Abstract
Objectives
Oestrogen receptor (ER) is a common nucleus receptor that is essential for the regulation of cell growth, proliferation and differentiation. This study was to examine whether ERα can affect the proliferation and odonto/osteogenic differentiation of stem cells from apical papilla (SCAPs).
Materials and Methods
Stem cells from apical papillas were isolated, purified and then transfected with ERα lentiviruses. The proliferation capacity was investigated by cell counting kit‐8 (CCK‐8) assay and flow cytometry. The odonto/osteogenic differentiation ability was analysed by alkaline phosphatase (ALP) activity, alizarin red staining, western blot assay (WB) and real‐time RT‐PCR. MAPK pathway and its downstream transcriptional factors were explored by WB assay.
Results
As indicated by CCK‐8 assay and flow cytometry, ERα had no significant effect on the proliferation of SCAPs. When ERα was overexpressed, the ALP activity and the formation of calcified nodules were significantly enhanced in SCAPs. Moreover, the odonto/osteogenic markers (DMP1/DMP1, DSPP/DSP,RUNX2/RUNX2, OCN/OCN) in SCAPs were significantly up‐regulated at both mRNA and protein levels. On the contrary, the odonto/osteogenic differentiation ability of SCAPs was remarkably inhibited after suppression of ERα. Mechanistically, the protein levels of phosphorylated ERK and JNK significantly increased after ERα overexpression. Moreover, some downstream transcriptional factors of MAPK pathway were simultaneously activated by ERα overexpression.
Conclusions
Together, the data accumulated here indicated that ERα can enhance the odonto/osteogenic differentiation of SCAPs via ERK and JNK MAPK pathways.
1. INTRODUCTION
Regenerative medicine is recently concentrating on guiding differentiation of stem cells into lineage‐specific, functional cells which can create and repair damaged tissues.1 Stem cell therapies are attractive for treating a large amount of diseases, including oral diseases.2 Stem cells from apical papilla (SCAPs) are a population of stem/progenitor cells residing in the apical papilla of developing human permanent teeth.3 They can differentiate into neurocyte, adipocyte and osteoblast lineages in vitro under proper conditions, and has been reported to have effects on neuronal repair.4 SCAPs have been shown to form more uniform dentin‐like tissue and possess much higher proliferation rate, dentinogenic capacity than other dental stem cells, being potentially a preferable cell source for tooth regeneration.5, 6 SCAPs can give rise to primary odontoblasts, which are responsible for the formation of primary root dentin during the odontogenesis.5, 6, 7 Apical infection caused by dental caries, trauma and periodontal diseases may block the tooth development.8 Some documents have found that SCAPs may still exist and work at the site of the root development process during infections.9 In vivo studies, SCAPs have been proved to form a dentin/pulp complex when transplanted with HA/TCP particles in immunodeficient mice.5 It is reported that SCAPs could also be used in combination with periodontal ligament stem cells to form a bioroot.10
A wide array of biochemical (growth factors, hormones and microRNAs) and biophysical (electrical, magnetic and ultraviolet ray) elements are currently being investigated in vitro for their capacity to aid stem cell regeneration.11 It is well established that oestrogen plays an important role in affecting growth, differentiation and function in multiple systems and organs in the organism.12 The predominant biological effects of oestrogen are generally exerted by activation of oestrogen receptor (ER).13 ER is a member of the receptor superfamily, and it is an oestrogen‐dependent transcription factor which accounts for the regulation of bone metabolism.14 Some studies have found that the expression of ER is up‐regulated during the osteogenic differentiation of rat calvaria osteoblasts.15 ER is mainly divided into 2 types (ie ERα and ERβ) and ERβ exhibits a more limited expression pattern.16 ERα has the ability to decrease bone turnover and increase trabecular bone volume in male mice.17 Oestrogen and ER axis is multiply reported to have effects on the proliferation, apotosis and differentiation of mesenchymal stem cells (MSCs).18 Some studies have proved that ER can activate related signalling pathways (Wnt/β‐catenin, mTOR and PI3K/Akt/STAT3) to regulate the proliferation‐associated genes of MSCs.19, 20 Researches suggest that oestrogen is involved in the differentiation of bone marrow MSCs13 and dental MSCs‐mediated morphogenesis of tooth.21
Our previous studies have detected the increased expression of ERα in the process of oestrogen‐mediated differentiation of SCAPs.22 However, the exact effects of ERα on SCAPs and the underlying mechanisms have not been elucidated. In this work, we investigated the effects of ERα in SCAPs and demonstrated that ERα can promote the odonto/osteogenic differentiation of SCAPs by activating ERK and JNK MAPK pathways.
2. MATERIALS AND METHODS
2.1. Cell isolation and culture
Human third molars with immature roots were collected from donors (17‐20 years old) at the Oral and Maxillofacial Surgery Department of Jiangsu Provincial Stomatological Hospital. The experimental procedures were approved by the Ethical Committee of Stomatological School of Nanjing Medical University and performed with the informed consent of the patients. The apical papilla was softly isolated from surfaces of roots and then minced and digested in a solution of 3 mg/mL type I collagenase (Gibco, Life Technologies, MA, USA) and 4 mg/mL trypsin (Gibco, Life Technologies) for 1 hour at 37°C. The dissolved tissues were evenly plated into 6 cm culture dishes, then incubated in alpha minimum essential medium (α‐MEM, Gibco, Life Technologies) supplemented with 10% foetal bovine serum (FBS, Gibco, Life Technologies), 100 g/mL streptomycin and 100 U/mL penicillin at 37°C in 5% CO2. The media were changed after 24 hours thereafter every 2 days. After 7‐10 days, the attached cells were collected and purified by the procedure of magnetic activated cell sorting as previously described.23 Cells were passaged by 0.25% trypsin at a 1:3 split ratio after the cell density came up to 80%‐85%. The experiments were carried out by using SCAPs within 3‐5 passages.
2.2. Flow cytometry
Enzyme digestion was used to collect SCAPs at the density of 1 × 104 cells/well. Then, cells were rinsed with 0.01 mol/L PBS twice and resuspended in 100 μL wash buffer. After adding the primary antibodies (STRO‐1, CD34, CD45, CD73, CD90, CD105) into each solution, the samples were incubated for 15 minutes at room temperature in the dark. Stained cells were washed twice with 0.01 mol/L PBS and analysed by FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). To investigate the effects of ERα on the proliferation of SCAPs, transfected cells were collected by using trypsin (Beyotime, Haimen, China) and fixed with 75% ice‐cold ethanol at 4°C for 24 hours away from the light. Then, each sample was analysed by FACScan flow cytometer (BD Biosciences).
2.3. Plasmid construction
The PLKO.1/ERα‐shRNA plasmid, envelope plasmid (VSVG) and packaging plasmid (drpr) were mixed according to the ratio of 6.67: 2: 5 μg, and then transfected 293T cells. After 48 hours, the viral supernatant was collected, centrifugated for 5 minutes at the speed of 2000 g, and at last stored under −70°C. The PMSCV‐GFP/ERα plasmid, envelope plasmid (VSVG) and packaging plasmid (drpr) were mixed according to the ratio of 1.08 μg: 0.27 μg: 0.81 μg, and then transfected 293T cells. After 72 hours, the viral supernatant was collected, centrifugated for 5 minutes at the speed of 2000 g, and at last stored under −70°C.
2.4. Cell transfection
SCAPs cultured at 60%‐70% confluence were transfected with the lentiviruses in 1 mL α‐MEM supplemented with 10% FBS and 8 μg/mL polybrene (Santa Cruz, Delaware Ave, Santa Cruz, CA, USA). 10 hours later, the infected cells were incubated in replaced conventional media. After 3 days, the antibiotic‐resistant transfected cells were selected by applying puromycin in culture medium and passaged at the ratio of 1:3 for further use.
2.5. Cell counting kit‐8 assay
The proliferation ability of transfected SCAPs was detected by using the cell counting kit‐8 (CCK‐8, Dojindo, Tokyo, Japan) according to the manufacturer's protocol. Cells were cultured in a 96‐well plate at an initial density of 2 × 103 cells per well. Then, the complete medium was changed for the serum‐free medium when cells adhered to the wall. At day 0, 1, 3, 5, 7 and 9, SCAPs were treated with CCK‐8 regents at 37°C for 2 hours, and then the OD values were determined at 450 nm by a microplate reader (Bio‐Tek, Vermont, USA).
2.6. Alkaline phosphatase activity
Transfected SCAPs were plated at a density of 1 × 104 cells/well into 6‐well plates (Corning, Life Sciences, NY, USA). Cells were cultured for 5 days and changed for fresh medium every other day. Then, they were rinsed with PBS for 3 times and incubated with 50 μL 0.2% Triton X‐100 for 2 hours at 37°C. The ALP activity was detected by using an ALP activity assay kit (Jiancheng, Nanjing, China) according to the manufacturer's instructions. The normalization of ALP activity to total protein was determined with a BCA kit (Beyotime, Shanghai, China).
2.7. Alizarin red staining
The mineralization ability of transfected SCAPs in osteogenic induction group and uninduced group was investigated. Briefly, well‐grown cells were plated into 6‐well plates (Corning, Life Sciences). Then, cells were cultured in a mineralization‐inducing medium containing α‐MEM, 10% FBS, 100 U/mL penicillin, 100 g/mL streptomycin, 2 mmol/L l‐glutamine (Sigma‐Aldrich, Munich, Germany), 50 mg/L ascorbic acid (Sigma‐Aldrich), 10 mmol/L β‐glycerophosphate (Sigma‐Aldrich) and 10 nmol/L dexamethasone (Sigma‐Aldrich). Cells were fixed in ice‐cold 70% ethyl alcohol for 30 minutes at room temperature after 2 weeks. Then cells were rinsed with deionized water and dyed with 100 μmol L−1 alizarin red (pH = 4.2, Sigma‐Aldrich) for 2 minutes. To quantify the mineralization nodules, the alizarin red was dissolved with 10% cetylpyridinium chloride (CPC, Sigma‐Aldrich) in 10 mmol/L sodium phosphate for 30 minutes at 25°C. The absorbances were respectively read at a wavelength of 560 nm using a microplate reader (Bio‐Tek). The final calcium concentrations in each group were normalized with the total protein concentrations.
2.8. Real‐time reverse transcription polymerase chain reaction
Total RNA was extracted from SCAPs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The mRNA was subjected to reverse transcription by a PrimeScript RT Master Mix kit (TaKaRa, Dalian, China). Real‐time RT‐PCR was performed to analyse gene expression changes using SYBR Premix Ex Taq kit (TaKaRa Bio, Otsu, Japan) and ABI 7300 real‐time PCR system. All primers were synthesized by the same manufacturer (Sangon Biotech, Shanghai, China). Table 1 showed the sense and antisense primers used for detection of the mRNA expression levels of ERα, OCN, RUNX2, DSPP and DMP1. Real‐time RT‐PCR reaction conditions were: 95°C for 30 s; followed by 40 cycles of 95°C for 5 s; 60°C for 31 s. mRNA levels were normalized to the amount of GAPDH (glyceralde‐hyde‐3‐phosphate dehydrogenase). Relative gene expression values were calculated by the 2−ΔΔCt method as previously reported.21, 22 Each experiment was repeated independently at least 3 times.
Table 1.
Sense and antisense primers for real‐time reverse transcription polymerase chain reaction
| Genes | Primers | Sequences (5′‐3′) |
|---|---|---|
| ERα | Forward | ACTCAACAGCGTGTCTCCG |
| Reverse | GGGCTCGTTCTCCAGGTAG | |
| DMP1 | Forward | TTTTAGGAAGTCTCGCATCT |
| Reverse | TGGGACCATCTACGTTTT | |
| DSPP | Forward | CGTTCAGGGAGTCCTAGCGGGAACG |
| Reverse | GTGACTCTCCCTTCCATCTCCTG | |
| RUNX2 | Forward | GAATGCCTCTGCTGTTATG |
| Reverse | ACTCTTGCCTCGTCCACT | |
| OCN | Forward | AGCAAAGGTGCAGCCTTTGT |
| Reverse | GCGCCTGGGTCTCTTCACT | |
| GAPDH | Forward | GAAGGTGAAGGTCGGAGTC |
| Reverse | GAGATGGTGATGGGATTTC |
2.9. Western blot
To measure the expression of MAPK pathway‐related proteins, transfected SCAPs at 48 hours were collected to obtain the cytoplasmic and nuclear protein with a Keygen Kit (Keygen Bio‐Tech, Nanjing, China). To examine effects of ERα on the odonto/osteogenic differentiation of SCAPs, transfected cells were collected at day 0, 3 and 7 respectively. After washing thrice by PBS, SCAPs were lysed in RIPA buffer (Beyotime). The same amount of protein was subject to a 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene fluoride (PVDF, Millipore, Darmstadt, Germany) membranes. The transblotted membranes were blocked in 5% BSA for 2 hours at room temperature followed by an incubation with primary antibodies including DMP1 (NBP1‐45525, Novus, Littleton, USA), RUNX2 (ab76956, Abcam, Cambridge, UK), DSP (sc‐33586, Santa Cruz), OCN (ab93876, Abcam), ERK (#4695, Cell Signaling Technology), p‐ERK (#4370, Cell Signaling Technology, Danvers, MA, USA), JNK (#9252, Cell Signaling Technology), p‐JNK (#9255, Cell Signaling Technology), P38 (#8690, Cell Signaling Technology), p‐P38 (#4511, Cell Signaling Technology), c‐Fos (#2250, Cell Signaling Technology), p‐c‐Fos (#5348, Cell Signaling Technology), c‐Jun (#9165, Cell Signaling Technology), p‐c‐Jun (#9261, Cell Signaling Technology), Elk1 (#9182, Cell Signaling Technology), p‐Elk1 (#9186, Cell Signaling Technology), H3 (#9728, Cell Signaling Technology) and β‐ACTIN (AP0060, Bioworld, Nanjing, China) at 4°C overnight. After rinsed thrice in TBST for 30 minutes, the membranes were incubated with secondary antibodies for 1 hour at 4°C, and antibody‐antigen reaction was detected using Western Blotting Imaging System.
2.10. Statistical analysis
Experiments were performed in triplicate and repeated more than 3 times independently. The results were presented as mean ± SD and analysed using spss 17.0 software (SPSS, Chicago, IL, USA). Two‐tailed Student's t‐test was used for comparison of pairs. A P value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Characterization of SCAPs and cell transfection
There was a high expression of MSC markers (STRO‐1, CD73, CD90 and CD105), while the hematopoietic makers (CD34 and CD45) were expressed at a low level in SCAPs, as indicated by flow cytometry (Figure 1A).
Figure 1.
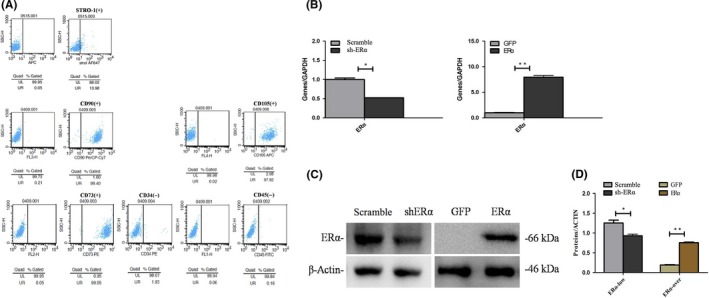
Characterization of SCAPs and ERα expression in transfected SCAPs. A, Flow cytometry revealed that SCAPs were positive for STRO‐1, CD73, CD90 and CD105 and negative against CD34 and CD45. B, The ERα expression at day 3 in Scramble group, sh‐ERα group, GFP group and ERα group respectively. *P < .05 or **P < .01. C, The ERα expression at day 3 in Scramble group, sh‐ERα group, GFP group and ERα group respectively. *P < .05 or **P < .01. D, Greyscale analyses of (C) by Image‐Pro Plus 5.0 software
To detect the transfection efficiency, ERα expression in the transfected SCAPs using ERα overexpression or inhibition lentiviruses was measured by real‐time RT‐PCR and western blot assay at day 3. The results demonstrated a significant increase of ERα expression in ERα group as compared with that in GFP group (P < .05 or P < .01, Figure 1B‐D), while the expression level of ERα was significantly decreased in sh‐ERα group as compared with that in Scramble group (P < .05 or P < .01, Figure 1B‐D).
3.2. ERα had no influence on the proliferation of SCAPs
CCK‐8 assay and flow cytometry were performed to investigate whether ERα can exert an effect on the proliferation ability of SCAPs. CCK‐8 assay indicated that there was no significant difference (P > .05, Figure 2A) in cell growth between sh‐ERα group and Scramble group, while ERα group also presented no distinct difference (P > .05, Figure 2A) as compared with GFP group. FCM analyses further confirmed that no significant difference (P > .05) was detected between sh‐ERα group (23.16%, Figure 2B) and Scramble group (22.59%, Figure 2B), as indicated by the proliferation index (PI = G2M+S). Meanwhile, there was no significant difference in proliferation index (P > .05) between ERα group (10.78%) and GFP group (9.15%, Figure 2B). Therefore, it was concluded that ERα was of no influence on the proliferation capacity of SCAPs.
Figure 2.
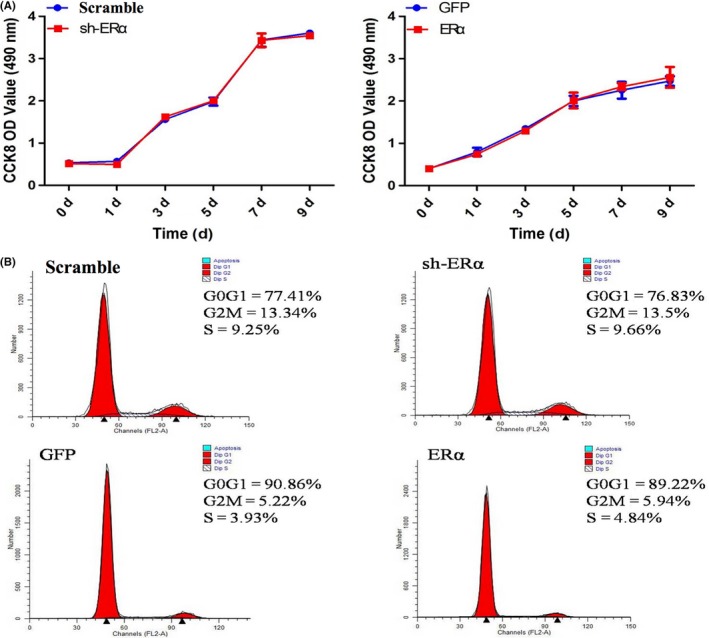
Effects of ERα on the proliferation of SCAPs. A, CCK‐8 assay for Scramble group, sh‐ERα group, GFP group and ERα group at day 0, 1, 3, 5, 7 and 9 respectively. B, Cell cycle phases in Scramble group, sh‐ERα group, GFP group and ERα group respectively
3.3. ERα enhanced ALP activity and mineralization capacity of SCAPs
After suppression of ERα in SCAPs, ALP activity was obviously down‐regulated as compared with that in Scramble group (P < .01, Figure 3A) at day 5. Meanwhile, SCAPs generated more mineralization nodules in Scramble group as compared with that in sh‐ERα group under osteogenic conditions (Figure 3B,C). CPC assay further demonstrated that the calcium concentration in Scramble group was significantly higher than that in sh‐ERα group in osteogenic media (P < .05, Figure 3D). On the contrary, both ALP activity and mineralized nodules remarkably increased in ERα group as compared with those in GFP group (P < .05, Figure 3A‐D) after overexpression of ERα in SCAPs.
Figure 3.
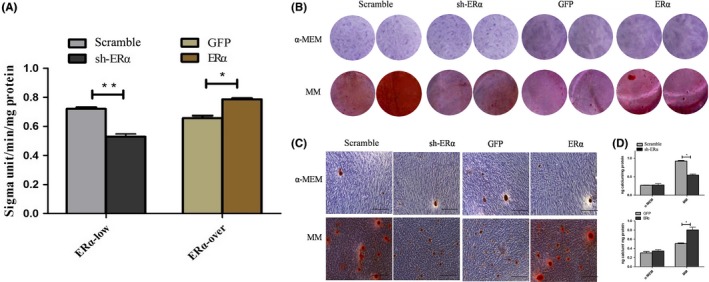
Effects of ERα on ALP and mineralization capacity of SCAPs. A, ALP activity of SCAPs in Scramble, sh‐ERα, GFP and ERα groups at day 5 respectively. *P < .05 or **P < .01. B, The formation of mineralized nodules in SCAPs in 4 groups at day 14. C, Mineralized nodules under the inverted microscope in 4 groups. Scale bars = 100 μm. D, Calcium contents in 4 groups by CPC assay. *P < .05
3.4. ERα promoted the odonto/osteogenic differentiation of SCAPs
After suppression of ERα in SCAPs, the mRNA levels of odonto/osteoblastic marker genes (DMP1, RUNX2, DSPP and OCN) were down‐regulated at day 3 or 7 (P < .05, Figure 4A), while the protein levels of DMP1, RUNX2, DSP and OCN also obviously decreased at day 3 or 7 (P < .05 or P < .01, Figure 4C,D). On the contrary, the mRNA expression of DMP1, RUNX2, DSPP and OCN was up‐regulated at day 3 or 7 (P < .05 or P < .01, Figure 4B), while protein levels of DMP1, RUNX2, DSP and OCN also increased at day 3 or 7 (P < .05 or P < .01, Figure 4E,F). Collectively, the above findings indicated that ERα can regulate the odonto/osteogenic differentiation of SCAPs.
Figure 4.
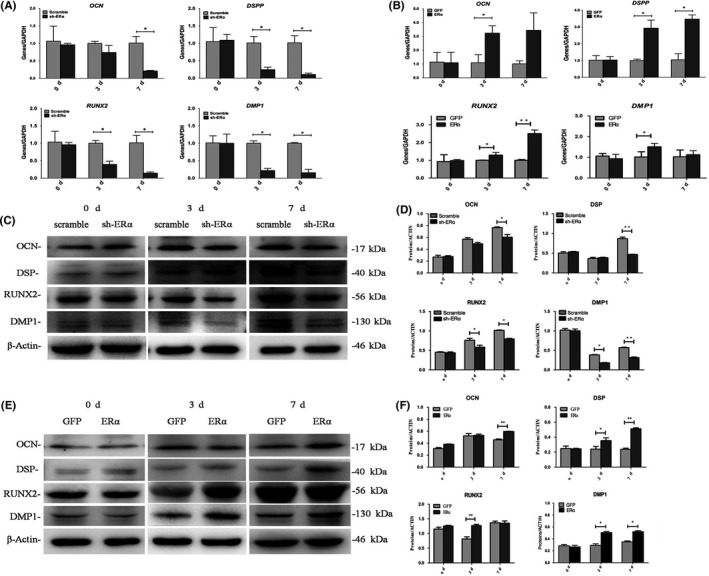
Effects of ERα on the odonto/osteogenic differentiation of SCAPs. A, Real‐time RT‐PCR analysis of DMP1, RUNX2, DSPP and OCN gene levels in Scramble and sh‐ERα groups at day 0, 3 and 7 respectively. *P < .05. B, Real‐time RT‐PCR analysis of DMP1, RUNX2, DSPP and OCN gene levels in GFP and ERα groups at day 0, 3 and 7 respectively. *P < .05 or **P < .01. C, The protein levels of OCN, DSP, RUNX2 and DMP1 in Scramble and sh‐ERα groups at day 0, 3 and 7 respectively. D, Grayscale analyses of (C). *P < .05 or **P < .01. E, The protein levels of OCN, DSP, RUNX2 and DMP1 in GFP and ERα groups at day 0, 3 and 7 respectively. F, Grayscale analyses of (E). *P < .05 or **P < .01
3.5. ERα activated ERK and JNK MAPK pathways of SCAPs
Protein levels of p38, p‐p38, ERK, p‐ERK, JNK and p‐JNK, as measured by western blot, are shown in Figure 5A. No change in p38 and p‐p38 expression was observed (Figure 5B). When ERα was repressed, the protein level of p‐ERK was significantly down‐regulated while the ERK expression was up‐regulated. The expression level of JNK was down‐regulated, while the p‐JNK expression decreased more. The ratios of p‐ERK/ERK and p‐JNK/JNK had statistically significant differences between Scramble and sh‐ERα groups (P < .05 or P < .01, Figure 5B). When ERα was overexpressed, the protein level of ERK was constant, while the p‐ERK expression was significantly up‐regulated. The expression level of JNK was down‐regulated while p‐JNK significantly increased. The ratios of p‐ERK/ERK and p‐JNK/JNK had statistically significant differences between GFP and ERα groups (P < .01, Figure 5B). It is suggested that ERα activated ERK and JNK MAPK pathways.
Figure 5.
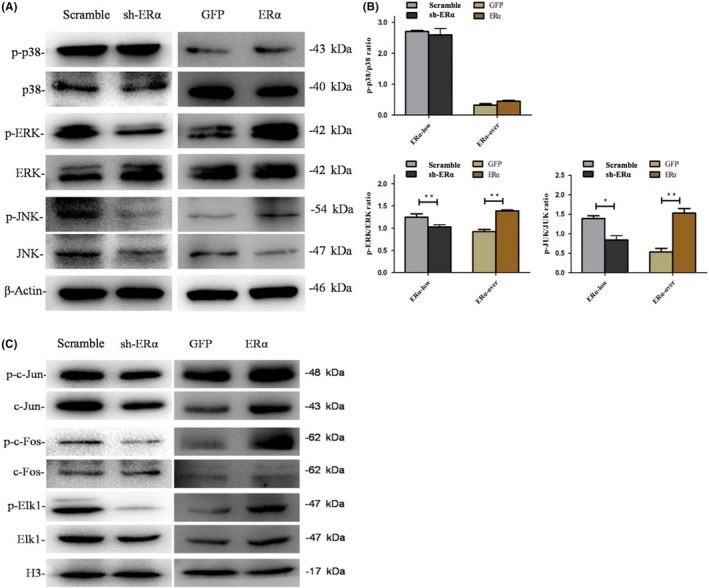
Effects of ERα on MAPK pathway and its downstream transcription factors of SCAPs. A, Protein levels of p38, p‐p38, ERK, p‐ERK, JNK and p‐JNK in the cytoplasm of transfected SCAPs in Scramble, sh‐ERα, GFP and ERα groups at 48 hours. β‐ACTIN served as an internal control. B, Quantitative analysis for the ratios of p‐p38/p38, p‐ERK/ERK and p‐JNK/JNK. *P < .05 or **P < .01. C, Protein levels of c‐Fos, p‐c‐Fos, c‐Jun, p‐c‐Jun, Elk1 and p‐Elk1 in the nuclei of transfected SCAPs in Scramble, sh‐ERα, GFP and ERα groups at 48 hours. H3 served as an internal control
To further explore the role of MAPK pathway in ERα‐mediated odonto/osteogenic differentiation of SCAPs, the downstream transcriptional factors of MAPK pathway were examined. The detected transcriptional factors expressed differently, as shown in Figure 5C. When ERα was repressed, the protein level of p‐c‐Jun was obviously decreased. The protein level of p‐c‐Fos was obviously down‐regulated while the c‐Fos expression had no change. p‐Elk1 expression level was reduced, while the protein level of Elk1 was unaltered. When ERα was overexpressed, the expression of p‐c‐Jun increased. The protein level of p‐c‐Fos was significantly up‐regulated, while the c‐Fos expression was not altered. The protein level of p‐Elk1 increased, while the Elk1 expression had no change. Our results demonstrated that ERα activated these transcriptional factors (c‐Jun, c‐Fos and Elk1).
4. DISCUSSION
Various research studies have investigated the effects of oestrogen on the osteogenesis and osteogenic differentiation of MSCs.24, 25 Oestrogen deficiency may result in the damage of periodontium and abnormal tooth development.26, 27 It is recently reported that the concentration of ER plays a decisive role in the cellular responses to oestrogen.28, 29 To date, little information is available concerning the effects of ERα on the committed differentiation of MSCs. The current study reveals for the first time that ERα can enhance the odonto/osteogenic differentiation capacity of SCAPs via ERK and JNK MAPK pathways.
In this study, ERα had no effect on the proliferation ability of SCAPs. Repression of ERα can decrease the number of mineralized nodules as well as the ALP activity of SCAPs. On the contrary, the formation of the mineralized nodules and the ALP activity of SCAPs were up‐regulated when ERα was overexpressed. ALP is an early marker during the osteoblast differentiation.23 It is an enzyme protein secreted by osteoblasts, which can raise the local concentration of inorganic phosphate and accelerate the precipitation of calcium by hydrolysis of multiple phosphate.21 Among the odonto/osteogenic markers concerned, DSP and DSPP are specific biomarkers of tooth, which are highly expressed in mature odontoblasts and play an important role in the formation and mineralization of dentin, growth and development of tooth germ.30, 31 DMP1 is a downstream factor of DSPP and regulated by it during the formation of dentin.32 RUNX2 is a vital nuclear transcription regulator of odonto/osteogenic differentiation.30 It has been reported that RUNX2‐knockout mice present the defective odontogenic ability while overexpression of RUNX2 can induce bone regeneration.33, 34 OCN is a small γ‐carboxyglutamate protein which is a marker of late phases of osteogenic differentiation.35 Our results showed that the expression of odonto/osteogenic markers was decreased after repression of ERα at both mRNA and protein levels while the expression of these markers was up‐regulated as ERα was overexpressed.
In terms of mechanisms, ERα has complex signalling pathways for different genes in different cell types in which the classic mechanism of ERα action is ligand‐dependent.14 This model states that in the presence of oestrogen, the binding of ligand induces high affinity within ERα, which can promote its binding to specific DNA response elements‐oestrogen receptor element (ERE) on the promoter region of target genes, thereby regulating the gene transcription level.18 Except the classic genomic way, ligand‐independent activation (cross‐talk with peptide growth factors) and ERE‐independent genomic actions of ER (combining with specific transcriptional factors) are also well documented.18 Additionally, ER possesses a nongenomic effect which can induce biological effects in several minutes or even seconds via signal transduction pathways.36, 37
Oestrogen membrane receptors have been reported to be involved in delivering mitogen‐activated protein kinase (MAPK)‐mediated phosphorylation, whereas the activator is likely to be trace of oestrogen in serum.14 MAPK is an important protein kinase in cells, which plays an important role in transmitting signals between the membrane and the nucleus and subsequently regulating cell proliferation, differentiation, survival, and apoptosis.38 MAPKs mainly include 3 parallel signalling pathways, ie extracellular signal‐regulated kinase (ERK), c‐Jun N‐terminal kinase (JNK) and p38.39 Blockage of the ERK pathway can inhibit the RUNX2‐mediated osteogenic differentiation,40 while JNK pathway is closely related to cell differentiation and apoptosis.41 Our data demonstrated that the changes of ERα expression brought about the corresponding changes in the phosphorylation level of ERK and JNK, indicating an activation of ERK and JNK MAPK pathways by ERα overexpression.
To further explore the effect of MAPK pathway in the odonto/osteogenic differentiation of SCAPs, the expression of downstream transcription factors of MAPK pathway was then investigated. Our results revealed that c‐Fos and c‐Jun were phosphorylated after overexpression of ERα. It is reported that ERK and JNK pathways are likely to be responsible for the stimulation of c‐Fos and c‐Jun, which can then form AP‐1 complex and thereby lead to activation of different target genes.42 The phosphorylation level of Elk‐1 increased after the activation of JNK in this study, suggesting that the Elk‐1 phosphorylation may correlate with the activation of JNK. Some studies have proved that ERα can directly interact with some nuclear transcription factors including AP‐1, forming a ERα/AP‐1 complex which can binds to the specific GC‐rich promoter sequences and then activates the expression of several osteogenic genes.14, 43 Moreover, ERα has many phosphorylation sites (Ser118), and phosphorylation state can control the responses to transcription events.44 Taken together, ERα carries out the functions in the differentiation process of SCAPs via the utilization of synergistic effects.
5. CONCLUSIONS
This work identifies an important role of ERα in regulating the odonto/osteogenic differentiation of SCAPs by activating ERK and JNK MAPK pathways as well as their downstream transcriptional factors. These findings not only contribute to a more comprehensive understanding of the mechanism of stem cells differentiation but also provide potential therapeutic strategies for the tissue regeneration.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Yanqiu Wang, Yadie Lu, Jinhua Yu: Study design. Yixiang Zhou, Yongchun Gu, Xiyao Pang, Jintao Wu, Zehan Li, Romila Gobin: Data collection. Yanqiu Wang, Yadie Lu: Manuscript preparation.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (81371144), Medical Talent Project of Jiangsu Province (ZDRCA2016086), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2014‐37), and Science and Technology Development Project of Jiangsu Province (BE2017731).
Wang Y, Lu Y, Li Z, et al. Oestrogen receptor α regulates the odonto/osteogenic differentiation of stem cells from apical papilla via ERK and JNK MAPK pathways. Cell Prolif. 2018;51:e12485 10.1111/cpr.12485
Yanqiu Wang, Yadie Lu, and Zehan Li contributed equally to this manuscript.
REFERENCES
- 1. Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. Pediatr Dent. 2013;35:129‐140. [PubMed] [Google Scholar]
- 2. Yang J, Yuan G, Chen Z. Pulp regeneration: current approaches and future challenges. Front Physiol. 2016;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu J, Huang GT, He W, et al. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod. 2012;38:614‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao L, Nasu M. From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning. 2014;7:89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell‐mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abe S, Yamaguchi S, Watanabe A, Hamada K, Amagasa T. Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun. 2008;371:90‐93. [DOI] [PubMed] [Google Scholar]
- 8. Huang GT. A paradigm shift in endodontic management of immature teeth: conservation of stem cells for regeneration. J Dent. 2008;36:379‐386. [DOI] [PubMed] [Google Scholar]
- 9. Huang GT. Apexification: the beginning of its end. Int Endod J. 2009;42:855‐866. [DOI] [PubMed] [Google Scholar]
- 10. Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol. 2000;2012(59):203‐227. [DOI] [PubMed] [Google Scholar]
- 11. Amrollahi P, Shah B, Seifi A, Tayebi L. Recent advancements in regenerative dentistry: a review. Mater Sci Eng C Mater Biol Appl. 2016;69:1383‐1390. [DOI] [PubMed] [Google Scholar]
- 12. Lu Y, Jin L, Lei G, Fu Y, Wang Y, Yu J. Estrogen‐mediated dental tissue regeneration. Histol Histopathol. 2016;31:1281‐1289. [DOI] [PubMed] [Google Scholar]
- 13. Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem Suppl 2001:36:144‐155. [DOI] [PubMed] [Google Scholar]
- 14. Saville B, Wormke M, Wang F, et al. Ligand‐, cell‐, and estrogen receptor subtype (alpha/beta)‐dependent activation at GC‐rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379‐5387. [DOI] [PubMed] [Google Scholar]
- 15. Bodine PV, Henderson RA, Green J, et al. Estrogen receptor‐alpha is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression. Endocrinology. 1998;139:2048‐2057. [DOI] [PubMed] [Google Scholar]
- 16. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor‐alpha (ERalpha) and estrogen receptor‐beta (ERbeta) messenger ribonucleic acid in the wild‐type and ERalpha‐knockout mouse. Endocrinology. 1997;138:4613‐4621. [DOI] [PubMed] [Google Scholar]
- 17. Sims NA, Dupont S, Krust A, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors‐beta in bone remodeling in females but not in males. Bone. 2002;30:18‐25. [DOI] [PubMed] [Google Scholar]
- 18. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869‐36872. [DOI] [PubMed] [Google Scholar]
- 19. Zhu Y, Shen J, Gao L, Feng Y. Estrogen promotes fat mass and obesity‐associated protein nuclear localization and enhances endometrial cancer cell proliferation via the mTOR signaling pathway. Oncol Rep. 2016;35:2391‐2397. [DOI] [PubMed] [Google Scholar]
- 20. Yin X, Wang X, Hu X, Chen Y, Zeng K, Zhang H. ERbeta induces the differentiation of cultured osteoblasts by both Wnt/beta‐catenin signaling pathway and estrogen signaling pathways. Exp Cell Res. 2015;335:107‐114. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Yan M, Yu Y, Wu J, Yu J, Fan Z. Estrogen deficiency inhibits the odonto/osteogenic differentiation of dental pulp stem cells via activation of the NF‐kappaB pathway. Cell Tissue Res. 2013;352:551‐559. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Yan M, Wang Z, et al. 17beta‐estradiol promotes the odonto/osteogenic differentiation of stem cells from apical papilla via mitogen‐activated protein kinase pathway. Stem Cell Res Ther. 2014;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halling Linder C, Ek‐Rylander B, Krumpel M, et al. Bone alkaline phosphatase and tartrate‐resistant acid phosphatase: potential co‐regulators of bone mineralization. Calcif Tissue Int. 2017;101:92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ejiri S, Tanaka M, Watanabe N, et al. Estrogen deficiency and its effect on the jaw bones. J Bone Miner Metab. 2008;26:409‐415. [DOI] [PubMed] [Google Scholar]
- 25. Qu Q, Perala‐Heape M, Kapanen A, et al. Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone. 1998;22:201‐209. [DOI] [PubMed] [Google Scholar]
- 26. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu T, Yan M, Wang Y, et al. Estrogen deficiency reduces the dentinogenic capacity of rat lower incisors. J Mol Histol. 2014;45:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Xin Q, Wang X, et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre‐ovulatory oocytes in mammals. Cell Death Dis. 2017;8:e2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mcrobb LS, Mcgrath KCY, Tsatralis T, et al. Estrogen receptor control of atherosclerotic calcification and smooth muscle cell osteogenic differentiation. Arterioscler Thromb Vasc Biol. 2017;37:1127‐1137. [DOI] [PubMed] [Google Scholar]
- 30. Chen S, Gluhak‐Heinrich J, Wang YH, et al. Runx2, osx, and dspp in tooth development. J Dent Res. 2009;88:904‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iejima D, Sumita Y, Kagami H, Ando Y, Ueda M. Odontoblast marker gene expression is enhanced by a CC‐chemokine family protein MIP‐3alpha in human mesenchymal stem cells. Arch Oral Biol. 2007;52:924‐931. [DOI] [PubMed] [Google Scholar]
- 32. Gibson MP, Zhu Q, Wang S, et al. The rescue of dentin matrix protein 1 (DMP1)‐deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis. J Biol Chem. 2013;288:7204‐7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi T. Overexpression of Runx2 and MKP‐1 stimulates transdifferentiation of 3T3‐L1 preadipocytes into bone‐forming osteoblasts in vitro. Calcif Tissue Int. 2011;88:336‐347. [DOI] [PubMed] [Google Scholar]
- 34. D'souza RN, Aberg T, Gaikwad J, et al. Cbfa1 is required for epithelial‐mesenchymal interactions regulating tooth development in mice. Development 1999:126 :2911‐:2920. [DOI] [PubMed] [Google Scholar]
- 35. Neugebauer BM, Moore MA, Broess M, Gerstenfeld LC, Hauschka PV. Characterization of structural sequences in the chicken osteocalcin gene: expression of osteocalcin by maturing osteoblasts and by hypertrophic chondrocytes in vitro. J Bone Miner Res. 1995;10:157‐163. [DOI] [PubMed] [Google Scholar]
- 36. Edwards DP, Boonyaratanakornkit V. Rapid extranuclear signaling by the estrogen receptor (ER): MNAR couples ER and Src to the MAP kinase signaling pathway. Mol Interv. 2003;3:12‐15. [DOI] [PubMed] [Google Scholar]
- 37. Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y, Lacasa D. Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein‐1, and cAMP response element binding protein in rat white adipocytes. Endocrinology. 2002;143:930‐940. [DOI] [PubMed] [Google Scholar]
- 38. Zhou C, Steplowski TA, Dickens HK, et al. Estrogen induction of telomerase activity through regulation of the mitogen‐activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS ONE. 2013;8:e55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schindeler A, Little DG. Ras‐MAPK signaling in osteogenic differentiation: friend or foe? J Bone Miner Res. 2006;21:1331‐1338. [DOI] [PubMed] [Google Scholar]
- 40. Zhang P, Wu Y, Jiang Z, Jiang L, Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2‐Runx2 signaling. Int J Mol Med. 2012;29:1083‐1089. [DOI] [PubMed] [Google Scholar]
- 41. Mu C, Lv T, Wang Z, et al. Mechanical stress stimulates the osteo/odontoblastic differentiation of human stem cells from apical papilla via erk 1/2 and JNK MAPK pathways. Biomed Res Int. 2014;2014:494378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Minden A, Lin A, Smeal T, et al. c‐Jun N‐terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen‐activated protein kinases. Mol Cell Biol. 1994;14:6683‐6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Babu RL, Naveen Kumar M, Patil RH, Devaraju KS, Ramesh GT, Sharma SC. Effect of estrogen and tamoxifen on the expression pattern of AP‐1 factors in MCF‐7 cells: role of c‐Jun, c‐Fos, and Fra‐1 in cell cycle regulation. Mol Cell Biochem. 2013;380:143‐151. [DOI] [PubMed] [Google Scholar]
- 44. Denton RR, Koszewski NJ, Notides AC. Estrogen receptor phosphorylation. Hormonal dependence and consequence on specific DNA binding. J Biol Chem. 1992;267:7263‐7268. [PubMed] [Google Scholar]


