.80 Through 15‐LOX‐1, IL‐13, which is up‐regulated in approximately 50% of asthmatic patients, was found to induce the formation of esterified 15‐HETE (15‐HETE‐PE) which contributed to the increase in mucin MUC5AC expression in human airway epithelial cells.81, 82 Similarly, in vitro activation of human lung macrophages by exposure to IL‐4/IL‐13 was associated with an increase in the expression of 15‐LOX‐1.83 Using both in vivo and in vitro models of human airway epithelial cells, IL‐13‐induced 15‐LOX‐1 was shown to interact with phosphatidylethanolamine‐binding protein 1 (PEBP1) to displace Raf‐1 and activate MAPK/ERK pro‐inflammatory signalling pathway, and induce MUC5AC expression.84 In another study, after IL‐13 treatment, binding of 15‐LOX‐1 with PEBP1 was shown to contribute the desensitization of β2‐Adrenergic receptor (β2AR) through release of G protein receptor kinase 2 (GRK2), which may lead to loss of therapeutic effects in patients with airway diseases.82 Therefore, 15‐LOX‐1 and PEBP1 interaction has been suggested as a potential target in patients with airway diseases, such as asthma, for restoring β2AR sensitization to enhance adenylate cyclase activity and cAMP synthesis, which can promote smooth muscle relaxation in airways and blood vessels as well as enhance ciliary beating and mucin clearance.82 More recently, in silico analysis of the interaction between 15‐LOX‐1 and PEBP1 in humans was predicted to modify the 15‐LOX‐1 protein in a way that would allow the enzyme to efficiently oxygenate esterified PUFAs. In human airway epithelial cells, PEBP1 and 15‐LOX‐1 were shown to co‐localize and enhance the formation of oxidized lipid species, which, in the absence of an efficient antioxidant system (such as GPX4) could trigger cell death via ferroptosis.85 In a diabetic retinopathy model, a hyperglycaemia‐induced reduction in 15‐LOX‐1 levels and downstream production of resolvin D1 (RvD1) was reported, while activation of β2AR signalling pathway rescued these decreases in 15‐LOX‐1 and RvD1.86 Therefore, the biological action of 15‐LOX‐1 in inflammation can be different in airway epithelial cells when compared to retinal cells. It should be also noted that several mediators that elevate the inflammatory phase of inflammation can simultaneously initiate an active resolution program, which can be specific to associated pathologies,65 with context specific molecular interactions and signalling networks.
Atherosclerosis is accepted as an inflammatory disease involving the vascular wall.87 LOXs are involved in the pathogenesis of atherosclerosis as reviewed previously.88, 89 15‐LOX is one of the enzymes that catalyses the formation of oxidized low‐density lipoprotein (ox‐LDL), a major cause for atherosclerosis,90 and polymorphisms in ALOX15 gene was found to be associated with coronary artery disease in human.26, 91, 92, 93, 94 ox‐LDL and/or reduced cholesterol efflux leads to the deposition of esterified cholesterol in the cytoplasm of macrophages and generation of foam cells which then accumulate in the subendothelial space of the arteries and lead to atherosclerotic plaque formation.95 In atherosclerotic lesions in humans, 15‐LOX was observed to co‐localize with LDL in macrophage‐rich areas.96, 97, 98, 99, 100 Furthermore, Makheja et al found an increased formation of 15‐HETE in aortas from cholesterol fed Watanabe heritable hyperlipidaemic (WHHL) rabbits, an indication of elevated 15‐LOX activity.101
15‐LOX‐1 is also associated with endothelial cell (EC) function in the pathogenesis of atherosclerosis (for details please see Figure 3). Ox‐LDL, formed through 15‐LOX activity, was shown to enhance the expression of its receptor Lectin‐like oxidized LDL receptor‐1 (LOX‐1) via the activation of NF‐κB and p38 MAPK signalling pathways and ligand‐receptor interaction was shown to enhance the expression of intercellular cell adhesion molecule‐1 (ICAM‐1) in ECs. Transient overexpression of 15‐LOX in bovine aortic ECs induced the expression vascular cell adhesion molecule 1 (VCAM‐1) by inflammatory stimulation.102 Similarly, the 15‐LOX‐1 metabolite 15‐HpETE was found to induce a subset of cell adhesion molecules (CAMs): ICAM‐1, endothelial cell leukocyte adhesion molecule‐1 (ELAM‐1) and VCAM‐1 in human umbilical vein endothelial cells (HUVECs) and increase transendothelial migration of monocyte‐like cells through platelet endothelial cell adhesion molecule‐1 (PECAM‐1).103 Treatment of HUVECs with Atorvastatin, a cholesterol‐lowering medication, suppressed the up‐regulation of the adhesion molecules whereas overexpression of 15‐LOX‐1 partially eliminated this effect.104 More recently, Liu et al demonstrated that chronic hypoxia increased the expression of CAMs in pulmonary arterial endothelium cells via a positive interaction between 15‐LOX/15‐HETE and NF‐κB,105 underlying the influence of inflammation in the etiology of pulmonary diseases. On the other hand, the increase in expression of CAMs through 15‐LOX‐1‐related metabolites have the potential to render endothelial cells responsive to inflammatory activation for antitumour immunity (discussed later).
Figure 3.
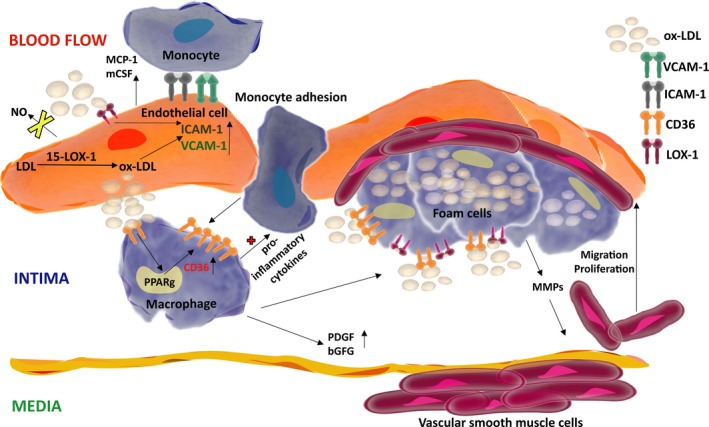
Involvement of 15‐LOX‐1 in the formation of atherosclerotic lesions. Formation of atherosclerotic lesions represents a series of highly dynamic events145 initiated by endothelial dysfunction, generally accompanied by a loss of production of nitric oxide (NO). Ox‐LDL generated from LDL by 15‐LOX‐1 activity in endothelial cells (ECs) induces the expression of cell surface adhesion molecules ICAM‐1 and VCAM‐1, enhancing the adhesive properties of endothelium. At early stages of plaque formation, expression of the major Ox‐LDL receptor, Lectin‐like oxidized LDL receptor‐1 (LOX‐1), increases in ECs. Moreover, Ox‐LDL stimulates ECs to secrete cytokines that promote the recruitment of monocytes from blood flow into the endothelial wall (intima), and differentiate into macrophages. These macrophages express several scavenger receptors such as cluster of differentiation 36 (CD36) and LOX‐1. Internalized Ox‐LDL may enhance CD36 expression (via PPARγ) which in turn facilitates the internalization of more Ox‐LDL, forming foam cells. ECs and macrophages also enhance platelet‐derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) expression and secretion which induce vascular smooth muscle cell (VSMC) proliferation. Foam cells secrete matrix metallopeptidases (MMPs) that trigger VSMCs migration from the media into the intima via the degradation of the extracellular matrix (ECM). Later in the process, VSMCs can produce extracellular matrix factors, including interstitial collagen and elastin, and form a fibrous cap that covers the plaque, which are not shown here
Taken together, these data imply that 15‐LOX‐1 pathway can both stimulate inflammation or resolve inflammation. These opposed effects may depend on cell type and also substrate available in the cell at a specific time. Therefore, re‐modelling of cellular characteristics by 15‐LOX‐1 action may modulate inflammatory cell actions in a context‐dependent manner.
6. 15‐LOX‐1 IN THE TUMOUR MICROENVIRONMENT
The tumour microenvironment (TME) consists of a variety of stromal cells, including noncancerous immune cells, within the extracellular matrix (ECM) that provides for sustained growth, angiogenesis, invasion and metastasis of the tumour cells.106 Infiltration of immune cells in the TME may be a double‐edged sword. Thus, while the activation of pro‐inflammatory transcription factors such as NF‐κB and STAT‐3 and their transcriptional targets IL‐6 and TNFα are known to enhance proliferation and tumour progression,107 immunogenic tumour cells can enhance the infiltration of CD8+ T cells and Natural Killer (NK) cells, which in turn have the potential to restrict tumour growth.108 The TME is generally tumour suppressive, with recruitment of cells such as tumour associated macrophages, myeloid derived suppressor cells (MDSCs) and regulatory T cells and the exclusion of inflammatory TH1 type cells.109 As discussed in the previous section, 15‐LOX‐1 appears to play an important role in inflammation and its resolution through metabolism of the PUFAs: AA, LA, DHA and EPA. Since both epithelial and immune cells (such as macrophages) are known to express 15‐LOX‐1, it is likely that the composition of bioactive lipids in the TME may guide tumour progression. Although most of the studies examining the role of 15‐LOX‐1 in cancer have focused on expression in the epithelial cells, emerging data indicate that both epithelial and stromal derived bioactive lipids can influence the recruitment of different cell types to the TME.
6.1. Modulation of cellular motility in the microenvironment
Metastasis is a complex process that requires cancer cells to undergo epithelial to mesenchymal transformation, detach from the resident tumour, migrate through the blood vessels to a secondary tumour site where the cells extravasate into the tissue where the cells undergo transformation to a more epithelial phenotype and promote the secondary tumour formation.110 The TME, particularly the composition of ECM, is a significant determinant of whether a tumour can successfully metastasize to distant organs.111 Recent data indicate that 15‐LOX‐1 may have an antimetastatic role, although the detailed mechanisms and specific interactions with the TME need to be elucidated.
We have shown that 15‐LOX‐1 re‐expression in the colon cancer cell lines HCT‐116 and HT‐29 can enhance adhesion of cells to fibronectin, reduce cellular motility, wound healing, migration and invasion in vitro7 through the reduced expression of MTA‐1 (Metastasis‐associated protein 1), a master regulator of metastasis in many tumours including CRC.112, 113 Functionally, the reduced motility seen in CRC cell lines overexpressing 15‐LOX‐1 was rescued when MTA‐1 was overexpressed.113 Other studies have indicated that the mechanism behind reduced motility in CRC cell lines expressing 15‐LOX‐1 could also be attributed to a loss of VEGF expression and secretion.114
Data on the role of 15‐LOX‐1 expression of the TME in metastatic spread is relatively limited. Activation of PPAR‐γ by 13‐HODE was shown to inhibit metastasis to the peritoneal cavity in an in vivo mouse gastrointestinal peritoneal metastasis model and reduced motility in human colon and gastric cancer cell lines.115 A transgenic mouse model that over‐expressed 15‐LOX‐1 in endothelial cells under the control of the murine preproendothelin‐1 promoter showed reduced tumour metastasis in both mammary and Lewis lung cancer models.116 The same study also reported decreased expression of EGF and increased expression of apoptosis marker Bax indicating an overall tumour suppressive effect.116 Future studies are necessary to tease out the delicate balance between different 15‐LOX‐1 derived bioactive lipids in the TME and their role in epithelial‐stromal cell interactions.
6.2. Modulation of vasculature in the microenvironment
The vasculature plays a crucial role in determining the composition of the TME. Tumour associated vasculature is usually chaotic and generally unable to meet the requirements of the growing tumour, generating pockets of hypoxia.109 These angiogenic cues result in the expression of growth factors such as VEGF that stimulate the sprouting of new blood vessels in a haphazard and disorderly manner. Moreover, these blood vessels are often leaky with uneven blood flow and are unable to carry nutrients or drugs efficiently to the TME.117
A number of recent studies have examined the role of 15‐LOX‐1 expression on the vasculature. The enzyme has been implicated as a factor that both activates and inhibits angiogenesis in different in vitro and in vivo models (Figure 4). In a hypoxia‐induced retinal neovascularization (RNV) model, 15‐LOX‐1 expression showed anti‐angiogenic properties by inhibiting the expressions of VEGF‐A, vascular endothelial growth factor receptor‐2 (VEGF‐R2) and endothelial nitric oxide synthase (eNOS) in rat microvascular endothelial cells (RMVECs) in vitro.118 Corroborating this, intravitreous injection of adenoviral‐15‐LOX‐1 in a mouse model of oxygen‐induced retinopathy (OIR) significantly inhibited RNV and down‐regulated VEGF‐A expression.119 Mechanistically, the inhibition of RNV in the presence of 15‐LOX‐1 was reported to be via the up‐regulation of PPAR‐γ and down‐regulation of VEGFR‐2 expressions.120 Similarly, in a rabbit skeletal muscle system, an anti‐angiogenic effect was ascribed to 15‐LOX‐1 via the inhibition of capillary perfusion, vascular permeability, vasodilatation, increase in capillary number due to the reduced mRNA expressions of VEGF‐A165, PlGF‐2 and VEGF‐R2 together with reduced NO bioactivity.121
Figure 4.
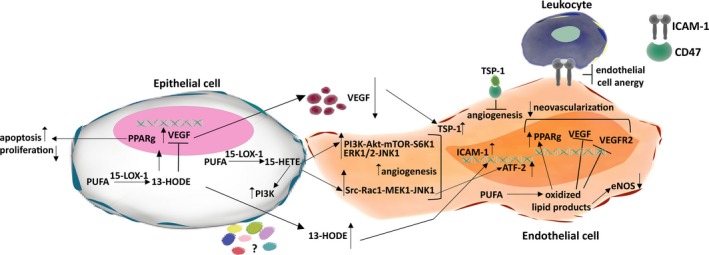
Implications of 15‐LOX‐1 in angiogenesis in different models. 15‐LOX‐1 has been implicated as a factor that both activates and inhibits angiogenesis. Expression of 15‐LOX‐1 in ECs reduced angiogenesis by reducing VEGF‐A, VEGF‐R2 and eNOs mRNA, and NO bioactivity, in vitro. In vivo, down‐regulation of VEGFR‐2 expression was via the upregulation of PPAR‐γ. 13‐HODE produced by 15‐LOX‐1 expressing cancer cells was shown to inhibit angiogenesis through enhanced expression of thrombospondin‐1 (TSP‐1) and ICAM‐1 in ECs. Increased ICAM‐1 expression may increase the recruitment of leukocytes and reduce endothelial cell anergy. On the other hand, 15‐HETE was shown as a tumourigenic factor in epithelial cells, and a pro‐angiogenic factor in ECs by activating the PI3K pathway. 15‐HETE was also found to mediate EC migration, tube formation and angiogenesis through enhanced ATF‐2 activity via the mitogen‐activated protein kinase (MAPK, Src, Rac1, MEK1, JNK1) cascade. The other PUFA metabolites generated by 15‐LOX‐1 activity need to be determined in order to clarify the context‐dependent action of the enzyme
An increasing amount of evidence supports that lipid products of the 12/15‐LOX pathway in mice (which includes a mixture of 12‐HETE and 15‐HETE using AA as substrate) are involved in the pathogenesis of retinal microvascular dysfunction thereby implicating a role in angiogenesis. 12/15‐LOX plays a role in the development of pathological RNV, which was reduced in mice lacking 12/15‐LOX or treated with the LOX inhibitor baicalein.122 In diabetic mice, 12‐HETE and 15‐HETE were indicated to activate vascular NADPH oxidase, leading to overproduction of ROS, with subsequent activation of VEGF‐R2 signalling and disruption of retinal endothelial cell barrier.123 15‐HETE was also implicated as a pro‐angiogenic factor by stimulating human dermal microvascular endothelial cell (HDMVEC) tube formation and migration in vitro and angiogenesis in vivo via the PI3K‐Akt‐mTOR‐S6K1 signalling axis.124 In addition, under hypoxic conditions, 15‐HETE production was higher in human neonatal vessels compared to the normoxic condition.125 Further studies showed that hypoxia could induce the expression of 15‐LOX‐1 and the production of 15‐HETE, which in turn stimulated the migration and tube formation of human retinal microvascular endothelial cells (HRMVEC) through Src‐dependent Rac1‐mediated MEK1 stimulation.126, 127 Additionally, Singh et al have shown that by inducing the expression of HMG‐CoA reductase, 15‐HETE (produced through the activity of 12/15 LOX in mice) could facilitate the farnesylation and translocation of Rac1 to the plasma membrane of human dermal microvascular endothelial cell (HDMVECs) where it became activated and induced angiogenesis in response to hind‐limb ischaemia.128 15‐HETE was also shown to induce angiogenesis in endothelial cells derived from adipose tissue by up‐regulating the production of CD31 and VEGF through PI3K/Akt/mTOR signalling in rats.129 However, 15‐oxo‐ETE, the 15‐LOX‐1 metabolite that is produced from 15‐HETE by macrophage‐derived 15‐Hydroxyprostaglandin dehydrogenase (15‐PGDH), was shown to inhibit the proliferation of human vascular vein endothelial cells by suppressing DNA synthesis, implicating a potential angiostatic role.130
The effects of 15‐LOX‐1 expression on neo‐angiogenesis have been recently examined in different cancer types. The overexpression of 15‐LOX‐1 in endothelial cells was shown to inhibit tumour growth and metastasis in a transgenic mouse model of mammary and Lewis lung carcinoma.116 Additionally, the re‐expression of 15‐LOX‐1 in HCT116, HT29 and LoVo colon cancer cells inhibited epithelial VEGF expression and the conditioned medium (CM) from these cells inhibited endothelial cell tube formation through, at least in part, a decrease in the expression and stability of HIF‐1α.114 On the other hand, 15‐LOX‐1 over‐expression in the human prostate cancer cell line PC‐3 was shown to increase the expression of VEGF in vitro and increase angiogenesis in subcutaneous xenografts.131 Complicating the story even further, recently, 15‐LOX‐1 over‐expression in PC‐3 cells was shown to reduce angiogenesis through a ubiquitin‐mediated enhanced degradation of HIF‐1α and a consequent lower expression of VEGF‐A mRNA.132 Supporting the anti‐angiogenic role of 15‐LOX‐1, we have recently shown that re‐expression of 15‐LOX‐1 in HCT‐116 and SW‐480 CRC cell lines and LNCaP prostate cancer cells reduced the expression and secretion of VEGF‐A in both normoxic and hypoxic conditions and CM from these cells decreased endothelial cell motility and tube formation.133 Moreover, 15‐LOX‐1 CM treated mouse aortic rings showed significantly less sprouting with a more organized (less chaotic) structure of vascular network.133 Mechanistically, endothelial cells treated with CM from 15‐LOX‐1 overexpressing CRC cell lines showed enhanced expression of Thrombospondin‐1 (TSP‐1), a matrix glycoprotein known to strongly inhibit neovascularization.133 TSP‐1 interacts with a vast number of different proteins, many of which play important roles in extracellular matrix remodelling, interactions between cells and the cell and extracellular matrix, motility and vascular normalization.134 The same cells also showed enhanced expression of the cell adhesion molecule ICAM‐1. Endothelial cell‐leukocyte interactions are mediated by the expression of CAMs, which may be reduced in tumour associated endothelial cells.135, 136 A reduction in adhesion proteins such as ICAM‐1 can lead to endothelial cell anergy, causing reduced recruitment of immune cells to the TME and thereby immune suppression.137 Treatment of HUVECs with 13‐HODE mimicked most of the anti‐angiogenic effects of 15‐LOX‐1.133 Therefore, these data suggest that 15‐LOX‐1 related metabolites have the potential to be used as a therapeutic option to drive antitumour immunity through immune cell infiltration.
7. FUTURE PERSPECTIVES
To date, the 15‐LOX‐1 field has attributed both pro‐ and anti‐inflammatory as well as pro‐ or anti‐tumourigenic roles to the enzyme. Such conflicting observations may due to the different biological effects of the many lipid mediators generated by the 15‐LOX‐1 pathway, which have not yet been fully elucidated.138 It should also be noted that separate 12‐LOX and 15‐LOX enzymes do not exist in rodents, and 12/15‐LOX, the mouse homolog of 15‐LOX‐1, has opposing functions with 15‐LOX‐1. Therefore, 12/15‐LOX gene knockouts are likely to give limited and controversial results in human disease models. Additionally, the controversies between data published from different labs, or even from the same group may have resulted from differences in the expression of the enzyme and availability of substrates, which may affect the type of bioactive lipids produced in different tissues in a spatial and temporal manner. Furthermore, attempts to re‐express the enzyme should also be context, tissue‐type and disease dependent. For instance, lack of 15‐LOX‐1 expression in cancer cells is associated with their ability to escape terminal differentiation, while 15‐LOX‐1 and its downstream pathways are involved in the differentiation of nasal epithelial cells into a goblet cell/mucus‐producing epithelium, and therefore contribute to pathology of chronic inflammatory airway diseases.139, 140
Considering that the expression of 15‐LOX‐1 is lost in many different tumour types and strong data in recent years establishing an anti‐tumourigenic role of 15‐LOX‐1, it is not surprising that there has been a lot of interest to understand the differential regulation of ALOX15 in various types of cancers. However, the results are far from sufficient to indicate a general mechanism of repression of gene expression or to explain the interplay between the regulatory factors. Furthermore, the exact mechanisms underlying the transcriptional reactivation of 15‐LOX‐1 remains unknown in terms of the transcription factors that bind to the 15‐LOX‐1 promoter in different types of tumours. The direct effect of STAT‐6 on 15‐LOX‐1 transcription is also questionable, since STAT‐6 does not contribute to transcriptional reactivation by HDACi's in colon cancer cell lines.141 At this point, identification of the specific components of HDAC‐containing complexes, determination of the specific functions of HDACs within these complexes, and the molecular and biological consequences of pharmacological inhibition of these HDACs are necessary for different tumour types. For example, while existing HDACi's are effective for the treatment of a relatively small population of patients with defined haematological malignancies, they are not useful for the treatment of most other tumours.142 Therefore, generation of target specific HDACi's with increased delivery and diffusion properties and less toxicity is needed. Moreover, use of methylation inhibitors together with specific HDACis might be an effective strategy for the re‐expression of 15‐LOX‐1 in certain types of tumours, like CRC.
To the best of our knowledge, post‐transcriptional regulation of 15‐LOX‐1 in human tumour has not been studied yet. Additionally, translational regulation of human 15‐LOX‐1 is not known although these mechanisms are well defined for rabbit 15‐LOX‐1.143, 144 Better understanding of the regulation of 15‐LOX‐1 expression in transcriptional, post‐transcriptional and translational levels might help to understand the specific molecular mechanisms that control 15‐LOX‐1 expression in different types of human cancers. On the other hand, detailed analysis of ALOX15 gene polymorphisms and mutations located in the coding or regulatory region of the gene and the contribution of these SNPs and/or mutations towards the expression of 15‐LOX‐1 in a cell type specific manner need to be examined. These data may help to develop proper diagnostic and therapeutic strategies in the treatment of human diseases, especially cancer.
8. CONCLUSIONS
There is no doubt that lipids are crucial in signalling and cellular physiology in both health and disease. The importance of lipid metabolizing LOXs in the generation of pro‐inflammatory mediators and more recently the pro‐resolution mediators have firmly established these enzymes as important players in determining disease outcomes and re‐establishing homeostasis.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The work was supported by the TÜBİTAK projects 113S935, 113S063 and 11S308 and BAGEP (Science Academy of Turkey Young Investigators Award) to SB.
Çolakoğlu M, Tunçer S, Banerjee S. Emerging cellular functions of the lipid metabolizing enzyme 15‐Lipoxygenase‐1. Cell Prolif. 2018;51:e12472 10.1111/cpr.12472
The authors Melis Çolakoğlu and Sinem Tunçer have contributed equally to this study.
REFERENCES
- 1. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871‐1875. [DOI] [PubMed] [Google Scholar]
- 2. Kelavkar U, Lin Y, Landsittel D, Chandran U, Dhir R. The yin and yang of 15‐lipoxygenase‐1 and delta‐desaturases: dietary omega‐6 linoleic acid metabolic pathway in prostate. J Carcinog. 2006;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serhan CN. Resolution phase of inflammation: novel endogenous anti‐inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101‐137. [DOI] [PubMed] [Google Scholar]
- 4. Mao F, Wang M, Wang J, Xu W‐R. The role of 15‐LOX‐1 in colitis and colitis‐associated colorectal cancer. Inflamm Res. 2015;29:2359‐2370. [DOI] [PubMed] [Google Scholar]
- 5. Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15‐lipoxygenase‐1 (ALOX15). Gene. 2015;573:1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dobrian AD, Lieb DC, Cole BK, Taylor‐Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12‐ and 15‐lipoxygenases. Prog Lipid Res. 2011;50:115‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Çimen I, Tunçay S, Banerjee S. 15‐Lipoxygenase‐1 expression suppresses the invasive properties of colorectal carcinoma cell lines HCT‐116 and HT‐29. Cancer Sci. 2009;100:2283‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev. 2011;30:277‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford‐Hutchinson AW. Arachidonate 15‐lipoxygenase; characteristics and potential biological significance. Eicosanoids. 1991;4:65‐74. [PubMed] [Google Scholar]
- 10. Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S‐lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148‐6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillmor SA, Villaseñor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15‐lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol. 1997;4:1003‐1009. [DOI] [PubMed] [Google Scholar]
- 12. Walther M, Anton M, Wiedmann M, Fletterick R, Kuhn H. The N‐terminal domain of the reticulocyte‐type 15‐lipoxygenase is not essential for enzymatic activity but contains determinants for membrane binding. J Biol Chem. 2002;277:27360‐27366. [DOI] [PubMed] [Google Scholar]
- 13. Nigam S, Schewe T. Phospholipase A2s and lipid peroxidation. Biochim Biophys Acta ‐ Mol Cell Biol Lipids. 2000;1488:167‐181. [DOI] [PubMed] [Google Scholar]
- 14. Mahipal SVK, Subhashini J, Reddy MC, et al. Effect of 15‐lipoxygenase metabolites, 15‐(S)‐HPETE and 15‐(S)‐HETE on chronic myelogenous leukemia cell line K‐562: reactive oxygen species (ROS) mediate caspase‐dependent apoptosis. Biochem Pharmacol. 2007;74:202‐214. [DOI] [PubMed] [Google Scholar]
- 15. Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15‐lipoxygenases: structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263‐290. [DOI] [PubMed] [Google Scholar]
- 16. Zhao J, Minami Y, Etling E, et al. Preferential generation of 15‐HETE‐PE induced by IL‐13 regulates goblet cell differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2017;57:692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spanbroek R, Hildner M, Köhler A, et al. IL‐4 determines eicosanoid formation in dendritic cells by down‐regulation of 5‐lipoxygenase and up‐regulation of 15‐lipoxygenase 1 expression. Proc Natl Acad Sci U S A. 2001;98:5152‐5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15‐lipoxygenase and generate 15(S)‐hydroxy‐5,8,11‐cis‐13‐trans‐eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roy B, Cathcart MK. Induction of 15‐lipoxygenase expression by IL‐13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J Biol Chem. 1999;273:32023‐32029. [DOI] [PubMed] [Google Scholar]
- 20. Nassar GM, Morrow JD, Roberts LJ, Lakkis FG, Badr KF. Induction of 15‐lipoxygenase by interleukin‐13 in human blood monocytes. J Biol Chem. 1994;269:27631‐27634. [PubMed] [Google Scholar]
- 21. Brinckmann R, Topp MS, Zalán I, et al. Regulation of 15‐lipoxygenase expression in lung epithelial cells by interleukin‐4. Biochem J. 1996;318:305‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen X, Ji N, Qin N, et al. 1,6‐O, O‐diacetylbritannilactone inhibits eotaxin‐1 and ALOX15 expression through inactivation of STAT6 in A549 cells. Inflammation. 2017;40:1967‐1974. [DOI] [PubMed] [Google Scholar]
- 23. Shankaranarayanan P, Chaitidis P, Kuhn H, Nigam S. Acetylation by histone acetyltransferase CREB‐binding protein/p300 of STAT6 is required for transcriptional activation of the 15‐lipoxygenase‐1 gene. J Biol Chem. 2001;276:42753‐42760. [DOI] [PubMed] [Google Scholar]
- 24. Kelavkar UP, Wang S, Badr KF. Ku autoantigen (DNA helicase) is required for interleukins‐13/‐4‐induction of 15‐lipoxygenase‐1 gene expression in human epithelial cells. Genes Immun. 2000;1:237‐250. [DOI] [PubMed] [Google Scholar]
- 25. Xu B, Bhattacharjee A, Roy B, et al. Interleukin‐13 induction of 15‐lipoxygenase gene expression requires p38 mitogen‐activated protein kinase‐mediated serine 727 phosphorylation of Stat1 and Stat3. Mol Cell Biol. 2003;23:3918‐3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wittwer J, Marti‐Jaun J, Hersberger M. Functional polymorphism in ALOX15 results in increased allele‐specific transcription in macrophages through binding of the transcription factor SPI1. Hum Mutat. 2006;27:78‐87. [DOI] [PubMed] [Google Scholar]
- 27. Shureiqi I, Zuo X, Broaddus R, et al. The transcription factor GATA‐6 is overexpressed in vivo and contributes to silencing 15‐LOX‐1 in vitro in human colon cancer. FASEB J. 2007;21:743‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu C, Xu D, Han H, et al. Transcriptional regulation of 15‐Lipoxygenase expression by histone H3 lysine 4 methylation/demethylation. PLoS ONE. 2012;7:e52703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamitani H, Geller M, Eling T. Expression of 15‐lipoxygenase by human colorectal carcinoma Caco‐2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569‐21577. [DOI] [PubMed] [Google Scholar]
- 31. Kamitani H, Taniura S, Ikawa H, Watanabe T, Kelavkar UP, Eling TE. Expression of 15‐lipoxygenase‐1 is regulated by histone acetylation in human colorectal carcinoma. Carcinogenesis. 2001;22:187‐191. [DOI] [PubMed] [Google Scholar]
- 32. Hsi LC, Xi X, Lotan R, Shureiqi I, Lippman SM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis via induction of 15‐lipoxygenase‐1 in colorectal cancer cells. Cancer Res. 2004;64:8778‐8781. [DOI] [PubMed] [Google Scholar]
- 33. Zuo X, Morris JS, Broaddus R, Shureiqi I. 15‐LOX‐1 transcription suppression through the NuRD complex in colon cancer cells. Oncogene. 2009;28:1496‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuo X, Shen L, Issa J‐P, et al. 15‐Lipoxygenase‐1 transcriptional silencing by DNA methyltransferase‐1 independently of DNA methylation. FASEB J. 2008;22:1981‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu C, Xu D, Sjoberg J, Forsell P, Bjorkholm M, Claesson HE. Transcriptional regulation of 15‐lipoxygenase expression by promoter methylation. Exp Cell Res. 2004;297:61‐67. [DOI] [PubMed] [Google Scholar]
- 36. Kelavkar UP, Badr KF. Effects of mutant p53 expression on human 15‐lipoxygenase‐promoter activity and murine 12/15‐lipoxygenase gene expression: evidence that 15‐lipoxygenase is a mutator gene. Proc Natl Acad Sci U S A. 1999;96:4378‐4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moussalli MJ, Wu Y, Zuo X, et al. Mechanistic contribution of ubiquitous 15‐lipoxygenase‐1 expression loss in cancer cells to terminal cell differentiation evasion. Cancer Prev Res. 2011;4:1961‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cimen I, Astarci E, Banerjee S. 15‐lipoxygenase‐1 exerts its tumor suppressive role by inhibiting nuclear factor‐kappa B via activation of PPAR gamma. J Cell Biochem. 2011;112:2490‐2501. [DOI] [PubMed] [Google Scholar]
- 39. Yuan H, Li M‐Y, Ma LT, et al. 15‐Lipoxygenases and its metabolites 15(S)‐HETE and 13(S)‐HODE in the development of non‐small cell lung cancer. Thorax. 2010;65:321‐326. [DOI] [PubMed] [Google Scholar]
- 40. Jiang WG, Watkins G, Douglas‐Jones A, Mansel RE. Reduction of isoforms of 15‐lipoxygenase (15‐LOX)‐1 and 15‐LOX‐2 in human breast cancer. Prostaglandins Leukot Essent Fat Acids. 2006;74:235‐245. [DOI] [PubMed] [Google Scholar]
- 41. Hennig R, Kehl T, Noor S, et al. 15‐lipoxygenase‐1 production is lost in pancreatic cancer and overexpression of the gene inhibits tumor cell growth. Neoplasia. 2007;9:917‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Philips BJ, Dhir R, Hutzley J, Sen M, Kelavkar UP. Polyunsaturated fatty acid metabolizing 15‐Lipoxygenase‐1 (15‐LO‐1) expression in normal and tumorigenic human bladder tissues. Appl Immunohistochem Mol Morphol. 2008;16:159‐164. [DOI] [PubMed] [Google Scholar]
- 43. Viita H, Pacholska A, Ahmad F, et al. 15‐Lipoxygenase‐1 induces lipid peroxidation and apoptosis, and improves survival in rat malignant glioma. In Vivo (Brooklyn). 2012;26:1‐8. [PubMed] [Google Scholar]
- 44. Shureiqi I, Wojno KJ, Poore JA, et al. Decreased 13‐S‐hydroxyoctadecadienoic acid levels and 15‐lipoxygenase‐1 expression in human colon cancers. Carcinogenesis. 1999;20:1985‐1995. [DOI] [PubMed] [Google Scholar]
- 45. Heslin MJ, Hawkins A, Boedefeld W, et al. Tumor‐associated down‐regulation of 15‐lipoxygenase‐1 is reversed by celecoxib in colorectal cancer. Ann Surg. 2005;241:941‐946. 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nixon JB, Kim KS, Lamb PW, Bottone FG, Eling TE. 15‐Lipoxygenase‐1 has anti‐tumorigenic effects in colorectal cancer. Prostaglandins Leukot Essent Fat Acids. 2004;70:7‐15. [DOI] [PubMed] [Google Scholar]
- 47. Wu Y, Fang B, Yang XQ, et al. Therapeutic molecular targeting of 15‐lipoxygenase‐1 in colon cancer. Mol Ther. 2008;16:886‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shureiqi I, Chen D, Lee JJ, et al. 15‐LOX‐1: a novel molecular target of nonsteroidal anti‐inflammatory drug‐induced apoptosis in colorectal cancer cells. J Natl Cancer Inst. 2000;92:1136‐1142. [DOI] [PubMed] [Google Scholar]
- 49. Yuri M, Sasahira T, Nakai K, Ishimaru S, Ohmori H, Kuniyasu H. Reversal of expression of 15‐lipoxygenase‐1 to cyclooxygenase‐2 is associated with development of colonic cancer. Histopathology. 2007;51:520‐527. [DOI] [PubMed] [Google Scholar]
- 50. Kelavkar UP, Parwani AV, Shappell SB, Martin WD. Conditional expression of human 15‐lipoxygenase‐1 in mouse prostate induces prostatic intraepithelial neoplasia: the FLiMP mouse model. Neoplasia. 2006;8:510‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelavkar UP, Cohen C. 15‐Lipoxygenase‐1 expression upregulates and activates insulin‐like growth factor‐1 receptor in prostate cancer cells. Neoplasia. 2004;6:41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Flaherty JT, Hu Y, Wooten RE, et al. 15‐Lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PLoS ONE. 2012;7:e45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salceda S, Caro J. Hypoxia‐inducible factor 1α (HIF‐1α) protein is rapidly degraded by the ubiquitin‐proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox‐induced changes. J Biol Chem. 1997;272:22642‐22647. [DOI] [PubMed] [Google Scholar]
- 54. Ma J, Zhang L, Zhang J, et al. 15‐lipoxygenase‐1/15‐hydroxyeicosatetraenoic acid promotes hepatocellular cancer cells growth through protein kinase B and heat shock protein 90 complex activation. Int J Biochem Cell Biol. 2013;45:1031‐1041. [DOI] [PubMed] [Google Scholar]
- 55. Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ou Y, Wang S‐J, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53‐mediated ferroptotic responses. Proc Natl Acad Sci. 2016;113:E6806‐E6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seiler A, Schneider M, Förster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15‐lipoxygenase dependent‐ and AIF‐mediated cell death. Cell Metab. 2008;8:237‐248. [DOI] [PubMed] [Google Scholar]
- 58. Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612‐619. [DOI] [PubMed] [Google Scholar]
- 60. Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2015;7:a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rahman M, Selvarajan K, Hasan MR, et al. Inhibition of COX‐2 in colon cancer modulates tumor growth and MDR‐1 expression to enhance tumor regression in therapy‐refractory cancers in vivo. Neoplasia. 2012;14:624‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zuo X, Peng Z, Wu Y, et al. Effects of gut‐targeted 15‐LOX‐1 transgene expression on colonic tumorigenesis in mice. J Natl Cancer Inst. 2012;104:709‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mao F, Xu M, Zuo X, et al. 15‐Lipoxygenase‐1 suppression of colitis‐associated colon cancer through inhibition of the IL‐6/STAT3 signaling pathway. FASEB J. 2015;29:2359‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saraiva TD, Morais K, Pereira VB, et al. Milk fermented with a 15‐lipoxygenase‐1‐producing Lactococcus lactis alleviates symptoms of colitis in a murine model. Curr Pharm Biotechnol. 2015;16:424‐429. [DOI] [PubMed] [Google Scholar]
- 65. Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immuno. 2016;7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fierro IM, Colgan SP, Bernasconi G, et al. Lipoxin A4 and aspirin‐triggered 15‐epi‐lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688‐2694. [DOI] [PubMed] [Google Scholar]
- 68. Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Traf D. Lipoxin A4 inhibits lipopolysaccharide‐induced production of inflammatory cytokines in keratinocytes by up‐regulating SOCS2. J Huazhong Univ Sci Technolog Med Sci. 2015;35:426‐431. [DOI] [PubMed] [Google Scholar]
- 70. Zhang C, Yu H, Ni X, Shen S, Das UN. Growth inhibitory effect of polyunsaturated fatty acids (PUFAs) on colon cancer cells via their growth inhibitory metabolites and fatty acid composition changes. PLoS ONE. 2015;10:e0123256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15‐lipoxygenase and endogenous anti‐inflammatory lipid mediators. J Immunol. 2003;171:6856‐6865. [DOI] [PubMed] [Google Scholar]
- 72. Akagi Y, Isaka Y, Arai M, et al. Inhibition of TGF‐β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148‐155. [DOI] [PubMed] [Google Scholar]
- 73. Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)‐13 and interferon gamma and inhibits tumor necrosis factor alpha‐induced IL‐8 release. J Exp Med. 1998;187:1285‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Munger KA, Montero A, Fukunaga M, et al. Transfection of rat kidney with human 15‐lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1999;96:13375‐13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Han H, Liang X, Ekberg M, et al. Human 15‐lipoxygenase‐1 is a regulator of dendritic‐cell spreading and podosome formation. FASEB J. 2017;31:491‐504. [DOI] [PubMed] [Google Scholar]
- 76. Raszeja‐Wyszomirska J, Safranow K, Milkiewicz M, Milkiewicz P, Szynkowska A, Stachowska E. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat. 2012;99:51‐56. [DOI] [PubMed] [Google Scholar]
- 77. Claesson HE. On the biosynthesis and biological role of eoxins and 15‐lipoxygenase‐1 in airway inflammation and Hodgkin lymphoma. Prostaglandins Other Lipid Mediat. 2009;89:120‐125. [DOI] [PubMed] [Google Scholar]
- 78. James A, Daham K, Backman L, et al. The influence of aspirin on release of eoxin C4, Leukotriene C4 and 15‐HETE, in eosinophilic granulocytes isolated from patients with asthma. Int Arch Allergy Immunol. 2013;162:135‐142. [DOI] [PubMed] [Google Scholar]
- 79. Larsson N, Lundström SL, Pinto R, et al. Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans. Eur Respir J. 2014;43:453‐463. [DOI] [PubMed] [Google Scholar]
- 80. Liu C, Xu D, Liu L, et al. 15‐Lipoxygenase‐1 induces expression and release of chemokines in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L196‐L203. [DOI] [PubMed] [Google Scholar]
- 81. Zhao J, Maskrey B, Balzar S, et al. Interleukin‐13‐induced MUC5AC is regulated by 15‐lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Albano GD, Zhao J, Etling EB, et al. IL‐13 desensitizes β2‐adrenergic receptors in human airway epithelial cells through a 15‐lipoxygenase/G protein receptor kinase 2 mechanism. J Allergy Clin Immunol. 2015;135:1144‐1153. e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abrial C, Grassin‐Delyle S, Salvator H, Brollo M, Naline E, Devillier P. 15‐Lipoxygenases regulate the production of chemokines in human lung macrophages. Br J Pharmacol. 2015;172:4319‐4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao J, O'Donnell VB, Balzar S, St. Croix CM, Trudeau JB, Wenzel SE. 15‐Lipoxygenase 1 interacts with phosphatidylethanolamine‐binding protein to regulate MAPK signaling in human airway epithelial cells. Proc Natl Acad Sci. 2011;108:14246‐14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wenzel SE, Tyurina YY, Zhao J, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi H, Carion TW, Jiang Y, Chahine A, Steinle JJ, Berger EA. A regulatory role for β‐adrenergic receptors regarding the resolvin D1 (RvD1) pathway in the diabetic retina. PLoS ONE. 2017;12:e0185383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kita T, Kume N, Minami M, et al. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci. 2001;947:199‐206. [DOI] [PubMed] [Google Scholar]
- 88. Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clin Chem Lab Med. 2010;48:1063‐1073. [DOI] [PubMed] [Google Scholar]
- 89. Wittwer J, Hersberger M. The two faces of the 15‐lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fat Acids. 2007;77:67‐77. [DOI] [PubMed] [Google Scholar]
- 90. Takeda S, Hirayama A, Urata S, et al. Cannabidiol‐2′,6′‐dimethyl ether as an effective protector of 15‐lipoxygenase‐mediated low‐density lipoprotein oxidation in vitro. Biol Pharm Bull. 2011;34:1252‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.‐292C>T promoter polymorphism increases reticulocyte‐type 15‐lipoxygenase‐1 activity and could be atheroprotective. Clin Chem Lab Med. 2007;45:487‐492. [DOI] [PubMed] [Google Scholar]
- 92. Assimes TL, Knowles JW, Priest JR, et al. A near null variant of 12/15‐LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198:136‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang K, Wang Y, Liu Q, et al. Two single nucleotide polymorphisms in ALOX15 are associated with risk of coronary artery disease in a Chinese Han population. Heart Vessels. 2010;25:368‐373. [DOI] [PubMed] [Google Scholar]
- 94. McCaskie PA, Beilby JP, Hung J, et al. 15‐Lipoxygenase gene variants are associated with carotid plaque but not carotid intima‐media thickness. Hum Genet. 2008;123:445‐453. [DOI] [PubMed] [Google Scholar]
- 95. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage‐mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20:17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hiltunen T, Luoma J, Nikkari T, Yla‐Herttuala S. Induction of 15‐lipoxygenase mRNA and protein in early atherosclerotic lesions. Circulation. 1995;92:1012‐3297. [DOI] [PubMed] [Google Scholar]
- 97. Ylä‐Herttuala S, Rosenfeld ME, Parthasarathy S, et al. Colocalization of 15‐lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage‐rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990;87:6959‐6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kühn H, Belkner J, Zaiss S, Fährenklemper T, Wohlfeil S. Involvement of 15‐lipoxygenase in early stages of atherogenesis. J Exp Med. 1994;179:1903‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yla‐Herttuala S, Luoma J, Viita H, Hiltunen T, Sisto T, Nikkari T. Transfer of 15‐lipoxygenase gene into rabbit iliac arteries results in the appearance of oxidation‐specific lipid‐protein adducts characteristic of oxidized low density lipoprotein. J Clin Invest. 1995;95:2692‐2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ylä‐Herttuala S, Rosenfeld ME, Parthasarathy S, et al. Gene expression in macrophage‐rich human atherosclerotic lesions: 15‐lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid‐protein adducts. J Clin Invest. 1991;87:1146‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Makheja AN, Bloom S, Muesing R, Simon T, Bailey JM. Anti‐inflammatory drugs in experimental atherosclerosis: 7. Spontaneous atherosclerosis in WHHL rabbits and inhibition by cortisone acetate. Atherosclerosis. 1989;76:155‐161. [DOI] [PubMed] [Google Scholar]
- 102. Wolle J, Welch KA, Devall LJ, Cornicelli JA, Saxena U. Transient overexpression of human 15‐lipoxygenase in aortic endothelial cells enhances tumor necrosis factor‐induced vascular cell adhesion molecule‐1 gene expression. Biochem Biophys Res Commun. 1996;220:310‐314. [DOI] [PubMed] [Google Scholar]
- 103. Sultana C, Shen Y, Rattan V, Kalra VK. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte‐like HL‐60 cells is linked to protein kinase C activation. J Cell Physiol. 1996;167:477‐487. [DOI] [PubMed] [Google Scholar]
- 104. Zhang P, Xing X, Hu C, et al. 15‐Lipoxygenase‐1 is involved in the effects of atorvastatin on endothelial dysfunction. Mediators Inflamm. 2016;2016:6769032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li J, Rao J, Liu Y, et al. 15‐lipoxygenase promotes chronic hypoxia‐induced pulmonary artery inflammation via positive interaction with nuclear factor‐κB. Arterioscler Thromb Vasc Biol. 2013;33:971‐979. [DOI] [PubMed] [Google Scholar]
- 106. Moore GY, Pidgeon GP. Cross‐talk between cancer cells and the tumour microenvironment: the role of the 5‐lipoxygenase pathway. Int J Mol Sci. 2017;18:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615‐629. [DOI] [PubMed] [Google Scholar]
- 108. Parcesepe P, Giordano G, Laudanna C, Febbraro A, Pancione M. Cancer‐associated immune resistance and evasion of immune surveillance in colorectal cancer. Gastroenterol Res Pract. 2016;2016:6261721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hui L, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368:7‐13. [DOI] [PubMed] [Google Scholar]
- 110. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679‐695. [DOI] [PubMed] [Google Scholar]
- 111. Hill BS, Pelagalli A, Passaro N, Zannetti A. Tumor‐educated mesenchymal stem cells promote pro‐metastatic phenotype. Oncotarget. 2017;8:73296‐73311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tuncay Cagatay S, Cimen I, Savas B, Banerjee S. MTA‐1 expression is associated with metastasis and epithelial to mesenchymal transition in colorectal cancer cells. Tumour Biol. 2013;34:1189‐1204. [DOI] [PubMed] [Google Scholar]
- 113. Tuncer S, Tuncay Cagatay S, Keskus AG, Colakoglu M, Konu O, Banerjee S. Interplay between 15‐lipoxygenase‐1 and metastasis‐associated antigen 1 in the metastatic potential of colorectal cancer. Cell Prolif. 2016;49:448‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu Y, Mao F, Zuo X, et al. 15‐LOX‐1 suppression of hypoxia‐induced metastatic phenotype and HIF‐1α expression in human colon cancer cells. Cancer Med. 2014;3:472‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sasaki T, Fujii K, Yoshida K, et al. Peritoneal metastasis inhibition by linoleic acid with activation of PPARgamma in human gastrointestinal cancer cells. Virchows Arch. 2006;448:422‐427. [DOI] [PubMed] [Google Scholar]
- 116. Harats D, Ben‐Shushan D, Cohen H, et al. Inhibition of carcinogenesis in transgenic mouse models over‐expressing 15‐lipoxygenase in the vascular wall under the control of murine preproendothelin‐1 promoter. Cancer Lett. 2005;229:127‐134. [DOI] [PubMed] [Google Scholar]
- 117. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873‐887. [DOI] [PubMed] [Google Scholar]
- 118. Yan Y, He T, Shen Y, et al. Adenoviral 15‐lipoxygenase‐1 gene transfer inhibits hypoxia‐induced proliferation of retinal microvascular endothelial cells in vitro. Int J Ophthalmol. 2012;5:562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li Z, He T, Du K, Xing YQ. Overexpression of 15‐lipoxygenase‐1 inhibits oxygen‐induced ischemic retinopathy inhibits retinal neovascularization via downregulation of vascular endothelial growth factor‐A expression. Mol Vis. 2012;18:2847‐2859. [PMC free article] [PubMed] [Google Scholar]
- 120. Li Z, He T, Du K, et al. Inhibition of oxygen‐induced ischemic retinal neovascularization with adenoviral 15‐lipoxygenase‐1 gene transfer via up‐regulation of PPAR‐γ and down‐regulation of VEGFR‐2 expression. PLoS ONE. 2014;9:e85824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Viita H, Markkanen J, Eriksson E, et al. 15‐Lipoxygenase‐1 prevents vascular endothelial growth factor a‐ and placental growth factor‐induced angiogenic effects in rabbit skeletal muscles via reduction in growth factor mRNA levels, NO bioactivity, and downregulation of VEGF receptor 2 expression. Circ Res. 2008;102:177‐184. [DOI] [PubMed] [Google Scholar]
- 122. Al‐Shabrawey M, Mussell R, Kahook K, et al. Increased expression and activity of 12‐lipoxygenase in oxygen‐induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011;60:614‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Othman A, Ahmad S, Megyerdi S, et al. 12/15‐Lipoxygenase‐derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS ONE. 2013;8: e57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang B, Cao H, Rao GN. 15(S)‐hydroxyeicosatetraenoic acid induces angiogenesis via activation of PI3K‐Akt‐mTOR‐S6K1 signaling. Cancer Res. 2005;65:7283‐7291. [DOI] [PubMed] [Google Scholar]
- 125. Setty BN, Ganley C, Stuart MJ. Effect of changes in oxygen tension on vascular and platelet hydroxyacid metabolites. II. Hypoxia increases 15‐hydroxyeicosatetraenoic acid, a proangiogenic metabolite. Pediatrics. 1985;75:911‐915. [PubMed] [Google Scholar]
- 126. Bajpai AK, Blaskova E, Pakala SB, et al. 15(S)‐HETE production in human retinal microvascular endothelial cells by hypoxia: novel role for MEK1 in 15(S)‐HETE‐induced angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:4930‐4938. [DOI] [PubMed] [Google Scholar]
- 127. Zhao T, Wang D, Cheranov SY, et al. A novel role for activating transcription factor‐2 in 15(S)‐hydroxyeicosatetraenoic acid‐induced angiogenesis. J Lipid Res. 2009;50:521‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Singh NK, Kundumani‐Sridharan V, Rao GN. 12/15‐Lipoxygenase gene knockout severely impairs ischemia‐induced angiogenesis due to lack of Rac1 farnesylation. Blood. 2011;118:5701‐5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Soumya SJ, Binu S, Helen A, Reddanna P, Sudhakaran PR. 15(S)‐HETE‐induced angiogenesis in adipose tissue is mediated through activation of PI3K/Akt/mTOR signaling pathway. Biochem Cell Biol. 2013;91:498‐505. [DOI] [PubMed] [Google Scholar]
- 130. Wei C, Zhu P, Shah SJ, Blair IA. 15‐oxo‐Eicosatetraenoic acid, a metabolite of macrophage 15‐hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol. 2009;76:516‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15‐lipoxygenase‐1 in PC‐3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765‐1773. [DOI] [PubMed] [Google Scholar]
- 132. Zhong H, Wang R, Kelavkar U, Wang CY, Simons J. Enzyme 15‐lipoxygenase 1 promotes hypoxia‐inducible factor 1α turnover and reduces vascular endothelial growth factor expression: implications for angiogenesis. Cancer Med. 2014;3:514‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tunçer S, Keşküş AG, Çolakoğlu M, et al. 15‐Lipoxygenase‐1 re‐expression in colorectal cancer alters endothelial cell features through enhanced expression of TSP‐1 and ICAM‐1. Cell Signal. 2017;39:44‐54. [DOI] [PubMed] [Google Scholar]
- 134. Narizhneva NV, Razorenova OV, Podrez EA, et al. Thrombospondin‐1 up‐regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005;19:1158‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692‐704. [DOI] [PubMed] [Google Scholar]
- 136. Hellebrekers DMEI, Castermans K, Viré E, et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule‐1 by histone modifications. Cancer Res. 2006;66:10770‐10777. [DOI] [PubMed] [Google Scholar]
- 137. Dirkx AEM. Anti‐angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte‐endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621‐630. [DOI] [PubMed] [Google Scholar]
- 138. Weibel GL, Joshi MR, Alexander ET, Zhu P, Blair IA, Rothblat GH. Overexpression of human 15(S)‐lipoxygenase‐1 in RAW macrophages leads to increased cholesterol mobilization and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2009;29:837‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hill EM, Eling T, Nettesheim P. Differentiation dependency of eicosanoid enzyme expression in human tracheobronchial cells. Toxicol Lett. 1998;96–97:239‐244. [DOI] [PubMed] [Google Scholar]
- 140. Kim KS, Chun HS, Yoon JH, Jeung GL, Lee JH, Yoo JB. Expression of 15‐lipoxygenase‐1 in human nasal epithelium: its implication in mucociliary differentiation. Prostaglandins Leukot Essent Fat Acids. 2005;73:77‐83. [DOI] [PubMed] [Google Scholar]
- 141. Zuo X, Morris JS, Shureiqi I. Chromatin modification requirements for 15‐lipoxygenase‐1 transcriptional reactivation in colon cancer cells. J Biol Chem. 2008;283:31341‐31347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ostareck DH, Ostareck‐Lederer A, Shatsky IN, Hentze MW. Lipoxygenase mRNA silencing in erythroid differentiation: the 3'UTR regulatory complex controls 60S ribosomal subunit joining. Cell. 2001;104:281‐290. [DOI] [PubMed] [Google Scholar]
- 144. Reimann I, Huth A, Thiele H, Thiele BJ. Suppression of 15‐lipoxygenase synthesis by hnRNP E1 is dependent on repetitive nature of LOX mRNA 3’‐UTR control element DICE. J Mol Biol. 2002;315:965‐974. [DOI] [PubMed] [Google Scholar]
- 145. Leiva E, Wehinger S, Guzmán L, Orrego R. Role of oxidized LDL in atherosclerosis. Hypercholesterolemia. London: InTech; 2015. pp 61‐65. [Google Scholar]


