Abstract
Objectives
Thymidine kinase 1 (TK1) is one of the salvage enzymes engaged in the synthesis of DNA. Although a pro‐carcinogenetic role of TK1 has been reported in various types of cancers, its role in pancreatic ductal adenocarcinoma (PDAC) is still unknown. The study is aimed to elaborate the function of TK1 in PDAC and the potential mechanisms in the following study.
Materials and methods
TK1 expression was analysed by immunohistochemistry, real‐time PCR and Western blot, and its relationship with clinicopathological characteristics of PDAC patients was further investigated. To verify the function of TK1 and potential mechanism, TK1 siRNA was used to transfect PDAC cells and performed a series of assays in cell and animal models.
Results
The level of TK1 expression was higher in cancerous tissues compared with matched adjacent tissues. TK1 overexpression was associated with progression of PDAC and poor prognosis. Knockdown of TK1 could suppress cell proliferation via inducing S phase arrest mediated by upregulation of P21. Further mechanism investigation suggested that transcription factor E2F‐1 could directly regulate the TK1 and promote tumour proliferation.
Conclusions
The results suggested that TK1 might be involved in the development and progression of PDAC by regulating cell proliferation and show that TK1 may work as a promising therapeutic target in patients with PDAC.
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive digestive malignancies and is the fourth most common cause of cancer‐related death in the United States. Patients diagnosed with PDAC have a poor prognosis with an overall 5 year survival rate of only 8% and a median survival of 6 months.1 Although the morbidity of this malignant tumour has been increased over the last several years, there is no obvious improvement in the survival rate. Surgical resection may prolong survival and provide a unique curative option. Unfortunately, the surgical resection rate is <20% because of difficulty for diagnosis at early stage.2 Therefore, an increasing understanding of the complex molecular basis of PDAC and exploring novel therapeutic targets may contribute to the clinical treatment of this aggressive malignancy.
Thymidine kinase 1 (TK1), a cell cycle‐dependent marker, is a pyrimidine salvage enzyme involved in the synthesis and repair of DNA. Thus, TK1 is closely correlated with cell proliferation and cell cycle activity.3, 4 It is reported that wide distribution and overexpression of TK1 are found in most neoplastic cells.5 Progression and development of cancer are complex biological processes. Although the causes and exact mechanisms are still unknown, the anomaly of the cell cycle and abnormal regulation of cell proliferation are recognized to be elementary events in any malignant transformation. Numerous studies have documented the TK1 protein expression patterns could act as clinicopathological predictive markers in various types of cancers like breast cancer,6 kidney cancer,7, 8 lung cancer,9 and gastrointestinal cancer;10 however, little has been explored about the expression and biological function of TK1 in PDAC. To further identify the role of TK1 in PDAC, we evaluated the expression patterns in PDAC cell lines and tissues and investigated the possible molecular mechanism.
2. MATERIALS AND METHODS
2.1. Tissue microarray
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. TK1 mRNA expression of 165 patients with pancreatic cancer was downloaded from The Cancer Genome Atlas (TCGA, USA) data portal (http://cancergenome.nih.gov/). Seventy‐two pancreatic cancer tissues and matched adjacent tissues microarray were obtained from Shanghai Zhuoli Biotechnology Co., Ltd (Zhuoli Biotechnology Co, Shanghai, China). All tissue samples applied in this study were fixed in formalin, embedded paraffin and further used for IHC.
2.2. Immunohistochemical staining and analysis
Formalin‐fixed paraffin‐embedded tissues were sliced into 5 μm thickness, and then deparaffinized with xylene, and gradually rehydrated in grated ethanol solutions. Next, the slides were incubated with the anti‐TK1 antibody (1:150; Abcam, Cambridge, MA, UK) at 4°C overnight, followed by incubation with biotinylated goat anti‐rabbit serum streptavidin–peroxidase conjugate at room temperature for 30 minutes. Finally, the sections were developed with diaminobenzidine and counterstained with haematoxylin. Two independent pathologists who were blinded to assess the positivity and intensity of the tissue sections. The percentage of TK1‐positive staining cells was scored as 0 (0%), 1 (1%), 2 (2%) … 99 (99%), 100 (100%). The staining intensity was given 0 (none), 1 (low), 2 (medium) and 3 (high) scores, respectively. A combination of the two scores was calculated by the following formula for all cases: IHC score = positive rate score × intensity score.11
2.3. RNA isolation and quantitative real‐time PCR
Total RNA was isolated from cell pellets using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instruction. The total RNA was then reverse‐transcribed with PrimeScript RT Master Mix (Takara, Tokyo, Japan). For detection of the mRNA expression, qPCR was performed on the Step One Plus Real‐Time PCR system (Applied Biosystems, Carlsbad, CA, USA) with FastStart Universal SYBR Green Master. Three independent experiments were performed with the following condition: 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 15 seconds and 60°C for 1 minute. β‐actin was used as an internal control. The specific primers of human TK1, E2F‐1 and β‐actin were designed by Primer Premier 5 and checked by Oligo 6. The 2−ΔΔCt method was used to analyse the relative expression.
2.4. Cell lines and cell culture
Human PDAC cell lines (MiaPaCa‐2, BxPc‐3, CFPAC‐1, SW1990 and PANC‐1) and the normal human pancreatic ductal cell line hTERT‐HPNE were all purchased from American Type Culture Collection (ATCC). hTERT‐HPNE cells were cultured in keratinocyte serum‐free medium supplemented with epidermal growth factor and bovine pituitary extract according to the recommendation of ATCC. PDAC cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific, New York, USA) supplemented with 10% foetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
2.5. Transfection of small interfering RNA
The siRNA and the scrambled negative control of TK1 and E2F‐1 used in this study were synthesized and purchased from GenePharma (Shanghai, China). The siRNA and the scrambled negative control sequences were as follows: TK1 siRNA#1, 5′‐GG UG AUCAAGUAUGCCAAATT‐3′ (sense) and 5′ UUUGGCAUACUUGAUCACCTT‐3′(antisense); TK1 siRNA#2, 5′‐CCUUCCAGAGGAAGCCAUUTT‐3′ (sense) and 5′‐AAUGGCUUCCUCUGGAAGGTT‐3′ (antisense); TK1 siRNA#3, 5′‐GGC CG GA CAACAAAGAGAATT‐3′ (sense) and 5′‐UUCUCUUUGUUG UCCGG CCTT‐3′ (antisense); negative control, 5′‐UUCUCCGAACGUGUCACGUTT‐3′ (sense) and 5′ ACGUGACACGUUCGGAGAATT‐3′(antisense). PANC‐1 and CFPAC‐1 cells were respectively transfected with siRNA (final concentration: 20 nmol/L) and the scrambled negative control using jetPRIME® transfection reagent (Polyplus, ILLKIRCH, France). The efficiency of all siRNAs was detected by qRT‐PCR and Western blot.
2.6. Western blot analysis
A Total Protein Extraction Kit (Keygen BioTECH, Nanjing, China) was used to extract protein from collected cells which contains PMSF, protease inhibitors and phosphatase inhibitors. Extracted protein was mixed with 5× SDS‐PAGE and further boiled for 5 minutes. Standard methods of Western blot were used to analysis each protein expression,12 β‐actin protein was used as a endogenous reference.
2.7. Proliferation assay
2.7.1. Cell proliferation assay
The PDAC cells were seeded into 96 well plates at a density of 2.5 × 103 cells per well after co‐incubation with siRNA for TK1. 10 μL of Cell Counting Kit‐8 reagent (Dojindo, Tokyo, Japan) and 100 μL complete medium were mixed and added to each well to detect the cell viability. After incubation in dark at 37°C for 3 hours, each well was analysed at 450 nm absorbance.
2.7.2. Clone formation assay
Human pancreatic cancer cell lines treated with or without siRNA of TK1 were plated at a density of 1 × 103 cells/well in 6 well plates. Cells were cultured for 10 days in DMEM supplemented with 10% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) in a 37°C humidified atmosphere of 5% CO2. The colonies were stained with crystal violet solution, and then the number was counted.
2.7.3. Cell cycle analysis
PANC‐1 and CFPAC‐1 cells were harvested after transfection of siRNA for TK1 and negative controls for 72 hours and fixed with 70% ethanol overnight at 4°C, and then incubated with 500 μL PI/RNase staining buffer at 37°C for 15 minutes. Cell cycle was analysed by flow cytometer (Gallios, Beckman Coulter, Brea, CA, USA).
2.7.4. Apoptosis detection
Pancreatic ductal adenocarcinoma cells were harvested after transfection for 48 hours and resuspended in 500 μL Annexin V binding buffer. 5 μL PE Annexin V and 7‐AAD Viability Staining Solution were used to stain tumour cells. After incubation in dark at room temperature for 15 minutes, cells were analysed by flow cytometer (Gallios, Beckman Coulter).
2.7.5. Transwell assay
Cell migration and invasion were respectively performed using Matrigel‐coated and non‐Matrigel‐coated transwell chambers purchased from BD Biosciences (Franklin Lakes, NJ, USA). For both invasion and migration assays, 2.5 × 104 cells in 200 μL serum‐free DMEM after transfection with siRNA or negative controls were seeded in the upper chambers, the lower chambers were filled with 10% FBS DMEM. After cultured 24 and 48 hours, cells on the underside of the chamber were counted.
2.7.6. Wound‐healing assay
Wound‐healing assays were performed in 6 well plates to assess cell migration. A scratch was made 24 hours after transfection. The cells were then cultured in serum‐free DMEM for 24 hours. To assess migrated cells and wound area, pictures were taken at 0, 12 and 24 hours.
2.7.7. Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation (ChIP) was carried out with the Magna Chromatin Immunoprecipitation kit (Millipore, Darmstadt, Germany). Immunoprecipitations were performed with anti‐E2F‐1 antibodies. ChIP products and genomic input DNA were analysed by qRT‐PCR (E2F‐1 primer sequences: forward, 5′‐CCTGGAATTATTCTATCTTGCAGAA‐3′; reverse, 5′‐AATGAATGAA ATGCAGCTTTTTAAC‐3′, TK‐1 primer sequences: forward, 5′‐GCAATCCACCTACCTCTG‐3′; reverse, 5′‐CTCCAAGGCC GTCCCG CAGT‐3′). Data of ChIP are shown as the percentage of input normalized to control purifications.
2.7.8. Animal studies
Animal ethics was approved by Animal Core Facility of Nanjing medical university. Five‐week‐old male BALB/c nude mice were obtained from Model Animal Research Center of Nanjing University. To evaluate the proliferative function of TK1, each mouse was implanted subcutaneously with 1 × 106 PANC‐1 cells. A total of 12 mice were randomly divided into two groups after 4 weeks of tumour injection. siRNA of TK1 or negative control injected into the tumour once every 48 hours. 4 weeks later, the nude mice were sacrificed, and the tumours were excised and photographed.
2.8. Statistical analysis
Statistical analysis was performed by SPSS 20.0 software. A two‐tailed Mann–Whitney U test and Student's t test were used to analysing the statistical differences of proliferative activity and motility of the cells. Survival analysis was assessed by Kaplan–Meier plots. Cox proportional hazard regression model was used to evaluate the independent prognostic factors. Results were presented as the mean ± SD. A significant difference was considered statistically with P‐value <.05.
3. RESULTS
3.1. Expression of TK1 in PDAC
Generally, expression of TK1 mRNA in PDAC tissues (n = 165) was observed based on the data provided by TCGA (Figure 1A). The correlation between TK1 expression and the clinicopathologic characteristics in PDAC from all 165 patients in TCGA were shown in Table 1. It was shown that high level of TK1 was significantly correlated with higher T staging and positively correlated with Ki67 expression (Figure 1F). Furthermore, the Kaplan–Meier survival curves showed that PDAC patients with high level of TK1 have a shorter survival (Figure 1D,E). Cox proportional hazard regression model suggested that TK1 was an independent prognostic factor for PDAC patients (Table 2).
Figure 1.
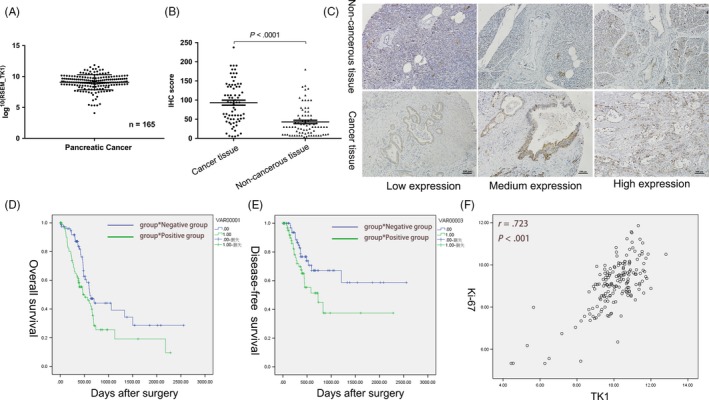
Expression and roles of thymidine kinase 1 (TK1) in pancreatic ductal adenocarcinoma (PDAC). A, TK1 mRNA expression in 165 PDAC samples obtained from TCGA was shown. B, C, Higher expression of TK1 was observed in pancreatic cancer than adjacent non‐tumour tissues by IHC. D, E, Patients with relatively high expression of TK1 have a shorter overall survival and disease‐free survival. F, The level of TK1 expression was positively correlated with Ki67
Table 1.
Association of TK1 expression with clinicopathological features of PDAC
| TK1 | ||||
|---|---|---|---|---|
| Low | High | P | ||
| Histology stage | Well or moderate | 61 | 55 | .253 |
| Poor | 21 | 28 | ||
| N stage | Absent | 22 | 26 | .608 |
| Present | 60 | 57 | ||
| T stage | T1 or T2 | 18 | 9 | .042* |
| T3 or T4 | 64 | 74 | ||
| TNM stage | I‐IIA | 19 | 25 | .313 |
| IIB‐IV | 63 | 58 | ||
PDAC, pancreatic ductal adenocarcinoma; TK1, thymidine kinase 1; TMN, tumour‐node‐metastasis.
*P<.05
Table 2.
Univariate and multivariate analyses
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| TK1 | 1.734 | 1.116‐2.693 | .014 | 1.588 | 1.005‐2.510 | .048 |
| Age | 1.456 | 0.942‐2.250 | .091 | |||
| Gender | 0.934 | 0.608‐1.434 | .755 | |||
| HS | 1.556 | 0.990‐2.445 | .055 | |||
| N stage | 2.001 | 1.193‐3.358 | .009 | 1.113 | 0.352‐3.5166 | .856 |
| T stage | 2.396 | 1.186‐4.838 | .015 | 1.287 | 0.585‐2.832 | .53 |
| TMN stage | 2.323 | 1.333‐4.046 | .003 | 1.806 | 0.513‐6.357 | .357 |
CI, confidence interval.
To further detect the TK1 protein expression in PDAC, 72 pancreatic cancer tissues and matched adjacent tissues were analysed, which revealed that protein levels of TK1 were significantly higher in cancerous tissues compared to that in adjacent non‐cancerous tissues (Figure 1B,C). We also analysed the association between TK1 expression and clinicopathological characteristics in these patients, and TK1 overexpression was correlated with the TNM stage and histological grade (Table 3).
Table 3.
Relationship between TK1 expression and patient characteristics
| Characteristics | TK1 IHC score | |
|---|---|---|
| Standard deviation | P | |
| Gender | ||
| Male | 48.44 ± 43.524 | .640 |
| Female | 36.46 ± 29.277 | |
| Age (year) | ||
| ≤65 | 44.33 ± 35.860 | .631 |
| >65 | 36.46 ± 29.277 | |
| TNM stage | ||
| I‐IIA | 66.44 ± 43.880 | .034 |
| IIB‐IV | 39.60 ± 37.200 | |
| Diameter (cm) | ||
| ≤2 | 72.34 ± 48.937 | .051 |
| >2 | 40.96 ± 37.220 | |
| Location | ||
| Head | 40.14 ± 35.841 | .174 |
| Body, tail | 56.45 ± 47.449 | |
| T stage | ||
| T1 or T2 | 44.65 ± 40.563 | .724 |
| T3 or T4 | 42.50 ± 30.856 | |
| N stage | ||
| Absent | 49.09 ± 41.213 | .091 |
| Present | 33.27 ± 33.482 | |
| M stage | ||
| Absent | 45.91 ± 40.858 | .631 |
| Present | 28.33 ± 12.910 | |
| Blood vessel invasion | ||
| Absent | 49.60 ± 45.346 | .572 |
| Present | 37.62 ± 29.585 | |
| Histological grade | ||
| I/I‐II/II | 38.38 ± 39.396 | .009 |
| II‐III/III | 59.17 ± 36.754 | |
3.2. Knockdown of TK1 expression by siRNA
TK1 expression was also measured in five PDAC cell lines (MiaPaCa‐2, SW1990, BxPc‐3, CFPAC‐1 and PANC‐1) and a normal human pancreatic ductal cell line (hTERT‐HPNE) by qRT‐PCR and Western blot. Compared to hTERT‐HPNE, TK1 expression was higher in all cancer cell lines (Figure 2A,B). CFPAC‐1 and PANC‐1 were chosen to further study due to the relatively higher expression of TK1. CFPAC‐1 and PANC‐1 were used to conduct siRNA‐related assays. The interference efficiency of three siRNAs (labelled as siRNA#1, siRNA#2 and siRNA#3) was detected at both mRNA and protein levels. siRNA#3 with the highest efficiency was chosen for further studies (Figure 2C,D).
Figure 2.
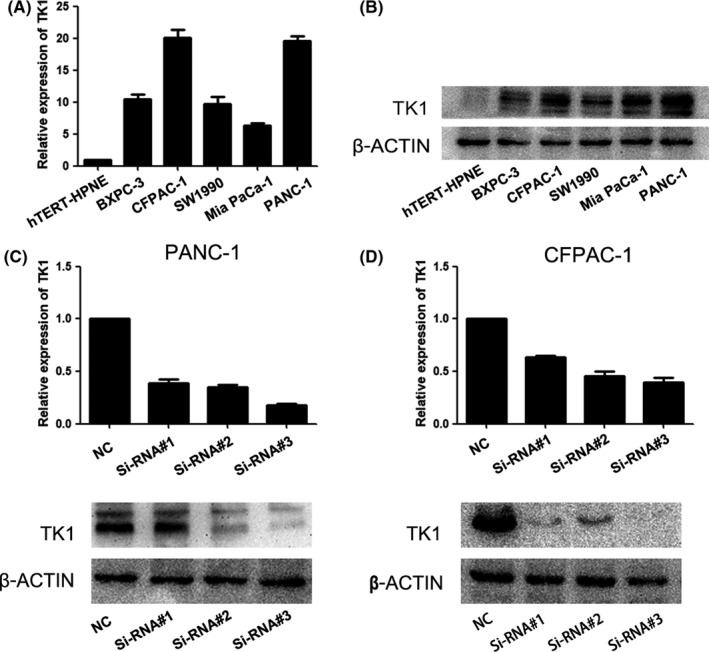
Knockdown of thymidine kinase 1 (TK1) by siRNA. A, B, Relative expression of TK1 in different PDAC cell lines was detected by Western blot. C, D, The different interference efficiency of three siRNAs for TK1 was evaluated by qRT‐PCR and Western blot. Each error bar indicates the variation between the means of three independent experiments
3.3. Effects of gene silencing of TK1 on cell proliferation, apoptosis and cell cycle
We supposed that TK1 played a vital role in the proliferation of PDAC cells because of its correlation with Ki67. Therefore, CCK‐8 and clone formation assays were performed to investigate the effect of TK1 on PDAC cell proliferation. As shown in Figure 3, after transfection with siRNA of TK1, the proliferation of PDAC cells was remarkably decreased than the control group. Moreover, the cell clone formation assay showed the similar result. The number of cell colonies was decreased significantly in TK1‐knockdown cells group than the control group.
Figure 3.
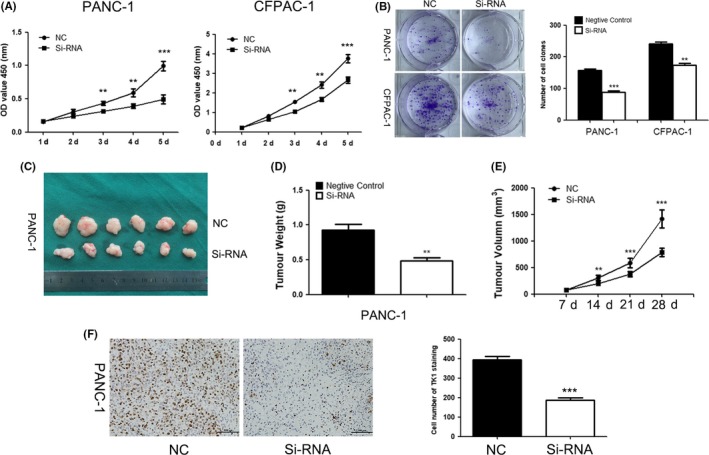
Effects of thymidine kinase 1 (TK1) downregulation on cell proliferation (A) CCK‐8 assay showed that inhibition of TK1 expression suppressed PDAC cell proliferation. (B) The colon formation assay was performed to verify that gene silencing of TK1 suppressed cell proliferation. (C) Tumour formation after injecting siRNA of TK1 or negative control in nude mice. (D, E) The tumour volume was measured and calculated everyone week after injection, the weights of tumours obtained from the animal model were decreased after TK1 downregulation by siRNA. (F) Ki‐67 index of tumours in TK1 downregulation group or negative control group ** represents P < .01, and *** represents P < .001
To investigate the effect of TK1 knockdown on proliferation of PDAC cells in vivo, a xenograft model of subcutaneous tumour in nude mice was applied. Subcutaneous tumours were harvested and taken images (Figure 3C). The volume and weight of tumours from the TK1‐interfered group were lower than the negative control group (Figure 3D,E). We further detected the expression of Ki‐67 by IHC, and decreased Ki‐67 expression was observed in the TK1‐knockdown group (Figure 3F). These results above revealed that gene interfering of TK1 might attenuate the proliferation of PDAC cells in vivo and in vitro.
No significant statistical difference of the percentage of apoptotic cells was observed between TK1 downregulation group and negative control group (Figure 4A). Meanwhile, there was little change in expression of the apoptosis‐related protein, apoptosis may not be involved in proliferation change of TK1 downregulation. As shown in Figure 4C, downregulation of TK1 induced an increased cell percentage in S phase compared to control group. These results indicated that interference of TK1 could suppress PDAC cell proliferation by S phase arrest.
Figure 4.
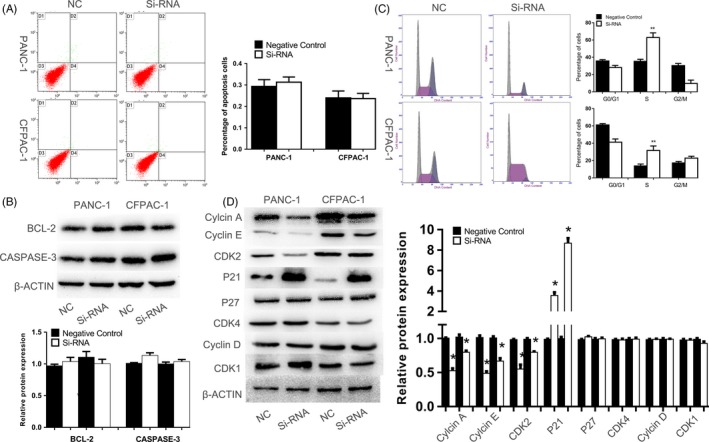
Effects of gene silencing of thymidine kinase 1 (TK1) on apoptosis and cell cycle. A, B, There was no statistical difference on the percentage of apoptotic cells and apoptosis‐related proteins between two groups. C, D, Western blot indicated increased P21 was responsible for S phase arrest which was induced by TK1 knockdown. * represents P < .05, and ** represents P < .01
Western blot was used to further examine the effect of downregulation of TK1 on the expression of cell cycle and apoptotic related proteins. Several proteins (cyclin A, cyclin B1, cyclin D1, cyclin E, CDK2, CDK4, CDK1, P21, P27 which might play a fundamental role in S phase) were further assessed by Western blot. It has been proven that Cyclin E and CDK2 could regulate normal S phase progression,13, 14 and we found downregulation of TK1 decreased the expression levels of cyclin E and CDK2 and increased the expression of P21. The result indicated that downregulation of TK1 in PDAC cells may induce S phase arrest by upregulating P21.
3.4. Downregulation of TK1 exhibit no effect on migration and invasion
We conducted migration and invasion assay in vitro to explore the effects of TK1 knockdown on migration and invasion of PDAC cells. As shown in Figure S1, downregulation of TK1 had no impact on migration and invasion of PDAC cells. The result was also confirmed by detecting the key proteins involved in migration and invasion of cancer cells with Western blot (Figure S1).
3.5. Expression of TK1 was directly regulated by E2F‐1
As an essential gene involved in DNA replication and synthesis, it is reported that TK1 was directly regulated by E2F‐1 in several studies.15, 16, 17, 18 To find out whether E2F‐1 regulates the TK1 transcription in PDAC cells, qRT‐PCR, ChIP and Western blot assays were conducted. Firstly, the interference efficiency of three siRNAs (labelled as siRNA#1, siRNA#2 and siRNA#3) was detected by qRT‐PCR and Western blot; siRNA#1 with the highest efficiency was chosen for further studies (Figure 5A). qRT‐PCR and Western blot assay showed that the expression of TK1 was decreased in E2F‐1 knockdown cells at mRNA and protein levels (Figure 5B). Besides, the results of ChIP assay indicated that E2F‐1 could bind to the promoter of TK1 and the binding efficiency was decreased in E2F‐1 knockdown cells (Figure 5C). Furthermore, the proliferation of PDAC cells was inhibited after E2F‐1 knockdown (Figure 5D). These results suggested that E2F‐1 might directly regulate the transcription of TK1 to further affect the cell proliferation in PDAC cells.
Figure 5.
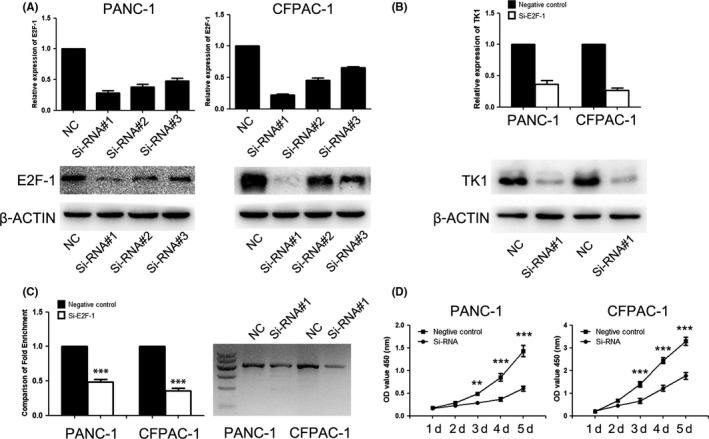
Expression of TK1 was directly regulated by E2F‐1. A, Western blot and RT‐PCR were used to validate the interference efficiency of three siRNAs for E2F‐1. B, The expression of TK1 was downregulated by siRNA for E2F‐1. C, The promoter of TK1 could be combined with E2F‐1, and TK1 expression was decreased in E2F‐1 knockdown group. D, The proliferation PDAC cells were affected after E2F‐1 knockdown. ** represents P < .01, and *** represents P < .001
4. DISCUSSION
In this study, we have identified high expression of TK1 was tightly correlated with the progression and prognosis of PDAC. In addition, E2F‐1 could directly regulate TK1 transcription and promote PDAC cell proliferation. As far as we know, this study first identified aggressive characters of TK1 in PDAC, which suggested that TK1 could function as a prognostic and therapeutic target for PDAC.
It was generally known that plentiful oncogenes and complex mechanisms played a vital role in development and progression of PDAC. TK1 was a type of TK which plays a significant role in mammalian cell cycle due to its vital function in DNA replication and repair. Increasing activity of TK1 during S phase could promote cell cycle from S phase to G2/M phase.19 TK1 was overexpressed in mitotic or malignant cells, but absent in quiescent cells.20, 21, 22 Lots of studies have focused on the diagnostic and prognostic value of TK1 in many tumours.23 Mao et al6 discovered the application of TK1 as a cell proliferation marker in human breast cancer. Kruck et al8 observed that high TK1 expression has been described to be associated with a higher stage, and grade in renal cell carcinoma. Moreover, TK1 has been assessed as a marker for prediction of biochemical recurrence in prostate cancer.24 However, the expression pattern and function of TK1 in PDAC are not elucidated. In this study, relationship between TK1 expression and patient characteristics analysis indicated that high‐TK1 expression correlated with Ki67, higher T stage (from TCGA data statistics), and advanced tumour stage (from IHC data statistics). We also observed that overexpression of TK1 in PDAC was associated with a shorter OS time and DFS time of PDAC patients. Further analysis identified that TK1 was an independent risk factor for poor prognosis.
Furthermore, we found that downregulation of TK1 could inhibit cell proliferation in vivo and in vitro, and the mechanisms involved in this phenomenon were still unclear. Cell cycle and apoptosis were confirmed to be involved in the process of proliferation.25, 26 Therefore, we supposed that whether these processes contribute to the proliferation of PDAC cells. Although no obvious changes were observed in apoptosis, S phase arrest was observed in PDAC cells by TK1 knockdown. We found that the inhibition of S phase was concomitant with decreased CDK2, cyclin A and cyclin E expressions in TK1 knockdown PDAC cell, as well as increased P21 expression. P21, known as an inhibitor of the cyclin‐dependent kinase, could inhibit the activation of CDK1, CDK2 and CDK4.27, 28, 29, 30 TK1 might interact with P21 by combining with the C‐terminal domain of P21.31 Our data showed that CDK2 was obviously downregulated after interfering TK1; however, CDK1 and CDK4 expressions might not be affected by the change of TK1. PDAC cell cycle arrest after TK1 downregulation might be mediated by P21/CDK2 interaction.
No evidence has shown that overexpression of TK1 was correlated with blood vessel invasion in clinicopathologic characteristics of PDAC patient. Accordingly, migration, invasion and Western blot assays further proved that there was no relation between TK1 overexpression and migration or invasion of PDAC cells.
We have identified the downstream genes regulated by TK1, and the upstream regulatory factors for TK1 were further investigated in our study. E2F‐1, as an important transcription factor, is required for timely control of plentiful genes involved in DNA replication, repair and cell cycle.15 The previous study has reported that E2F‐1 could directly bind to the promoter of TK1.15, 16, 17, 18 We also confirmed that E2F‐1 was an upstream regulatory gene of TK1 in PDAC. Furthermore, there were some other factors that could regulate TK1, including C‐MYC,32 FHIT,33 SP134 and Prohibition,17 which might relate to potential pathways and involved in regulation of TK1. Further studies should be designed to explore the mechanism of TK1 in PDAC.
To the best of our current knowledge, this study first revealed the overexpression of TK1 in PDAC. Abnormal expression of TK1 is associated with progression of PDAC and a poor prognosis of PDAC patients. Downregulation of TK1 impedes PDAC proliferation both in vitro and in vivo experiments. As a transcription factor, E2F‐1 could directly mediate expression of TK1. Therefore, TK1 could serve as a new anti‐cancer target for treating PDAC.
ACKNOWLEDGEMENTS
This work was supported by Talents planning of six summit fields of Jiangsu Province (WSW‐021), the Natural Science Foundation of Jiangsu Province (BK20151027) and the Innovation Capability Development Project of Jiangsu Province (No. BM2015004).
CONFLICT OF INTEREST
The authors confirm that there is no conflict of interest.
Supporting information
Zhu X, Shi C, Peng Y, et al. Thymidine kinase 1 silencing retards proliferative activity of pancreatic cancer cell via E2F1‐TK1‐P21 axis. Cell Prolif. 2018;51:e12428 10.1111/cpr.12428
XZ, CS and YP contributed equally to this work.
Contributor Information
Qiang Li, Email: liqiang020202@163.com.
Yi Miao, Email: miaoyi@njmu.edu.cn.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21. [DOI] [PubMed] [Google Scholar]
- 3. Bello LJ. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974;89:263. [DOI] [PubMed] [Google Scholar]
- 4. Johnson LF, Rao LG, Muench AJ. Regulation of thymidine kinase enzyme level in serum‐stimulated mouse 3T6 fibroblasts. Exp Cell Res. 1982;138:79‐85. [DOI] [PubMed] [Google Scholar]
- 5. Arnér ESJ, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155‐186. [DOI] [PubMed] [Google Scholar]
- 6. He Q, Wu JP, Mao RY, Wang N, He LX, Skog S. The clinical significance of thymidine kinase 1 measurement in patients with breast cancer using anti‐TK1 antibody. Int J Biol Markers. 2000;15:139. [DOI] [PubMed] [Google Scholar]
- 7. Gakis G, Hennenlotter J, Scharpf M, et al. XPA‐210: a new proliferation marker to characterize tumor biology and progression of renal cell carcinoma. World J Urol. 2011;29:801‐806. [DOI] [PubMed] [Google Scholar]
- 8. Kruck S, Hennenlotter J, Vogel U, et al. Exposed proliferation antigen 210 (XPA‐210) in renal cell carcinoma (RCC) and oncocytoma: clinical utility and biological implications. BJU Int. 2012;109:634. [DOI] [PubMed] [Google Scholar]
- 9. Xu Y, Liu B, Shi QL, et al. Thymidine kinase 1 is a better prognostic marker than Ki‐67 for pT1 adenocarcinoma of the lung. Int J Clin Exp Med. 2014;7:2120‐2128. [PMC free article] [PubMed] [Google Scholar]
- 10. Kameyama R, Yamamoto Y, Izuishi K, Sano T, Nishiyama Y. Correlation of 18F‐FLT uptake with equilibrative nucleoside transporter‐1 and thymidine kinase‐1 expressions in gastrointestinal cancer. Nucl Med Commun. 2011;32:460‐465. [DOI] [PubMed] [Google Scholar]
- 11. Peng YP, Zhu Y, Yin LD, et al. PEG10 overexpression induced by E2F‐1 promotes cell proliferation, migration, and invasion in pancreatic cancer. J Exp Clin Cancer Res. 2017;36:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang JJ, Zhu Y, Xie KL, et al. Yin Yang‐1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C‐dependent mechanism. Mol Cancer. 2014;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malumbres M, Barbacid M, Malumbres M. Barbacid MCell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153‐166. [DOI] [PubMed] [Google Scholar]
- 15. Adrian P, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409. [DOI] [PubMed] [Google Scholar]
- 16. Ishida S, Huang E, Zuzan H, et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koushyar S, Economides G, Zaat S, Jiang W, Bevan CL, Dart DA. The prohibitin‐repressive interaction with E2F1 is rapidly inhibited by androgen signalling in prostate cancer cells. Oncogenesis. 2017;6:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren B, Cam H, Takahashi Y, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chabes A, Thelander L. DNA building blocks at the foundation of better survival. Cell Cycle. 2003;2:171. [DOI] [PubMed] [Google Scholar]
- 20. Gross MK, Merrill GF. Thymidine kinase synthesis is repressed in nonreplicating muscle cells by a translational mechanism that does not affect the polysomal distribution of thymidine kinase mRNA. Proc Natl Acad Sci USA. 1989;86:4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kit S. Thymidine kinase, DNA synthesis and cancer. Mol Cell Biochem. 1976;11:161‐182. [DOI] [PubMed] [Google Scholar]
- 22. Piper AA, Tattersall MH, Fox RM. The activities of thymidine metabolising enzymes during the cell cycle of a human lymphocyte cell line LAZ‐007 synchronised by centrifugal elutriation. Biochem Biophys Acta. 1980;633:400. [DOI] [PubMed] [Google Scholar]
- 23. Aufderklamm S, Todenhöfer T, Gakis G, et al. Thymidine kinase and cancer monitoring. Cancer Lett. 2012;316:6. [DOI] [PubMed] [Google Scholar]
- 24. Aufderklamm S, Hennenlotter J, Todenhoefer T, et al. XPA‐210: a new proliferation marker determines locally advanced prostate cancer and is a predictor of biochemical recurrence. World J Urol. 2012;30:547. [DOI] [PubMed] [Google Scholar]
- 25. Blagosklonny MV. Cell cycle checkpoints and cancer. Protein Nucleic Acid & Enzyme. 2002;54:556‐560. [PubMed] [Google Scholar]
- 26. Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251. [DOI] [PubMed] [Google Scholar]
- 27. Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Wgjr K. Identification of a cyclin‐cdk2 recognition motif present in substrates and p21‐like cyclin‐dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623‐6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Detjen KM, Brembeck FH, Kaiser A, Riecken EO, Rosewicz S. Protein kinase C α inhibits growth of human pancreatic carcinoma cells via P21 WAF ‐mediated inhibition of CDK2 activity. Gastroenterology. 1998;114(Suppl 1):A1138‐A1139. [Google Scholar]
- 29. Labaer J, Garrett MD, Stevenson LF, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847‐862. [DOI] [PubMed] [Google Scholar]
- 30. Göke R, Barth P, Schmidt A, Samans B, Lankat‐Buttgereit B. Programmed cell death protein 4 suppresses CDK1/cdc2 via induction of p21(Waf1/Cip1). Am J Physiol Cell Physiol. 2004;287:C1541. [DOI] [PubMed] [Google Scholar]
- 31. Huang DY, Chang ZF. Interaction of human thymidine kinase 1 with p21(Waf1). Biochem J. 2001;356:829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pusch O, Soucek T‐OE, Bernaschek G, Hengstschlager M. Cellular targets for activation by c‐Myc include the DNA metabolism enzyme thymidine kinase. DNA Cell Biol. 1997;16:737‐747. [DOI] [PubMed] [Google Scholar]
- 33. Kiss DL, Waters CE, Ouda IM, et al. Identification of Fhit as a post‐transcriptional effector of thymidine kinase 1 expression. Biochim Biophys Acta. 2017;1860:374‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karlseder J, Rotheneder H, Wintersberger E. Interaction of Sp1 with the growth‐ and cell cycle‐regulated transcription factor E2F. Mol Cell Biol. 1996;16:1659‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


