Abstract
Objectives
Despite improvements in diagnosis and treatment, preeclampsia (PE) continues to pose a significant risk of maternal and foetal morbidity and mortality if not addressed promptly. An increasing number of studies have suggested that tissue factor pathway inhibitor 2 (TFPI2) acts as a suppressor gene, possibly inhibiting multiple serine proteases affecting cell proliferation and migration. It plays an essential role in the occurrence and development of PE, but the pathogenesis remains unclear.
Materials and methods
In our research, we performed western blotting, immunohistochemistry and qPCR assays to investigate TFPI2 and miR‐616‐3p expression in preeclamptic placental tissues. Cell assays were performed in HTR‐8/SVneo and JEG3 cell lines. Cell proliferation and migration events were investigated by MTT, EdU and transwell assays. In conjunction with bioinformatics analysis, luciferase reporter assays were performed to elucidate the mechanism by which miR‐616‐3p binds to TFPI2 mRNA.
Results
We established that TFPI2 protein levels were significantly upregulated in PE placental tissues. In addition, we found that miR‐616‐3p binds specifically to the 3′‐UTR region of TFPI2 mRNA. Furthermore, miR‐616‐3p knockdown or TFPI2 overexpression substantially impaired cell growth and migration, whereas miR‐616‐3p upregulation or TFPI2 knockdown stimulated cell proliferation and migration. This miR‐616‐3p/TFPI2 axis was also found to affect the epithelial‐mesenchymal transition process in PE.
Conclusions
Our results demonstrated that TFPI2 plays a vital role in the progression of PE and might provide a prospective therapeutic strategy to mitigate the severity of the disorder.
Abbreviations
- miRNA
microRNA
- PE
preeclampsia
- TFPI2
tissue factor pathway inhibitor 2
1. INTRODUCTION
Preeclampsia (PE) is a pregnancy‐specific syndrome characterized by gestational kidney disease involving glomerular endothelial injury. It induces severe clinical maternal hypertension and proteinuria.1 It has been reported that PE affects 2%‐8% of pregnancies worldwide.2 When severe, uncontrolled symptoms of the disorder can result in significant morbidity and mortality of both mother and foetus. As would be expected, the disease induces a series of typical complications, including refractory hypertension, kidney damage and acute pulmonary oedema.3 Currently, preventative measures for the disorder include aspirin therapy, low‐intake calcium supplementation and treatment of prior hypertension with medication.2, 4, 5. Despite these efforts, delivery of the baby and placenta remains the most effective treatment for patients with PE.6, 7 Though recent developments have improved its treatment, the disease mechanism still remains unclear. It has been reported that tissue factor pathway inhibitor 2 (TFPI2) may play a role in numerous biological behaviours, such as cell proliferation, differentiation and apoptosis.8, 9, 10 As a suppressor gene, TFPI2 is known to be dysregulated in multiple human disorders, including PE11 and various cancers.8, 10 Although the expression levels of TFPI2 have been characterized, the potential mechanisms of upregulated TFPI2 expression have not yet been explored, especially in severe cases of PE.
MicroRNAs (miRNAs) are a class of small and endogenous RNAs of 21‐25 nucleotides (nts) in length.12, 13 A single miRNA can target multiple genes and several miRNAs can regulate the same gene. This complex network regulates the expression of multiple genes through a single miRNA. Several combinations of miRNAs can also serve to modulate the expression of a gene.14 MiRNAs are transcribed by RNA polymerase II as part of capped and polyadenylated primary transcripts (pri‐miRNAs).15 It may play particularly important roles in restraining the main function of target gene by directly interacting with its mRNA 3ʹ‐untranslated region (3ʹ‐UTR), with transcriptional degradation/translational repression. Moreover, several mRNAs may have multiple binding sites for different miRNAs, resulting in a complex regulatory network.16 Altogether, miRNAs are known to be involved in many pathological and physiological processes, such as cellular growth, organogenesis, invasion, migration and apoptosis.17 The accumulated evidence suggests that the analysis of miRNAs will prove to be useful in studying a variety of diseases, ranging from diabetes18 to tumourgenesis.19, 20 Also, the study of miRNAs has expedited technological advancements in RNA‐based treatments. MiRNAs are now being studied for their potential as next‐generation drugs. A study of the miRNA expression profile in placentas of PE patients explored many PE‐associated, differentially expressed miRNAs.21 To date, several studies have also revealed that miRNAs play a specific role in placental development and that more miRNAs could be candidates for biomarkers in early screening of PE.22, 23, 24
In this report, we established that the level of TFPI2 was significantly upregulated in PE placental tissues compared with that in normal tissues. Furthermore, knockdown of TFPI2 promoted cell growth and migration in trophoblast cell lines. Mechanistic investigation revealed that miR‐616‐3p binds to the 3′‐UTR region of wild‐type TFPI2 mRNA in cytoplasm. Therefore, our findings show an miR‐616‐3p‐TFPI2‐associated mechanism in PE, thus providing novel insights for the development of clinical predictive analytics, molecular diagnostics and targeted therapy for future PE treatments.
2. MATERIALS AND METHODS
2.1. Tissue collect and Ethics statement
Study participants were PE patients from the obstetrical department of the First Affiliated Hospital of Nanjing Medical University. Written informed consent was provided by all participants. This study was approved by the Ethics Board of the First Affiliated Hospital of Nanjing Medical University, China, and it was performed in compliance with the Declaration of Helsinki Principles.
2.2. Cell line and culture
HTR‐8/SVneo cells were kindly supplied by Dr. Charles Graham, Queen's University, Canada and the JEG3 cell line was purchased from the Institute of the Chinese Academy of Sciences (Shanghai, China). HTR‐8/SVneo and JEG3 were cultured as previously reported in Xu et al25 HTR‐8/SVneo and JEG3 were maintained at 37°C with 5% CO2 in RPMI 1640 (GIBCO, Nanjing, China) and MEM (GIBCO, Nanjing, China), respectively, to which we added 10% FBS (GIBCO, Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Invitrogen).
2.3. Cell transfection
HTR‐8/SVneo was cultured in 6‐well plates and then transfected with siTFPI2 (10 μL), siCon (10 μL), or plasmid vectors (4 μg) with 10 μL Lipofectamine 2000 (Invitrogen). Plasmid vectors (pcDNA3.1 + TFPI2 and pcDNA) were extracted using DNA Midiprep kit (Qiagen, Hilden, Germany). The DNA sequences of siRNAs for TFPI2 were acquired from Invitrogen. MiR‐616‐3p mimics and miR‐616‐3p inhibitor were obtained from GeneCopoeia (Guangzhou, China). At 48 hours post‐transfection, cells were harvested for further experiments. MicroRNA NC CAGUACUUUUGUGUAGUACAA MiR‐616‐3p mimics AGUCAUUGGAGGGUUUGAGCAG TFPI2 siRNA sense 5′‐AGCCCAUACAAGUAGCUUCAUCUGG‐3′ antisense 5′‐CCAGAUGAAGCUACUUGUAUGGGCU‐3′.
2.4. Plasmid construction
To ectopically upregulate TFPI2 expression, TFPI2 plasmid (2444 bp, NM_006528.3, synthesized by Realgene, Nanjing, China) was subcloned into pcDNA3.1(+) vector (Invitrogen), following standard protocols. TFPI2 plasmids as well as empty vector were transfected into HTR‐8/SVneo on 6‐well plates and/or 24‐well plates.
2.5. RNA extraction and qRT‐PCR analyses
RNA extraction and qRT‐PCR assays were performed as previously reported in Xu et al25 Total RNA from tissues and cells was extracted by using the TRIzol reagent (Invitrogen) and qRT‐PCR analyses were performed by using the SYBR® Green Master Mix (TaKaRa BIO INC, Otsu, Japan) following standard protocols. Results were normalized to the level of GAPDH. For miRNA quantification, miScript reverse transcription kit (Qiagen, Valencia, CA, USA) was used to reverse transcribe miRNA into cDNA. Primers for miR‐616‐3p and U6 were purchased from GeneCopoeia (Rockville, MD, USA). Gene‐specific primers sequences were as follows: TFPI2 Forward‐1 5′‐CTGGGGCTGTCGATTCTGC‐3′ Reverse‐1 5′‐TCTCCGCGTTATTTCCTGTTG‐3′; Forward‐2 5′‐CAGATGAAGCTACTTGTATGGGCTTC‐3′ Reverse‐2 5′‐ GGCAAAGCGAAGCTTTGGCATC‐3′; Forward‐3 5′‐ CTGGGGCTGTCGATTCTGC‐3′ Reverse‐3 5′‐ TCTCCGCGTTATTTCCTGTTG‐3′; GAPDH Forward 5′‐GGGAGCCAAAAGGGTCAT‐3′ Reverse 5′‐GAGTCCTTCCACGATACCAA‐3′; U6 Forward 5′‐CTCGCTTCGGCAGCACA‐3′ Reverse 5′‐AACGCTTCACGAATTTGCGT‐3′. cDNA was amplified on a 7500 Real‐Time PCR System (7500, Applied Biosystems, Foster City, CA, USA) with SYBR Premix Ex Taq (Takara, Dalian China).
2.6. Cell viability assays
To assess the cell growth ability of HTR‐8/SVneo and JEG3 cell lines, MTT and EdU assays were conducted. These experiments were carried out as previously reported.25 For the MTT assays, HTR‐8/SVneo and JEG3 transfected with mimics (NC or miRNA‐616‐3p and/or siTFPI2 containing 3500 cells/well) were plated in 96‐well plates with 5 duplicates. Cell viability was measured every 24 hours, following the manufacturer's instructions. The absorbance was detected at 490 nm with ELx‐800 University Microplate Reader (BioTek, Winooski, VT, USA). For the EdU assays, cells were plated into 24 wells with 5 × 104 cells/well. We then used the 5‐ethynyl‐2‐deoxyuridine labelling/detection kit (Ribobio, Guangzhou, China) to estimate cell viability, according to the manual.
2.7. Quantitative cell migration assays
This experiment was carried out as previously described.26 Briefly, HTR‐8/SVneo and JEG3 cell lines, transfected with specific siRNAs and/or plasmid, were re‐suspended in RPMI 1640 containing 1% FBS and then added to the upper chamber. The lower chamber was filled with 700 μL RPMI 1640 containing 10% FBS. After incubation for 24 hours at 37°C, cells inside the upper chamber were removed before fixing. Cells on the bottom membrane surface were fixed with methanol and then stained with a 0.5% crystal violet solution. Three to five randomly dispersed fields were counted per well.
2.8. Western blotting analysis
These assays were performed to detect protein levels as previously described in Xu et al.26 Five to 15 μL of each sample was loaded onto a 10% SDS gel, then analysed by western blotting. The samples were incubated with specific antibodies (GAPDH, TFPI2, E‐cadherin, N‐cadherin and vimentin, purchased from CST or Proteintech) at 1:1000 concentration. GAPDH was used as a loading control. The intensity of autoradiogram protein bands was quantified using Quantity One software (Bio‐Rad, Hercules, CA, USA).
2.9. Luciferase reporter assays
The complementary DNA fragment containing the wild‐type and mutant TFPI2 3′‐UTR was linked into the pGL3‐basic vector (Promega). HTR‐8/SVneo cells were co‐transfected with either the wild‐type or mutant 3′‐UTR of the TFPI2 vector, along with a miR‐616‐3p mimic or miR‐616‐3p inhibitor in the 6‐wells. Forty‐eight hours after transfection, the luciferase activity for each group was assessed using the Dual‐Luciferase Kit (Promega). Renilla luciferase was used as the control. All experiments were repeated in triplicate.
2.10. Statistical analysis
All statistical analyses and figure generation were performed using GraphPad Prism version 7.0 and Adobe Photoshop CC2015. Data were analysed using a 2‐tailed Student t test. Statistical significance was ascribed at P < .05(*) or P < .01(**). Each experiment was independently repeated at least 3 times.
3. RESULTS
3.1. TFPI2 protein levels are upregulated in PE placental tissues
The levels of TFPI2 in PE placental tissues and normal pregnancy placental tissues were analysed by western blotting. In contrast to normal pregnancy placental tissues, TFPI2 protein levels were noticeably upregulated in PE placental tissues (Figure 1A,B). We also performed immunohistochemistry assays to estimate TFPI2 protein levels, which were expressed predominantly in PE placental tissues rather than normal tissues, and primarily distributed in the cytoplasm (Figure 1C). The detailed clinical characteristics of the patients who met the criteria of the study are listed in Table 1.
Figure 1.
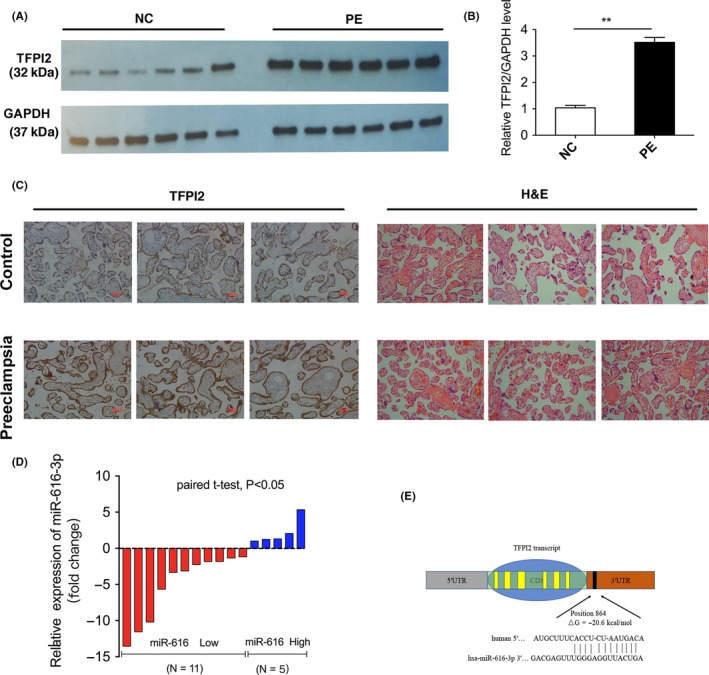
The expression of tissue factor pathway inhibitor 2 (TFPI2) and miR‐616‐3p in human PE placenta tissues. A, Western blotting analysis of TFPI2 levels in PE placenta tissues compared with the normal (n = 6). B, Quantitative analysis of A; C, Images of HE staining and immunohistochemistry of the pregnancy placenta tissues. TFPI2 protein levels in placenta tissues as evaluated by immunohistochemistry. D, Relative levels of miR‐616‐3p in PE placenta tissues and the normal pregnancy placenta tissues (n = 16). The blue column indicates the upregulated expression of miR‐616‐3p, and red column indicates the downregulated level of miR‐616‐3p. E, The predicted base‐pairing interaction between miR‐616‐3p and TFPI2 mRNA. NC represents the group of the normal pregnancy. Numbers are mean ± SD. **indicates P < .01; *indicates P < .05
Table 1.
Clinical characteristics of preeclampsia and normal pregnancies
| Variable | PE (N = 16) | Normal (N = 16) | P valuea Normal vs P |
|---|---|---|---|
| Maternal age (y) | 28.67 ± 1.902 | 29.33 ± 2.6 | P > .05 |
| Maternal weight (kg) | 69.4167 ± 9.252 | 68.333 ± 2.773 | P > .05 |
| Smoking | 0 | 0 | P > .05 |
| Systolic blood pressure (mm Hg) | 169 ± 8.5188 | 114.33 ± 7.044 | P < .01 |
| Diastolic blood pressure (mm Hg) | 105.667 ± 4.1633 | 71.5 ± 4.268 | P < .01 |
| Proteinuria (g/d) | >0.3 g | <0.3 g | P < .05 |
| Body weight of infant (g) | 2305 ± 748.88 | 3416.67 ± 176.608 | P < .05 |
| Gestational age (wk) | 31.833 ± 1.456 | 39.333 ± 0.873 | P < .05 |
Emerging evidence has confirmed that miRNAs in cytoplasm mediate mRNA expression, thus further affecting cellular phenotype. The protein levels of TFPI2 were upregulated in PE placental tissues and predominantly distributed in the cytoplasm, indicating that the level of TFPI2 might be regulated at a post‐transcriptional level.
3.2. MiR‐616‐3p is downregulated in PE placenta tissues and directly targets TFPI2
Based on bioinformatics analysis (included RNA22, RNAhybrid, Targetscan, miRMap and miRWalk.), we found that miR‐616‐3p was predicted to bind a region in the 3′ UTR of TFPI2 mRNA (Figure 1E). Previous studies have confirmed that abnormally expressed miR‐616 is involved in various biological processes in a multitude of diseases.27, 28, 29 Ma et al29 have also reported that miR‐616 could induce androgen‐independent growth of prostate cancer cells by regulating expression of TFPI2. Therefore, we postulate that TFPI2 was regulated by miR‐616‐3p in PE placental tissues. We conducted qRT‐PCR to evaluate the expression of miR‐616‐3p in PE placental tissues and normal pregnancy placental tissues. As shown in Figure 1D, the expression level of miR‐616‐3p was significantly decreased in PE placental tissues compared with normal placental tissues. Since miR‐616‐3p might act as an essential regulator of TFPI2 in trophoblast cells, we chose miR‐616‐3p for further experiments to validate its specific binding to TFPI2.
In order to explore the direct interaction between miR‐616‐3p and TFPI2, various luciferase genes, including the 3′‐UTR of TFPI2 and the mutated 3′‐UTR sequence, were cloned and then co‐transfected with miR‐616‐3p mimics and inhibitors in HTR‐8/SVneo and HEK‐293T cell lines, respectively. Intriguingly, we found that the relative luciferase activity of the reporters of miR‐616‐3p mimics and 3′UTR of TFPI2 were significantly decreased compared with the control (Figure 2A,B). In contrast, the relative luciferase activity of the reporters of mutant 3′UTR of TFPI2 showed no difference after transfection with miR‐616‐3p mimics (Figure 2A,B).
Figure 2.
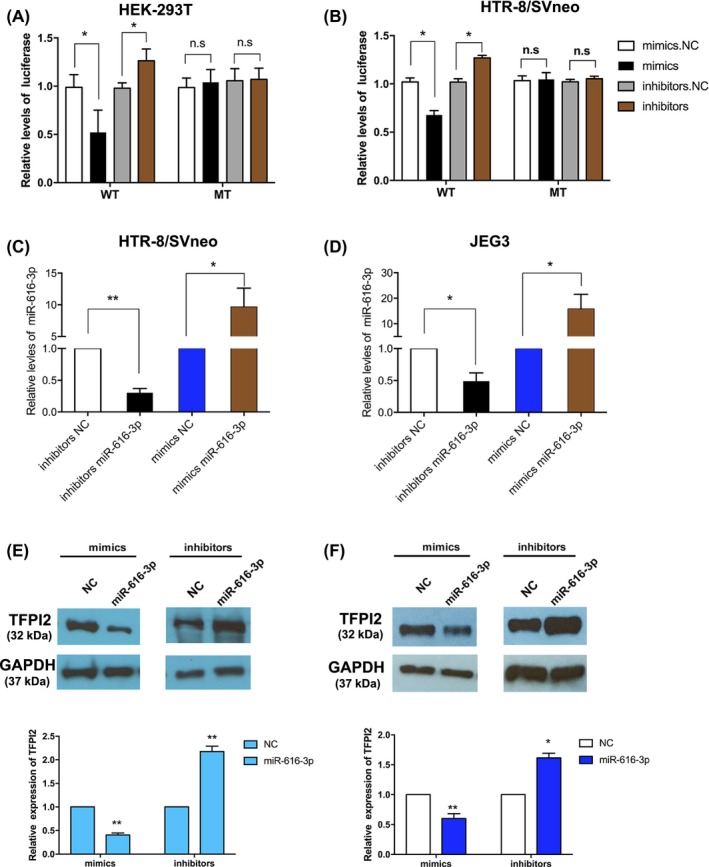
MiR‐616‐3p regulates tissue factor pathway inhibitor 2 (TFPI2) expression in trophoblast cell lines. (A and B) Direct recognition of TFPI2 by miR‐616‐3p. HTR‐8/SVneo and HEK‐293T cells were co‐transfected with luciferase reporters containing WT and/or mutant TFPI2 3′‐UTR with miR‐616‐3p mimics and inhibitors, NC represents the control. (C and D) qRT‐PCR analysis of miR‐616‐3p expression in HTR‐8/SVneo and JEG3 cells transfected with mimics or inhibitors. (E and F) The suppression of TFPI2 protein levels by miR‐616‐3p in HTR‐8/SVneo and JEG3 cell lines (n = 3). NC represents the controls (**indicates P < .01, *indicates P < .05, n.s. represents no statistical significance.)
We further explored the potential biological function of miR‐616‐3p in trophoblast cells. Overexpression and knockdown studies of miR‐616‐3p were conducted after treatment with miR‐616‐3p mimics and miR‐616‐3p inhibitors in HTR‐8/SVneo and JEG3 cell lines, respectively. The expression level of miR‐616‐3p was evaluated by qRT‐PCR (Figure 2C,D). The expression of TFPI2 was significantly decreased after transfection with miR‐616‐3p mimics, while it was increased after transfection with miR‐616‐3p inhibitors (Figure 2E,F). Therefore, our resulting data demonstrate that miR‐616‐3p could downregulate TFPI2 expression by binding to the 3′UTR region of TFPI2 mRNA.
3.3. Upregulation of miR‐616‐3p promotes the growth and migration of HTR‐8/SVneo and JEG3 cell lines
To explore the potential biological behaviours of miR‐616‐3p and TFPI2 on trophoblast cells, we performed MTT and EdU assays to estimate cell growth ability in HTR‐8/SVneo and JEG3 cell lines. As shown in Figure 3A‐C, knockdown of miR‐616‐3p could promote cell proliferation, whereas the upregulation of miR‐616‐3p could result in increased cell proliferation. Next, we conducted transwell assays to evaluate the migration ability of HTR‐8/SVneo and JEG3 cells after transfection with mimics and/or inhibitors. The results showed that silencing of miR‐616‐3p significantly inhibited cell migration compared to the controls. Conversely, the overexpression of miR‐616‐3p could stimulate cell migration (Figure 3D‐F). These results suggest that if miR‐616‐3p can stimulate cell proliferation and migration capacity in HTR‐8/SVneo and JEG3 cells, it could play a vital role in the progression of PE.
Figure 3.
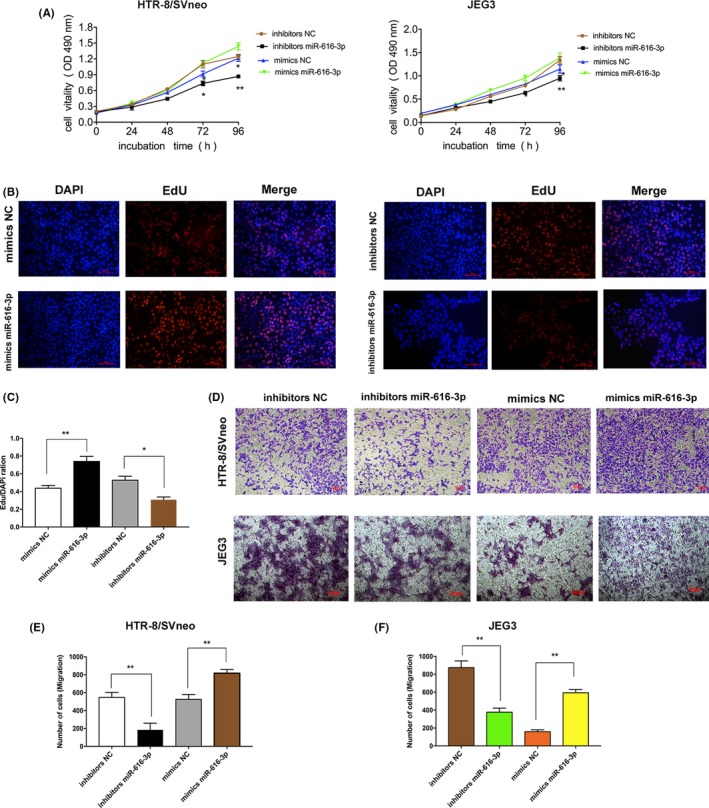
MiR‐616‐3p regulates the cell growth and migration in HTR‐8/SVneo and JEG3 cell lines. (A and B) MTT assays and EdU assays show that miR‐616‐3p promotes the growth of trophoblast cells. Upregulation of miR‐616‐3p stimulates cell proliferation, whereas cell proliferation capacity was impaired after transfecting with miR‐616‐3p inhibitors. (C) Quantitative analysis of B; (D) Transwell assays exhibit that miR‐616‐3p induces the migration of trophoblast cells. Silence of miR‐616‐3p could repress trophoblast cell migrate ability, whereas overexpression of miR‐616‐3p promote cell migration in vitro (n = 3). (E and F) Quantitative analysis of D. (**P < .01, *P < .05)
3.4. Effects of TFPI2 on trophoblast cells proliferation and migration
To investigate the biological effects of TFPI2 on trophoblast cells, we designed and synthesized the specific siRNAs and plasmids promoting overexpression to exogenously decrease/increase the expression of TFPI2 in HTR‐8/SVneo cells. qRT‐PCR and western blot analysis indicated that TFPI2 expression was significantly silenced/increased after transfection with specific siRNAs/overexpression plasmids in HTR‐8/SVneo cells (Figure 4A,B). We then performed EdU and transwell assays to assess the function of TFPI2. As shown in Figure 4C,E, silencing of TFPI2 expression significantly promoted cell proliferation and migration, while TFPI2 overexpression inhibited cell growth and migration (Figure 4C‐F). Zhou et al30 have reported that TFPI2 decreased cell proliferation and migration, promoting cell apoptosis in JEG3 and BeWo cell lines. In parallel, rescue experiments also found that TFPI2 can rescue the inhibition effect of cell proliferation and migration of miR‐616‐3p (Figure 5A,B). Consequently, TFPI2 might act as an important suppressor in PE, while the aberrant expression of TFPI2 might induce the growth and migration of trophoblast cells involved in the pathogenesis of PE.
Figure 4.
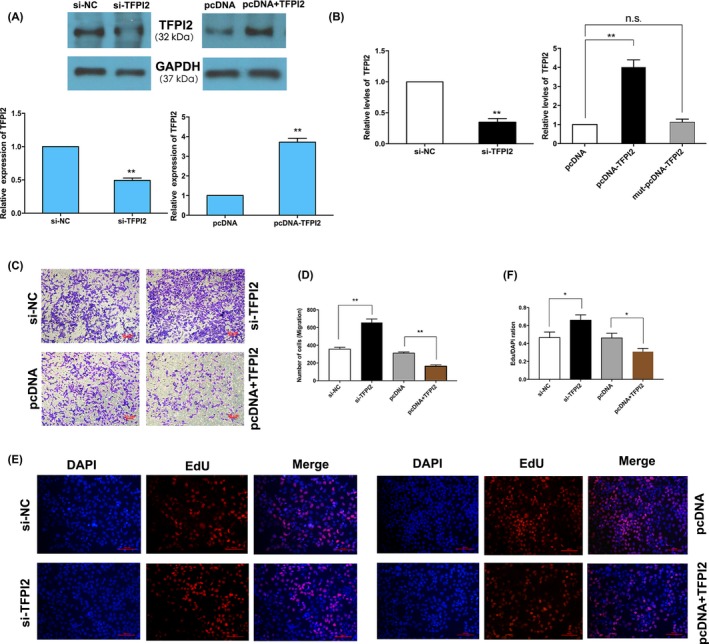
Effects of tissue factor pathway inhibitor 2 (TFPI2) downregulating and upregulating in HTR‐8/SVneo cells, identification of TFPI2 as a suppressor in trophoblast cells. A, TFPI2 protein levels were detected after transfected with TFPI2 siRNAs or TFPI2 plasmid in HTR‐8/SVneo cells, the following is the quantitative. B, The TFPI2 mRNA expression were detected by qRT‐PCR. C, Transwell assays display that silence of TFPI2 promotes cell migration of HTR‐8/SVneo cells (n = 3). D, Quantitative analysis of C. E, EdU assays reveal that upregulation of TFPI2 inhibits cell growth, whereas downregulation of TFPI2 promotes cell proliferation of HTR‐8/SVneo cells. F, Quantitative analysis of E (**P < .01, *P < .05)
Figure 5.
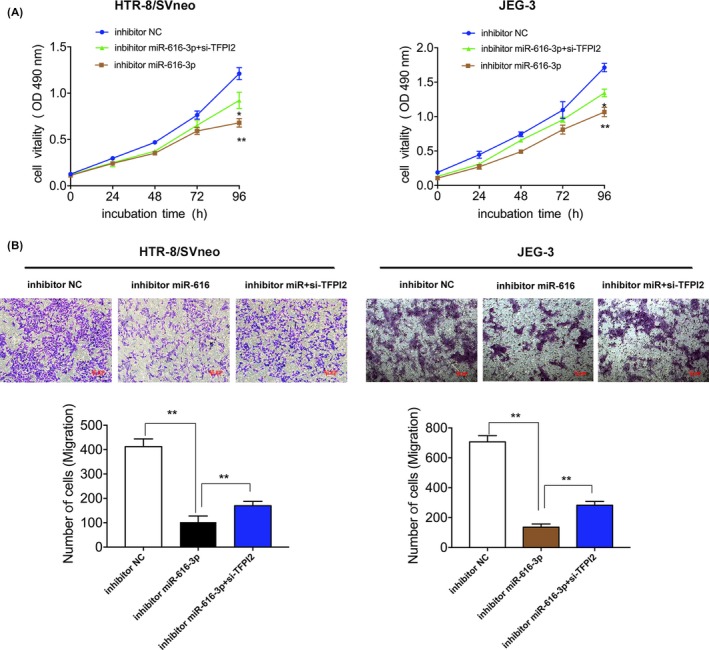
miR‐616‐3p stimulates trophoblast cell growth and migration by inhibiting tissue factor pathway inhibitor 2 (TFPI2) in trophoblast cells. A, For cell growth analysis, MTT assays were performed to assess the cell viability for inhibitors miR‐616‐3p and si‐TFPI2 co‐transfected trophoblast cells. B, For cell migration analyses, migration was allowed to occur for 24 h. Quantitative analysis is below. Numbers are mean ± SD. **P < .01, *P < .05
3.5. Effects of miR‐616‐3p‐TFPI2 pathway on the EMT‐associated markers in vitro
Previous studies have reported that epithelial‐mesenchymal transition might be involved in cell migration and invasion.31, 32 We further explored the function of the miR‐616‐3p‐TFPI2‐associated pathway in trophoblast cells. Epithelial and mesenchymal‐induced markers, including E‐cadherin, N‐cadherin and vimentin, were tested with western blot assays. Our results indicated that E‐cadherin expression was higher after miR‐616‐3p upregulation or TFPI2 knockdown, and N‐cadherin and vimentin expression was lower (Figure 6A,B). In contrast, the opposite occurred after transfection with miR‐616‐3p inhibitors or TFPI2 overexpresses (Figure 6A,B).
Figure 6.
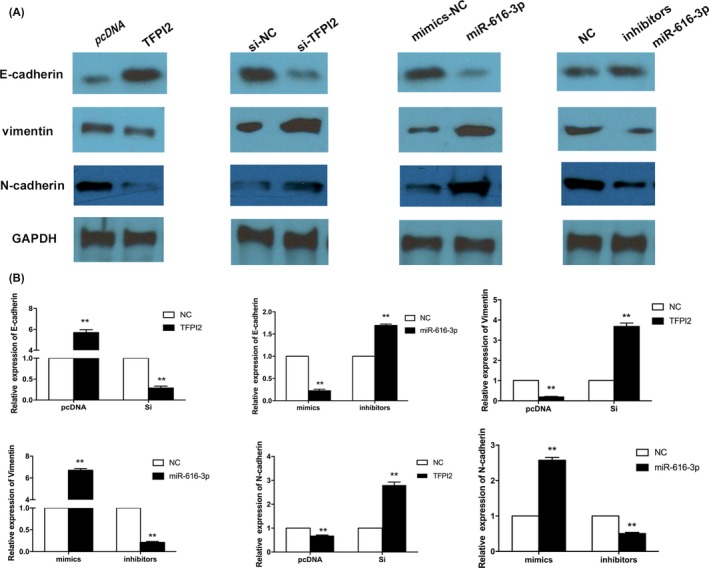
Effects of miR‐616‐3p‐TFPI2 pathway on the EMT‐associated markers in vitro. A, Western blotting analysis of E‐cadherin, N‐cadherin and vimentin levels in HTR‐8/SVneo cells which transfected with miR‐616‐3p mimics, miR‐616‐3p inhibitors, tissue factor pathway inhibitor 2 (TFPI2) siRNAs and TFPI2 overexpression plasmid, respectively. B, Quantitative analysis of A. (values are mean ± SD, **P < .01.)
4. DISCUSSION
Recently, an increasing number of studies have indicated that miRNAs are involved in varies human diseases,33, 34 including PE.35, 36, 37 Our previous research38 has reported that an aberrant level of the miR‐101 could affect trophoblast cell apoptosis, migration and invasiveness to induce impaired spiral artery remodelling, thus contributing to the occurrence and development of PE. Previous studies have also demonstrated that miRNAs in cytoplasm could sponge various genes to further affect cellular biological phenotypes. For example, miR‐3174 contributes to apoptosis and autophagic cell death defects in gastric cancer cells by targeting ARHGAP10.39 MicroRNA‐136 inhibits growth and stimulates apoptosis and radiosensitivity of cervical carcinoma via the NF‐κB pathway by targeting E2F1.40 Thus, the identification and enhancement of the connection between miRNA and its target genes will serve to emphasize the roles of miRNA in multiple biological process.
TFPI2, a kind of serine proteinase inhibitor which located on chromosome 7q22,41, 42 is abundantly expressed abundantly in multiple human organs and can reduce cell growth,10 apoptosis,8 metastasis and invasion.43, 44, 45 Moreover, many studies have confirmed that TFPI2 might essentially regulate plasminogen activator inhibitor, urokinase plasminogen activator, and the process of proteolysis, and prevent ECM degradation to affect cell migration and invasive capacity.44, 45 Previous studies have revealed that TFPI2 acts as a suppressor gene in a variety of human diseases, including PE.46, 47, 48 For example, Zhou et al30 found that TFPI2 expression caused impairment of invasion and proliferation, and induced apoptosis in BeWo and JEG3 cells. Xiao et al11 have also reported overexpression of TFPI2 and aberrant promoter methylation status presented in PE placentas, suggesting that an epigenetic mechanism might result in the pathogenesis of PE. However, the role of TFPI2 in the pathogenesis of PE is still unknown.
In our study, we found that TFPI2 protein levels were significantly upregulated in PE placental tissues, while miR‐616‐3p transcriptional expression levels were clearly decreased. MiR‐616‐3p mRNA level was negatively correlated with TFPI2 protein level. Bioinformatics analysis and a dual‐luciferase activity assay have confirmed that miR‐616‐3p could bind to the 3′‐UTR region of wild‐type TFPI2 mRNA, but not the mutant construct. Also, miR‐616‐3p mimics resulted in downregulated TFPI2 protein levels, while miR‐616‐3p inhibitors contributed to upregulate TFPI2 expression in HTR‐8/SVneo and JEG3. Subsequently, functional experiments indicated that miR‐616‐3p overexpression could stimulate trophoblast cell growth and migration in HTR‐8/SVneo and JEG3. In contrast, downregulated miR‐616‐3p might decrease cell proliferation and migration. Silencing of TFPI2 could stimulate the effects of miR‐616‐3p mimics on the phenotype of HTR‐8/SVneo; furthermore, TFPI2 overexpression yielded the same phenotypes as those of the miR‐616‐3p inhibitors. These results establish that miR‐616‐3p could sponge TFPI2, which functions as a suppressor in PE. Finally, the association of miR‐616‐3p and TFPI2 with epithelial‐mesenchymal markers highlights their potential biological functions in PE.
To sum up, our findings provide further evidence of an miR‐616‐3p/TFPI2‐associated mechanism in PE, thus offering novel insights for the development of methods for latent diagnosis and therapeutic targets for PE. However, there are still many gaps in our current understanding of TFPI2 function and its biological mechanism in PE. Further studies are needed to elucidate a TFPI2‐associated mechanism of PE.
ACKNOWLEDGEMENTS
This study was supported by the National Scientific Foundation of China (No. 81471508 and No. 81771603), the traditional Chinese medicine project of Jiangsu Province (NO. ZX2016D2), and the Natural Science Foundation of Jiangsu Province (Project numbers: BK20161061 and BK20171502), Key Disciplines of 13th Fifth‐year Strong and Healthy Engineering in Jiangsu Province, and the Research Team of Female Reproductive Health and Fertility Preservation (NO. SZSM201612065).
CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Yetao Xu, Dan Wu and Ziyan Jiang performed most of the experiments. Yuanyuan Zhang, Sailan Wang and Jing Wang collected clinical tissues and analysed data. Zhonghua Ma, Bingqing Hui and Weiping Qian conducted some experiments. Yetao Xu, Zhiping Ge and Lizhou Sun designed the project and edited the manuscript.
Xu Y, Wu D, Jiang Z, et al. MiR‐616‐3p modulates cell proliferation and migration through targeting tissue factor pathway inhibitor 2 in preeclampsia. Cell Prolif. 2018;51:e12490 10.1111/cpr.12490
Yetao Xu, Dan Wu and Ziyan Jiang contributed equally to this work.
Contributor Information
Zhiping Ge, Email: gzp88142@163.com.
Lizhou Sun, Email: sunlizhou@njmu.edu.cn.
REFERENCES
- 1. Kurtz WS, Glueck CJ, Hutchins RK, Sisk RA, Wang P. Retinal artery and vein thrombotic occlusion during pregnancy: markers for familial thrombophilia and adverse pregnancy outcomes. Clin Ophthalmol. 2016;10:935‐938. 10.2147/OPTH.S106164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO recommendations for Prevention and Treatment of Pre‐Eclampsia and Eclampsia WHO Guidelines Approved by the Guidelines Review Committee. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 3. Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131‐138. 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 4. Henderson JT, et al. Low‐dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 160, 695‐703, 10.7326/M13-2844 (2014). [DOI] [PubMed] [Google Scholar]
- 5. Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low‐dose aspirin for the prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695‐703. 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 6. Myatt L, Redman CW, Staff AC. Strategy for standardization of preeclampsia research study design. Hypertension. 2014;63:1293‐1301. 10.1161/HYPERTENSIONAHA.113.02664. [DOI] [PubMed] [Google Scholar]
- 7. Stella CL, Sibai BM. Preeclampsia: diagnosis and management of the atypical presentation. J Matern Fetal Neonatal Med. 2006;19:381‐386. 10.1080/14767050600678337. [DOI] [PubMed] [Google Scholar]
- 8. Feng C, Ho Y, Sun C, Xia G, Ding Q, Gu B. TFPI‐2 expression is decreased in bladder cancer and is related to apoptosis. J BUON. 2016;21:1518‐1523. [PubMed] [Google Scholar]
- 9. Sun FK, Sun Q, Fan YC, et al. Methylation of tissue factor pathway inhibitor 2 as a prognostic biomarker for hepatocellular carcinoma after hepatectomy. J Gastroenterol Hepatol. 2016;31:484‐492. 10.1111/jgh.13154. [DOI] [PubMed] [Google Scholar]
- 10. Zhao B, Luo X, Shi H, Ma D. Tissue factor pathway inhibitor‐2 is downregulated by ox‐LDL and inhibits ox‐LDL induced vascular smooth muscle cells proliferation and migration. Thromb Res. 2011;128:179‐185. 10.1016/j.thromres.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 11. Xiao X, Tao X, Wang Y, et al. Hypomethylation of tissue factor pathway inhibitor 2 in human placenta of preeclampsia. Thromb Res. 2017;152:7‐13. 10.1016/j.thromres.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 12. Esmaili MH, Pleuvry BJ, Healy TE. Interactions between atracurium and vecuronium on indirectly elicited muscle twitch in vitro. Eur J Anaesthesiol. 1986;3:469‐472. [PubMed] [Google Scholar]
- 13. Fujita Y, Kojima K, Ohhashi R, et al. MiR‐148a attenuates paclitaxel resistance of hormone‐refractory, drug‐resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem. 2010;285:19076‐19084. 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lakhter AJ, Pratt RE, Moore RE, et al. Beta cell extracellular vesicle miR‐21‐5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61:1124‐1134. 10.1007/s00125-018-4559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hummel R, Hussey DJ, Michael MZ, et al. MiRNAs and their association with locoregional staging and survival following surgery for esophageal carcinoma. Ann Surg Oncol. 2011;18:253‐260. 10.1245/s10434-010-1213-y. [DOI] [PubMed] [Google Scholar]
- 16. Wu L, Song WY, Xie Y, et al. miR‐181a‐5p suppresses invasion and migration of HTR‐8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 2018;9:16 10.1038/s41419-017-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sergi CM, Caluseriu O, McColl H, Eisenstat DD. Hirschsprung's disease: clinical dysmorphology, genes, micro‐RNAs, and future perspectives. Pediatr Res. 2017;81:177‐191. 10.1038/pr.2016.202. [DOI] [PubMed] [Google Scholar]
- 18. Amr KS, Abdelmawgoud H, Ali ZY, Shehata S, Raslan HM. Potential value of circulating microRNA‐126 and microRNA‐210 as biomarkers for type 2 diabetes with coronary artery disease. Br J Biomed Sci. 2018;75:82‐87. 10.1080/09674845.2017.1402404. [DOI] [PubMed] [Google Scholar]
- 19. Farran B, Dyson G, Craig D, et al. A study of circulating micrornas identifies a new potential biomarker panel to distinguish aggressive prostate cancer. Carcinogenesis. 2018;39:556‐561. 10.1093/carcin/bgy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Haidari A, Algaber A, Madhi R, Syk I, Thorlacius H. MiR‐155‐5p controls colon cancer cell migration via post‐transcriptional regulation of Human Antigen R (HuR). Cancer Lett. 2018; 421:145‐151. 10.1016/j.canlet.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 21. Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200:661.e661‐661.e667. 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 22. Niu ZR, Han T, Sun XL, Luan LX, Gou WL, Zhu XM. MicroRNA‐30a‐3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF‐1. Am J Obstet Gynecol. 2018;218:249.e241‐249.e212, 10.1016/j.ajog.2017.11.568. [DOI] [PubMed] [Google Scholar]
- 23. Cronqvist T, Saljé K, Familari M, et al. Syncytiotrophoblast vesicles show altered micro‐RNA and haemoglobin content after ex‐vivo perfusion of placentas with haemoglobin to mimic preeclampsia. PLoS ONE. 2014;9:e90020 10.1371/journal.pone.0090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noack F, Ribbat‐Idel J, Thorns C, et al. miRNA expression profiling in formalin‐fixed and paraffin‐embedded placental tissue samples from pregnancies with severe preeclampsia. J Perinat Med. 2011;39:267‐271. 10.1515/JPM.2011.012. [DOI] [PubMed] [Google Scholar]
- 25. Xu Y, Lian Y, Zhang Y, et al. The long non‐coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J Cell Mol Med. 2018;22:1272‐1282. 10.1111/jcmm.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Ge Z, Zhang E, et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8:e3104 10.1038/cddis.2017.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bai QL, Hu CW, Wang XR, Shang JX, Yin GF. MiR‐616 promotes proliferation and inhibits apoptosis in glioma cells by suppressing expression of SOX7 via the Wnt signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:5630‐5637. 10.26355/eurrev_201712_14006. [DOI] [PubMed] [Google Scholar]
- 28. Wang D, Cao Q, Qu M, Xiao Z, Zhang M, Di S. MicroRNA‐616 promotes the growth and metastasis of non‐small cell lung cancer by targeting SOX7. Oncol Rep. 2017;38:2078‐2086. 10.3892/or.2017.5854. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Ma S, Chan YP, Kwan PS, et al. MicroRNA‐616 induces androgen‐independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI‐2. Cancer Res. 2011;71:583‐592. 10.1158/0008-5472.CAN-10-2587. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Q, Xiong Y, Chen Y, et al. Effects of tissue factor pathway inhibitor‐2 expression on biological behavior of BeWo and JEG‐3 cell lines. Clin Appl Thromb Hemost. 2012;18:526‐533. 10.1177/1076029611429785. [DOI] [PubMed] [Google Scholar]
- 31. Kiesslich T, Pichler M, Neureiter D. Epigenetic control of epithelial‐mesenchymal‐transition in human cancer. Mol Clin Oncol. 2013;1:3‐11. 10.3892/mco.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karamitopoulou E. Role of epithelial‐mesenchymal transition in pancreatic ductal adenocarcinoma: is tumor budding the missing link? Front Oncol. 2013;3:221 10.3389/fonc.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alfonsi R, Grassi L, Signore M, Bonci D. The double face of exosome‐carried microRNAs in cancer immunomodulation. Int J Mol Sci. 2018;19:1183 10.3390/ijms19041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arroyo AB, de Los Reyes‐García AM, Teruel‐Montoya R, et al. microRNAs in the haemostatic system: more than witnesses of thromboembolic diseases? Thromb Res. 2018;166:1‐9. 10.1016/j.thromres.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 35. Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep. 2015;12:527‐534. 10.3892/mmr.2015.3414. [DOI] [PubMed] [Google Scholar]
- 36. Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88:130 10.1095/biolreprod.113.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laresgoiti‐Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94:247‐257. 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 38. Zou Y, Jiang Z, Yu X, et al. MiR‐101 regulates apoptosis of trophoblast HTR‐8/SVneo cells by targeting endoplasmic reticulum (ER) protein 44 during preeclampsia. J Hum Hypertens. 2014;28:610‐616. 10.1038/jhh.2014.35. [DOI] [PubMed] [Google Scholar]
- 39. Li B, Wang L, Li Z, et al. miR‐3174 contributes to apoptosis and autophagic cell death defects in gastric cancer cells by targeting ARHGAP10. Mol Ther Nucleic Acids. 2017;9:294‐311. 10.1016/j.omtn.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Lu HJ, Jin PY, Tang Y, et al. microRNA‐136 inhibits proliferation and promotes apoptosis and radiosensitivity of cervical carcinoma through the NF‐kappaB pathway by targeting E2F1. Life Sci. 2018;199:167‐178. 10.1016/j.lfs.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 41. Miyagi Y, Yasumitsu H, Eki T, et al. Assignment of the human PP5/TFPI‐2 gene to 7q22 by FISH and PCR‐based human/rodent cell hybrid mapping panel analysis. Genomics. 1996;35:267‐268. 10.1006/geno.1996.0353. [DOI] [PubMed] [Google Scholar]
- 42. Sprecher CA, Kisiel W, Mathewes S, Foster DC. Molecular cloning, expression, and partial characterization of a second human tissue‐factor‐pathway inhibitor. Proc Natl Acad Sci U S A. 1994;91:3353‐3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang S, Xiao X, Zhou X, et al. TFPI‐2 is a putative tumor suppressor gene frequently inactivated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer. 2010;10:617 10.1186/1471-2407-10-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sierko E, Wojtukiewicz MZ, Kisiel W. The role of tissue factor pathway inhibitor‐2 in cancer biology. Semin Thromb Hemost. 2007;33:653‐659. 10.1055/s-2007-991532. [DOI] [PubMed] [Google Scholar]
- 45. Hirata M, Sato T, Tsumagari M, Hashizume K, Ito A. Discoordinate regulation of expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases‐3 in bovine endometrial stromal cells on type‐I collagen gel. Biol Pharm Bull. 2003;26:1013‐1017. [DOI] [PubMed] [Google Scholar]
- 46. Xiong Y, Zhou Q, Jiang F, et al. Changes of plasma and placental tissue factor pathway inhibitor‐2 in women with preeclampsia and normal pregnancy. Thromb Res. 2010;125:e317‐e322. 10.1016/j.thromres.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 47. Kempaiah P, Chand HS, Kisiel W. Identification of a human TFPI‐2 splice variant that is upregulated in human tumor tissues. Mol Cancer. 2007;6:20 10.1186/1476-4598-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Udagawa K, Miyagib Y, Hirahara F, et al. Specific expression of PP5/TFPI2 mRNA by syncytiotrophoblasts in human placenta as revealed by in situ hybridization. Placenta. 1998;19:217‐223. [DOI] [PubMed] [Google Scholar]


