Abstract
Background
Fibrosis involves the activation of inflammatory cells, leading to a decrease in physiological function of the affected organ or tissue.
Aims
To update and synthesize relevant information concerning fibrosis into a new hypothesis to explain the pathogenesis of fibrosis and propose potential novel therapeutic approaches.
Materials and Methods
Literature was reviewed and relevant information is discussed in the context of the pathogenesis of fibrosis.
Results
A number of cytokines and their mRNA are involved in the circulatory system and in organs of patients with fibrotic tissues. The profibrotic cytokines are generated by several activated immune cells, including fibroblasts and mast cells (MCs), which are important for tissue inflammatory responses to different types of injury. MC‐derived TNF, IL‐1, and IL‐33 contribute crucially to the initiation of a cascade of the host defence mechanism(s), leading to the fibrosis process. Inhibition of TNF and inflammatory cytokines may slow the progression of fibrosis and improve the pathological status of the affected subject. IL‐37 is generated by various types of immune cells and is an IL‐1 family member protein. IL‐37 is not a receptor antagonist; it binds IL‐18 receptor alpha (IL‐18Rα) and delivers the inhibitory signal by using TIR8. It has been shown that IL‐37 can be protective in inflammation and injury, and inhibits both innate and adaptive immunity.
Discussion
IL‐37 may be useful for suppression of inflammatory diseases induced by inhibiting MyD88‐dependent TLR signalling. In addition, IL‐37 downregulates NF‐κB induced by TLR2 or TLR4 through a mechanism dependent on IL‐18Rα.
Conclusion
This review summarizes current knowledge on the role of MC in inflammation and tissue/organ fibrosis, with a focus on the therapeutic potential of IL‐37‐targeting cytokines.
Keywords: cytokine, fibrosis, IL‐37, immunity, mast cells
1. INTRODUCTION
Tissue damage and inflammation can be caused by different acute and chronic stimuli, chemical, physical and/or biological. Among the biological damages that cause tissue dysregulation are: ischaemia, autoimmune diseases, infections and toxic biological substances including inflammatory cytokines, such as IL‐1, which is generated by infiltrated and resident immune cells in inflamed tissue. In fact, in 1986, we reported that IL‐1 represents a family of polypeptides with a wide range of biological activities and plays an important role in the pathogenesis of various infectious and inflammatory, neoplastic and immunological disorders.1, 2
Fibrosis is a dysregulation in collagen synthesis, with increased deposition of collagens, (primarily types I and III), and other extracellular matrix proteins in a number of organs, with the involvement of inflammatory cytokines, and dysfunction of the microvasculature and immunological parameters with a mechanism that is thought to be an overactive wound healing process.3
Fibrosis can affect nearly all tissues and organ systems, but often is most present in: inflammatory vessels, skin disease, interstitial lung disease, liver cirrhosis, kidney disease, heart disease, diseases of the eye, atherosclerosis and scleroderma.4
In fibrosis, the reparative process is characterized by a connective tissue that replaces normal parenchymal tissue and sometimes can cause morbidity. Approximately 45% mortality in the Western world is due to this disease.5 Fibrosis begins as a healing process but can subsequently be pathogenic as it remodels the tissue with a badly functioning scarring process.6 However, the exact mechanisms that cause tissue/organ fibrosis remain not completely understood.
The affected organ normally presents an inflammatory state mediated by immune cells such as macrophages, lymphocytes CD4+, eosinophils, plasma cells, CD8+ T cells, fibroblasts and mast cells (MCs).7 These cells, once activated, generate inflammatory products including proteolytic enzymes, growth factors and cytokines, which participate in the formation of connective tissue destroying the physiological one.7 Increased proteins and mRNAs for multiple profibrotic cytokines and chemokines have been revealed in several tissues/organs and the circulatory system.8
Type 2 immunity is mediated by MCs, eosinophils, basophils and T helper 2 cells, and by cytokines IL‐4, IL‐5, IL‐9, IL‐13, IL‐25 and IL‐33, while that of type 1 is characterized by macrophages, mast cells and neutrophils.7 Recently, it has been seen that these two immunities work and collaborate in a crossover way.9 In fibrosis, there is initially a rapid inflammatory response due to TH1 cytokines and subsequently a TH2 immune response which is established with production of IL‐13 and TGF‐β, promoting fibroblast proliferation and fibrinogenesis.9
In chronic damage, CD4+ lymphocytes activate and produce cytokines that stimulate macrophages and other inflammatory cells characteristic of the chronic reaction. The latter also produce cytokines that stimulate the lymphocytes, thus establishing an adaptive immune response and an inflammatory circuit.10
Degradation or necrosis products can be associated with “danger‐associated molecular patterns” or DAMPs that have receptors called “pattern recognition receptors” (PRRs). These receptors also include toll‐like receptors (TLRs) which play a crucial role in innate and adaptive immunity, and the activation of TLR2, TLR3 or TLR4 upregulates the production of inflammatory mediators in human diseases. The adaptive immune responses are regulated by STAT4, which is a major transcription factor that can be also detrimental in several immune disorders.11
Stimulation of TLR4 increases the production of intercellular adhesion molecule‐1, monocyte chemoattractant protein‐1 (MCP‐1) and inflammatory cytokines. Knockdown of myeloid differentiation factor 88 (MyD88) affects inflammatory mediator production following TLR4 stimulation.12
Organ/tissue fibrosis is also mediated by the cytokines TGF‐β1 and 2, which are the major proteins involved in this disease.13
Thus, fibrinogenesis is correlated with the CD4+ lymphocytes which, once activated, produce cytokines such as, IL‐4, IL‐5, IL‐13 and IFNγ.14 The activation of TH1 cells generates cytokines that activate Th2 cells to produce IL‐13 (the major profibrotic mediator) which causes the synthesis of collagen with fibrosis formation and extracellular matrix remodelling as part of an attempted reparative process after injury.15
Type 1 or type 2 immunity may be protective or may play a pathogenic role. Type 2 immunity with IL‐4 and IL‐13 production is directly involved in the regeneration and repair of tissues damaged by environmental insults.14, 15
During the inflammatory response and neovascularization, MCs, as well as monocyte/macrophages, are important for their expression of fibroblast growth factor (FGF) at both mRNA and protein levels, which contribute to cell proliferation, fibrosis and angiogenesis16 (Figure 1).
Figure 1.
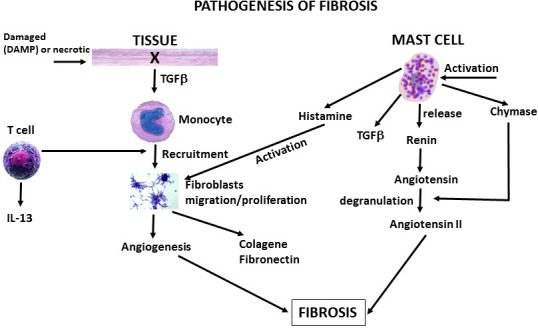
Mast cell‐mediated tissue fibrosis. This figure depicts a tissue which releases TGF‐β, activating monocyte which participate in angiogenesis and fibrosis. In addition, activated mast cells release chemical mediators, TGF‐β and angiotensin II, which also participate in fibrotic processes
Fibroblasts are also fundamental cells of the connective tissue, located in every tissue and organ and produce numerous substances such as fibronectin, proteoglycans, collagen and cytokines. Fibroblasts remodel extracellular molecules (ECM) by collaborating with IL‐13 lymphocytes, monocytes and MC products. These cells express different receptors including PDGF(R) and 5‐lipoxygenase(R) which produces chemoattractant and inflammatory leukotrienes.17
Mast cells participate in the fibrosis process by producing TGF‐β (which induces fibroblast proliferation and migration), IL‐33, IL‐1 mRNA, IL‐18 and other inflammatory cytokines, and also collagen.
2. MAST CELLS
Mast cells are of haematopoietic origin, characterized by prominent cytoplasmic granules, and derive from CD34+ haematopoietic stem cells.18 MCs are derived from circulating progenitors which migrate to tissues where they finally differentiate, mature and reside virtually in all vascularized tissues.18 They are abundant in the skin, in close proximity to blood vessels, lymphatics, nerves, secretory glands and mucosal body surfaces where, along with other immune cells, such as dendritic cells (DCs), macrophages, fibroblasts and lymphocytes, they play a role of “sentinels” against the environmental challenges.19, 20
Among the many receptors expressed by MCs, there is the important receptor for IgE, the FcεRI which when activated releases important chemical and lipid mediators of de novo synthesis and cytokines and chemokines induced by “alarmins”.21
“Alarmins” are factors induced by DAMPs and released from damaged cells. They have the ability to immediately recruit innate immune inflammatory cells such as monocytes/macrophages, dendritic cells and MCs.22 DAMPs are involved in inflammation by binding the receptor of chemokines on immune cells.
Mast cells mediate innate and adaptive immunity, and cause inflammation and tissue remodelling collaborating in fibrous tissues with fibroblasts.23
Innate immunity mediated by TLRs leads to the generation of inflammatory cytokines including IL‐1, IL‐18, IL‐33, IL‐36 among others.24
In tissue damage, increased IL‐1, IL‐33 and IL‐18 levels are directly correlated with increased acute inflammation modulated via TLR4 signalling.25
In chronic allergic disorders and MC studies in vitro, there is an involvement of mediation, development and activation of fibroblasts.26 MCs that physiologically perform a protective beneficial function can also contribute to the fibrotic process and tissue dysfunction and damage.27 MC numbers and their mediators, such as histamine, tryptase and chymase, are elevated in tissue and organs affected by fibrosis.28 This number increases along with the augmentation of the number of fibroblasts and their infiltration, tightly attached to fibroblasts, is associated with the degree of fibrosis which can occur in various diseases.29, 30
In fact, asthmatic patients may present fibrotic events with activation of fibroblasts due to the interaction with MCs that can release various growth factors (TGF‐β, SCF, NGF, VEGF, FGF) which participate in wound healing and promote cell growth of keratinocytes and collagen.31
In cirrhosis of the liver, fibrosis is characterized by accumulation of extracellular matrix rich in collagen I and III, accompanied by liver failure, hypertension and risk of neoplastic formation.32
In experimental models of fibrosis in rodents, it has been reported that MCs can release renin with generation of angiotensin which is degraded by MC chymase, leading to the formation of angiotensin II and then to the fibrosis process. In addition, the histamine released by the MCs also activates the fibroblasts by participating in tissue fibrosis.31
In turn, fibroblasts influence the development and activation of MCs and their FcεRI receptor, releasing IL‐3 and SCF which act by binding to the tyrosine kinase receptor c‐Kit (CD117), expressed by both precursors and mature MCs.33 In addition, the products released by activated fibroblast allow the survival of MCs and also cause the release of histamine and the chemokine eotaxin generation, which chemoattracts immune cells.31
From these observations, we can surely deduce that MCs and fibroblast cross‐talk influence each other in fibrotic diseases. Therefore, MCs play an important role in tissue remodelling and fibrosis.
3. MACROPHAGES
Activated macrophages are crucial in the pathogenesis of fibrosis, since they carry out phagocitic activity on tissue debris and dead cells. They also release cytokines/chemokines which recruit and activate the profibrotic Th2 cell‐derived IL‐13.7 In local milieu, macrophages are influenced by several factors and undergo phenotypic and metabolic changes, directly influencing the fibrogenesis process through the secretion of matrix metalloproteinase (MMP) and other factors.
Activation of macrophages secretes IL‐4 and IL‐13 which also provokes the release of MMP which may result in an increase in fibrotic activity. The inhibition of TNF and other proinflammatory cytokines/chemokines generated by macrophages may prevent fibrinogenesis.7
In fibrosis, TGF‐β, along with IL‐10, is released by macrophages which promote the differentiation of Treg cells, modulating dendritic cells and Th2 cells. In addition, activated fibroblasts generate immature TGF‐β which, through the integrin receptors and other factors, provokes the release of mature TGF‐β, a crucial factor for the pathogenic profibrotic activity and tissue repair.7
4. IL‐1
Inflammasome activation, which regulates the release of IL‐1, IL‐18 and IL‐33, together with IL‐17A is a central driver of fibrosis and can mediate this process with the involvement of TGF‐β and the rise of cytokines IL‐4 and IL‐13.34, 35 Inflammasome and inflammatory IL‐1 family members can act synergistically in the fibrotic process.
IL‐1 family members consist of seven agonists, IL‐1α, IL‐1β, IL‐18, IL‐33, IL‐36α, IL‐36β and IL‐36γ, three antagonists, IL‐1Ra, IL‐36Ra and IL‐38, and one anti‐inflammatory cytokine IL‐37. Inhibition of IL‐1 can attenuate inflammation and fibrosis by inhibiting other inflammatory and fibrogenic mediators.36, 37
IL‐1 is involved in the promotion of collagen synthesis and in the increase in expression of transforming growth factor (TGF‐β), which is an important profibrotic growth factor in fibrinogenesis.38, 39 Cytokines, including TGF‐β, may stimulate fibroblast proliferation and collagen deposition which are important in tissue remodelling. Furthermore, TNF, IL‐1 and IL‐33 also augment fibroblast proliferation and collagen synthesis leading to tissue fibrosis.38 In addition, TGF‐β, which is increased by angiotensin II, inhibits matrix metalloproteinase (MMP‐1)‐induced degradation of collagen.40
Mast cells secrete high levels of TGF‐β, TNF and IL‐6, and low levels of IL‐1; on the other hand, they can be activated with several cytokines including IL‐6, SCF, TNF, IL‐33 and IL‐1.41
The profibrotic cytokines, such as IL‐1, TNF, IL‐33 and others, increase fibroblast proliferation leading to greater collagen synthesis and then fibrosis.4
Inflammatory IL‐1 family members play an important role in the innate response to infections and in acute and chronic inflammation and are profibrotic cytokines. The abnormal expression of IL‐1 that promotes fibroblast proliferation, and the generation of IL‐33 can contribute to fibrosis, whereas IL‐6 stimulated by IL‐1 and PDGFα could be involved in procollagen type I production. IL‐33 also increases collagen VI, III and MMP‐1.42
Mast cells not only produce TNF at the transcriptional and translational level, but are the only known cells able to prestore TNF in their granules, which can be released in seconds after activation.43
5. TNF AND FIBROSIS
The exposure of the organism to the pathological damaging insults can induce a pathological response with acute inflammation of the organ and accumulation of inflammatory cells, including MCs, with TNF release.44 It is well known that TNF is linked to a number of inflammatory diseases inducing recruitment of immune cells, inflammation, hyper‐responsiveness and tissue remodelling. TNF generated by macrophages and MCs, along with proinflammatory IL‐1 family members are the crucial modulators of inflammation that initiate and drive many pathological disorders.45 TNF can provoke differentiation at sites of injury, necrosis, apoptosis, oxidative stress, tissue remodelling, cell proliferation, cachexia, cytotoxicity, angiogenesis and it is involved in thickening of the airway walls.
TNF is generated by its precursor transmembrane TNF (26 kDa), which is cleaved by a converting enzyme in mature TNF (17 kDa) biologically active soluble form.46
TNF has been linked to a number of diseases including interstitial tissue fibrosis and acute and chronic inflammation.43, 47 In addition, TNF upregulates the expression of adhesion molecules, and inflammatory cytokines and increases the generation of MMP‐1, promoting tissue damage, and remodelling via NFκB transcription activation.48
Tissue fibrosis is characterized by a decline in organ function with increased levels of TNF, suggesting a key role of this cytokine in fibrinogenesis.
Lack of TNFRI, II and III receptors, can have a protective effect and attenuate histopathological alterations in organ fibrosis.49
Inhibiting MC‐derived TNF and histamine, which may contribute to fibrin formation by increasing vascular permeability and downregulating cytokine/chemokine generation, may have a therapeutic effect, demonstrating an important role of TNF and other cytokines in fibrinogenesis induced in the organism by external environmental insults.50
The administration of anti‐inflammatory cytokines or anti‐mast cell chemical mediators may have an anti‐fibrotic therapeutic effect, by restoring the functionality of the tissues and/or organs.51
Therefore, the inhibition of inflammatory IL‐1 family members could ameliorate a number of inflammatory diseases including tissue fibrosis.
6. IL‐33/ST2 AND FIBROSIS
IL‐33 is located in humans on chromosome 9, and the size of full‐length protein is about 30 kDa, which translocates to the nucleus where it has intracrine gene regulatory functions.52
IL‐33 is produced by epithelial cells, fibroblasts, endothelial cells, macrophages, mast cells and others. It responds to microbial, allergic and inflammatory stimuli, even if it is not clear how it is secreted by the cells. This inflammatory cytokine has also been defined as an “alarmin” signal in response to external stimulus. The IL‐33 full‐length protein is split by caspase‐1 into mature forms that bind to its ST2 receptor, previously known as an orphan receptor of the IL‐1 receptor family, and activates MyD88.
IL‐33 is mainly an important initiator and maintainer of type 2‐associated mucosal inflammation and immunity.53
IL‐33 exerts its biological effects on many tissues and cells, including MCs, and plays an important role in adaptive immunity and allergy. It is important in the physiological remodelling of tissues, in cell hyperplasia and in the production of other cytokines such as IL‐5 and IL‐13. In addition, IL‐33 stimulates the production of IL‐6, TNF and IL‐1 in APC cells, while on TH2 lymphocytes it stimulates the generation of TH2 cytokines.
IL‐33 induces chemokine CCL11 (eotaxin), IL‐8 and MCP‐1, and the cytokine IL‐6 in human fibroblasts and increases IL‐6 and TNF in keratinocytes.54
IL‐33 is elevated in the serum level of patients affected by organ fibrosis and can be a serum marker for vascular dysfunctions, although sometimes, for unknown reasons, IL‐33 in fibrosis is not high. IL‐33 is involved in collagen generation and mast cell recruitment and activation, as well as in induction of IL‐4, IL‐5 and IL‐13. IL‐33 and its receptor ST2 are upregulated in myofibroblasts where it stimulates TGF‐β1, IL‐1 and TNF. It has been reported that IL‐33 polarizes M2 macrophages and upregulates the transcription of TGF‐β1.42 Tissue fibrosis is associated with the biological inflammatory effects of IL‐33 produced by macrophages and CD8+ T cells as well as by MCs present in the tissue. Activation of MCs leads to the generation and release of IL‐33 that is related to collagen synthesis, and mice that are genetically free of IL‐33 (IL‐33−/−) are largely protected from experimentally induced fibrosis. This is because IL‐33 may induce IL‐13, IL‐6 and TGF‐β.32 Therefore, high serum levels of ST2 or IL‐33 are considered a fibrinogenic prognostic factor. In addition, activated MCs release chemical mediators and cytokines, which provoke VEGF generation, stimulate vascular cells, and participate in angiogenesis.55
IL‐33 is a fundamental cytokine in allergic and inflammatory diseases and it is a profibrotic protein. Targeting IL‐33 is important to ameliorate progressive type 2‐driven diseases, including fibrosis of tissues and organs.
7. IL‐37 INHIBITS FIBROSIS
IL‐37 (or ILF7), which is located in the cell nucleus, is present in inflammatory processes and is an inhibitor of the inflammatory response.52, 56 IL‐37 is made of 5 proteins, of which IL‐37b is the most important and most studied.57 The inhibition of IL‐37 leads to an increase in stimulation of macrophage cells in vitro to produce more inflammatory cytokines. This cytokine, which uses IL‐18 receptor alpha (IL‐18Rα), does not act as an anti‐receptor, but as an endocellular inhibitor acting on TIR8.
IL‐37 downregulates and silences inflammatory cytokines in monocytes and other cells.58 IL‐37 transgenic mice are protected against LPS treatment, present less inflammation and are more resistant to infection, cardiovascular and liver diseases.58 This is due to its effect on the inhibition of inflammatory cytokines and chemokines, along with the stimulation of IL‐10 which is also an anti‐inflammatory cytokine. IL‐37 is not only an inhibitor of innate immunity but also inhibits adaptive immunity, since it downregulates antigen presenting cells (APC) and the activation of regulatory T cells (Tregs). The inhibitory effect of IL‐37 is due to its suppression of MyD88‐mediated inflammatory responses and, consequently, to the inhibition of NF‐κB induced through TLR2 or TLR4, a mechanism dependent of IL‐18Rα.59 Therefore, IL‐37 may be useful for suppression of inflammatory diseases induced by MyD88‐dependent TLR signalling7 (Figure 2).
Figure 2.
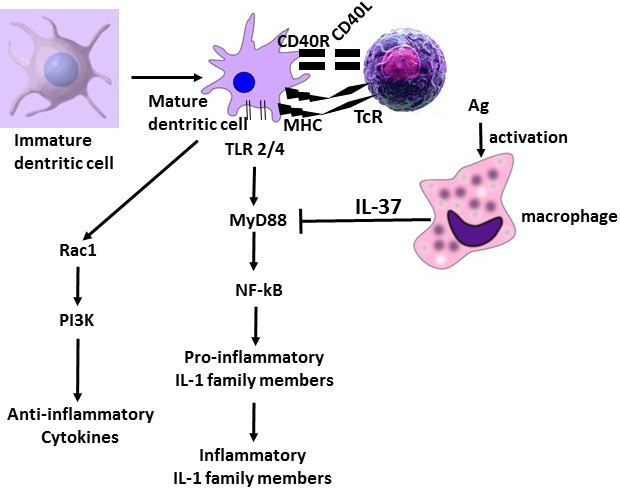
Production of anti‐inflammatory and proinflammatory cytokines. Macrophage activation releases IL‐37 which inhibits MyD88. Dendritic cells activated after maturation release proinflammatory and anti‐inflammatory cytokines. Proinflammatory IL‐1 family members are inhibited by IL‐37 generated by activated macrophage
In the inflammatory response, macrophages generate IL‐1 which activates mast cells to produce inflammatory IL‐6 and TNF in the site.60 IL‐37 may play a role in this activation and in certain infections and inflammatory disorders by inhibiting the production of proinflammatory cytokines, including IL‐1 MC release and TNF.61 IL‐37 protects against septic shock and colitis and suppresses inflammatory responses to Toll‐like receptor (TLR) agonists.56 In addition, IL‐37 inhibits the production of inflammatory cytokine IL‐1 family members induced by TLR4. In IL‐37 knockdown mice there is an increase in IL‐1 produced by TLR4.56
Therefore, inflammatory cytokines are suppressed by IL‐37 by inhibiting MyD88, which is a TLR2‐dependent factor, and reduce inflammatory mediator production following stimulation of TLR2 and TLR4.7
Targeting proinflammatory IL‐1 family members in innate immunity, IL‐37 may be useful in anti‐inflammatory therapy since rodents treated with this cytokine express lower recurrent inflammatory events.
Recently, it was found that subjects with lesions and alcoholic hepatic inflammation have a reduced expression of IL‐37. Exogenous administration of recombinant IL‐37 improves hepatic inflammation.62 This shows that certain compounds, as in the case of alcohol, can endogenously suppress IL‐37, making patients more vulnerable to inflammatory diseases and their consequences. In addition, the exogenous therapeutic administration of IL‐37 may help in the resolution of inflammatory disease.
Overexpression of IL‐37 may lead to a reduction in the incidence and severity of collagen‐induced arthritis, whereas lacking IL‐37 may instigate an earlier onset of collagen‐induced arthritis. Administration of IL‐37 may prevent tissue damage and an increase in collagen deposition.
IL‐37 can be used as a therapeutic cytokine to treat patients with innate immune diseases, such as Crohn's disease, rheumatoid arthritis, atherosclerosis and psoriasis, even if its toxicity is still unknown.
It has also been reported that IL‐1 and its receptor are paracrine mediators of fibroblast growth in fibrosis that can be experimentally inhibited by treating mice with IL‐1 receptor antagonist (IL‐1RA).63, 64
IL‐37 is mostly expressed in various immune cells and tissue upon stimulation by IL‐1β or TLR. Cytokines and other inflammatory stimulii activate the production of IL‐37 to protect and prevent the inflammatory responses and tissue damage. Recently, IL‐37‐deficient mice have been generated for the study of cytokines.36
Today, transgenic mice expressing the human IL‐37 gene (IL‐37‐tg) have been generated, and while these mice are protected from various proinflammatory stimulii, their treatment with exogenous recombinant IL‐37 can induce immunosuppression with the related pathological consequences.36 However, the role of IL‐37 in fibrosis has not yet been studied, and this is the first paper on this topic.
8. CONCLUSION
Here, in the light of the latest findings, we report the cross‐talk between mast cells and the cytokine/chemokine network in tissue fibrosis, offering opportunities for the design of new therapeutic interventions.
However, the role of IL‐37 in diseases, including fibrosis, as well as its toxicity, is still unknown and for these reasons more research is needed before its entry into therapeutic trials.
CONFLICT OF INTEREST
The authors report no conflicts of interest relevant to this article. The authors alone are responsible for the content and writing of the article.
Conti P, Caraffa AL, Mastrangelo F, et al. Critical role of inflammatory mast cell in fibrosis: Potential therapeutic effect of IL‐37. Cell Prolif. 2018;51:e12475 10.1111/cpr.12475
REFERENCES
- 1. Dinarello CA, Conti P, Mier JW. Effects of human interleukin‐1 on natural killer cell activity: is fever a host defense mechanism for tumor killing? Yale J Biol Med. 1986;59:97‐106. [PMC free article] [PubMed] [Google Scholar]
- 2. Robuffo I, Toniato E, Tettamanti L, et al. Mast cell in innate immunity mediated by proinflammatory and antiinflammatory IL‐1 family members. J Biol Regul Homeost Agents. 2017;31:837‐842. [PubMed] [Google Scholar]
- 3. Iannitti RG, Napolioni V, Oikonomou V, et al. IL‐1 receptor antagonist ameliorates inflammasome‐dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7:10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Artlett CM. The IL‐1 family of cytokines. Do they have a role in scleroderma fibrosis? Immunol Lett. 2017;195:30‐37. 10.1016/j.imlet.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 5. Mack M. Inflammation and fibrosis. Matrix Biol. 2017; Nov 28. pii: S0945‐053X(17)30375‐X. doi: 10.1016/j.matbio.2017.11.010. [Epub ahead of print] Review. 10.1016/j.matbio.2017.11.010. [DOI] [Google Scholar]
- 6. Ho CY, López B, Coelho‐Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552‐563. 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62‐76. [DOI] [PubMed] [Google Scholar]
- 8. Hügle T. Beyond allergy: the role of mast cells in fibrosis. Swiss Med Wkly. 2014;144:w13999. [DOI] [PubMed] [Google Scholar]
- 9. Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276‐281. [DOI] [PubMed] [Google Scholar]
- 10. Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti‐cytokine therapy ‐ novel treatments for asthma? Br J Pharmacol. 2011;163:81‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkhordarian A, Thames AD, Du AM, et al. Viral immune surveillance: toward a TH17/TH9 gate to the central nervous system. Bioinformation. 2015;11:47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhan Q, Zeng Q, Song R, et al. IL‐37 suppresses MyD88‐mediated inflammatory responses in human aortic valve interstitial cells. Mol Med. 2017. Mar 27;23. doi: 10.2119/molmed.2017.00022 . [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viera MH, Vivas AC, Berman B. Update on Keloid management: clinical and basic science advances. Adv Wound Care (New Rochelle). 2012;1:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrell AM, Antrobus P, Simpson D, Powell S, Chapel HM, Ferry BL. A rapid flow cytometric assay to detect CD4+ and CD8+ T‐helper (Th) 0, Th1 and Th2 cells in whole blood and its application to study cytokine levels in atopic dermatitis before and after cyclosporin therapy. Br J Dermatol. 2001;144:24‐33. [DOI] [PubMed] [Google Scholar]
- 15. Wei L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation‐mediated cardiac fibrosis. Exp Mol Pathol. 2011;90:74‐78. [DOI] [PubMed] [Google Scholar]
- 16. Qu Z, Liebler JM, Powers MR, et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564‐573. [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenkranz S. TGF‐beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423‐432. [DOI] [PubMed] [Google Scholar]
- 18. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257‐265. [DOI] [PubMed] [Google Scholar]
- 19. Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38:581‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toniato E, Frydas I, Robuffo I, et al. Activation and inhibition of adaptive immune response mediated by mast cells. J Biol Regul Homeost Agents. 2017;31:543‐548. Review. [PubMed] [Google Scholar]
- 21. Aoki R, Kawamura T, Goshima F, et al. The alarmin IL‐33 derived from HSV‐2‐infected keratinocytes triggers mast cell‐mediated antiviral innate immunity. J Invest Dermatol. 2016;136:1290‐1292. [DOI] [PubMed] [Google Scholar]
- 22. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:1885‐1886. [DOI] [PubMed] [Google Scholar]
- 23. Sismanopoulos N, Delivanis DA, Mavrommati D, Hatziagelaki E, Conti P, Theoharides TC. Do mast cells link obesity and asthma? Allergy. 2013;68:8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Presta I, Guadagno E, Di Vito A, et al. Innate immunity may play a role in growth and relapse of chordoid meningioma. Int J Immunopathol Pharmacol. 2017;30:429‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conti P, Caraffa AI, Kritas SK, et al. Mast cell, pro‐inflammatory and anti‐inflammatory: Jekyll and Hyde, the story continues. J Biol Regul Homeost Agents. 2017;31:263‐267. [PubMed] [Google Scholar]
- 26. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140:335‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167‐255. [DOI] [PubMed] [Google Scholar]
- 28. Atamas SP, White B. (Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537‐550. [DOI] [PubMed] [Google Scholar]
- 29. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513‐521. [PubMed] [Google Scholar]
- 30. Gerges Geagea A, Rizzo M, Eid A, et al. Tea catechins induce crosstalk between signaling pathways and stabilize mast cells in ulcerative colitis. J Biol Regul Homeost Agents. 2017;31:865‐877. [PubMed] [Google Scholar]
- 31. Landolina N, Gangwar RS, Levi‐Schaffer F. Mast cells' integrated actions with eosinophils and fibroblasts in allergic inflammation: implications for therapy. Adv Immunol. 2015;125:41‐85. [DOI] [PubMed] [Google Scholar]
- 32. Sun Z, Chang B, Gao M, Zhang J, Zou Z. IL‐33‐ST2 Axis in liver disease: progression and challenge. Mediators Inflamm. 2017;2017:5314213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gangwar RS, Landolina N, Arpinati L, Levi‐Schaffer F. Mast cell and eosinophil surface receptors as targets for anti‐allergic therapy. Pharmacol Ther. 2017;170:37‐63. [DOI] [PubMed] [Google Scholar]
- 34. Lorenz G, Darisipudi MN, Anders HJ. Canonical and non‐canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2014;29:41‐48. [DOI] [PubMed] [Google Scholar]
- 35. Conti C, Caraffa A, Kritas SK, Ronconi G, Fulcheri M. Alexithymia and its relationships with inflammatory response mediated by IL‐1 family members. J Biol Regul Homeost Agents. 2017;31:21‐28. [PubMed] [Google Scholar]
- 36. Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome‐independent regulation of IL‐1‐family cytokines. Annu Rev Immunol. 2015;33:49‐77. [DOI] [PubMed] [Google Scholar]
- 37. Lin Q, Li Y, Zhang D, Jin H. Levels of circulating soluble receptor activator of NF‐κB and interleukins‐1 predicting outcome of locally advanced basal cell carcinoma. Int J Immunopathol Pharmacol. 2016;29:784‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lonnemann G, Shapiro L, Engler‐Blum G, Müller GA, Koch KM, Dinarello CA. Cytokines in human renal interstitial fibrosis. I. Interleukin‐1 is a paracrine growth factor for cultured fibrosis‐derived kidney fibroblasts. Kidney Int. 1995;47:837‐844. [DOI] [PubMed] [Google Scholar]
- 39. Rajan TS, Diomede F, Bramanti P, Trubiani O, Mazzon E. Conditioned medium from human gingival mesenchymal stem cells protects motor‐neuron‐like NSC‐34 cells against scratch‐injury‐induced cell death. Int J Immunopathol Pharmacol. 2017;30:383‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Wu J, Dong H, Khan SA, Chu ML, Tsuda T. Fibulin‐2 deficiency attenuates angiotensin II‐induced cardiac hypertrophy by reducing transforming growth factor‐β signalling. Clin Sci (Lond). 2014;126:275‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. Targeting IL‐33 in autoimmunity and inflammation. J Pharmacol Exp Ther. 2015;354:24‐31. [DOI] [PubMed] [Google Scholar]
- 42. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF‐alpha. Nature. 1996;381:77‐80. [DOI] [PubMed] [Google Scholar]
- 44. Theoharides TC, Stewart JM, Tsilioni I. Tolerability and benefit of a tetramethoxyluteolin‐containing skin lotion. Int J Immunopathol Pharmacol. 2017;30:146‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Xu Y, Zhang Y, Si Y, Jing L, Bao H. The effect of adiponectin on LPS‐induced inflammation via autophagy in RAW 264.7 macrophages. Eur J Inflamm. 2017;15:160‐167. [Google Scholar]
- 46. Taracanova A, Alevizos M, Karagkouni A, et al. SP and IL‐33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci U S A. 2017;114:E4002‐E4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang S, Xie H, Wang Y, et al. Enriched environment improves behavioral performance and attenuates inflammatory response induced by TNF‐α in healthy adult mice. Eur J Inflamm. 2017;15:200‐209. [Google Scholar]
- 48. Cortez DM, Feldman MD, Mummidi S, et al. IL‐17 stimulates MMP‐1 expression in primary human cardiac fibroblasts via p38 MAPK‐ and ERK1/2‐dependent C/EBP‐beta, NF‐kappaB, and AP‐1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356‐H3365. [DOI] [PubMed] [Google Scholar]
- 49. Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll‐like receptor 2‐mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330‐1340. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pérez L, Muñoz‐Durango N, Riedel CA, et al. Endothelial‐to‐mesenchymal transition: cytokine‐mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017;33:41‐54. [DOI] [PubMed] [Google Scholar]
- 51. Aquili A, Farinelli L, Bottegoni C, Antonicelli L, Gigante A. The effect of anti‐IgE therapy in knee osteoarthritis: a pilot observational study. J Biol Regul Homeost Agents. 2017;31(4 S1):1‐5. [PubMed] [Google Scholar]
- 52. Garlanda C, Dinarello CA, Mantovani A. The interleukin‐1 family: back to the future. Immunity. 2013;39:1003‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Theoharides TC, Zhang B, Kempuraj D, et al. IL‐33 augments substance P‐induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448‐4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borthwick LA. The IL‐1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol. 2016;38:517‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boesiger J, Tsai M, Maurer M, et al. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E‐dependent upregulation of fc epsilon receptor I expression. J Exp Med. 1998;188:1135‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dinarello CA, Bufler P. Interleukin‐37. Semin Immunol. 2013;25:466‐468. [DOI] [PubMed] [Google Scholar]
- 57. Conti P, Ronconi G, Caraffa A, Lessiani G, Duraisamy K. IL‐37 a New IL‐1 family member emerges as a key suppressor of asthma mediated by mast cells. Immunol Invest. 2017;46:239‐250. [DOI] [PubMed] [Google Scholar]
- 58. Nold MF, Nold‐Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL‐37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luo Y, Cai X, Liu S, et al. Suppression of antigen‐specific adaptive immunity by IL‐37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci U S A. 2014;111:15178‐15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kandere‐Grzybowska K, Letourneau R, Kempuraj D, et al. IL‐1 induces vesicular secretion of IL‐6 without degranulation from human mast cells. J Immunol. 2003;171:4830‐4836. [DOI] [PubMed] [Google Scholar]
- 61. Huang Z, Gao C, Chi X, et al. IL‐37 Expression is upregulated in patients with tuberculosis and induces macrophages towards an M2‐like phenotype. Scand J Immunol. 2015;82:370‐379. [DOI] [PubMed] [Google Scholar]
- 62. Grabherr F, Grander C, Adolph TE, et al. Ethanol‐mediated suppression of IL‐37 licenses alcoholic liver disease. Liver Int. HYPERLINK \l “Liver Int. 2017. Nov 29. doi: 10.1111/liv.13642. [Epub ahead of print]. 10.1111/liv.13642. [DOI] [PubMed] [Google Scholar]
- 63. Nakagawa S. Identification of biomarkers for tubular injury and interstitial fibrosis in chronic kidney disease. Yakugaku Zasshi. 2017;137:1355‐1360. Japanese. [DOI] [PubMed] [Google Scholar]
- 64. Marinari S, De Iuliis V, Dadorante V, et al. Cytokine modulation in patients with idiopathic pulmonary fibrosis undergoing treatment with steroids, immunosuppressants, and IFN‐γ 1b. J Biol Regul Homeost Agents. 2017;31:59‐69. [PubMed] [Google Scholar]


