Abstract
Long non‐coding RNAs (lncRNAs), a group of non‐protein‐coding RNAs with more than 200 nucleotides in length, are involved in multiple biological processes, such as the proliferation, apoptosis, migration and invasion. Moreover, numerous studies have shown that lncRNAs play important roles as oncogenes or tumour suppressor genes in human cancers. In this paper, we concentrate on actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1), a well‐known long non‐coding RNA that is overexpressed in various tumour tissues and cell lines, including oesophageal cancer, pancreatic ductal adenocarcinoma, nasopharyngeal carcinoma, lung cancer, hepatocellular carcinoma, ovarian cancer, colorectal cancer, biliary tract cancer and gastric cancer. Moreover, high expression of AFAP1‐AS1 was associated with the clinicopathological features and cancer progression. In this review, we sum up the current studies on the characteristics of AFAP1‐AS1 in the biological function and mechanism of human cancers.
1. INTRODUCTION
Nowadays, cancer is one of the major causes of death all over the world.1, 2 According to mortality data collected by the National Center for Health Statistics, it is estimated that 1 688 780 new cancer cases and 600 920 cancer deaths will take place in the United States in 2017.3 In order to fight against cancer effectively, we should make a great effort to find more precise diagnostic biomarkers and effective therapeutic targets.
Recently, increasing evidence has shown that the non‐coding portion of the genome has a crucial functional importance in both normal physiology and diseases.4, 5, 6 Long non‐coding RNAs (lncRNAs), a group of non‐protein‐coding RNAs with more than 200 nucleotides in length, play a vital role in regulating significant cellular functions, including cell proliferation, differentiation, apoptosis, invasion, metabolism, developmental timing and immune responses.7, 8, 9, 10, 11, 12 However, mutation of lncRNAs will cause cancer initiation and promote the metastasis of malignancy.13, 14, 15 For instance, lncRNA metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) was first found in lung cancer metastasis. Many studies had demonstrated that lncRNA MALAT1 was overexpressed in various cancer, and it might act as a potential biomarker and therapeutic target in cancer treatment.16, 17, 18 LncRNA MALAT1 might function as an oncogene through controlling alternative splicing process in breast cancer, influencing the expression of N‐cadherin and E‐cadherin in bladder cancer, combining with a multifunctional RNA‐binding protein in colorectal cancer (CRC) and osteosarcoma.19, 20, 21, 22 LncRNA HOX antisense intergenic RNA (HOTAIR) induced cancer invasion and metastasis by regulating PRC2 target genes in breast cancer and epithelial‐mesenchymal transition (EMT) programme in gastric cancer (GC).23, 24 LncRNA H19 was upregulated in GC and associated with miR‐675, p53 and Isthmin1 that improved cells proliferation, migration and invasion.25, 26, 27, 28, 29 Among so many cancer‐related lncRNAs, actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) was initially discovered in oesophageal adenocarcinoma in 2013.30 Then, numerous recent studies had focused on lncRNA AFAP1‐AS1 and demonstrated that it was upregulated in many cancers and played an important role in tumour progression. A meta‐analysis had shown that high expression of AFAP1‐AS1 in human cancers was closely related to poor clinical outcome such as lymph node metastasis and distant metastasis.31 Hence, we chose it as the main research object to summarize its characteristics in the biological function and mechanism of human cancers.
2. IDENTIFICATION OF AFAP1‐AS1
Actin filament‐associated protein 1 (formerly AFAP‐110), an actin cross‐linking protein and a cSrc‐binding partner, is a member of the AFAP family which includes AFAP1, AFAP1 like‐1 and AFAP1 like‐2/XB‐130.32, 33 There are 2 pleckstrin homology domains in AFAP1, and one of them involves a protein kinase C‐binding site and carboxy‐terminal domains.33, 34 On the basis of multimerization associated with its leucine zipper and binding to actin filaments through its carboxy‐terminal actin filament‐binding domain, AFAP1 can crosslink actin filaments.34
Long non‐coding RNA AFAP1‐AS1 with 6810 bp in length is mapped to the 4p16.1 region of human chromosome 4. Moreover, AFAP1‐AS1 is transcribed from the AFAP1 gene in the antisense direction, containing several overlapping and complementary regions among the exons of AFAP1‐AS1 and AFAP135 (Figure 1). Antisense lncRNAs like AFAP1‐AS1 are oriented in an antisense direction regard to a protein‐coding gene in the opposite strand, and AFAP1‐AS1 can affect the expression of AFAP1.36, 37, 38, 39 Further experiments have demonstrated that AFAP1‐AS1 was overexpressed in cancer tissues and cell lines, such as oesophageal cancer, pancreatic ductal adenocarcinoma (PDAC), nasopharyngeal carcinoma (NPC) and lung cancer. In addition, overexpression of AFAP1‐AS1 was closely associated with tumour size, lymphatic metastasis, distant metastasis, tumour‐node‐metastasis (TNM) stage and poor prognosis of cancer patients. Using siRNA to impair the expression of AFAP1‐AS1 inhibited cell proliferation, migration and invasion and induced cell apoptosis through regulating related genes or signalling pathways.30, 35, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 In this review, the related clinicopathological characteristics and molecular functions of this lncRNA in cancers are presented in Tables 1 and 2.
Figure 1.
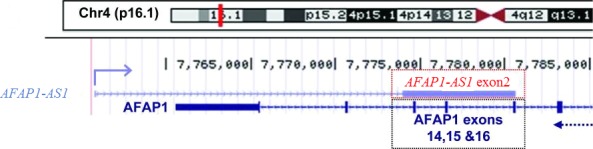
Long non‐coding RNAs (lncRNA) actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) is mapped to the 4p16.1 region of human chromosome 4. AFAP1‐AS1 is transcribed from AFAP1 gene in the antisense direction, containing several overlapping and complementary regions among the exons of AFAP1‐AS1 and AFAP1
Table 1.
Overexpression of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) is associated with clinicopathological features
| Cancer types | Clinicopathological features | References |
|---|---|---|
| Oesophageal cancer | Tumour size, tumour depth, lymphatic metastasis, distant metastasis, TNM stage, poor prognosis, shorter progression‐free survival and overall survival chemoradioresistance | 40, 41 |
| Pancreatic ductal adenocarcinoma | Lymph node metastasis, perineural invasion, poor survival overall survival, progression‐free survival | 42 |
| Nasopharyngeal carcinoma | Lymph node metastasis, distant tumour metastasis, TNM stage, poor prognosis, EBV infection poor overall survival, poor relapse‐free survival | 35, 43, 44 |
| Lung cancer | Clinical stage, smoking history, infiltration degree, lymph node metastasis, distant metastasis, poor prognosis tumour progression, poor survival | 46, 47, 48 |
| Hepatocellular carcinoma | Tumour size, TNM stage, lymph‐vascular space invasion poor prognosis | 49, 50 |
| Ovarian cancer | Resistance response, FIGO stage | 51 |
| Colorectal cancer | Tumour size, TNM stage, distant metastasis, poor prognosis poor overall survival and disease‐free survival | 52, 53, 54 |
| Biliary tract cancers | Tumour size, vascular invasion, TNM stage, poor prognosis, poor overall survival | 55, 56, 57 |
| Gastric cancer | Poor survival | 58 |
TNM: tumour‐node‐metastasis; EBV: Epstein‐Barr virus; FIGO: International Federation of Gynecology and Obstetrics.
Table 2.
Functional characterization of the actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) in tumours
| Cancer types | Expression | Effects | Related gene | Role | References |
|---|---|---|---|---|---|
| Oesophageal cancer | Upregulated | Hypomethylation proliferation colony formation, migration, invasion, apoptosis, cycle arrest | — | Oncogene | 30, 40, 41 |
| Pancreatic ductal adenocarcinoma | Upregulated | Proliferation, cycle arrest migration, invasion, EMT process | E‐cadherin, N‐cadherin, vimentin, Slug, Snail1 | Oncogene | 42 |
| Nasopharyngeal carcinoma | Upregulated | Migration, invasion | AFAP1 protein, GTPase family, Pfn1, Lasp1, PD‐1, lncRNA MALAT1, lncRNA AL359062 | Oncogene | 35, 43, 44 |
| Lung cancer | Upregulated | Proliferation, apoptosis migration, invasion | AFAP1 protein, GTPase family, Pfn1 Lasp1 | Oncogene | 45, 46, 47, 48 |
| Hepatocellular carcinoma | Upregulated | Proliferation, migration, invasion, apoptosis, cycle arrest | PCNA, MMP‐9, cyclin D1, Bax, RhoA/Rac2, Ki67, Bcl‐2 | Oncogene | 49, 50 |
| Ovarian cancer | Upregulated | Proliferation, apoptosis | Oncogene | 51 | |
| Colorectal cancer | Upregulated |
EMT process, proliferation, cycle arrest colony formation migration, invasion |
E‐cadherin, N‐cadherin, vimentin, fibronectin, MMP‐9, AFAP1 | Oncogene | 52, 53, 54 |
| Biliary tract cancers | Upregulated |
EMT process, proliferation colony formation, cell cycle migration, invasion |
Twist1, vimentin, E‐cadherin, C‐myc, cyclin D1, MMP‐2, MMP‐9, AFAP1 | Oncogene | 55, 56, 57 |
| Gastric cancer | Upregulated |
Proliferation apoptosis |
PTEN/p‐AKT, Bcl‐2, PARP, Caspase 3, Caspase 9, Bax | Oncogene | 58 |
EMT: epithelial‐mesenchymal transition; GTP: guanosine triphosphate; Pfn1: profilin 1; Lasp1: LIM and SH3 protein 1; PD‐1: programmed death 1; MALAT1: metastasis‐associated lung adenocarcinoma transcript 1; PCNA: proliferating cell nuclear antigen; MMP: matrix metalloproteinase; Bcl‐2: B‐cell CLL/lymphoma 2 protein; Bax: BCL2‐associated X protein; RhoA: ras homologue family member A; Rac2: rac family small GTPase 2; Ki67: Antigen Ki‐67; C‐myc: MYC proto‐oncogene, bHLH transcription factor; PTEN: phosphatase and tensin homologue; AKT: AKT serine/threonine kinase 1; PARP: poly‐ADP‐ribose polymerase.
3. AFAP1‐AS1 IN VARIOUS CANCERS
3.1. Oesophageal cancer
Oesophageal cancer is the eighth most frequent types of cancer and is the sixth leading cause of tumour‐related death all over the world.59, 60 There are 2 primary histological subtypes of oesophageal cancer, including oesophageal adenocarcinoma (OAC) and oesophageal squamous cell carcinoma (OSCC).61, 62 OSCC accounts for more than 95% of oesophageal cancer.63 OAC is one of the fastest growing cancers in the Western world, while OSCC is the main subtype of oesophageal cancer in Asia.3, 64 Although different kinds of treatment have been developed, including chemotherapy, radiotherapy and surgery, the long‐term survival rate of oesophageal cancer is still extremely low.64, 65 Because of the rising morbidity and poor prognosis of oesophageal cancer patients, it is urgent to look for new tumour markers and therapeutic targets for early diagnosis and advanced treatment of oesophageal cancer patients.
Wu et al30 reported that AFAP1‐AS1 was exceedingly hypomethylated and overexpressed in Barrett's oesophagus and OAC tissues and OAC cell lines. Further functional analyses demonstrated that AFAP1‐AS1 was an oncogene in the oesophagus cancer. Inhibition of AFAPA‐AS1 by siRNA in OAC cells reduced cell proliferation, colony formation, migration and invasion, increased apoptosis and induced G2/M phase arrest. However, the expression of AFAP1‐AS1 was irrelevant with AFAP1 expression. They drew a conclusion that the overexpression of AFAP1‐AS1, which exerted functional pro‐cancerous effects in oesophageal cells, was associated with hypomethylation.
Subsequent studies demonstrated that AFAP1‐AS1 was increased in OSCC, and high expression of AFAP1‐AS1 was closely associated with tumour size, tumour depth, lymphatic metastasis, distant metastasis and TNM stage. Moreover, those OSCC patients with increased AFAP1‐AS1 level have shorter progression‐free survival and overall survival. Overexpression of AFAP1‐AS1 will lead to tumour resistance to radiotherapy and chemotherapy in OSCC patients who received definitive chemoradiotherapy. Furthermore, knockdown of AFAP1‐AS1 in OSCC cells suppressed cell proliferation and colony formation and induced cell apoptosis.40, 41 Therefore, AFAP1‐AS1 may work as a novel prognostic marker and potential therapeutic target for oesophageal cancer.
3.2. Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma, one of the most aggressive solid malignancies, is the fourth leading cause of cancer‐related deaths all over the world.66, 67 PDAC is characterized by a fatal disease with early metastasis and resistance to chemotherapy and radiation therapy.7, 68 Although the study of PDAC has made rapid progress in the last decades, the 5‐year survival rate of PDAC patients is still only around 5%‐7%.69, 70 Therefore, it is crucial to identify reliable biomarkers for early diagnosis of PDAC patients.
Ye et al42 demonstrated that AFAP1‐AS1 was upregulated in PDAC tissues and cell lines compared with corresponding normal counterparts. Overexpression of AFAP1‐AS1 was associated with lymph node metastasis, perineural invasion, poor survival, overall survival and progression‐free survival of PDAC patients. In addition, knockdown of AFAP1‐AS1 reduced proliferation and induced G2/M phase arrest in PDAC cells. Knockdown of AFAP1‐AS1 in PDAC cells inhibited migration and invasion by influencing the expression of EMT‐related genes, including E‐cadherin, N‐cadherin, vimentin, Slug and Snail1. As we know, EMT is deemed to be the essential process of cancer progression, enhancing tumour migration, invasion and metastasis.71, 72, 73, 74 During the EMT process, epithelial cells lose epithelial status, apico‐basal polarity and cell‐cell adhesion so as to transform into mesenchymal cells.74, 75, 76 In order to distinguish diverse functions, EMT is classified into 3 types: primary/type 1, secondary/type 2 and tertiary/type 3. Type 1 is associated with implantation, embryogenesis and organogenesis. Type 2 takes part in wound healing, tissue regeneration and organ development. Type 3 promotes tumour metastasis.77, 78, 79 During cancer progression and metastasis, the expression of some EMT‐related genes is changed, such as mesenchymal genes (fibronectin, N‐cadherin and vimentin) are increased while epithelial genes (E‐cadherin and ZO‐1) are decreased.80, 81 Ye et al42 also found that inhibition of AFAP1‐AS1 reduced PDAC cell tumorigenicity in nude mice. However, amplification of AFAP1‐AS1 produced opposite effects (Figure 2). In conclusion, AFAP1‐AS1 has potential value as a prognostic biomarker and therapeutic target in PDAC.
Figure 2.
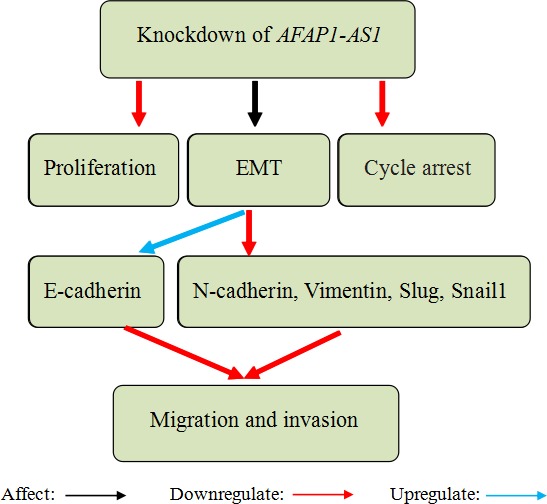
Knockdown of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) reduced proliferation and induced G2/M phase arrest in pancreatic ductal adenocarcinoma (PDAC) cells. Knockdown of AFAP1‐AS1 in PDAC cells inhibited migration and invasion by influencing the expression of epithelial‐mesenchymal transition (EMT)‐related genes (E‐cadherin, N‐cadherin, vimentin, Slug, Snail1)
3.3. Nasopharyngeal carcinoma
Nasopharyngeal carcinoma, a unique disease to Southeast Asia, is associated with the Epstein‐Barr virus (EBV).82, 83 About 75%‐90% of NPC cases are diagnosed at advanced stages due to the non‐specific symptoms at an early stage and poor accessibility for physical examination.84, 85 The main clinical treatment of NPC is radiotherapy over the past few decades, but many patients finally die because of recurrence and distant metastasis.86, 87 Therefore, it is essential to find therapeutic targets and prognostic biomarkers for accurate early diagnosis of high‐risk populations and evaluation of NPC treatment.
Bo et al35 reported that AFAP1‐AS1 was upregulated in NPC samples compared with non‐tumour nasopharyngeal epithelial samples, and amplification of AFAP1‐AS1 was associated with NPC metastasis and poor prognosis. Knockdown of AFAP1‐AS1 by siRNAs suppressed tumour cell migration and invasion. However, AFAP1‐AS1 has no effects on cell viability, cell cycle progression and apoptosis. Knockdown of AFAP1‐AS1 could increase AFAP1 protein level, induce the loss of stress filament integrity and influence the expression of many proteins related to small GTPase signalling Rho/Rac pathway in NPC cells. Hence, they suspected that AFAP1‐AS1 might promote cancer cell migration and invasion by interfering with AFAP1 expression, small GTPase signalling Rho/Rac pathway and the loss of stress filament integrity (Figure 3). They also carried out nude mouse experiments and discovered that knockdown of AFAP1‐AS1 inhibited NPC cell metastasis to mouse lungs. Based on the previous studies, Tang et al43 found that the expression of AFAP1‐AS1 was positively correlated with programmed death 1 (PD‐1), an immune escape marker. They concluded that AFAP1‐AS1 and PD‐1 were co‐expressed in infiltrating lymphocytes in NPC tissue and the co‐expression predicted poor prognosis of NPC. Moreover, overexpression of AFAP1‐AS1 or PD‐1 was correlated with distant metastasis at relapse. He et al44 identified that 3 circulating lncRNAs (MALAT1, AFAP1‐AS1 and AL359062) may act as potential biomarkers for NPC, and the three‐lncRNA signature could contribute to the identification of early NPC patients. Besides, high expression of these 3 lncRNAs was closely related to advanced NPC tumour node metastasis stages and EBV infection. These findings suggest that AFAP1‐AS1 may serve as a cancer‐promoting gene and a potential therapeutic target in NPC.
Figure 3.
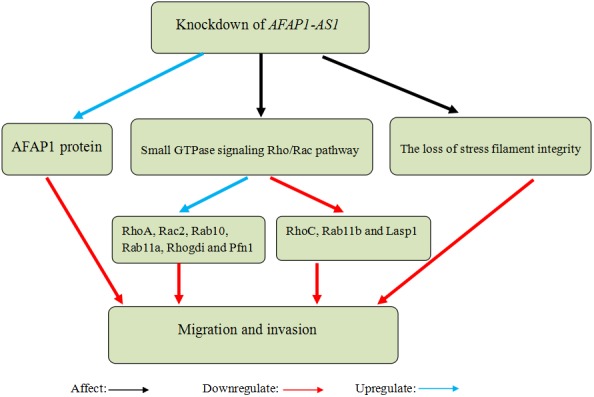
Knockdown of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) in nasopharyngeal carcinoma (NPC) cells suppressed migration and invasion by increasing AFAP1 protein levels, inducing the loss of stress filament integrity and influencing the expression of many proteins in the small GTPase signalling Rho/Rac pathway (RhoA, Rac2, Rab10, Rab11a, Rhogdi and Pfn1 were significantly upregulated, but RhoC, Rab11b and Lasp1 were significantly downregulated)
3.4. Lung cancer
Lung cancer is the leading cause of cancer‐related mortality all over the world.88, 89 According to histopathological presentation, lung cancer is divided into 4 primary histological subtypes: small cell lung cancer, squamous cell carcinoma (SCC), adenocarcinoma (ADC) and large cell carcinoma (LCC).90 SCC, ADC and LCC are collectively called non‐small cell lung cancer (NSCLC) that accounts for almost 80% of lung cancer.91 Despite recent progresses of surgical resection, chemoradiotherapy or target drugs, lung cancer patients have a poor prognosis due to metastasis and recurrence.92, 93, 94 The 5‐year overall survival rate of advanced lung cancer patients is less than 15%.95 Hence, it is very important to find adequate tumour biomarkers for early diagnosis and metastasis identification in lung cancers.
Yu et al45 used microarray gene expression analysis and quantitative real‐time polymerase chain reaction analysis to identify that 551 lncRNAs were upregulated in NSCLC tissues, and AFAP1‐AS1 expression changed the most among the upregulated lncRNAs. Deng et al46 confirmed the above results. They also found that augmented expression of AFAP1‐AS1 was closely associated with clinical stage, smoking history, infiltration degree, lymph node metastasis, distant metastasis and poor prognosis in NSCLC patients. Next, Zeng et al47 found that AFAP1‐AS1 was significantly upregulated in lung cancer, and AFAP1‐AS1 upregulation was associated with tumour progression and poor survival. In vitro experiments demonstrated that knockdown of AFAP1‐AS1 suppressed tumour cell migration and invasion. Silence of AFAP1‐AS1 also increased the levels of its antisense protein‐coding gene, AFAP1, but had no significantly effect on AFAP1 mRNA. In addition, repression of AFAP1‐AS1 influenced some Rho/Rac GTPase family members and actin cytokeratin signalling pathway. Therefore, they speculated that AFAP1‐AS1 might promote migration and invasion in lung cancer by interfering with the expression of AFAP1 and some small GTPases (Figure 4). Recently, Zhuang et al48 reported that AFAP1‐AS1 was overexpressed in ADC and associated with survival time. Knockdown of AFAP1‐AS1 suppressed cell growth, induced apoptosis and inhibited invasion. Taken together, these results suggest that AFAP1‐AS1 may function as an oncogenic lncRNA and a potential prognostic biomarker and therapeutic target in lung cancer.
Figure 4.
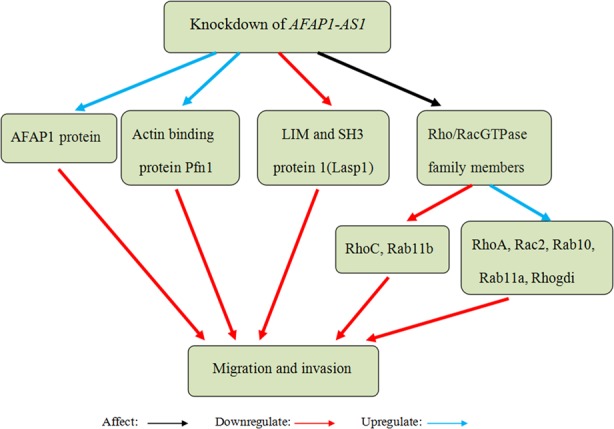
Knockdown of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) suppressed migration and invasion in lung cancer by increasing the levels of AFAP1 and influencing some Rho/Rac GTPase Rhogdi proteins and actin‐binding proteins (RhoA, Rac2, Rab10, Rab11a, Rhogdi and Pfn1 were upregulated, but RhoC, Rab11b and Lasp1 were downregulated)
3.5. Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), developing on the basis of pre‐existing chronic liver disease and cirrhosis, is the fifth most commonly diagnosed cancer and the third leading cause of cancer death all around the world.96, 97 Unlimited cell growth and invasion are the main characteristic of HCC. Because of delayed diagnosis and rapid metastasis, the treatment of advanced HCC is still in a dilemma, and the 5‐year survival rate of HCC patients is only about 7%.98, 99, 100 Lots of experiments have proved that HCC occurs in company with genetic or epigenetic mutation.101, 102, 103 Therefore, it is vital to find novel tumour biomarkers and understand the pathogenesis of HCC.
Zhang et al49 and Lu et al50 investigated the effects of AFAP1‐AS1 in HCC. Their findings demonstrated that AFAP1‐AS1, an independent predictor of overall survival, was apparently upregulated in HCC tissues compared with the adjacent non‐tumour tissues. Overexpression of AFAP1‐AS1 was associated with tumour size, TNM stage, lymph‐vascular space invasion and poor prognosis in HCC. Their results suggested that silencing of AFAP1‐AS1 impaired cell proliferation, migration and invasion through mediating some gene expressions related to proliferation and invasion in vitro. Moreover, Zhang et al49 reported that silencing of AFAP1‐AS1 promoted cell apoptosis and cycle arrest in S phase by upregulating the expression of Bax (BCL2‐associated X protein) and downregulating the expression of cyclin D1. Silencing of AFAP1‐AS1 also suppressed the activation of RhoA/Rac2 (ras homologue family member A/rac family small GTPase 2) signalling to decrease RhoA and Rac2 expression in HCC cells (Figure 5). Hence, they suspected that AFAP1‐AS1 may accelerate the progression and invasion in HCC by upregulating the RhoA/Rac2 signalling. Tumour xenograft studies showed that knockdown of AFAP1‐AS1 suppressed xenograft tumour growth in vivo. The above results suggest that AFAP1‐AS1 may play an important role in HCC development and serve as a therapeutic target of HCC.
Figure 5.
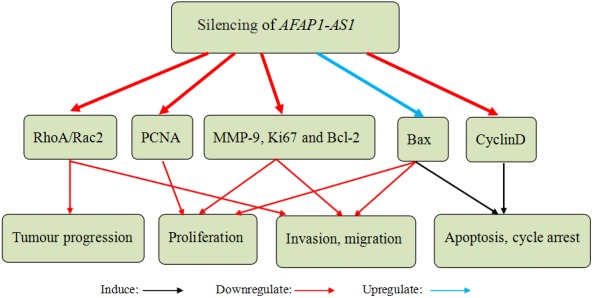
Silencing of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) in hepatocellular carcinoma (HCC) cells inhibited proliferation, migration and invasion through mediating proliferation‐ and invasion‐related gene expression in vitro (PCNA, MMP‐9, Ki67 and Bcl‐2 was downregulated, but Bax was upregulated). Silencing of AFAP1‐AS1 induced cell apoptosis and cycle arrest in S phase by upregulating the expression of Bax and downregulating the expression of cyclin D1. Silencing of AFAP1‐AS1 also suppressed the expression of RhoA and Rac2 to repress the progression and invasion in HCC
3.6. Ovarian cancer
Ovarian cancer (OC) is the third most widespread carcinoma of the female reproductive system.104 Despite the advances in surgery, diagnostic method and new chemotherapy, OC mortality rate is still high because most patients are diagnosed at an advanced stage.105, 106, 107 Therefore, it is exceedingly important to study its molecular mechanisms.
Yang et al51 reported that AFAP1‐AS1 was overexpressed in OC tissue samples and cell lines compared with corresponding normal counterparts. They found that high expression of AFAP1‐AS1 was obviously associated with aggressive clinicopathological parameters of OC, including resistance response and International Federation of Gynecology and Obstetrics (FIGO) stage. Then, knockdown of AFAP1‐AS1 suppressed cell proliferation and increased cell apoptosis. Therefore, their results indicate that AFAP1‐AS1 can serve as a novel oncogene and therapeutic target for OC.
3.7. Colorectal cancer
Colorectal cancer is the third most commonly diagnosed cancer and the second leading cause of cancer death worldwide.104, 108 Although advanced treatments, involving the combination of surgery, radiation therapy, chemotherapy and targeted therapy, are utilized to improve the prognosis of CRC patients, the recurrence and metastasis of CRC are still unavoidable.109, 110 The incidence and mortality of CRC will reduce by screening CRC from curable early stage, so we need to find a novel diagnostic and prognostic indicator for CRC.
Some experimental results proved that AFAP1‐AS1 was aberrantly overexpressed in CRC tissues and cells lines, and overexpression of AFAP1‐AS1 predicted poor prognosis of CRC patients.52, 53, 54 Wang et al52 found that upregulation of AFAP1‐AS1 was closely correlated with tumour size, TNM stage, distant metastasis, poorer overall survival and disease‐free survival. AFAP1‐AS1 inhibition suppressed cell proliferation, colony formation, migration and invasion. Moreover, suppression of AFAP1‐AS1 enhanced G0/G1 cell cycle arrest and the protein level of AFAP1 while having no effect on the mRNA level of AFAP1.52, 53 We suspected that AFAP1‐AS1 may affect some transcription factors expression related to AFAP1 protein, and AFAP1‐AS1 is irrelevant with AFAP1 transcription. Han et al53 found that knockdown of AFAP1‐AS1 inhibited tumour metastasis‐associated genes expression associated with EMT progression. Western blot results showed that the expression of E‐cadherin was elevated, but the expression of N‐cadherin, vimentin, fibronectin and matrix metalloproteinase 9 (MMP‐9) was reduced (Figure 6). They confirmed that knockdown of AFAP1‐AS1 inhibited tumour formation and hepatic metastasis of CRC cells in nude mice. In conclusion, these results suggest that AFAP1‐AS1 may act as an oncogene and a promising diagnostic and therapeutic target for CRC.
Figure 6.
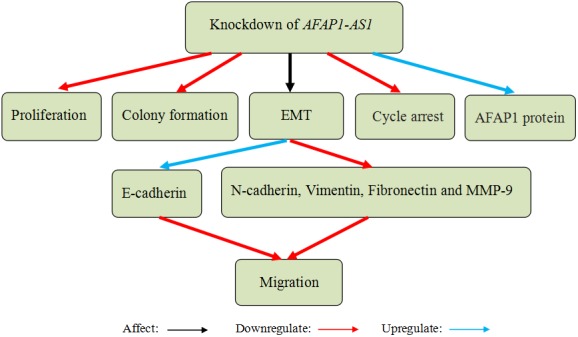
Actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) knockdown in colorectal cancer (CRC) cells suppressed cell proliferation, colony formation, migration and invasion, induced G0/G1 cell cycle arrest and improved the protein level of AFAP1. Knockdown of AFAP1‐AS1 inhibited tumour metastasis‐associated genes expression in terms of epithelial‐mesenchymal transition (EMT; the expression of E‐cadherin was elevated, but the expression of N‐cadherin, vimentin, fibronectin and MMP‐9 was reduced)
3.8. Biliary tract cancers
Biliary tract cancers (BTC) consist of gallbladder cancer (GBC) and cholangiocarcinoma (CCA). GBC accounts for 80%‐95% of BTC, while CCA makes up the rest. CCA is divided into intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma.111 CCA is one of the most aggressive and lethal tumours, originating from biliary epithelial cells lining the bile duct.112 GBC is a highly invasive malignancy neoplasm, and the overall 5‐year survival of GBC is less than 5%.113, 114 It is hard to diagnose BTC at an early stage because of non‐symptomatic manifestation and lack of sensitive biomarkers. Hence, finding early diagnostic markers and novel therapeutic targets is urgently needed.
Ma et al55 found that AFAP1‐AS1 was significantly elevated in GBC tissues and GBC cell lines and associated with tumour sizes and poor prognosis. Besides, knockdown of AFAP1‐AS1 suppressed cell growth and invasion. Further experiments demonstrated that knockdown of AFAP1‐AS1 impaired the EMT process in GBC cells via downregulating the transcription factor Twist1 and vimentin and upregulating the E‐cadherin. These findings showed that AFAP1‐AS1 may promote GBC cells invasion through accelerating EMT process (Figure 7).
Figure 7.
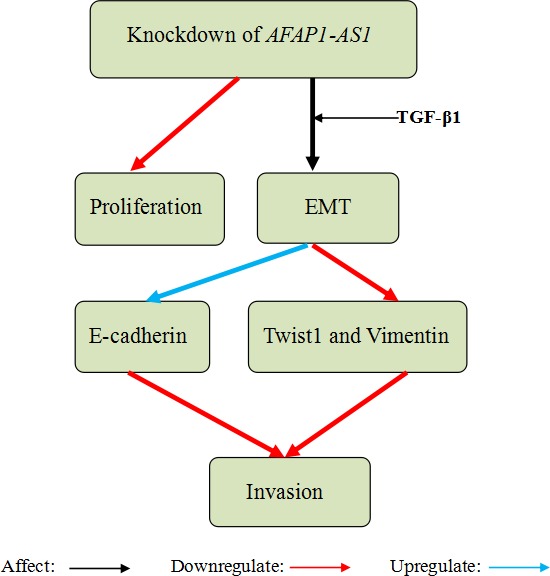
Knockdown of actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) in gallbladder cancer (GBC) cells suppressed cell proliferation and inhibited cell invasion through regulating the epithelial‐mesenchymal transition (EMT) process. Then, AFAP1‐AS1 was knockdown and simultaneously induced by TGF‐β1 treatment in GBC cells influencing EMT process by downregulating the transcription factor Twist1 and vimentin and upregulating the E‐cadherin
Shi et al56 and Lu et al57 investigated the effects of AFAP1‐AS1 in CCA at the same time. Their findings demonstrated that AFAP1‐AS1 was overexpressed in CCA tissues and cell lines, and AFAP1‐AS1 overexpression was associated with tumour size, vascular invasion, advance TNM stage, poor overall survival and prognosis. In addition, AFAP1‐AS1 knockdown in vitro suppressed cell proliferation and colony formation, induced G0/G1 cell cycle arrest and inhibited S‐G2/M transition. Moreover, AFAP1‐AS1 knockdown downregulated the expression of c‐Myc (MYC proto‐oncogene, bHLH transcription factor) and cyclin D1 that plays an important role in cell proliferation. Silence of AFAP1‐AS1 weakened cell migration and invasion by increasing AFAP1 mRNA and protein expression and reducing matrix metalloproteinase 2 (MMP‐2) and MMP‐9 expression in vitro. In addition, AFAP1‐AS1 inhibition reduced cell stress filament integrity and repressed CCA cell tumour growth and CCA metastasis in vivo (Figure 8). Taken together, these results suggest that AFAP1‐AS1 produces oncogenic effects in BTC and may become an effective diagnostic and therapeutic target for BTC.
Figure 8.
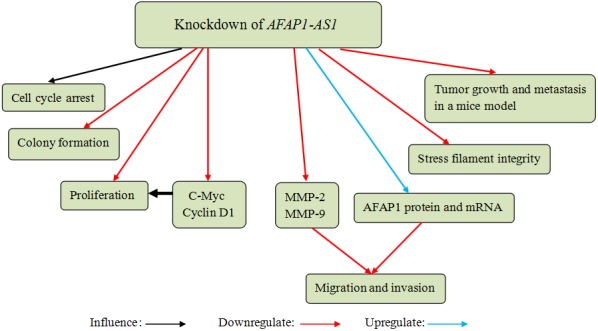
Actin filament‐associated protein 1‐antisense RNA 1 (AFAP1‐AS1) knockdown in cholangiocarcinoma (CCA) cells suppressed cell proliferation and colony formation and induced cell cycle arrest. AFAP1‐AS1 knockdown downregulated the expression of c‐Myc and cyclin D1, which played an important role in cell proliferation. AFAP1‐AS1 knockdown also inhibited cell migration and invasion by increasing AFAP1 protein and mRNA expression and reducing MMP‐2 and MMP‐9 expression. Moreover, AFAP1‐AS1 knockdown reduced cell stress filament integrity and repressed CCA cell tumour growth and CCA metastasis in a mice model
3.9. Gastric cancer
Gastric cancer, the fourth most commonly diagnosed cancer, is one of the major causes of cancer‐related death all over the world.115, 116 Although surgery and chemotherapy for GC have made great progress, GC patients at an advanced stage remain a poor prognosis, having an extremely low 5‐year survival rate.117, 118 Thus, it is urgent to find a new biomarkers for diagnosis and prognosis of GC.
Guo et al58 reported that AFAP1‐AS1 was overexpressed in GC tissues and cells compared with corresponding normal counterparts. AFAP1‐AS1 suppression inhibited cell proliferation through modulating phosphatase and tensin homologue (PTEN)/p‐AKT. In addition, decreased expression of AFAP1‐AS1 could impair the protein level of p‐AKT (AKT serine/threonine kinase 1) and strengthen the expression of PTEN. Moreover, AFAP1‐AS1 knockdown promoted cell apoptosis through decreasing the protein level of Bcl‐2 (B‐cell CLL/lymphoma 2 protein) and increasing the protein level of cleaved PARP (poly‐ADP‐ribose polymerase), Caspase 3, Caspase 9 and Bax. In conclusion, their results support that AFAP1‐AS1 may play a significant role as an indicator of poor survival and a therapeutic target for GC.
4. CONCLUSION
Long non‐coding RNA AFAP1‐AS1 plays an important role in cancer development and serves as an oncogenic lncRNA, which is overexpressed in all kinds of cancers, including oesophageal cancer, PDAC, NPC, lung cancer, HCC, OC, CRC, BTC and GC. High expression level of AFAP1‐AS1 in tumour tissues is correlated with clinicopathological characteristics, such as tumour size, lymphatic metastasis, distant metastasis, TNM stage, poor prognosis, overall survival and disease‐free survival. However, the precise concentration and detection method of AFAP1‐AS1 in the blood of cancer patients and healthy person are still unclear, impeding the clinical applications of AFAP1‐AS1. In order to carry out more deeper research and draw a more accurate conclusion, more cancer patients should be involved in the AFAP1‐AS1 study. Functional experiments demonstrated that AFAP1‐AS1 could promote tumour cell proliferation, migration and invasion and inhibit apoptosis. In addition, the involvement of some related genes or signalling pathways in the oncogenic function of AFAP1‐AS1 has been proved, such as EMT‐related genes and small GTPase signalling Rho/Rac pathway, but its particular upstream and downstream molecular mechanisms need to be systematically analysed in the future. Compared with other well‐studied lncRNAs such as MALAT1 and H19, the studies of AFAP1‐AS1 are not enough. So far, none of AFAP1‐AS1‐related miRNAs or mRNAs was found, and AFAP1‐AS1 research is still at an early stage. The relationship between AFAP1‐AS1 and proteins, miRNAs, mRNAs, ceRNAs and other lncRNAs should be better understood and investigated. Moreover, what is the role of AFAP1‐AS1 in the common pathogenesis of cancer such as chromosome abnormalities, DNA modification and histone modification? Previous studies also showed that knockdown of AFAP1‐AS1 increased the expression of AFAP1 in some cancers, but the combined actions, specific functions and regulatory molecules in tumour progression should be explored in great depth.
In conclusion, the recent studies suggest that lncRNA AFAP1‐AS1 produces oncogenic effects in human cancer and may become an effective diagnostic and therapeutic target for human cancer. With the development of AFAP1‐AS1 study, lncRNA AFAP1‐AS1 may be applied in clinical detection and treatment in the future.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Key Basic Research Program of China (2014CB745201), National Natural Science Foundation of China [81402103], International S&T Cooperation program of China (ISTCP) (2014DFA31050), the National Science Foundation Projects of Guangdong Province (2014A030313717).
Zhang F, Li J, Xiao H, Zou Y, Liu Y, Huang W. AFAP1‐AS1: A novel oncogenic long non‐coding RNA in human cancers. Cell Prolif. 2018;51:e12397 10.1111/cpr.12397
These authors FZ, JL, HX and YZ have contributed equally to this work.
REFERENCES
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 2. Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118‐128. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Mercer TR, Dinger ME, Mattick JS. Long non‐coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155‐159. [DOI] [PubMed] [Google Scholar]
- 5. Zheng Y, Liu L, Shukla GC. A comprehensive review of web‐based non‐coding RNA resources for cancer research. Cancer Lett. 2017;407:1‐8. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Zhuang C, Liu Y, et al. Synthetic tetracycline‐controllable shRNA targeting long non‐coding RNA HOXD‐AS1 inhibits the progression of bladder cancer. J Exp Clin Cancer Res. 2016;35:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629‐641. [DOI] [PubMed] [Google Scholar]
- 8. Seton‐Rogers S. Non‐coding RNAs: the cancer X factor. Nat Rev Cancer. 2013;13:224‐225. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Xie H, Zou Y, et al. Tetracycline‐controllable artificial microRNA‐HOTAIR + EZH2 suppressed the progression of bladder cancer cells. Mol BioSyst. 2017;13:1597‐1607. [DOI] [PubMed] [Google Scholar]
- 10. Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non‐coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer‐related long non‐coding RNA. Cell Prolif. 2017;50 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Zeng Y, Liu L, et al. Synthesizing AND gate genetic circuits based on CRISPR‐Cas9 for identification of bladder cancer cells. Nat Commun. 2014;5:5393. [DOI] [PubMed] [Google Scholar]
- 15. Li J, Zhuang C, Liu Y, et al. shRNA targeting long non‐coding RNA CCAT2 controlled by tetracycline‐inducible system inhibits progression of bladder cancer cells. Oncotarget. 2016;7:28989‐28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji P, Diederichs S, Wang W, et al. MALAT‐1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early‐stage non‐small cell lung cancer. Oncogene. 2003;22:8031‐8041. [DOI] [PubMed] [Google Scholar]
- 17. Gutschner T, Hammerle M, Diederichs S. MALAT1–a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl). 2013;91:791‐801. [DOI] [PubMed] [Google Scholar]
- 18. Wu Y, Huang C, Meng X, Li J. Long noncoding RNA MALAT1: insights into its biogenesis and implications in human disease. Curr Pharm Des. 2015;21:5017‐5028. [DOI] [PubMed] [Google Scholar]
- 19. Ellis MJ, Ding L, Shen D, et al. Whole‐genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Y, Shen B, Tan M, et al. TGF‐beta‐induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531‐1541. [DOI] [PubMed] [Google Scholar]
- 21. Ji Q, Zhang L, Liu X, et al. Long non‐coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111:736‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang D, Yang H, Lin J, et al. 17beta‐estradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating miR‐9 and thus degrades MALAT‐1 in osteosarcoma cell MG‐63 in an estrogen receptor‐independent manner. Biochem Biophys Res Commun. 2015;457:500‐506. [DOI] [PubMed] [Google Scholar]
- 23. Gupta RA, Shah N, Wang KC, et al. Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu ZY, Yu QM, Du YA, et al. Knockdown of long non‐coding RNA HOTAIR suppresses tumor invasion and reverses epithelial‐mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song H, Sun W, Ye G, et al. Long non‐coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med. 2013;11:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318‐2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhuang M, Gao W, Xu J, Wang P, Shu Y. The long non‐coding RNA H19‐derived miR‐675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315‐322. [DOI] [PubMed] [Google Scholar]
- 28. Yang F, Bi J, Xue X, et al. Up‐regulated long non‐coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159‐3165. [DOI] [PubMed] [Google Scholar]
- 29. Xiang W, Ke Z, Zhang Y, et al. Isthmin is a novel secreted angiogenesis inhibitor that inhibits tumour growth in mice. J Cell Mol Med. 2011;15:359‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu W, Bhagat TD, Yang X, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1‐AS1, in Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144:956‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu FT, Xue QZ, Zhu PQ, Luo HL, Zhang Y, Hao T. Long noncoding RNA AFAP1‐AS1, a potential novel biomarker to predict the clinical outcome of cancer patients: a meta‐analysis. Onco Targets Ther. 2016;9:4247‐4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flynn DC, Leu TH, Reynolds AB, Parsons JT. Identification and sequence analysis of cDNAs encoding a 110‐kilodalton actin filament‐associated pp60src substrate. Mol Cell Biol. 1993;13:7892‐7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC. The carboxy terminus of AFAP‐110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res. 2000;255:102‐113. [DOI] [PubMed] [Google Scholar]
- 34. Qian Y, Baisden JM, Cherezova L, et al. PC phosphorylation increases the ability of AFAP‐110 to cross‐link actin filaments. Mol Biol Cell. 2002;13:2311‐2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bo H, Gong Z, Zhang W, et al. Upregulated long non‐coding RNA AFAP1‐AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6:20404‐20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carrieri C, Cimatti L, Biagioli M, et al. Long non‐coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454‐457. [DOI] [PubMed] [Google Scholar]
- 37. Sehgal L, Mathur R, Braun FK, et al. FAS‐antisense 1 lncRNA and production of soluble versus membrane Fas in B‐cell lymphoma. Leukemia. 2014;28:2376‐2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Pang WJ, Wei N, et al. Identification, stability and expression of Sirt1 antisense long non‐coding RNA. Gene. 2014;539:117‐124. [DOI] [PubMed] [Google Scholar]
- 39. Yuan SX, Tao QF, Wang J, et al. Antisense long non‐coding RNA PCNA‐AS1 promotes tumor growth by regulating proliferating cell nuclear antigen in hepatocellular carcinoma. Cancer Lett. 2014;349:87‐94. [DOI] [PubMed] [Google Scholar]
- 40. Zhou XL, Wang WW, Zhu WG, et al. High expression of long non‐coding RNA AFAP1‐AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog. 2016;55:2095‐2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo HL, Huang MD, Guo JN, et al. AFAP1‐AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5:2879‐2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye Y, Chen J, Zhou Y, et al. High expression of AFAP1‐AS1 is associated with poor survival and short‐term recurrence in pancreatic ductal adenocarcinoma. J Transl Med. 2015;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang Y, He Y, Shi L, et al. Co‐expression of AFAP1‐AS1 and PD‐1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:39001‐39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He B, Zeng J, Chao W, et al. Serum long non‐coding RNAs MALAT1, AFAP1‐AS1 and AL359062 as diagnostic and prognostic biomarkers for nasopharyngeal carcinoma. Oncotarget. 2017;8:41166‐41177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu H, Xu Q, Liu F, Ye X, Wang J, Meng X. Identification and validation of long noncoding RNA biomarkers in human non‐small‐cell lung carcinomas. J Thorac Oncol. 2015;10:645‐654. [DOI] [PubMed] [Google Scholar]
- 46. Deng J, Liang Y, Liu C, He S, Wang S. The up‐regulation of long non‐coding RNA AFAP1‐AS1 is associated with the poor prognosis of NSCLC patients. Biomed Pharmacother. 2015;75:8‐11. [DOI] [PubMed] [Google Scholar]
- 47. Zeng Z, Bo H, Gong Z, et al. AFAP1‐AS1, a long noncoding RNA upregulated in lung cancer and promotes invasion and metastasis. Tumour Biol. 2016;37:729‐737. [DOI] [PubMed] [Google Scholar]
- 48. Zhuang Y, Jiang H, Li H, et al. Down‐regulation of long non‐coding RNA AFAP1‐AS1 inhibits tumor cell growth and invasion in lung adenocarcinoma. Am J Transl Res. 2017;9:2997‐3005. [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang JY, Weng MZ, Song FB, et al. Long noncoding RNA AFAP1‐AS1 indicates a poor prognosis of hepatocellular carcinoma and promotes cell proliferation and invasion via upregulation of the RhoA/Rac2 signaling. Int J Oncol. 2016;48:1590‐1598. [DOI] [PubMed] [Google Scholar]
- 50. Lu X, Zhou C, Li R, et al. Critical role for the long non‐coding RNA AFAP1‐AS1 in the proliferation and metastasis of hepatocellular carcinoma. Tumour Biol. 2016;37:9699‐9707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Yang SL, Lin RX, Si LH, Cui MH, Zhang XW, Fan LM. Expression and functional role of long non‐coding RNA AFAP1‐AS1 in ovarian cancer. Eur Rev Med Pharmacol Sci. 2016;20:5107‐5112. [PubMed] [Google Scholar]
- 52. Wang F, Ni H, Sun F, Li M, Chen L. Overexpression of lncRNA AFAP1‐AS1 correlates with poor prognosis and promotes tumorigenesis in colorectal cancer. Biomed Pharmacother. 2016;81:152‐159. [DOI] [PubMed] [Google Scholar]
- 53. Han X, Wang L, Ning Y, Li S, Wang Z. Long non‐coding RNA AFAP1‐AS1 facilitates tumor growth and promotes metastasis in colorectal cancer. Biol Res. 2016;49:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Q, Dai Y, Wang F, Hou S. Differentially expressed long non‐coding RNAs and the prognostic potential in colorectal cancer. Neoplasma. 2016;63:977‐983. [DOI] [PubMed] [Google Scholar]
- 55. Ma F, Wang SH, Cai Q, Zhang MD, Yang Y, Ding J. Overexpression of LncRNA AFAP1‐AS1 predicts poor prognosis and promotes cells proliferation and invasion in gallbladder cancer. Biomed Pharmacother. 2016;84:1249‐1255. [DOI] [PubMed] [Google Scholar]
- 56. Shi X, Zhang H, Wang M, et al. LncRNA AFAP1‐AS1 promotes growth and metastasis of cholangiocarcinoma cells. Oncotarget. 2017;8:58394‐58404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lu X, Zhou C, Li R, Deng Y, Zhao L, Zhai W. Long Noncoding RNA AFAP1‐AS1 Promoted Tumor Growth and Invasion in Cholangiocarcinoma. Cell Physiol Biochem. 2017;42:222‐230. [DOI] [PubMed] [Google Scholar]
- 58. Guo JQ, Li SJ, Guo GX. Long Noncoding RNA AFAP1‐AS1 Promotes Cell Proliferation and Apoptosis of Gastric Cancer Cells via PTEN/p‐AKT Pathway. Dig Dis Sci. 2017;62:2004‐2010. [DOI] [PubMed] [Google Scholar]
- 59. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400‐412. [DOI] [PubMed] [Google Scholar]
- 60. Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63:232‐248. [DOI] [PubMed] [Google Scholar]
- 61. Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction‐Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304‐317. [DOI] [PubMed] [Google Scholar]
- 62. Barton MK. Smoking found to increase the rate of progression of Barrett esophagus to adenocarcinoma. CA Cancer J Clin. 2012;62:215‐216. [DOI] [PubMed] [Google Scholar]
- 63. Yuequan J, Shifeng C, Bing Z. Prognostic factors and family history for survival of esophageal squamous cell carcinoma patients after surgery. Ann Thorac Surg. 2010;90:908‐913. [DOI] [PubMed] [Google Scholar]
- 64. Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98‐100. [DOI] [PubMed] [Google Scholar]
- 65. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241‐2252. [DOI] [PubMed] [Google Scholar]
- 66. Garrido‐Laguna I, Hidalgo M. Pancreatic cancer: from state‐of‐the‐art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319‐334. [DOI] [PubMed] [Google Scholar]
- 67. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73‐85. [DOI] [PubMed] [Google Scholar]
- 68. Frampton AE, Castellano L, Colombo T, et al. Integrated molecular analysis to investigate the role of microRNAs in pancreatic tumour growth and progression. Lancet. 2015;385(suppl 1):S37. [DOI] [PubMed] [Google Scholar]
- 69. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet. 2017;389:1011‐1024. [DOI] [PubMed] [Google Scholar]
- 71. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97‐110. [DOI] [PubMed] [Google Scholar]
- 72. Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341:9‐15. [DOI] [PubMed] [Google Scholar]
- 73. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. [DOI] [PubMed] [Google Scholar]
- 74. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21‐45. [DOI] [PubMed] [Google Scholar]
- 75. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139:871‐890. [DOI] [PubMed] [Google Scholar]
- 76. Santamaria PG, Moreno‐Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11:718‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest. 2009;119:1420‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial‐mesenchymal transition. Sci Signal. 2014;7:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Singh M, Yelle N, Venugopal C, Singh SK. EMT: mechanisms and therapeutic implications. Pharmacol Ther. 2017. 10.1016/j.pharmthera.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 80. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Du B, Shim JS. Targeting epithelial‐mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 83. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012‐1024. [DOI] [PubMed] [Google Scholar]
- 84. Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008;44:617‐627. [DOI] [PubMed] [Google Scholar]
- 85. Yan Q, Zeng Z, Gong Z, et al. EBV‐miR‐BART10‐3p facilitates epithelial‐mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma by targeting BTRC. Oncotarget. 2015;6:41766‐41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou Y, Zeng Z, Zhang W, et al. Lactotransferrin: a candidate tumor suppressor‐Deficient expression in human nasopharyngeal carcinoma and inhibition of NPC cell proliferation by modulating the mitogen‐activated protein kinase pathway. Int J Cancer. 2008;123:2065‐2072. [DOI] [PubMed] [Google Scholar]
- 87. Yang Y, Liao Q, Wei F, et al. LPLUNC1 inhibits nasopharyngeal carcinoma cell growth via down‐regulation of the MAP kinase and cyclin D1/E2F pathways. PLoS ONE. 2013;8:e62869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374‐1403. [DOI] [PubMed] [Google Scholar]
- 89. Barton MK. Integration of lung cancer screening into practice is lacking. CA Cancer J Clin. 2015;65:255‐256. [DOI] [PubMed] [Google Scholar]
- 90. Rami‐Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer–major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138‐155. [DOI] [PubMed] [Google Scholar]
- 91. He M, Xue Y. MicroRNA‐148a suppresses proliferation and invasion potential of non‐small cell lung carcinomas via regulation of STAT3. Onco Targets Ther. 2017;10:1353‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non‐small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29‐33. [DOI] [PubMed] [Google Scholar]
- 93. Barton MK. Encouraging long‐term outcomes reported in patients with stage I non‐small cell lung cancer treated with stereotactic ablative radiotherapy. CA Cancer J Clin. 2017;67:349‐350. [DOI] [PubMed] [Google Scholar]
- 94. Barton MK. Local consolidative therapy may be beneficial in patients with oligometastatic non‐small cell lung cancer. CA Cancer J Clin. 2017;67:89‐90. [DOI] [PubMed] [Google Scholar]
- 95. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735‐742. [DOI] [PubMed] [Google Scholar]
- 96. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 97. Au JS, Frenette CT. Erratum: management of hepatocellular carcinoma: current Status and future directions. Gut Liv. 2015;9:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol. 2015;7:1020‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ilikhan SU, Bilici M, Sahin H, et al. Assessment of the correlation between serum prolidase and alpha‐fetoprotein levels in patients with hepatocellular carcinoma. World J Gastroenterol. 2015;21:6999‐7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394‐399. [DOI] [PubMed] [Google Scholar]
- 101. Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54:757‐759. [DOI] [PubMed] [Google Scholar]
- 102. Dong QZ, Zhang XF, Zhao Y, et al. Osteopontin promoter polymorphisms at locus ‐443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013;57:1024‐1034. [DOI] [PubMed] [Google Scholar]
- 103. Wang B, Fang J, Qu L, Cao Z, Zhou J, Deng B. Upregulated TRIO expression correlates with a malignant phenotype in human hepatocellular carcinoma. Tumour Biol. 2015;36:6901‐6908. [DOI] [PubMed] [Google Scholar]
- 104. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 105. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3. [DOI] [PubMed] [Google Scholar]
- 106. Rustin G, van der Burg M, Griffin C, Qian W, Swart AM. Early versus delayed treatment of relapsed ovarian cancer. Lancet. 2011;377:380‐381. [DOI] [PubMed] [Google Scholar]
- 107. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. [DOI] [PubMed] [Google Scholar]
- 109. Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975‐2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490‐1502. [DOI] [PubMed] [Google Scholar]
- 111. Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249‐273. [DOI] [PubMed] [Google Scholar]
- 112. Wang WT, Ye H, Wei PP, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lazcano‐Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349‐364. [DOI] [PubMed] [Google Scholar]
- 114. Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211‐12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. [DOI] [PubMed] [Google Scholar]
- 117. Lim SM, Lim JY, Cho JY. Targeted therapy in gastric cancer: personalizing cancer treatment based on patient genome. World J Gastroenterol. 2014;20:2042‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654‐2664. [DOI] [PubMed] [Google Scholar]


