Abstract
Objectives
Stem cell factor (SCF) is considered as a commonly indispensable cytokine for proliferation of haematopoietic stem cells (HSCs), which is used in large dosages during ex vivo culture. The work presented here aimed to reduce the consumption of SCF by sustained release but still support cells proliferation and maintain the multipotency of HSCs.
Materials and methods
Stem cell factor was physically encapsulated within a hyaluronic acid/gelatin double network (HGDN) hydrogel to achieve a slow release rate. CD34+ cells were cultured within the SCF‐loaded HGDN hydrogel for 14 days. The cell number, phenotype and functional capacity were investigated after culture.
Results
The HGDN hydrogels had desirable properties and encapsulated SCF kept being released for more than 6 days. SCF remained the native bioactivity, and the proliferation of HSCs within the SCF‐loaded HGDN hydrogel was not affected, although the consumption of SCF was only a quarter in comparison with the conventional culture. Moreover, CD34+ cells harvested from the SCF‐loaded HGDN hydrogels generated more multipotent colony‐forming units (CFU‐GEMM).
Conclusion
The data suggested that the SCF‐loaded HGDN hydrogel could support ex vivo culture of HSCs, thus providing a cost‐effective culture protocol for HSCs.
1. INTRODUCTION
Haematopoietic stem cells (HSCs) have attracted increasing attention over the past several decades because they play a critical role in the treatment of severe haematological diseases and cancers such as leukaemia, haemolytic anaemia and lymphoma.1, 2, 3 Nowadays, cord blood (CB) is considered as a promising source of HSCs for cell transplantation, but the small volume of HSCs collected from a single donor has greatly restricted the clinical application.4, 5, 6, 7 Indeed, HSCs grow in a specified niche, the so‐called “haematopoietic niche,” which has been identified to play an essential role in regulating behaviours of stem cell including quiescence, migration, proliferation and maturation.8, 9, 10, 11, 12 Therefore, ex vivo expansion is a desirable strategy for donated HSCs to achieve sufficient cells by simulating this microenvironment.13
These behaviours of HSCs are greatly influenced by cytokines during ex vivo culture.14 Meanwhile, among numerous cytokines utilized for proliferation of HSCs, stem cell factor (SCF) has been identified to serve as guidance cues that direct HSCs to the niche, and it is conducive to HSC maintenance.15 It has been emphasized that the insufficient supplement of SCF would result in impeding proliferation of HSCs, but the excessive amount of SCF also leads to loss of stemness.16 Additionally, SCF stimulates the proliferation of myeloid, erythroid and lymphoid progenitors and acts synergistically with colony‐stimulating factors.17 In spite of widespread usage of SCF during ex vivo culture of HSCs, nevertheless, an optimal feeding strategy has not yet been defined. In conventional culture conditions, SCF was usually supplemented continually with the combination of thrombopoietin (TPO) and Flt3‐Ligand (Flt‐3) during the whole culture process.18 However, SCF is an extraordinarily expensive and efficient cytokines and has low service efficiency in conventional culture conditions. Previous research has reported that SCF exerts its activity at the early stages of haematopoiesis.19 Thus, new feeding strategies of SCF with high efficiency are urgent to be developed. More recently, SCF was covalently immobilized on polymeric substrate materials such as poly (ethylene glycol) and gelatin. Although the consumption of SCF was reduced and selectivity for maintaining primitive HSCs was improved, proliferation capacity of cells decreased dramatically.20 Therefore, we aimed to develop a strategy to reduce consumption of SCF without affecting the proliferation of HSCs. To the best of our knowledge, there are few reports on in situ release of SCF from substrate materials. In this study, SCF is physically encapsulated within a porous scaffold and released via a straightforward process of diffusion, and the high bioactivity of SCF may be remained at the same time.
It has been proven that the physicochemical property of microenvironment is also a determinant for HSCs behaviours.21, 22, 23 A lot of efforts have been turned towards developing two‐ and three‐dimensional biomaterials such as microspheres, films, scaffolds and hydrogels for proliferation or differentiation of HSCs.24, 25, 26, 27 Among them, hydrogels are considered as promising candidates for cells culture due to their adjustable properties and high water content, providing an artificial microenvironment similar to the native Excetral Cellular Matrix (ECM).28, 29, 30 However, bare hydrogels are rarely utilized to load cytokines or growth factors, because their loose crosslinked networks usually result in a relatively rapid release rate. For instance, most of recombinant human interleukin‐2 (IL‐2) encapsulated in dextran hydrogels was released within initial 2 days.31 In the last decade, the double network (DN) hydrogel, which generally consists in the combination of a fragile network with a ductile one, has exhibited a higher mechanical strength.32 Moreover, the DN hydrogel with a tighter inner structure has been utilized for sustained release of peptides, proteins and drugs, which could significantly reduce release rate and undesired leakage.33 Thus, SCF encapsulated within DN hydrogels may achieve a suitable release for proliferation of HSCs.
Herein, we encapsulated SCF within a hyaluronic acid/gelatin double network (HGDN) hydrogel for ex vivo culture of CD34+ cells. Hyaluronic acid and gelatin were modified with a photo‐crosslinkable segment, and the HGDN hydrogel was fabricated via a two‐step photo‐crosslinking process (Figure 1). SCF was physically encapsulated within the hydrogel prior to the second photo‐crosslinking, and its release behaviour was further evaluated by ELISA kit. One parameter investigated was the impact of SCF released from hydrogels on survival of CD34+ cells. Moreover, after the end of culture, CD34+ cells were harvested to evaluate the multipotency.
Figure 1.
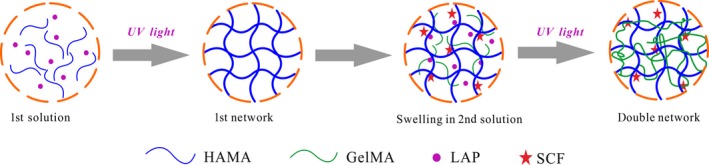
A schematic representation of the SCF‐loaded hyaluronic acid/gelatin double network (HGDN) hydrogel fabricated via a two‐step photo‐crosslinking process. SCF was encapsulated within the hyaluronic acid/gelatin double network (HGDN) hydrogel to achieve a sustained release and support proliferation of haematopoietic stem cells (HSCs)
2. MATERIALS AND METHODS
2.1. Isolation and culture of mononuclear cells (MNCs) and CD34+ cells
The experiments were approved by the State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology. Human cord blood samples were collected after written consent of the parents. MNCs were separated by density gradient centrifugation using Ficoll‐Paque (GE Healthcare, Stockholm, Sweden), and then paramagnetic microbeads and MiniMACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) were applied for further isolation of CD34+ cells. The purity of the freshly isolated cells was determined by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA, USA) and CD34+ cells with purity over 95% were utilized in the following experiments. CD34+ cells were cultured in serum‐free medium (SFM; Gibco, Paisley, UK) supplemented with a combination of recombinant human cytokines (Peprotech, London, UK), including TPO (20 ng/mL) and Flt3‐Ligand (Flt‐3, 50 ng/mL). SCF was supplemented according to the experiment. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 at all stages and half of the culture medium was exchanged every 3 days.
2.2. Hydrogel preparation
Methacrylated hyaluronic acid (HAMA) and methacrylated gelatin (GelMA) were synthesized by the esterification of sodium hyaluronate and gelatin with methacrylic anhydride, respectively. Meanwhile, a high‐efficient photo‐initiator, lithium phenyl‐2,4,6‐trimethylbenzoylphosphinate (LAP), was also synthesized (see Figure S1). The HAMA hydrogel was prepared by pouring pre‐gel solution of 3 wt% HAMA and 0.1 wt% LAP into a special round mold, and then irradiated by ultraviolet light (365 nm, 6 mW/cm2) for 30 seconds. The GelMA hydrogel was prepared by dissolving 5 wt% GelMA and 0.1 wt% LAP in phosphate buffer solution (PBS) at 37°C in the same manner. To prepare the HGDN hydrogel, the as‐prepared HAMA hydrogel as the first network was immersed in solution containing 5 wt% GelMA and 0.1 wt% LAP, and placed in a shaking bed at 37°C for 2 days to reach swelling equilibrium. Finally, the HGDN hydrogel was fabricated by UV light again for 30 seconds.
2.3. Hydrogel characterization
The microstructure of hydrogels was characterized by scanning electron microscope (SEM; S‐3400N, Hitachi, Tokyo, Japan). Freeze‐dried samples were sputtered with a thin layer of gold and imaged with an accelerating voltage of 15 kV.
The mechanical properties of hydrogels were determined by using a mechanical testing system (TCS‐2000, Gothch, Dongguan, China). Samples were prepared with a dimension of 10 mm × 5.0 mm (diameter × thickness), and then they were compressed with a crosshead at a rate of 1 mm/min. The elastic modulus was calculated as the slope of the linear region (initial 10% strain) of the stress‐strain curves.
Swelling behaviour of hydrogels was measured by weight. The dried hydrogel was weighed (m d) and immersed in PBS at room temperature. After each predetermined time interval, the excess PBS on surface was removed and the swelling hydrogel was weighed (m s). Swelling ratio (SR) of hydrogel was calculated as follows:
2.4. Release profile of SCF
To study release profile of SCF from a single network (SN) hydrogel, SCF (50 ng/mL) was directly mixed into pre‐gel solution to prepare SCF‐loaded hydrogels. For the SCF‐loaded HGDN hydrogel, SCF (500 ng/mL) was added to the pre‐gel solution in the formation of second network (Figure 1), and the SCF‐loaded HGDN hydrogel was prepared by UV light irradiation again. Here, the concentration of SCF in the HGDN hydrogel was fixed at 50 ng/mL. Then, hydrogels were immersed in PBS in a shaking bed at 37°C with an oscillation frequency of 80 rpm. After each predetermined time interval, a certain amount of supernatant was taken out and an equal volume of PBS was supplemented. The percentage of SCF released from hydrogels was determined using a sandwich ELISA kit according to the manufacturer's instructions.
2.5. Biocompatibility of hydrogels
The biocompatibility of hydrogels was evaluated by a direct contact between MNCs and hydrogels.26 In brief, MNCs were seeded in plates at a density of 5 × 104 cells per well without cytokines. Sterile hydrogels were directly incubated with MNCs for 14 days. After each predetermined time, cells were harvested and stained with 0.4% trypan blue. Unstained cells were indicative of living cells, thus cell proliferation and cell viability were evaluated, respectively.
2.6. Establishment of culture conditions
To study the SCF‐loaded HGDN hydrogel for cell culture, four culture conditions were designed. Condition I: CD34+ cells were cultured in SCF‐free medium. Condition II: CD34+ cells were cultured in medium containing SCF (50 ng/mL). Condition III: CD34+ cells were encapsulated within the HGDN hydrogels without SCF and cultured in SCF‐free medium. Condition IV: CD34+ cells and SCF were encapsulated within the HGDN hydrogel and cultured in SCF‐free medium. In brief, HAMA hydrogels were immersed into pre‐gel solution containing CD34+ cells (2 × 105 cells/mL) and SCF (500 ng/mL), and then irradiated by UV light for 30 seconds. The density of CD34+ cells in all conditions was fixed at 1 × 104 cells per well. According to the experiment instruction, the medium of Condition II was exchanged using fresh medium containing SCF (50 ng/mL), while the medium of other conditions (Conditions I, III and IV) was exchanged using SCF‐free medium. After 2 weeks, 50 U/mL collagenase type II and hyaluronidase (Sigma‐Aldrich, St. Louis, MO, USA) were utilized to digest the HGDN hydrogel to harvest cells, and the number of total nucleated cells (TNCs) was determined by cell counting.
2.7. Flow cytometry
The phenotype of cells was analysed by flow cytometry. Cells were harvested, rinsed and resuspended in PBS. Afterwards, cells were stained with anti‐human CD34‐PE/CD38‐FITC antibody (Becton Dickinson) or the respective isotype control at 4°C for 30 minutes in the dark, and then the percentage of CD34+ cells and CD34+ CD38− cells in total cell population was determined by flow cytometry. Data were analysed using Flow Jo software (FlowJo, LLC, Ashland, OR, USA).
2.8. Colony‐forming unit assay
Colony‐forming unit (CFU) assay was performed to evaluate the haematopoietic potency of expanded CD34+ cells. CD34+ cells were harvested on day 14, and then seeded in Iscove's modified Dulbecco's medium (IMDM; Gibco) supplemented with 1% methylcellulose medium, 30% foetal bovine serum, 1% bovine serum albumin, 5 × 10−5 mol/L 2‐mercaptoethanol, 50 ng/mL SCF, 20 ng/mL IL‐3, 20 ng/mL IL‐6, 20 ng/mL granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), 20 ng/mL granulocyte colony‐stimulating factor (G‐CSF) and 2 ng/mL erythropoietin at a density of 500 cells per well in 24 well plates. Cells were incubated for 14 days at 37°C in a humidified atmosphere of 5% CO2. On day 14, colonies were counted and morphologically identified according to the distinction between granulocytes‐macrophage colony‐forming unit (CFU‐GM), granulocyte‐erythroid‐macrophage‐megakaryocyte colony‐forming unit (CFU‐GEMM) and erythroid burst‐forming unit (BFU‐E).
2.9. Statistical analysis
All values were presented as mean ± standard deviation. For ex vivo experiments, average values were determined from at least 3 independent samples. Statistical significance was evaluated by ANOVA using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). P < .05 was considered to be statistically significant.
3. RESULTS
3.1. Hydrogel characterization
The hydrogels were successfully synthesized, which was confirmed by 1H‐NMR spectrum (Figure S1). The micromorphology of the HAMA hydrogel, GelMA hydrogel and HGDN hydrogel were characterized by SEM, as shown in Figure 2. All samples showed a highly ordered porous structure with the average pore size of 62.4 ± 2.8, 31.8 ± 3.7 and 26.7 ± 2.5 μm for the HAMA hydrogel, GelMA hydrogel and HGDN hydrogel, respectively. The porous structure of hydrogels could provide sufficient surface area for nutrient exchange and the growth of encapsulated cells.
Figure 2.
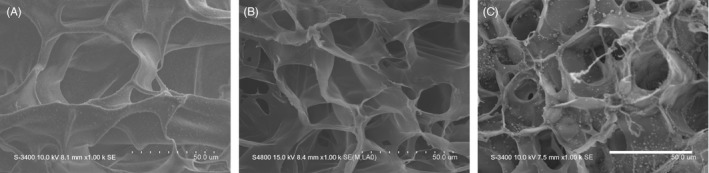
Scanning electron microscope (SEM) images of the Methacrylated hyaluronic acid (HAMA) hydrogel (A), methacrylated gelatin (GelMA) hydrogel (B) and hyaluronic acid/gelatin double network (HGDN) hydrogel (C), respectively. The scale bar was 50 μm
The mechanical property of hydrogels was evaluated by uniaxial compression test (Figure 3A). From stress‐strain curves, we found that all hydrogels exhibited a typical characteristic of viscoelastic materials. For SN hydrogels, the compressive modulus and failure stress of the HAMA hydrogel were both higher than those of the GelMA hydrogel even though the GelMA hydrogel contained higher polymer composition. Nevertheless, the GelMA hydrogel was tougher than the HAMA hydrogel, breaking at 75.12% ± 2.01% vs 65.45% ± 1.12%, respectively. Thus, to prepare a DN hydrogel, the stiffer HAMA was chosen as the first network, and the GelMA was used as the second one. The results showed that the HGDN hydrogel had significantly higher compressive modulus and failure stress.
Figure 3.
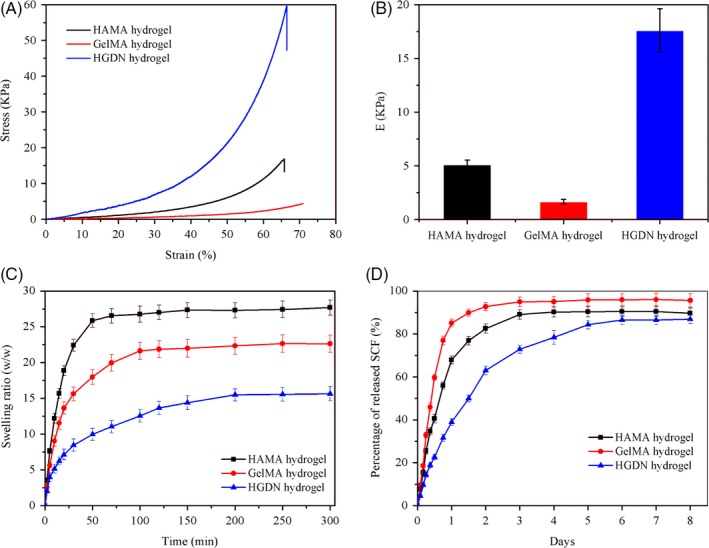
A, Representative stress‐strain curves of hydrogels under uniaxial compression. B, Calculated compressive modulus of curves at initial 10% strain. C, Swelling ratio of hydrogels in phosphate buffer solution (PBS). D, Release profiles of SCF from hydrogels at each specified time interval
In this study, the swelling behaviour of hydrogels was evaluated (Figure 3C). It could be clearly seen that all hydrogels possessed high ability of water absorption, but the HGDN hydrogel had a relatively lower SR.
3.2. Release profiles of SCF from hydrogels
SCF was physically encapsulated within hydrogels via a straightforward process, and SCF released from hydrogels was determined using ELISA assay as shown in Figure 3D. The release of SCF from the HGDN hydrogel was much slower than that from HAMA hydrogel and GelMA hydrogel, and the cumulative release amount of SCF from the HAMA hydrogel, GelMA hydrogel and HGDN hydrogel was 92.75% ± 1.86%, 82.53% ± 2.16% and 62.95% ± 2.06% within the first 2 days, respectively. Additionally, the release of SCF encapsulated within the HGDN hydrogel could sustain over 6 days, which could exactly meet the requirement of HSCs.
3.3. Biocompatibility of hydrogels
The biocompatibility was determined by seeding MNCs on hydrogels and plates without any cytokines and culturing for 14 days. It could be clearly seen that cells maintained a high cell viability (>90%) for all experimental groups (Figure 4A). An overall down‐regulation trend could be found for harvested MNCs, but there was no statistical significance compared to the control (Figure 4B). Therefore, the HGDN hydrogel showed a good biocompatibility, which was a potential biomaterial for ex vivo culture of CD34+ cells.
Figure 4.
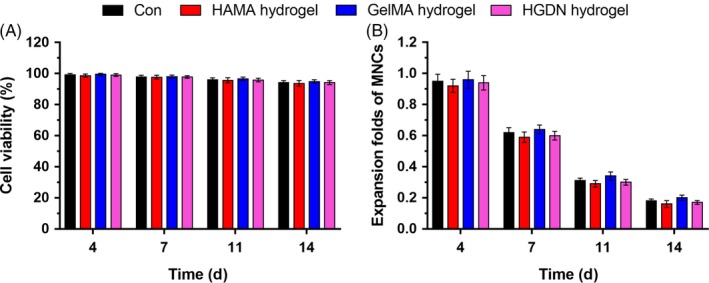
(A) Cell viability and (B) expansion folds of MNCs during the 14‐day incubation on methacrylated hyaluronic acid (HAMA) hydrogel, methacrylated gelatin (GelMA) hydrogel and hyaluronic acid/gelatin double network (HGDN) hydrogel. MNCs cultured on plates were served as the control
3.4. Proliferation and phenotype of CD34+ cells within the SCF‐loaded HGDN hydrogel
Freshly isolated CD34+ cells were cultured in plates or HGDN hydrogels supplemented with cytokine cocktails. At predetermined time, the number of total nucleated cells (TNCs) and the proportions of CD34+ cells and CD34+ CD38− cells were evaluated. Subsequently, the number of CD34+ cells and CD34+ CD38− cells was further calculated.
Obviously, as shown in Figure 5, without SCF in the cytokine cocktail (Conditions I and III), the number of TNCs showed only a trivial increase during the 14‐day culture. Oppositely, the proliferation of cells was greatly stimulated with the introduction of SCF (Conditions II and IV), indicating the promoted roles of SCF in proliferation of HSCs. Notably, HSCs cultured in plates and HGDN hydrogels showed equivalent increases in proliferation whether SCF being supplemented or not.
Figure 5.
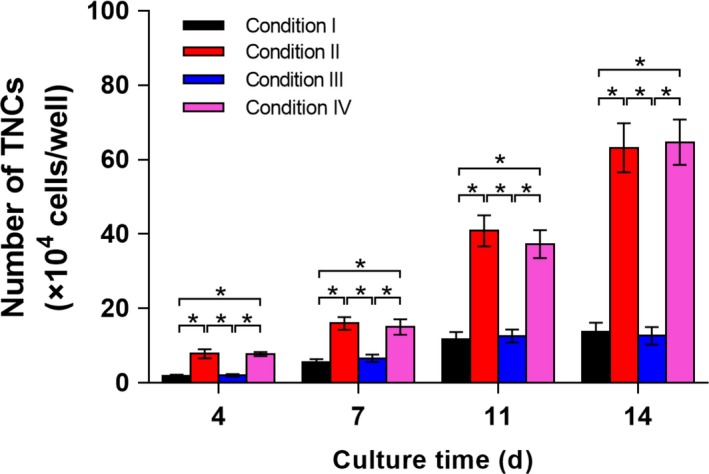
Numbers of TNCs resulted from CD34+ cells cultured in plates without SCF (Condition I) or supplemented with SCF (Condition II), hyaluronic acid/gelatin double network (HGDN) hydrogel without SCF (Condition III) and SCF‐loaded HGDN hydrogels (Condition IV) at regular intervals over a period of 14 days. (P < .05; n = 3)
CD34+ phenotype has been widely utilized in the identification of HSCs. Thus, the proportion of CD34+ cells under different culture conditions was determined using flow cytometry, and the representative density plots are shown in Figure 6A. The freshly isolated cells contained over 95% of CD34+ cells, while a significant decline in the proportion of was observed during ex vivo culture. Notably, cells cultured with SCF exhibited lower proportions of CD34+ cells level on day 7, in comparison with those cultured without SCF. Nevertheless, there was no significant difference in the proportion of CD34+ cells under different culture conditions on day 14. Additionally, the primitive HSCs with long‐term reconstituting capability reside in the CD34+ CD38− cell population. As such, the proportions of CD34+ CD38− cells were also analysed during ex vivo culture, and an overall down‐regulation trend in the proportion of CD34+ CD38− cells was observed in all culture conditions during ex vivo culture. The proportion of CD34+ CD38− cells was slightly higher under the culture conditions without SCF, which was similar to the results for CD34+ cells.
Figure 6.
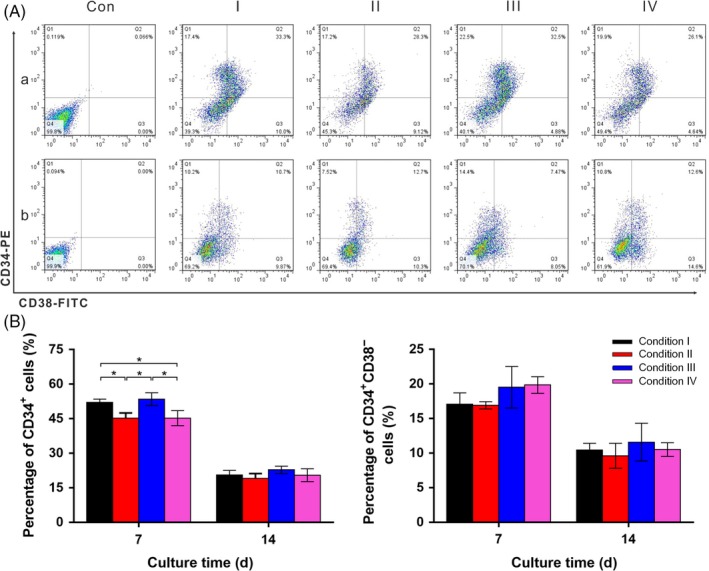
A, Representative density plots of CD34+ and CD34+ CD38− phenotype of harvest cells on day 7 and day 14. B, Percentages of CD34+ and CD34+ CD38− phenotype of cells cultured under 4 culture conditions (Conditions I‐IV). Isotype controls were used to establish the positive and negative quadrants (P < .05; n = 3)
The numbers of CD34+ cells and CD34+ CD38− cells under 4 culture conditions were further calculated and the results are illustrated in Figure 7. A continuous proliferation of CD34+ cells was obtained under 4 culture conditions. In addition, among the culture conditions with SCF, there was no significant difference detected in terms of the proliferation for CD34+ cells, although all were significantly higher than those cultured in medium without the addition of SCF. The relative proliferation profile of CD34+ CD38− cells followed almost identical trends as those observed from CD34+ cells.
Figure 7.

The number of (A) CD34+ cells and (B) CD34+ CD38− cells cultured under 4 culture conditions (Conditions I‐IV) was calculated on days 7 and 14 (P < .05; n = 3)
3.5. Haematopoietic functions of expanded CD34+ cells
Multipotency of cells was assessed through CFUs assays after being ex vivo expanded in plates or hydrogels. The number of each type of colony (CFU‐GM, CFU‐GEMM and BFU‐E) on day 14 was evaluated in 4 different culture conditions, and the results are shown in Figure 8. Cells cultured under the conditions with SCF generated higher total colony numbers compared to the conditions without SCF, which is consistent with the results for proliferation of total and CD34+ cells. Interestingly, cells cultured in the SCF‐loaded HGDN hydrogels generated more primitive CFU‐GEMM, compared to those cultured in plates with SCF. Therefore, the specific interaction between HSCs and SCF is favourable towards maintaining the multipotency of HSCs during the ex vivo culture of HSCs.
Figure 8.
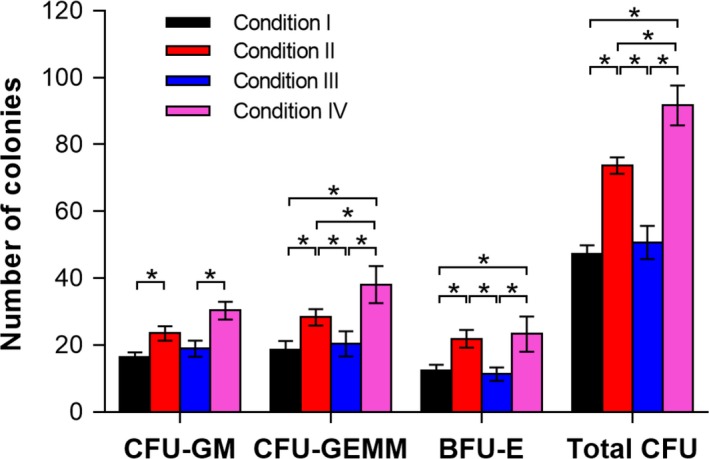
The number of colony‐forming unit (CFUs) granulocytes‐macrophage colony‐forming unit (CFU‐GM), granulocyte‐erythroid‐macrophage‐megakaryocytes colony‐forming unit (CFU‐GEMM), erythroid burst‐forming unit (BFU‐E) and total CFU) resulting from 500 CD34+ cells re‐isolated from expanded cells under 4 conditions for 14 days (P < .05; n = 3)
4. DISCUSSION
Haematopoietic stem cells are capable of long‐term self‐renewal in the native niche, while they are easily differentiated during ex vivo culture.21 Although a variety of conditions have been developed for ex vivo culture of HSCs, the benefits obtained are limited, and long‐term maintenance of HSCs with high self‐renewal capacity still remains a big challenge.34 Many reports have highlighted that the artificial environment was insufficient for preserving HSCs, thus a series of concentrations and combinations of cytokines have been tested to ex vivo culture HSC.35 However, all attempts could not really mimic the complicated microenvironment of the niche and could not well support proliferation of HSCs. SCF has been identified to be a critical component responsible for survival and maintenance of HSCs in the niche.36, 37, 38 However, SCF is a quite expensive and efficient cytokine and the high cost associated with its continuous supplement in the conventional culture is detrimental for stem cell engineering. Thus, we look for a new feeding strategy of SCF to reduce the consumption of SCF during ex vivo culture.
Our prior research has shown that the initial 4 days were the critical period for SCF to stimulate the proliferation of HSCs.39 Thus, we attempted to use hydrogel as the carrier to load SCF and achieve in situ release of SCF. Nevertheless, common hydrogels were hardly applied to sustained release of cytokines, owing to the quick diffusion of cytokines. From the release profiles of SCF, the experimental data also affirmed that the encapsulated SCF could be released from the HAMA hydrogel and GelMA hydrogel within 3 days, which was insufficient for proliferation of HSCs. Comparatively, based on the HGDN hydrogel, encapsulated SCF exhibited a much lower release rate and could continually release for more than 6 days, exactly meeting the requirement of HSCs. The decreased release rate may be resulted from the DN structure with a smaller pore size and higher polymer content with a relatively stronger polyion complexation.
In addition, hyaluronic acid and gelatin are the main components of the HGDN hydrogel, and they have been considered as desirable candidates for hydrogels. Hyaluronic acid is no antigenic, eliciting no inflammatory or foreign‐body reaction, and gelatin, a degradation product of collagen, is rich in peptide sequences, such as RGD, which have an advantageous effect on the proliferation of HSCs.40, 41, 42 The new microenvironment constructed by the HGDN hydrogel was a porous morphology, providing sufficient space for cells migration. Besides, the HGDN hydrogel provides a relatively higher compressive modulus. Although there was no agreement on optimal stiffness of biomaterials for HSC culture, it was reported that HSCs prefer a stiffer substance materials (12.2‐30.4 kPa), where the proliferation and migration of cells were promoted.43, 44
Introduction of SCF will significantly affect the balance between proliferation and differentiation of HSCs. In most cases, it is a conflict, because SCF can strongly stimulate proliferation of HSCs at the expense of losing desired phenotype. In order to promote the cost‐efficiency of SCF, they were covalently immobilized within a GelMA hydrogel. A critical finding was that it severely inhibited proliferation of cells, but selectively maintained a more primitive proportion of HSCs. Thus, not surprisingly, for the conditions supplemented with SCF (Conditions II and IV), the introduction of SCF led to increase of cell numbers, compared to the conditions without SCF (Conditions I and III). There was no difference in the number of TNCs between Conditions II and IV, indicating SCF encapsulated within the HGDN hydrogel still remained a high bioactivity. More importantly, for the consumption of SCF per well, Condition II required 200 ng of SCF over the entire culture period, while the Condition IV required 50 ng of SCF only. However, different with previous conclusions, HSCs cultured within the HGDN hydrogels did not show a decrease in proliferation in spite of a low availability of SCF in the matrix. Meanwhile, the results from phenotype analysis suggested supplement of SCF was adverse to maintenance of primitive phenotype of HSCs, but HSCs was not influenced by the forms of SCF. It is likely that the soluble and matrix encapsulated forms of SCF have distinct optimal concentrations required for proliferation of HSCs. Although HSCs cultured in plates or the HGDN hydrogels showed equivalent increases in proliferation after 2 weeks, but the SCF‐loaded HGDN hydrogels was demonstrated to be more favourable for maintenance of multipotency, and it was important to be noted that the former condition (Condition II) required a significantly greater SCF (SCF added to medium for repeated medium changes) total. Therefore, the SCF‐loaded HGDN hydrogel provided a preferable microenvironment for HSCs.
5. CONCLUSIONS
We have developed a SCF‐loaded HGDN hydrogel as a three‐dimensional biomimetic scaffold for ex vivo culture of CD34+ cells. The HGDN hydrogel was constructed from HAMA and GelMA via a DN strategy, and hydrogel with porous microstructure exhibited a suitable mechanical property and good biocompatibility for cell culture. In addition, SCF was physically encapsulated in the HGDN hydrogel via a straightforward process, and then it could be sustainedly released from the hydrogel for more than 6 days, exactly meeting the requirement of CD34+ cells. The HGDN hydrogel could support ex vivo proliferation of CD34+ cells in term of the number and phenotype, and CD34+ cells harvested from the SCF‐loaded HGDN hydrogel remained better haematopoietic functions. Moreover, the amount of SCF consumed in SCF‐loaded HGDN hydrogel was much less than manual feeding strategy, which greatly improved the cost‐efficiency of SCF. Therefore, the present model would provide a valuable platform for sustained release of cytokines to support cells culture.
Supporting information
Zhang Y, Pan X, Shi Z, Cai H, Gao Y, Zhang W. Sustained release of stem cell factor in a double network hydrogel for ex vivo culture of cord blood‐derived CD34+ cells. Cell Prolif. 2018;51:e12407 10.1111/cpr.12407
Funding information
This work was financially supported by the National Natural Science Foundation of China (grant number 21574039), Basic Research Project of Shanghai Science and Technology Commission (grant number 15JC1401402), and the Open Funding Project of the State Key Laboratory of Bioreactor Engineering.
Yuanhao Zhang and Xiuwei Pan are contributed equally to this paper.
Contributor Information
Haibo Cai, Email: caihaibo@ecust.edu.cn.
Weian Zhang, Email: wazhang@ecust.edu.cn.
REFERENCES
- 1. Chua K‐N, Chai C, Lee P‐C, et al. Surface‐aminated electrospun nanofibers enhance adhesion and expansion of human umbilical cord blood hematopoietic stem/progenitor cells. Biomaterials. 2006;27:6043‐6051. [DOI] [PubMed] [Google Scholar]
- 2. Thomas ED, Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 1999;5:341‐346. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Hu M, Zhang Y, Li H, Miao Z. CD29 of human umbilical cord mesenchymal stem cells is required for expansion of CD34+ cells. Cell Prolif. 2014;47:596‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen Y, Nagler A. Umbilical cord blood transplantation–how, when and for whom? Blood Rev. 2004;18:167‐179. [DOI] [PubMed] [Google Scholar]
- 5. Raic A, Rodling L, Kalbacher H, Lee‐Thedieck C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 2014;35:929‐940. [DOI] [PubMed] [Google Scholar]
- 6. Holmes T, Yan F, Ko KH, et al. Ex vivo expansion of cord blood progenitors impairs their short‐term and long‐term repopulating activity associated with transcriptional dysregulation of signalling networks. Cell Prolif. 2012;45:266‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee‐Thedieck C, Spatz JP. Biophysical regulation of hematopoietic stem cells. Biomater Sci. 2014;2:1548‐1561. [DOI] [PubMed] [Google Scholar]
- 8. Bonnet D. Biology of human bone marrow stem cells. Clin Exp Med. 2003;3:140‐149. [DOI] [PubMed] [Google Scholar]
- 9. Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003;425:778‐779. [DOI] [PubMed] [Google Scholar]
- 10. Leisten I, Kramann R, Ventura Ferreira MS, et al. 3D co‐culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials. 2012;33:1736‐1747. [DOI] [PubMed] [Google Scholar]
- 11. Ellis SL, Nilsson SK. The location and cellular composition of the hemopoietic stem cell niche. Cytotherapy. 2012;14:135‐143. [DOI] [PubMed] [Google Scholar]
- 12. Choi JS, Mahadik BP, Harley BAC. Engineering the hematopoietic stem cell niche: frontiers in biomaterial science. Biotechnol J. 2015;10:1529‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083‐6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Majka M, Janowska‐Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075‐3085. [DOI] [PubMed] [Google Scholar]
- 15. Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo‐osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387‐399. [DOI] [PubMed] [Google Scholar]
- 16. Wulf‐Goldenberg A, Eckert K, Fichtner I. Cytokine‐pretreatment of CD34+ cord blood stem cells in vitro reduces long‐term cell engraftment in NOD/SCID mice. Eur J Cell Biol. 2008;87:69‐80. [DOI] [PubMed] [Google Scholar]
- 17. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujimoto N, Fujita S, Tsuji T, et al. Microencapsulated feeder cells as a source of soluble factors for expansion of CD34(+) hematopoietic stem cells. Biomaterials. 2007;28:4795‐4805. [DOI] [PubMed] [Google Scholar]
- 19. Walenda T, Bokermann G, Ventura Ferreira MS, et al. Synergistic effects of growth factors and mesenchymal stromal cells for expansion of hematopoietic stem and progenitor cells. Exp Hematol. 2011;39:617‐628. [DOI] [PubMed] [Google Scholar]
- 20. Mahadik BP, Pedron Haba S, Skertich LJ, Harley BA. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials. 2015;67:297‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madlambayan GJ, Rogers I, Kirouac DC, et al. Dynamic changes in cellular and microenvironmental composition can be controlled to elicit in vitro human hematopoietic stem cell expansion. Exp Hematol. 2005;33:1229‐1239. [DOI] [PubMed] [Google Scholar]
- 22. Jiang J, Papoutsakis ET. Stem‐cell niche based comparative analysis of chemical and nano‐mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv Healthc Mater. 2013;2:25‐42. [DOI] [PubMed] [Google Scholar]
- 23. Jiang Y‐C, Jiang L, Huang A, Wang X‐F, Li Q, Turng L‐S. Electrospun polycaprolactone/gelatin composites with enhanced cell–matrix interactions as blood vessel endothelial layer scaffolds. Mater Sci Eng, C. 2017;71:901‐908. [DOI] [PubMed] [Google Scholar]
- 24. Higuchi A, Yang S‐T, Li P‐T, et al. Polymeric materials for ex vivo expansion of hematopoietic progenitor and stem cells. Polym Rev. 2009;49:181‐200. [Google Scholar]
- 25. Pan X, Sun Q, Cai H, Gao Y, Tan W, Zhang W. Encapsulated feeder cells within alginate beads for ex vivo expansion of cord blood‐derived CD34+ cells. Biomater Sci. 2016;4:1441‐1453. [DOI] [PubMed] [Google Scholar]
- 26. Pan X, Sun Q, Zhang Y, et al. Biomimetic macroporous pcl scaffolds for ex vivo expansion of cord blood‐derived CD34+ cells with feeder cells support. Macromol Biosci 2017;17 10.1002/mabi.201700054. [DOI] [PubMed] [Google Scholar]
- 27. Muller E, Grinenko T, Pompe T, Waskow C, Werner C. Space constraints govern fate of hematopoietic stem and progenitor cells in vitro. Biomaterials. 2015;53:709‐715. [DOI] [PubMed] [Google Scholar]
- 28. Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self‐renewal. J Biomed Mater Res A. 2006;79:1‐5. [DOI] [PubMed] [Google Scholar]
- 29. Kehr NS, Atay S, Ergun B. Self‐assembled monolayers and nanocomposite hydrogels of functional nanomaterials for tissue engineering applications. Macromol Biosci. 2015;15:445‐463. [DOI] [PubMed] [Google Scholar]
- 30. David L, Dulong V, Coquerel B, et al. Collagens, stromal cell‐derived factor‐1α and basic fibroblast growth factor increase cancer cell invasiveness in a hyaluronan hydrogel. Cell Prolif. 2008;41:348‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bos GW, Jacobs JJL, Koten JW, et al. In situ crosslinked biodegradable hydrogels loaded with IL‐2 are effective tools for local IL‐2 therapy. Eur J Hosp Pharm Sci. 2004;21:561‐567. [DOI] [PubMed] [Google Scholar]
- 32. Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double‐network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15:1155‐1158. [Google Scholar]
- 33. Fiorica C, Mauro N, Pitarresi G, Scialabba C, Palumbo FS, Giammona G. Double‐network‐structured graphene oxide‐containing nanogels as photothermal agents for the treatment of colorectal cancer. Biomacromol. 2017;18:1010‐1018. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira MS, Jahnen‐Dechent W, Labude N, et al. Cord blood‐hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials. 2012;33:6987‐6997. [DOI] [PubMed] [Google Scholar]
- 35. Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638‐2647. [DOI] [PubMed] [Google Scholar]
- 36. Du Z, Wang Z, Zhang W, Cai H, Tan WS. Stem cell factor is essential for preserving NOD/SCID reconstitution capacity of ex vivo expanded cord blood CD34(+) cells. Cell Prolif. 2015;48:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kishimoto S, Nakamura S, Hattori H, et al. Human stem cell factor (SCF) is a heparin‐binding cytokine. J Biochem. 2008;145:275‐278. [DOI] [PubMed] [Google Scholar]
- 38. Pati S, Kalra OP, Mukhopadhyay A. Foe turned friend: multiple functional roles attributable to hyper‐activating stem cell factor receptor mutant in regeneration of the haematopoietic cell compartment. Cell Prolif. 2011;44:10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Z, Cai H, Ye Z, Tan WS. Optimization of SCF feeding regimen for ex vivo expansion of cord blood hematopoietic stem cells. J Biotechnol. 2012;164:211‐219. [DOI] [PubMed] [Google Scholar]
- 40. Demange E, Kassim Y, Petit C, et al. Survival of cord blood haematopoietic stem cells in a hyaluronan hydrogel for ex vivo biomimicry. J Tissue Eng Regen Med. 2013;7:901‐910. [DOI] [PubMed] [Google Scholar]
- 41. Hutson CB, Nichol JW, Aubin H, et al. Synthesis and characterization of tunable poly (ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng Part A. 2011;17:1713‐1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gorgieva S, Kokol V. Preparation, characterization, and in vitro enzymatic degradation of chitosan‐gelatine hydrogel scaffolds as potential biomaterials. J Biomed Mater Res A. 2012;100:1655‐1667. [DOI] [PubMed] [Google Scholar]
- 43. Choi JS, Harley BAC. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials. 2012;33:4460‐4468. [DOI] [PubMed] [Google Scholar]
- 44. Kumar SS, Hsiao JH, Ling QD, et al. The combined influence of substrate elasticity and surface‐grafted molecules on the ex vivo expansion of hematopoietic stem and progenitor cells. Biomaterials. 2013;34:7632‐7644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


