Abstract
Objectives
To investigate the synergistic mechanisms of Paris Saponin II (PSII) and Curcumin (CUR) in lung cancer.
Materials and Methods
The combination changed the cellular uptake of CUR and PSII, apoptosis, cell cycle arrest and cytokine levels were analysed on different lung cancer cells.
Results
The combination displayed a synergistic anti‐cancer effect through promoting the cellular uptake of CUR on different lung cancer cells. Hoechst H33258 staining and FACS assay indicated that the combination of PSII and CUR induced cell cycle arrest and apoptosis. Western blot and cytokine antibody microarray suggested that the combination activated death receptors such as DR6, CD40/CD40L, FasL and TNF‐α to induce cancer cells apoptosis, and up‐regulated IGFBP‐1 leading to inhibition of PI3K/Akt pathway and increase of p21 and p27, which therefore induced a G2 phase arrest in NCI‐H446 cells. Meanwhile, the combination suppressed PCNA and NF‐κB pathway in 4 kinds of lung cancer cells. They activated the phosphorylation of p38 and JNK, and inhibited PI3K in NCI‐H460 and NCI‐H446 cells, enhanced the phosphorylation of JNK in NCI‐H1299 cells, and increased the phosphorylation of p38 and ERK, and suppressed PI3K in NCI‐H520 cells.
Conclusions
PSII combined with CUR had a synergistic anti‐cancer effect on lung cancer cells. These findings provided a rationale for using the combination of curcumin and PSII in the treatment of lung cancer in future.
Keywords: absorption, apoptosis, cell cycle arrest, curcumin, Paris saponin II
1. INTRODUCTION
Lung cancer divided into small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC) is one of the leading causes of cancer‐related mortality worldwide.1 The major causes of death in lung cancer include aberrations in cell cycle control, metastasis and so forth. Therefore, amounts of evidence indicated that targeting the intracellular signalling pathway regulating cell cycle progression and inducing apoptosis was an important strategy in lung cancer treatment.
As previous reported, paris saponin II (PSII) was isolated from Rhizoma Paridis saponins (RPS). Its anti‐tumour effect has been observed in several cultural cells and animal systems through inducing apoptosis by elevating pro‐apoptotic elements including Bax, cytosolic cytochrome C, activated‐caspase‐3, and activated‐caspase‐9,2 promoting S phase arrest,3 suppressing NF‐κB signalling4 and so forth. Meanwhile, curcumin (CUR) as a multi‐target agent in the spice turmeric exhibited anti‐inflammatory,5 anti‐proliferative,6 anti‐oxidant,7 pro‐apoptotic8 and so forth effects against a variety of cancer models. It also enhanced the efficacy of some chemotherapy drugs by improving their pharmacokinetics,9 inducing apoptosis10 and so on. However, poor oral bioavailability, glucuronide and sulphate conjugate in plasma account for its poor systemic bioavailability.11
Interestingly in our previous research, CUR not only alleviated the toxicity and gastric stimulus induced by RPS,12 but also improved the quality life of mice bearing tumour cells and enhanced their anti‐cancer effect.13, 14 With the widely application of complex mixtures in clinic, the aim of this study was to investigate the synergistic anti‐cancer effects of PSII and CUR in lung cancer cell lines. Taken together, these findings would provide the foundation for the use of CUR and RPS in future.
2. MATERIALS AND METHODS
2.1. Reagents
Paris Saponin II (PSII) was provided from National Institute for the Control of Pharmaceutical and Biological Products (purity>91.4%). Curcumin (CUR) was purchased from Zhongda Co. (China) (90% purity). The other reagents were commercially available and of analytic purity.
2.2. Cell culture
The normal human pulmonary epithelial cell (BEAS‐2B) and human lung cancer cells (NCI‐H1299, NCI‐H460, NCI‐H520 and NCI‐H446 as adenocarcinoma, large cell carcinoma, squamous carcinoma and SCLC, respectively) were acquired from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). These cells were cultured in RPMI‐1640 medium with 10% foetal bovine serum (Thermo, China) and 1% penicillin‐ streptomycin (Solarbio Science & Technology Co., Beijing, China) at 37°C in a humidified atmosphere (5% CO2).
2.3. Cell proliferation assays
Cell viability was determined by a colorimetric assay using MTT (Solarbio Science & Technology Co., China). Different cells were seeded at a density of 5 × 103/well in a complete growth medium in 96‐well plates. The cells were incubated with the test compounds for 24 hour before the MTT assay. Then, a fresh solution of MTT (0.5 mg/mL) was added to each single well of the 96‐well plate. The plate was incubated in a CO2 incubator for another 4 hour. Finally, the cells were dissolved with 100 μL of DMSO and then analysed in a multi‐wall plate reader (BioTek Instruments, Inc., Winooski, VT, USA).
2.4. Cell uptake of CUR
The cells were treated with CUR or the combination of PSII and CUR for 24 hour. The cells were washed in phosphate buffered saline (PBS) thrice and lysed with 1% Triton X‐100 for 30 minutes. The cellular uptake of CUR was measured by fluorescence spectrophotometer (λ excitation = 425 nm, λ emission = 515 nm) (BioTek Instruments, Inc. USA).
2.5. Cell uptake of PSII
The cells were treated with PSII or the combination of PSII and CUR for 24 hour. The cells were washed in PBS thrice and lysed with 1% Triton X‐100 for 30 minutes. The solution was deproteinated by adding 100 μL of acetonitrile and 100 μL of methanol followed by vortex mixing for 1 min, ice‐cooling and centrifugation (10 000 rpm, 10 minutes, 4°C). The supernatant was then transferred to a HPLC vial and volatilized to dryness with nitrogen prior to analysis. (λ = 203 nm). Samples were dissolved in 100 μL of 50% acetonitrile aqueous solution through 0.22 μm microporous membrane before use. The analytical conditions were an Agilent ZORBAX SB‐C18 column (250 × 4.6 mm i.d., 5 μm; Agilent, Palo Alto, CA, USA) and a solvent system composed of acetonitrile (A) and water (B) (condition: 30%‐60% A from 0 to 40 minutes; 60%‐30% A from 40 to 50 minutes) at a flow rate of 1 mL/min. The injection volume was 20 μL, and the detection was at 203 nm.
2.6. Median‐effect analysis
For combination studies, MTT assays of PSII and CUR at non‐fixed dose ratio were done on 24 h treatment. Results are expressed as the percentage of surviving cells determined by comparing the MTT of each sample relative to control samples considered 100% viable. The dose‐response curves of each agent for each cell line were obtained. The interactions between drugs were evaluated by calculating Chou‐Talalay combination indices (CI) using Compusyn software. Each experiment was replicated in triplicate.
2.7. Hoechst H33258 Staining
The cells were treated with PSII and CUR for 24 hour. The cells were washed in PBS thrice and then fixed in 100% ice‐cold methyl alcohol for 10 minutes. After treatment, the cells were washed in PBS thrice and their nuclei were stained with Hoechst H33258 (1 μg/mL) (Solarbio Science & Technology Co., China) for 15 minutes and examined by fluorescence microscopy.
2.8. FACS analysis
The cells were seeded at a density of 3 × 105/well in 6‐well plates and allowed to grow for 24 hour. After 24 hour of treatment with the test compounds, the cells were collected and fixed with ice‐cold 75% ethanol at −20°C overnight. Then, the cells were resuspended in PBS containing 200 μg/mL of DNase‐free RNase A and 50 μg/mL presidium iodide and incubated at 37°C for 30 min in the dark. Samples were analysed using a FACS (BD Accuri C6, USA). The cell cycle distribution was evaluated by counting 20 000 cells per sample.
2.9. Annexin‐V/PI double staining
A FITC annexin‐V Apoptosis Detection Kit I (Jiangsu KeyGEN BioTECH. LTD, Nanjing, China) was used to detect apoptosis in human lung cancer cells after exposure to PSII and CUR. Cells were washed in PBS thrice and resuspended in 100 μL of binding buffer with FITC annexin‐V and PI (1 μL each). The cell suspension was incubated for 15 min at room temperature in the dark, and analysed by flow cytometry (BD Accuri C6, USA) within 1 hour.
2.10. Western blot analyses
The harvested cells were washed by PBS and lysed with protein lysis buffer. The concentrations of the proteins were tested by BCA Protein Assay kit (Thermo Scientific, Waltham, MA, USA). Protein samples were separated by electrophoresis in Sodium Dodecyl Sulphonate (SDS)‐polyacrylamide gel and then transferred onto a PVDF membrane, and then incubated with primary antibody for 3 hour. The primary antibodies were Phospho‐PI3‐kinase p85α/β/γ (H‐274), PCNA, NF‐κB, Phospho‐AKT (phospho‐S473) (Bioworld Technology, St Louis, MN, USA), PI3‐Kinase p110β (C‐8), p38α/β (A‐12) and pro caspase‐3 (Santa Cruz Biotechnology, Dallas, TX, USA), JNK1/MAPK8, AKT1/PKBα and ERK1/2 (BOSTER, China), Phospho‐SAPK/JNK (Thr183/Tyr185), Phospho‐p38 MAP Kinase (Thr180/Tyr182) and Phospho‐p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signalling Technology, Danvers, MA, USA). After washed with TBS buffer solution for 3 times, PVDF membrane was incubated with second antibody for 1 hour. Then, the expression of protein was detected by Odyssey infrared imaging system (LI‐COR Biotechnology, USA).
2.11. Cytokine antibody microarray
The RayBio® Custom Cytokines Antibody Array kit was purchased from RayBio (RayBio, Shanghai, China) and used according to the manufacturer's instructions. Briefly, total proteins from lung cancer cells were isolated using RayBio® Cell Lysis Buffer (RayBio, Shanghai, China). After blocking with 1 × blocking buffer (provided by the manufacturer), membranes were incubated for 2 hours with 80 μL of each sample. Following wash steps, each array was incubated with 70 μL of biotin‐conjugated antibodies for 2 hour. Then, the membranes were washed again and incubated with 70 μL of 1500 fold diluted streptavidin‐conjugated fluorescent dye for 2 hours. Subsequently, cover the incubation chamber with adhesive film. Cover the plate with aluminium foil to avoid exposure to light and incubate in dark room temperature for 2 hours. Soon, incubation chambers were discarded and slides were aspirated, washed and dried completely in air at least 20 minutes. Dried slides were immediately scanned and imaged using a GenePix 4000B microarray scanner and GenePix 5.0 software (Axon Instruments, Union City, CA, USA). All fluorescence intensities in scanned images were reported as background corrected mean fluorescence intensities of the pixels within the spot ellipse.
2.12. Statistical analysis
Statistical evaluation was conducted by using the SPSS 17.0 for Windows package software. Data have been expressed as the means ± standard error mean (SEM). One‐way variance analysis and Duncan multiple range test were used to determine significantly different groups. P < .05 was considered as significant differences for all statistical calculations.
3. RESULTS
3.1. CUR and PSII inhibited the proliferation of lung cancer cell lines
MTT assay was performed to determine the effects of different concentrations of PSII and CUR on the cell viability in the normal human pulmonary epithelial cells (BEAS‐2B) and the lung cancer cells lines (NCI‐H1299, NCI‐H460, NCI‐H520 and NCI‐H446). As shown in Figure 1B, PSII and CUR treatment did not show cytotoxic to the normal human pulmonary epithelial cells (BEAS‐2B). However, they inhibited lung cancer cells viability in a concentration‐dependent manner. The inhibitory effect of CUR on SCLC was higher than that on NSCLC (IC50NCI‐H446 < IC50NCI‐H520 < IC50NCI‐H1299 < IC50NCI‐H460) for 24 hour treatment (Table 1 ).
Figure 1.
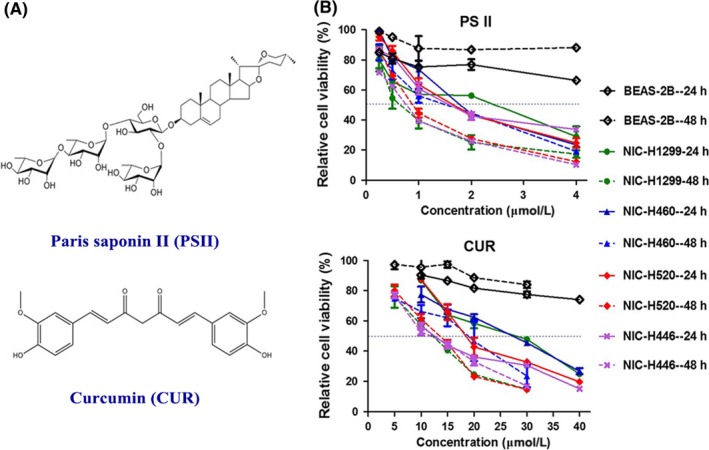
The inhibitory effect of PSII and CUR on lung cancer cells. (A) Structure of PSII and CUR. (B) The lung cell lines were treated with different concentrations of PSII and CUR for 24 and 48 hour. Relative cell viability was determined by MTT assay. Data were obtained from 3 independent experiments
Table 1.
The IC50 of PSII and CUR, respectively (μM)
| IC50 | NCI‐H460 | NCI‐H1299 | NCI‐H520 | NCI‐H446 | ||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| PSII | 2.54 ± 0.29 | 2.01 ± 0.45 | 2.50 ± 0.29 | 1.29 ± 0.24 | 2.24 ± 0.20 | 1.54 ± 0.05 | 2.21 ± 0.03 | 1.50 ± 0.23 |
| CUR | 29.7 ± 2.6a | 18.4 ± 2.1a | 26.5 ± 1.1a | 13.0 ± 1.7b | 22.9 ± 1.8b | 14.1 ± 0.7b | 17.8 ± 2.2c | 14.9 ± 1.6ab |
Different letters meant significant differences between 2 groups (P < .05).
3.2. The combination of PSII and CUR had synergistic anti‐cancer effects on lung cancer cells
PSII and CUR inhibited proliferation of human lung cancer cells. To test whether PSII and CUR exert synergic or additive effects on lung cancer cells, the dose‐effect curves of single and combined drug treatment were analysed by the median‐effect method, where the combination indexes (CI) of less than, equal to, or more than 1 indicated synergistic, additive or antagonistic activity, respectively. As a result, the dose‐effect curves, median‐effect plot and combination index plot of single and combined drug treatment were shown in Figure 2. CI values was less than 1 in combination of CUR and PSII at concentration ranging from 0.125 to 2.0 μM, indicating synergic effect of combined agents in 4 lung cancer cell lines (Table 2). CI value was ranging from 0.3 to 0.8, indicating synergic effects of combined agents in NCI‐H520, NCI‐H446 and NCI‐H1299. Meanwhile, CI value was ranging from 0.8 to 0.99, suggesting slightly synergic effect of combined agents in NCI‐H460.
Figure 2.
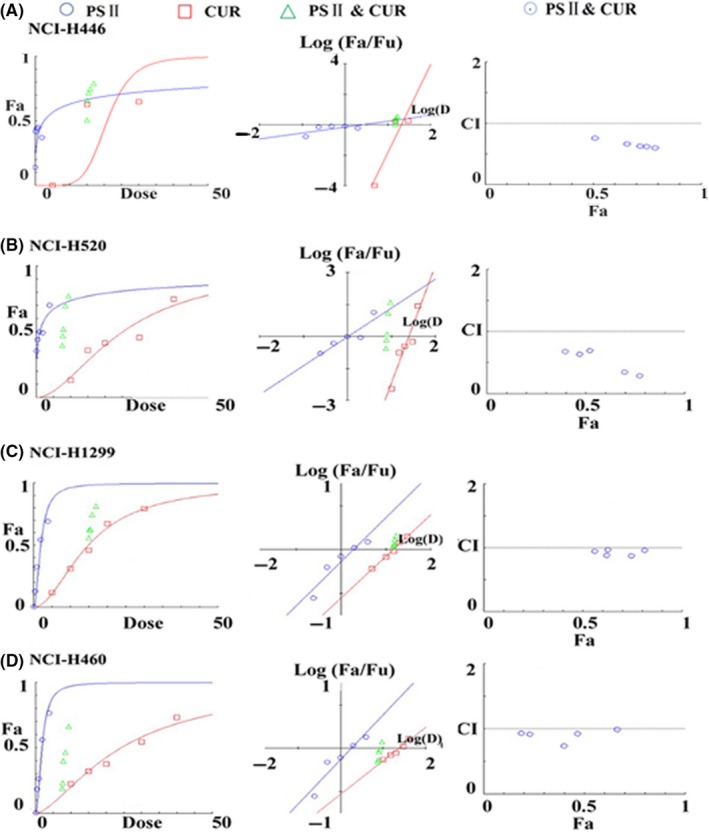
The combination indexes (CI) of RPS II and CUR in (A) NCI‐H446, (B) NCI‐H520, (C) NCI‐H1299 and (D) NCI‐H460 cancer cells
Table 2.
The CI values of PSII and CUR in lung cancer cell lines
| Concentration (μM) | NCI‐H446 | NCI‐H1299 | NCI‐H520 | NCI‐H460 | |||||
|---|---|---|---|---|---|---|---|---|---|
| PSII | CUR | Effect | CI | Effect | CI | Effect | CI | Effect | CI |
| 0.125 | 15 | 0.64 | 0.66 | 0.40 | 0.83 | 0.56 | 0.31 | 0.40 | 0.87 |
| 0.25 | 0.66 | 0.66 | 0.47 | 0.74 | 0.62 | 0.25 | 0.50 | 0.73 | |
| 0.50 | 0.70 | 0.64 | 0.52 | 0.75 | 0.63 | 0.30 | 0.47 | 0.98 | |
| 1.00 | 0.75 | 0.61 | 0.70 | 0.37 | 0.65 | 0.38 | 0.62 | 0.86 | |
| 2.00 | 0.79 | 0.59 | 0.77 | 0.30 | 0.70 | 0.44 | 0.78 | 0.72 | |
3.3. Combination of PSII and CUR promoted the cellular uptake of CUR and PSII
The cellular uptakes of CUR and PSII in lung cancer cells influenced their anti‐tumour activity. As shown in Figure 3, combination of PSII and CUR promoted singular cellular uptake of CUR and PSII in cancer cells. Meanwhile, the cellular uptake of CUR and PSII in SCLC (NCI‐H446) was more sensitive than that in other cells.
Figure 3.
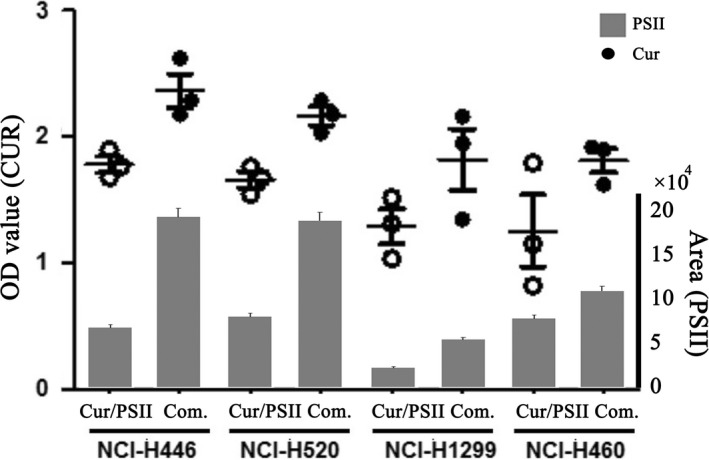
The cellular uptake of CUR and PSII in 4 lung cancer cells for 24 hour treatment. The globe represents CUR. The concentration of curcumin was measured by fluorescence spectrophotometer (λ excitation = 420 nm, λ emission = 540 nm). The white globe meant the cells treated with CUR. While the black one indicated the cells treated with the combination of CUR and PSII. The column stood for PSII. The concentration of PSII was measured by HPLC (λ = 203 nm)
3.4. The synergistic anti‐cancer effect of PSII and CUR on nucleus morphological changes
In order to further study the synergic anti‐cancer effect of PSII and CUR, the morphological changes in 4 lung cancer cells were detected by Hoechst 33258 staining. As shown in Figure 4, the viable cells exhibited bright blue, while the apoptotic cells showed deep white. The red arrows indicated chromatin condensation, while yellow arrows represented nucleus fragmentation. Compared with the control group, PSII and CUR alone increased the number of the chromatin compaction (Figure 4B,C). Meanwhile, the combination of PSII and CUR induced more evident apoptosis characteristics, including nuclear fragmentation and chromatin compaction of apoptotic appearance (Figure 4D). It indicated that the combination of PSII and CUR was more effective in pro‐apoptosis than any monomer alone.
Figure 4.
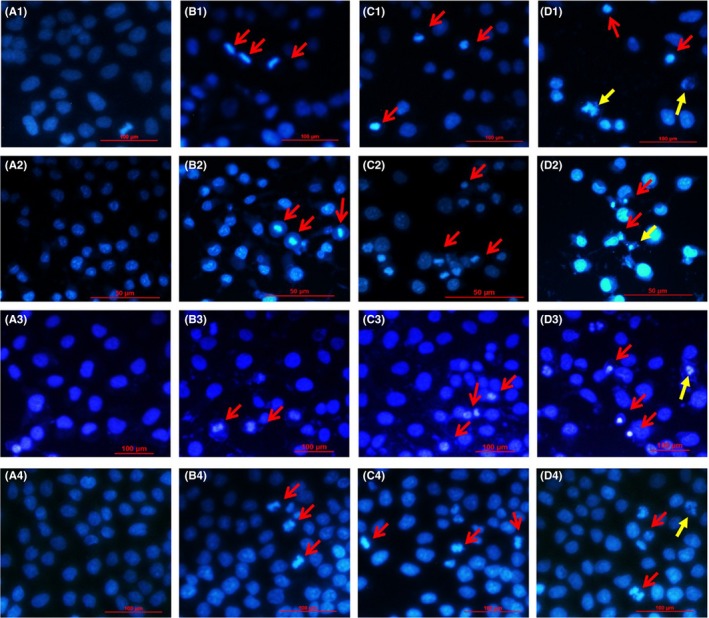
Morphological changes caused by PSII and CUR in (1) NCI‐H446, 2) NCI‐H520, 3) NCI‐H1299 and 4) NCI‐H460 human lung cancer cells for 24 hour (400×, final magnification). (A) Control, (B) PSII, (C) CUR and (D) combination groups. The viable cells exhibited bright blue, while the apoptotic cells showed deep white. The red arrows indicated chromatin condensation, while yellow arrows represented nucleus fragmentation
3.5. The synergistic anti‐cancer effect of PSII and CUR on cell apoptosis
To further characterize the synergistic anti‐cancer effect of PSII and CUR, Annexin‐V FITC/PI double staining was used to confirm this pro‐apoptotic phenomenon. As shown in Figure 5, the percentage of apoptotic cells treated by PSII was 21.6%, 0.07%, 2.52% and 21.2%, respectively. The percentage of apoptotic cells induced by CUR was 30.1%, 5.56%, 21.23% and 18.8%, respectively. When exposed to their combination, the percentage increased to 44.4%, 15.13%, 40.02% and 30.2% in NCI‐H446, NCI‐H520, NCI‐H1299 and NCI‐H460 human lung cancer cells. Therefore, the combination of PSII and CUR was more effective on pro‐apoptosis than any agent alone.
Figure 5.
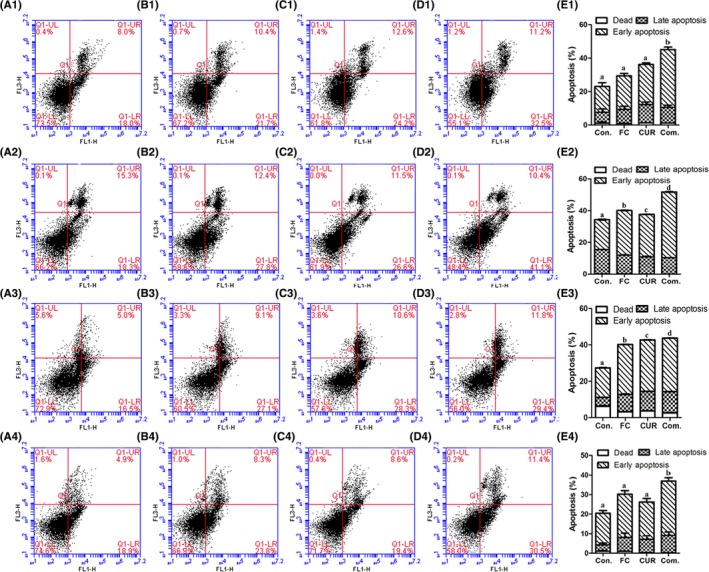
Effects of PSII and CUR on cell apoptosis in lung cancer cell lines including 1) NCI‐H446, 2) NCI‐H520, 3) NCI‐H1299 and 4) NCI‐H460 cancer cells for 24 hour. (A) Control, (B) PSII, (C) CUR, (D) combination groups and (E) statistical analysis. Different letters meant significant differences between 2 groups (p < .05)
3.6. The combination of PSII and CUR induced apoptosis via PI3K/AKT, MAPK and NF‐κB pathways
In order to understand the potential molecular mechanisms of PSII and CUR, several related pathways including phosphatidyl inositol 3 kinase (PI3K)/AKT, MAPK and nuclear factor‐kB (NF‐kB) signals were tested. As Figure 6 displayed, the expression of proliferating cell nuclear antigen (PCNA), p‐PI3K and NF‐κB was decreased and pro caspase 3 was increased in PSII‐ and CUR‐treated lung cancer cells (Figure 6A,B,D,F). Combination group increased phosphorylated level of p38MAPK in SCLC (NCI‐H446) and NSCLC (NCI‐H460 and NCI‐H520), c‐jun NH2‐terminal kinase (JNK) in SCLC (NCI‐H446) and NSCLC (NCI‐H1299 and NCI‐H460), and extracellular signal‐regulated kinase1/2 (ERK1/2) in NSCLC (NCI‐H520) (Figure 6C). In addition, combination group induced p‐AKT down in NCI‐H446 (Figure 6E). The results showed that the combination of PSII and CUR induced apoptosis through MAPK, PI3K and NF‐κB signalling pathways, which were slightly different in NSCLC and SCLC.
Figure 6.
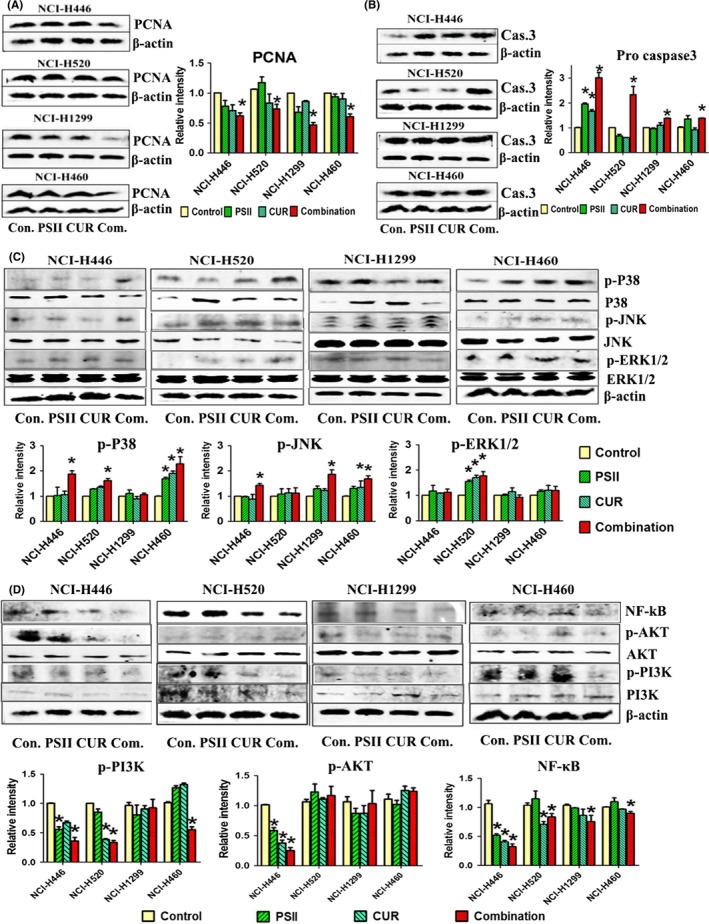
The combination of PSII and CUR inhibited PI3K/AKT, MAPK and NF‐κB signalling pathways. The expressions of (A) PCNA, (B) Pro caspase3, (C) MAPKs, (D) NF‐κB and PI3K/AKT were examined by Western blot assay. Protein levels were normalized with β‐actin levels (p < .05). *p < .05 compared with control group
3.7. The synergistic anti‐cancer effect of PSII and CUR on cell cycle arrest in NCI‐H446 cells
For they were more sensitive in SCLC (NCI‐H446) than that in other cells based on the above assays, further characterize the synergistic anti‐cancer effect of PSII and CUR based on the cell cycle arrest was carried out. As Figure 7 shown, an increasing accumulation of sub‐diploid cells displayed in those cells treated with combination of PSII and CUR for 24 hour compared with other groups. Meanwhile, cells were accumulated at G2‐phase in NCI‐H446 cells.
Figure 7.
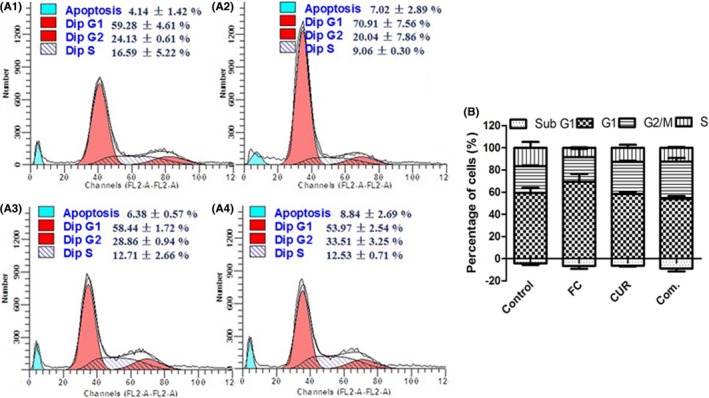
Effects of PSII and CUR on cell cycle distribution in NCI‐H446 cell including (A1) control, (A2) PSII, (A3) CUR and A4) Combination groups for 24 hour. (B) Column diagram of the cell cycle distribution
3.8. Screening of the content of differential cytokines after the combination of PSII‐ and CUR‐treated NCI‐H446 cells
In order to understand the combination effects of PSII and CUR on cell cycle and apoptosis in lung cancer cells, cytokine analyses were detected in the sensitive cells (NCI‐H446). As shown in Table 3, the combination of PSII and CUR significantly increased protein expression levels of mitochondria‐related proteins including BCL‐2/BCL‐XL‐associated death promoter (Bad), BCL2‐associated X protein (BAX) and Bcl‐2 (the ratio of Bad/Bcl‐2 was 1.0), death receptors such as CD40/CD40L, DR6, fatty acid synthetase ligand (FasL) and tumour necrosis factor (TNF)‐α, growth factor like insulin‐like growth factor‐binding protein 1 (IGFBP 1), and cell cycle‐related proteins containing p21 and p27. All these cytokines or receptors as initiators to induce tumour cell apoptosis indicated that the combination treatment induced death receptor‐related apoptosis pathway.
Table 3.
Representative apoptotic biomarkers in NCI‐H446 cells after drug treatment
| Biomarkers | Fold change | Pathway | ||
|---|---|---|---|---|
| PSII/Control | Cur/Control | Com./Control | ||
| Bad | — | — | ↑(1.30) | Mitochondria |
| Bax | — | — | ↑(1.32) | |
| Bid | ↓(0.74) | — | — | |
| Bcl‐2 | — | — | ↑(1.36) | |
| Bad/Bcl‐2 | 1.1 | 1.1 | 1.0 | |
| CD40 | — | — | ↑(1.26) | Death receptor |
| CD40L | — | — | ↑(1.32) | |
| DR6 | — | — | ↑(1.27) | |
| Fas | — | — | — | |
| FasL | — | — | ↑(1.25) | |
| TNF‐α | ↑(1.21) | ↑(1.36) | ↑(1.33) | |
| IGFBP‐1 | — | — | ↑(1.35) | IGF/IGFBP |
| IGF‐I | ↓(0.74) | — | — | |
| p21 | — | — | ↑(1.20) | Cell cycle arrest |
| p27 | — | — | ↑(1.20) | |
The biomarkers in one color shading indicated that they acted in the same pathway
4. DISCUSSION
Rhizoma paridis saponins (RPS) played a good anti‐tumour role in many clinical applications. Combination of RPS and CUR not only alleviated the toxicity and gastric stimulus induced by RPS,12 but also improved the quality life of mice bearing tumour cells and enhanced their anti‐cancer effect.13, 14 In our previous research, PSII (formosanin C) as one of the main effective compounds in RPS15 was absorbed in rat plasma after oral administration of RPS.16 Meanwhile, the anti‐tumour effect of PSII has been observed in several cultural cells and animal systems through inducing apoptosis,2 promoting S phase arrest3 and suppressing NF‐κB signalling.4 It was reported that CUR as a multi‐target agent enhanced the efficacy of some chemotherapy drugs by improving their pharmacokinetics9 and inducing apoptosis.10, 17 In addition, poor oral bioavailability of PSII18 and CUR11 alone promoted the application of their combination in clinic. This research was to investigate the synergistic anti‐cancer effects of PSII and CUR in lung cancer cell lines.
The present results demonstrated that PSII and CUR had synergic anti‐cancer effects on lung cancer cells (Figures 1 and 2). As our previous report, CUR promoted the absorption of RPS and thereby increased its anti‐tumour effect.13, 14 In this experiment, it also proved that the main compound of RPS, PSII combined with CUR promoted both of their cellular uptake and possibly enhanced their bioavailability. Furthermore, Hoechst 33258 staining assay and FACS analysis assay demonstrated that PSII and CUR had a synergistic anti‐cancer effect on the promotion of cancer cell apoptosis. The apoptotic pathway may be based on the activation of the phosphorylation of p38 and JNK, and inhibition of PI3K in NCI‐H460 and NCI‐H446 cells, enhancement of the phosphorylation of JNK in NCI‐H1299 cells, and increase of the phosphorylation of p38 and ERK, and suppression of PI3K in NCI‐H520 cells. Meanwhile, NF‐κB also modulated cellular growth, apoptosis and development.19 PCNA was regarded as a critical protein in regulating the growth of cancer cells and cell cycle arrest.20 Both of them were down‐regulated in cancer cells (Figure 6).
For they were more sensitive in SCLC (NCI‐H446) than that in other cells based on several assays, cell cycle assay and cytokine antibody microarray analyses in NCI‐H446 cancer cells were carried out. The main pathways involved in the cell apoptosis were the extrinsic death receptor pathway and intrinsic mitochondria pathway.21 According to the cytokine antibody microarray analyses in NCI‐H446 cancer cells, the combination of PSII and CUR mainly induced extrinsic death receptor pathway involved in the programmed cell death (Table 3). Combination treatment activated the death receptors such as TNF‐α, FasL, DR6 and CD40/CD40L to increase the protein expression of caspase 3 and then induced apoptosis (Figure 8).
Figure 8.
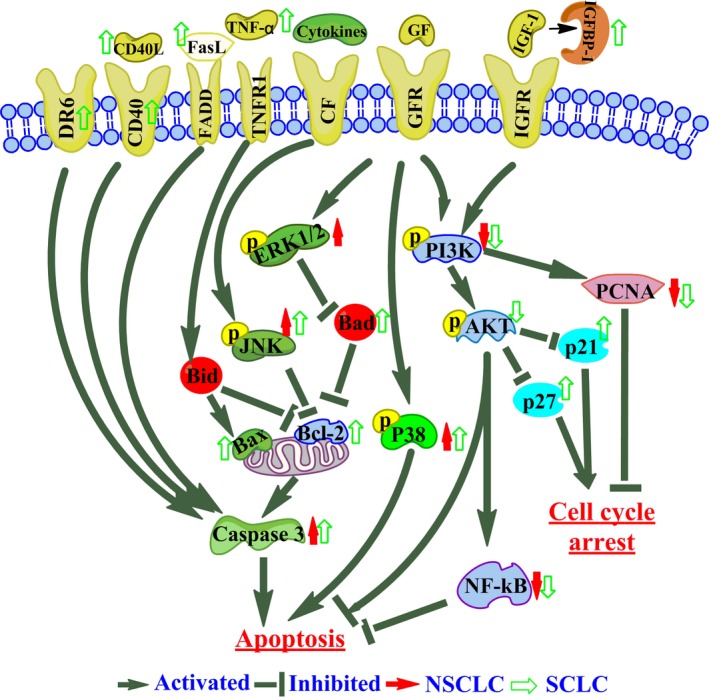
Schematic representation of proposed mechanism of action of PSII and CUR induced cell cycle arrest and apoptosis in lung cancer cells. Combination of PSII and CUR activated death receptors such as DR6, CD40/CD40L, FasL and TNF‐α to induce cell apoptosis. The up‐regulation of IGFBP‐1 decreased phosphorylation of PI3K to regulate cell cycle and pro‐apoptosis. Meanwhile, the down‐regulation of PI3K/Akt, NF‐κB pathways, PCNA and increasing the phosphorylation of P38 and JNK took part in the anti‐tumour effect of the combination treatment
As we know, the cell cycle was mediated by the activation of cyclin‐dependent kinase inhibitors (CKIs), such as p21 and p27.22 In this research, combination treatment suppressed cell cycle progression in the G2‐phase through decreasing p‐Akt and therefore up‐regulating expression of p27 and p21 in NCI‐H446 cells.23 Furthermore, combination treatment increased the level of IGFBP1 and subsequently inhibited the activity of IGF1 receptor/PI3K/Akt pathway,24 which was involved in the cellular differentiation, inflammatory responses, cell cycle arrest and apoptosis in cancer cells.25
In conclusion, the combination of PSII and CUR had a synergic anti‐cancer effect on lung cancer cells through the suppression of the NF‐κB pathway and PCNA expression in every cancer cells, activation of the phosphorylation of p38 and JNK, and inhibition of PI3K in NCI‐H460 and NCI‐H446 cells, enhancement of the phosphorylation of JNK in NCI‐H1299 cells, and increase of the phosphorylation of p38 and ERK, and suppression of PI3K in NCI‐H520 cells. The synergic effect displayed more sensitive in NCI‐H446 than that in other cells. The combination up‐regulated IGFBP‐1 to inhibit the PI3K/Akt pathway and then increased the expression of p21 and p27 to induce G2 phase arrest in NCI‐H446 cells. They also activated the death receptors such as DR6, CD40/CD40L, FasL and TNF‐α to induce cancer cells apoptosis. Therefore, PSII combined with CUR had a synergistic anti‐cancer effect on lung cancer cells, which provided the foundation for the use of CUR and RPS in future.
ACKNOWLEDGEMENTS
This work was supported by grants 81673647, 81673535, and 81503086 from National Natural Science Foundation of China, Drug Creation Project 2014ZX09301307‐018 from Science and Technology in China and 13JCQNJC13400 from Tianjin Natural Science Foundation in China.
Man S, Zhang L, Cui J, Yang L, Ma L, Gao W. Curcumin enhances the anti‐cancer effects of Paris Saponin II in lung cancer cells. Cell Prolif. 2018;51:e12458 10.1111/cpr.12458
Shuli Man and Long Ma are contributed equally to this paper.
Contributor Information
Shuli Man, Email: msl@tust.edu.cn.
Wenyuan Gao, Email: biochemgao@163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Xiao X, Zou J, Bui‐Nguyen TM, et al. Paris saponin II of Rhizoma Paridis – A novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6:201‐211. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Man S, Li J, et al. The antitumor effect of formosanin C on HepG2 cell as revealed by 1H‐NMR based metabolic profiling. Chem Biol Interact. 2014;220:193‐199. [DOI] [PubMed] [Google Scholar]
- 4. Yang M, Zou J, Zhu H, et al. Paris saponin II inhibits human ovarian cancer cell‐induced angiogenesis by modulating NF‐kappaB signaling. Oncol Rep. 2015;33:2190‐2198. [DOI] [PubMed] [Google Scholar]
- 5. Zhu H, Xu T, Qiu C, et al. Synthesis and optimization of novel allylated mono‐carbonyl analogs of curcumin (MACs) act as potent anti‐inflammatory agents against LPS‐induced acute lung injury (ALI) in rats. Eur J Med Chem. 2016;121:181‐193. [DOI] [PubMed] [Google Scholar]
- 6. Katsori AM, Chatzopoulou M, Dimas K, et al. Curcumin analogues as possible anti‐proliferative & anti‐inflammatory agents. Eur J Med Chem. 2011;46:2722‐2735. [DOI] [PubMed] [Google Scholar]
- 7. Tu ZS, Wang Q, Sun DD, Dai F, Zhou B. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur J Med Chem. 2017;134:72‐85. [DOI] [PubMed] [Google Scholar]
- 8. Liu GY, Sun YZ, Zhou N, Du XM, Yang J, Guo SJ. 3,3’‐OH curcumin causes apoptosis in HepG2 cells through ROS‐mediated pathway. Eur J Med Chem. 2016;112:157‐163. [DOI] [PubMed] [Google Scholar]
- 9. Cheng KW, Wong CC, Mattheolabakis G, Xie G, Huang L, Rigas B. Curcumin enhances the lung cancer chemopreventive efficacy of phospho‐sulindac by improving its pharmacokinetics. Int J Oncol. 2013;43:895‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan G, Graham K, Lanza‐Jacoby S. Curcumin enhances the anticancer effects of trichostatin a in breast cancer cells. Mol Carcinog. 2013;52:404‐411. [DOI] [PubMed] [Google Scholar]
- 11. Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Man S, Li J, Liu J, et al. Curcumin alleviated the toxic reaction of Rhizoma Paridis saponins in a 45‐day subchronic toxicological assessment of rats. Environ Toxicol. 2016;31:1935‐1943. [DOI] [PubMed] [Google Scholar]
- 13. Man S, Chai H, Qiu P, et al. Turmeric enhancing anti‐tumor effect of Rhizoma paridis saponins by influencing their metabolic profiling in tumors of H22 hepatocarcinoma mice. Pathol Res Pract 2015;211:948‐954. [DOI] [PubMed] [Google Scholar]
- 14. Man S, Li Y, Fan W, et al. Curcuma increasing antitumor effect of Rhizoma paridis saponins through absorptive enhancement of paridis saponins. Int J Pharm 2013;454:296‐301. [DOI] [PubMed] [Google Scholar]
- 15. Man S, Gao W, Zhang Y, et al. Characterization of steroidal saponins in saponin extract from Paris polyphylla by liquid chromatography tandem multi‐stage mass spectrometry. Anal Bioanal Chem. 2009;395:495‐505. [DOI] [PubMed] [Google Scholar]
- 16. Man S, Gao W, Zhang Y, Huang L, Liu C. Identification of chemical constituents in Rhizoma Paridis Saponins and their oral administration in rat plasma by UPLC/Q‐TOF/MS. Biomed Chromatogr. 2011;25:712‐719. [DOI] [PubMed] [Google Scholar]
- 17. Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL‐induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104‐112. [DOI] [PubMed] [Google Scholar]
- 18. Wang B, Ji S, Zhang H, et al. Liquid chromatography tandem mass spectrometry in study of the pharmacokinetics of six steroidal saponins in rats. Steroids. 2013;78:1164‐1170. [DOI] [PubMed] [Google Scholar]
- 19. Ma L, Liu H, Qin P, et al. Saponin fraction isolated from Conyza blinii H. Lev. demonstrates strong anti‐cancer activity that is due to its NF‐kappaB inhibition. Biochem Biophys Res Commun. 2017;483:779‐785. [DOI] [PubMed] [Google Scholar]
- 20. Zhao H, Ho PC, Lo YH, et al. Interaction of proliferation cell nuclear antigen (PCNA) with c‐Abl in cell proliferation and response to DNA damages in breast cancer. PLoS ONE. 2012;7:e29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stefanowicz‐Hajduk J, Bartoszewski R, Bartoszewska S, et al. Pennogenyl Saponins from Paris quadrifolia L. Induce Extrinsic and intrinsic pathway of apoptosis in human cervical cancer HeLa cells. PLoS ONE. 2015;10:e0135993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu J, Chen M, Chen N, et al. Glycyrrhetinic acid induces G1phase cell cycle arrest in human nonsmall cell lung cancer cells through endoplasmic reticulum stress pathway. Int J Oncol. 2015;46:981‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue B, Huang W, Yuan X, et al. YSY01A, a novel proteasome inhibitor, induces cell cycle arrest on G2 phase in MCF‐7 cells via ERalpha and PI3K/Akt pathways. J Cancer. 2015;6:319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fardini Y, Masson E, Boudah O, et al. O‐GlcNAcylation of FoxO1 in pancreatic beta cells promotes Akt inhibition through an IGFBP1‐mediated autocrine mechanism. FASEB J. 2014;28:1010‐1021. [DOI] [PubMed] [Google Scholar]
- 25. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol. 2014;90:197‐207. [DOI] [PubMed] [Google Scholar]


