Abstract
Objective
Despite a great number of studies analysing the effects of microgravity on stem cell proliferation and differentiation, few of them have focused on real‐time imaging estimates in space. Herein, we utilized the TZ‐1 cargo spacecraft, automatic cell culture equipment and live cell imaging techniques to examine the effects of real microgravity on the proliferation and differentiation of mouse embryonic stem cells (mESCs).
Materials and methods
Oct4‐GFP, Brachyury‐GFP mESC and Oct4‐GFP mESC‐derived EBs were used as experimental samples in the TZ‐1 spaceflight mission. These samples were seeded into chambers, cultured in an automatic cell culture device and were transported into space during the TZ‐1 mission. Over 15 days of spaceflight, bright field and fluorescent images of cell growth were taken in micrography, and the medium was changed every day. Real‐time image data were transferred to the ground for analysis.
Results
Space microgravity maintains stemness and long‐term survival of mESCs, promising 3D aggregate formation. Although microgravity did not significantly prevent the migration of EBs on the ECM substrate, it did prevent terminal differentiation of cells.
Conclusions
This study demonstrates that space microgravity might play a potential role in supporting 3D cell growth and maintenance of stemness in embryonic stem cells, while it may negatively affect terminal differentiation.
Keywords: differentiation, embryonic stem cell, microgravity, proliferation
1. INTRODUCTION
In a microgravity environment, a variety of fundamental physical phenomena become significantly altered, resulting in bone loss, immune alterations, muscle atrophy, etc.1 Recently, the influence of microgravity on cell growth and tissue regeneration has gained significant attention. Several studies investigating the role of microgravity on cell proliferation and differentiation have demonstrated that cells grown in a microgravity environment develop differently from those grown under 1 × g conditions, resulting in changes in spreading, migration, contraction and division.2
Embryonic stem cells (ESCs) have the capability to self‐renew and differentiate into all cell types and provide a great model for studying stem cell responses to microgravity, holding great promise as a robust cell source for tissue engineering in microgravity.3 Although a variety of Earth‐bound equipment has been used to study the effect of microgravity on proliferation and differentiation of ESCs (eg, the rotating wall bioreactor (RWB), the rotary cell culture system (RCCS) and the 3D clinostat),4, 5 results concerning the effect of microgravity on ESCs vary and are controversial. Some ground‐based results have demonstrated that mouse ESCs (mESCs) cultured in a simulated microgravity (SMG) bioreactor showed unique differentiation profiles, such as expression of specific haematopoietic genes6 and enhancement of hepatic differentiation.7 In contrast, other studies have suggested that SMG impairs ESC properties8 (eg, cell numbers, adhesion capabilities and apoptosis rates) and reduces their differentiation capabilities9 (eg, cardiomyogenesis). Thus, spaceflight experiments are a unique opportunity to assess the effect of real microgravity on cellular function. So far, a subset of spaceflight experiments has focused on studying the differentiation and proliferation capacity of adult stem cells. Exposure to microgravity to model in vivo mechanical unloading of bone reduces early mesenchymal and haematopoietic stem cell differentiation. Additionally, spaceflight increases the expression of genes related to nervous system development in bone marrow‐derived mesenchymal stem cells.10 Experiments on the STS‐63 and STS‐69 space missions found that spaceflight decreased total cell number and erythropoiesis while increasing macrophage differentiation in CD34+ bone marrow progenitor cells.11
Recently, Blaber et al reported using embryoid bodies (EBs) as a model to test the impacts of space microgravity on early lineage commitment of mESCs. They demonstrated that microgravity inhibited EB differentiation and maintained stemness of EBs by gene expression analysis and post‐microgravity cellular differentiation and viability assays when the samples returned to Earth.12 However, there was no system available that allowed long‐term culture of stem cells or real‐time imaging in space microgravity.
Previously, we investigated the role of rotary suspension culture in early differentiation of mouse embryoid bodies (mEBs). We found that the RCCS enhances mesendoderm differentiation of mESCs by modulating Wnt/β‐catenin signalling.13 In this study, through bright field and fluorescent imaging on the Chinese TZ‐1 cargo spacecraft, we report results of a fifteen‐day spaceflight on the proliferation of mESCs and the differentiation properties of mEBs in real time. We found that mESCs exhibit features of 3D growth, survive longer and retain enhanced marker for stemness in space microgravity. In addition, we noted that although mEBs cultured in attachment dishes were able to develop outgrowth on the ECM substrate under microgravity conditions, they expressed lower levels of Oct4 and higher levels of brachyury in a spontaneous differentiation system. Differentiated cells were still at the mesendoderm stage of differentiation after 15 days in culture. These findings may increase our understanding of the effect of space microgravity on the proliferation of mammalian ESCs and initiation of early differentiation.
2. MATERIALS AND METHODS
2.1. Cell culture
The Oct4‐GFP14 and Brachyury‐GFP15 reporter mESC line, as well as the Oct4‐GFP mESC‐derived EB line16, were used as experimental models in the TZ‐1 spaceflight mission. For ESC expansion and EB production on the ground, mESCs were maintained under standard conditions on inactivated mouse embryonic fibroblasts (MEFs) as we previously described.16 mESCs were passaged every 3 days onto inactivated MEFs. For mESC culture in space, we utilized a chemically defined system to culture ESCs instead of the conventional serum and LIF medium used in previous studies.17, 18 In brief, mESCs were cultured in N2B27 complete medium. One day before launch, Oct4‐GFP mESCs were seeded in matrigel‐coated space culture chambers at a density of 1000 cells per chamber, covered with 1.8 mL of N2B27 complete medium, and incubated at 37°C in a cell culture bioreactor.
2.2. EB formation and differentiation
For EB formation from mESCs, Oct4‐GFP and Brachyury‐GFP mESC colonies were treated as previously described, with some modifications.16 mESCs colonies were dissociated from dishes with 1 mg/mL collagenase IV (Invitrogen) at room temperature for 2 minutes to obtain ESC aggregates. Then, aggregates were further dissociated with 0.05% trypsin digestion to obtain single cell suspensions. Next, dissociated cells were incubated in 10‐cm dishes for 30 minutes at 37°C in 5% CO2 for further purification. The purified mESCs were forced to aggregate by hanging drop (1000 cells/10 μL) onto the lids of Petri dishes with EB differentiation medium. Dishes were incubated at 37°C for 24 hours. After incubation, cells had formed EBs that were uniform in size. EBs were washed off into Corning low attachment dishes with suspension culture, where they were kept until seeding into space culture chambers.
2.3. Hardware specifications
The bioreactor for stem cell culture was manufactured by the Shanghai Institute of Technical Physics of the Chinese Academy of Sciences to match the specifications and safety standards of the TZ‐1 cargo spacecraft mission. The bioreactor consisted of culture chambers, medium storage bags, cell culture modules (CCM) and an electronic container. Each culture chamber (44 mm × 22 mm × 10 mm) was loaded with 1.8 mL of medium. Two chambers were connected in series as a group that including an observation window (Figure 1A). Two medium storage bags, modified from blood bags, were used for medium storage and liquid waste recovery (Figure 1B). As a fully automated system, CCM provided temperature control, microscope imaging, medium recirculation and medium routing via micro peristaltic pumps (Figure 1C) and pinch valves. Medium nutrients were delivered through the micro peristaltic pump from the medium storage bag to the waste bag. Temperatures inside the cell culture chamber were 36.7 ± 0.5°C, and the storage bags were 4 ± 0.5°C. Images of cultures were obtained with a dual‐magnification microscope (10 × and 20 × focus) with programmable controls for automatic micrography.
Figure 1.

Establishment of an automated culture system to culture mouse embryonic stem cells (mESCs) and embryoid bodies (EBs). A, In our cell culture chamber, real‐time growth of cells can be monitored by microscopy through the window (white arrow). B, Pictures of the medium storage bags (red dotted line) and waste bags (white dotted line). C, Micro peristaltic pumps, which provide medium change with a flow rate of 400 μL/min in these experiments. D, Cell culture module (CCM), consisting of 2 connecting space culture chambers linked with the bags and with the pump. E, The electronic container, together with CCM, forms the complete experimental hardware, called the bioreactor. F, Representative bright field images of mESCs cultured with N2b27/2i medium using the automated culture system in ground conditions. Scale bars = 100 μm. G, Representative bright field images of EBs cultured with differentiation medium using the automated culture system in ground conditions. Scale bars = 200 μm
2.4. TZ‐1 spaceflight experiment: cell loading, module installation, check‐up and location on the cargo spacecraft
Cells were transported into space during the Chinese TZ‐1 mission, which was launched by rocket Long March 7 at the Wenchang cosmodrome on April 20, 2017. Thirty hours before launch, proliferation and differentiation media were inflated with a gas mixture (5% O2, 5% CO2 and 90% N2) for about two hours, and 6 cell culture chambers containing 1.8 mL of gas‐saturated medium were loaded with 3 different cell samples (Oct4‐GFP mESCs, Oct4‐GFP EBs, and Brachyury‐GFP EBs) and incubated at 37°C in 5% CO2 temporarily. Twenty hours before launch, the space cell culture chambers were assembled in CCM. Images of cells acquired 12 hour prior to launch were considered the initial states of cells on the ground. Six hours before launch, CCMs were installed into the electronic container, and the installed bioreactor was transferred onto the TZ‐1 cargo spacecraft. Two hours after the spacecraft entered orbit, the cell culture medium was changed automatically with a flow rate of 400 μL/min, and the first images in space were obtained. During 15 days of spaceflight, pictures of the cells were taken daily by micrography, and the medium was changed every day. Real‐time image data were transferred to the receiving station on the ground. Corresponding ground controls for the experiment were maintained at the laboratory in Beijing. As controls, experiments were performed following the same exact procedure on the ground in the spare cell culture bioreactor environment.
2.5. Cell area and fluorescence intensity analysis
For cell area measurement, the surface area of the colonies in each image was measured using image J software and normalized according to previously reported.19 We also analysed the cell fluorescence intensity of Oct4‐GFP using image J software as previously described.20, 21 Briefly, a cell expressing Oct4‐GFP was selected using any of the drawing/selection tools. From the analyse menu, “set measurements” was selected. Then, “Measure” was selected under the same menu. A box appeared with a stack of values for the first cell. Next, we selected a region next to the positive cell that had no fluorescence. This step was repeated for other cells in the field of view to measure. Once the fluorescence level of all cells was measured, we selected all of the data in the Results window and copied and pasted it into an excel spreadsheet. We used this formula to calculate the corrected total cell fluorescence (CTCF). Finally, we generated a graph for the fluorescent intensity data.
2.6. Statistical analysis
The cell area and fluorescence intensity of cells with GFP was analysed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Data are presented as the mean ± standard deviation (SD). Student's t test was used to determine the significance levels. Significantly different values are shown with asterisks (*P < .05; ** P < .01; ***P < .001).
3. RESULTS
3.1. Automated culture system and cell culture tests
Due to resource limitation and the non‐recoverability of the unmanned TZ‐1 cargo spacecraft, we were unable to use a conventional culture system with additional CO2 and manual image systems in our present study. Therefore, establishment of an automatic culture system without CO2 conditions is crucially important to study mESC development in space. To carry out the spaceflight experiments, we designed and developed a bioreactor for cell culturing. The bioreactor was composed of culture chambers, liquid storage vessels, cell culture modules (CCM) and an electronic container. Two cell culture chambers (44 × 22 × 10 mm) with an imaging window on each were connected in series as a group (Figure 1A). The medium storage bags were filled with the cell culture medium, and the empty bags were used for liquid waste recovery (Figure 1B). The tubing of the chambers connected the storage bags with the micro peristaltic pump (Figure 1C), allowing medium exchange. After cell seeding, the cell chambers and storage vessels were installed in the CCM (Figure 1D), the medium was transferred into the space cell chamber by those micro pumps, and the culture supernatant was driven into the empty bags. The assembled CCM was mounted into the electronic container (Figure 1E), which provided power and automatically maintained temperature control, changed medium and imaging. To determine the feasibility of this culture system, we seeded mESCs and EBs in separate cell culture chambers and incubated cells in the automatic bioreactor without CO2 supplement under normal 1 × g conditions. Time‐lapse microscopy revealed that mESCs cultured in matrigel‐coated chambers grew well and propagated. After 4 days in culture, mESCs were able to form colonies in a feeder free system with N2b27/2i medium (Figure 1F). We also observed growth of EBs in the automatic culture system. As shown in Figure 1G, EBs attached to the bottom of the chamber, gradually spread out, and flattened over 4 days of culturing. These results indicate that the automated culture system could be used to culture mESCs and EBs for the subsequent spaceflight experiment.
3.2. Spaceflight cultivation enhances cell survival, maintains stemness and forms 3D aggregates of mESCs
Cell samples were seeded into each chamber and launched into space on April 20, 2017 (Methods). Two hours after the spacecraft arrived in orbit, cell culture medium was changed automatically with a flow rate of 400 μL/min, and then the images were produced synchronously. Images of cells in the space culture chamber were daily acquired by micrography, and medium was changed every day during the 15‐day spaceflight (Figure 2A). As shown in Figure 2B and Figure 2C, mESCs exhibits a significant difference in cell morphology and characters between spaceflight group and normal 1 × g ground group. We found that cells exposed in microgravity were prone to propagate with multilayer colonies and confluence of a cell cluster after 6 days in culture, while cell in 1 × g appeared monolayer colonies on the surface of matrigel. We also quantified the growth surface area of mESCs on different conditions. As exemplified in Figure 2D,E, there were an obvious increase of cell growth area both in space and 1 × g condition during culturing, while, the cell area depicts significantly higher values for cells in space than those in 1 × g condition (Figure 2F). We also analysed the expression of Oct4‐GFP of mESCs. Purified Oct4‐GFP mESCs were prepared and seeded in each chamber (Figure 3A). Each chamber trapped 3 different positions for imaging through optical and fluorescence microscopes. Collected images were transmitted to Earth every day. Hundreds of high‐resolution mESC photos were received in this study. During the first few days of culture, we observed that mES colonies formed monolayers in both space and ground‐control cultures, and both groups displayed high Oct4‐GFP expression (Figure 3B,C). After 6 days in culture, most of the mESC colonies in space had aggregated and began to form a 3D structure (Figure 3B). Importantly, expression of Oct4 increased during spaceflight, as indicated by stronger GFP expression over time (Figure 3B,D). However, the number and size of mESCs in ground controls were reduced after 6 days in culture, and Oct4 expression declined during subsequent days (Figure 3C,D). It is noteworthy that mESCs cultured in space exhibited significantly higher expression of GFP than those cultured on the ground, particularly after 6 days of culture (Figure 3D). These results suggest that the space microgravity environment plays a role in long‐term survival and maintenance of stemness in mESCs, as well as 3D aggregate formation.
Figure 2.
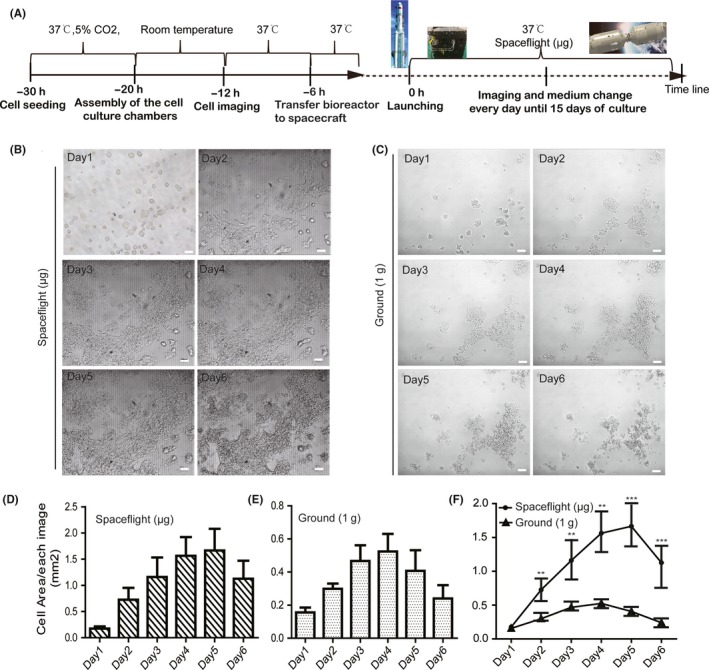
Comparison of mESCs growth in space microgravity and ground 1 g condition. A, Schematic illustration of procedure to load cells, install hardware and culture cells in the TZ‐1 spaceflight mission. Typical optical images showing the mESCs morphologies during 6 days of culture in spaceflight condition (B) or ground 1 × g condition (C). Scale bars = 100 μm. The growth areas of mESCs were measured for 3 typical images during 6 days of culture in spaceflight (D) and ground 1 × g condition (E). The results showed a notable increase in cell proliferation in a time‐dependent manner when cultured in spaceflight μg compared with 1 g ground condition (F). All results are shown as mean ± SD of with 3 independent images, with significance indicated by **P < .01 vs ground, ***P < .001 vs ground
Figure 3.
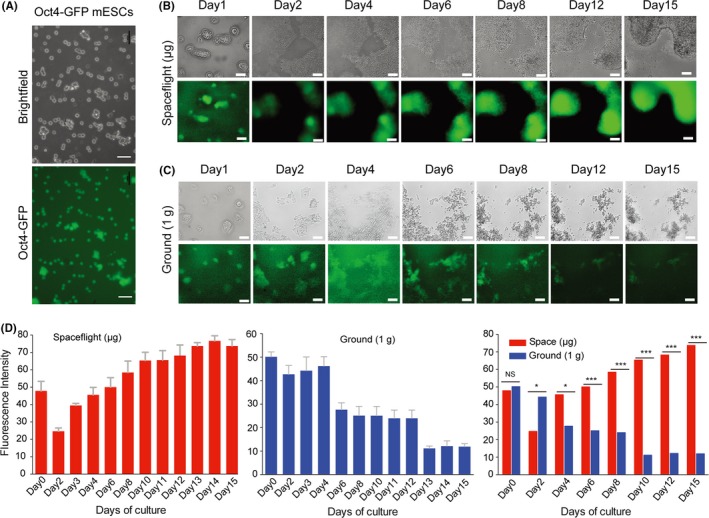
Effect of space microgravity on mESCs morphology and the expression of stem cell self‐renewal markers of mESCs. A, Purified Oct4‐GFP mESCs were prepared for seeding into chambers. Scale bars = 50 μm. B, C, Morphological characteristics, cell number and GFP expression in Oct4‐GFP transgenic mESCs by time‐lapse imaging over 15 days of culture in spaceflight and ground conditions. Scale bars are 100 μm. D, Relative mean fluorescence intensity between the spaceflight‐cultured and ground‐cultured groups over 15 days of culture. All results are shown as mean ± SD of at 3 independent images, with significance indicated by *P < .05 vs ground, ***P < .001 vs ground. NS=no significant
3.3. Outgrowth and differentiation of embryoid bodies during spaceflight
To explore the effects of spaceflight on ESC differentiation, we used the Oct4‐GFP mESC‐derived EB‐explant outgrowth culture as a model (Figure 4A). EBs in both the space and ground‐control groups successfully adhered to the chamber surface (on day 1), and cell outgrowth was clearly observed in both groups (on day 3). After 5 days in culture, some differentiated cells displaying a mesenchymal‐like morphology were found surrounding the adherent EBs (Figure 4B). After 8 days in culture, cells had evenly expanded throughout the chamber, reaching subconfluency on day 12 (Figure 4B). Interestingly, although in general the number of cells expressing GFP was significantly reduced in both the space and ground groups during the first 3 days (Figure 4C,D and E), some clusters of EBs in the space culture group retained obvious expression of Oct4‐GFP from days 5 to 15 (Figure 4C). Furthermore, we found that after 2 days of culture, Oct4‐GFP expression in EBs in the space culture group was significantly higher than EBs cultured in 1 × g ground conditions (Figure 4E). Therefore, outgrowth of EBs, alterations in cell morphology and decreases in Oct4 expression together suggest the occurrence of differentiation in space, but it seems that EB differentiation in space is slower than in normal 1 × g conditions. To confirm such outgrowth characteristics and differentiation patterns of EB in microgravity, we further cultured Brachyury‐EBs in the space environment and assessed their capacity for differentiation into mesendoderms. As expected, similar to the results seen with Oct4‐GFP EB differentiation, cell outgrowth was also clearly observed surrounding adherent EBs, both in space and 1 × g culture groups. After 5 days in culture, the cells at 1 × g had stably and evenly expanded throughout the culture chamber and reached confluency, while many cell aggregates were still observed in the space samples, and these cell masses became larger as they continued to grow (Figure 5A,B). Furthermore, as shown in Figure 5B, GFP‐expressing EBs were initially detected after 5 days of culture in space, while some Brachyury‐GFP cells were found on day 3 in ground‐cultured EBs (Figure 5C). Analysis of the mean fluorescence intensity showed that Brachyury‐GFP expression in space EBs was lower than in 1 × g at days 3 and 4, while this trend was reversed after 8 days in culture (Figure 5D). Together, these results suggest that although microgravity did not prevent the migration of EBs onto the ECM substrate, microgravity did inhibit effective terminal differentiation of mEBs.
Figure 4.
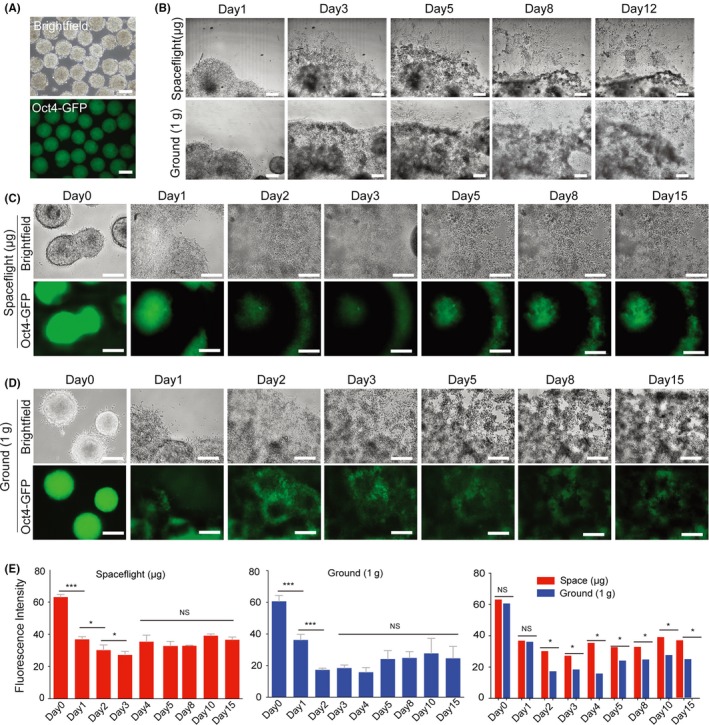
Cell adhesion, outgrowth and differentiation of Oct4‐GFP EBs cultured in space and ground environments. A, Formation of Oct4‐GFP EBs was used to analyse the characteristics of spontaneous differentiation during spaceflight. B, Representative bright field images at low magnification showing EB morphologies and outgrowth from day 1 to day 12 in culture. C, D, Phase contrast and GFP fluorescence images showing cell morphologies and Oct4 expression for the differentiation of Oct4‐GFP EBs over 15 days of culture in spaceflight and ground 1 × g conditions. All scale bars = 200 μm. E, Mean fluorescence intensity of spaceflight‐cultured and ground‐cultured OCT4‐GFP EBs. All results are shown as the mean ± SD of at 3 independent images. NS = no significant, (*) = P < .05, (***) = P < .001
Figure 5.
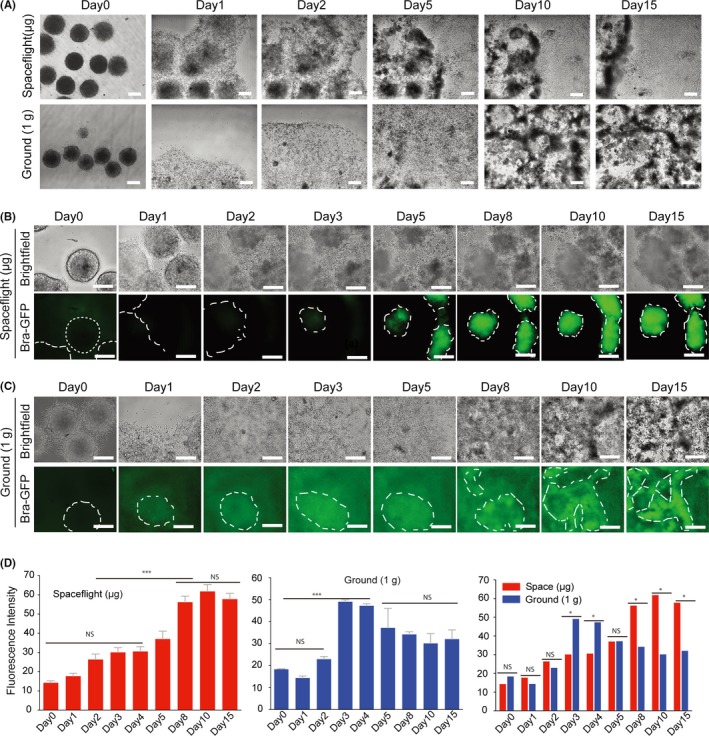
Cell adhesion, outgrowth and differentiation of Brachyury‐GFP EBs cultured in space and ground environment. A, Representative bright field images at low magnification showing EB morphologies and outgrowth from day 1 to day 15 in culture. B, C, Phase contrast and GFP fluorescence images showing cell morphologies and Brachyury expression for the differentiation of Brachyury‐GFP EBs over 15 days of culture in spaceflight and ground conditions. All scale bars = 200 μm. D, Mean fluorescence intensity of spaceflight‐cultured and ground‐cultured Brachyury‐GFP EBs. All results are shown as the mean ± SD of at 3 independent images. NS = no significant, (*) = P < .05, (***) = P < .001
4. DISCUSSION
Increasing attention has been paid to the influence of microgravity on stem cell growth and tissue regeneration. Exposure to microgravity may change cellular function, potentially causing severe deficits in mammalian stem cell‐based tissue regeneration, including altered adhesion properties, reduced cell numbers and inhibited cell differentiation.11, 22 Although some studies have focused on the proliferation and differentiation of stem cells in microgravity with spaceflight,23, 24 none of them acquired real‐time imaging of stem cells in long‐term space conditions. In the present study, we developed an automatic cell culture device and automated imaging technology to generate real‐time observation of mESC growth and EB differentiation in space for the first time. Because the TZ‐1 cargo spacecraft was unable to be recovered after the mission, all data had to be automatically collected and relayed to Earth before the spacecraft burned upon re‐entry.
The novelty of this work lies in the fact that we used a fluorescence reporter (GFP) on Oct‐4 as well as a Brachyury‐GFP fusion protein in mESCs, to monitor cell proliferation and differentiation. We found that mESCs exhibited stronger proliferative characteristics and longer survival probability under space microgravity conditions compared with ground‐cultured cells in this automated culture system. Of note, flat and well‐spread mESC colonies gradually transformed into dense 3D cell aggregates that exhibited high expression of Oct4 during incubation in space, while cells cultured on the ground exhibited monolayer growth patterns. This phenomenon is consistent with a study reporting that microgravity promotes formation of ball‐like ES cell colonies and maintains stem cell characteristics in the absence of LIF.25 Furthermore, EBs cultured in matrigel matrix‐coated cell chambers were capable of developing outgrowth onto the ECM substrate during differentiation under microgravity conditions. We also noticed decreased expression of Oct4‐GFP and increased expression of Brachyury in EBs cultured in space, indicating that EBs can be differentiated in microgravity. Interestingly, it seems that the EB morphology and GFP expression pattern in space may vary from those cultured in ground 1 × g controls, with our data indicating that the speed of EB differentiation in space is slower than in EBs differentiated in normal 1 × g conditions.
Observed differences in mESC proliferation and growth properties between ground and space environments could be attributed to not only biophysical factors but also biochemical factors. Regarding the strong self‐renewal ability and long‐term survival of stem cells and 3D structure formation in space, 1 explanation might be signalling pathway alternations, such as Wnt1, which involve cell fate determination during cell differentiation and proliferation that were specifically increased under space microgravity conditions.12 Another explanation is that mESCs might be able to more efficiently utilize nutrient bioavailability when exposed to microgravity conditions.26, 27, 28 Although CO2 gas was not provided by the spacecraft hardware in this study, media were initially inflated with a gas mixture (5% O2, 5% CO2 and 90% N2), and the medium was changed every day and stored at low temperature and sealed. We believe that enhanced nutrient utilization occurs in space μg environments compared to ground 1 × g environments. Further study is necessary to uncover the detailed mechanism as to why stem cells maintain an undifferentiated state and long‐term proliferation when exposed to a space environment.
Additionally, as reported by Blaber et al we found that space microgravity inhibits stem cell terminal differentiation. Despite the EBs developing outgrowths on matrigel substrates in the first 3 days in our study, EBs slowed their migration after 3 days, maintaining aggregates in the centre. These results strengthen the above‐mentioned hypothesis that microgravity triggers 3D cell growth.29, 30, 31 We found that the speed of EB differentiation at 1 × g on the ground was faster when compared with space. It is possible that increased flow shear forces on the ground when infused with fresh medium increased the differentiation of stem cells.32 In fact, microgravity reduces the migration of mesenchymal stem cells by remodelling the actin cytoskeleton and increasing cell stiffness.33 Reduced attachment/spreading on the substrate is associated with cell‐cell aggregation that promotes mesendoderm differentiation.34 Interestingly, we found that although EB could differentiate into the mesendoderm lineage in the space environment, these differentiated cells maintained high expression of Brachyury, even after 15 days of culture. This result confirmed that mechanical unloading of stem cells in microgravity inhibits later or terminal differentiation and decreases the activity of mature cells.12 Taken together, our results demonstrate that the space microgravity environment might play a potential role in supporting 3D growth of cells and maintenance of stemness in embryonic stem cells, while spaceflight in microgravity may have a negative effect on the terminal differentiation of ESCs.
DISCLOSURE
The authors have no financial conflicts of interest.
AUTHORS' CONTRIBUTIONS
X.L. and Y.C performed the experiments. X.L. collected data and prepared the manuscript. Y.Z., J.Q., J.Z., Q.Z. and Y.G. assisted in the experiments and analysed the data. F.L. and T.Z. developed the hardware for cell culture and imaging. X.L., G.X. and E.D. designed the study, analysed data and edited the manuscript.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China Grants (U1738103, 31600683), the TZ‐1 Application Program and the Strategically Guiding Scientific Special Project from the Chinese Academy of Sciences (XDA04020202‐20, XDA15014000).
Lei X, Cao Y, Zhang Y, et al. Effect of microgravity on proliferation and differentiation of embryonic stem cells in an automated culturing system during the TZ‐1 space mission. Cell Prolif. 2018;51:e12466 10.1111/cpr.12466
Contributor Information
Guoliang Xia, Email: glxiachina@sohu.com.
Enkui Duan, Email: duane@ioz.ac.cn.
REFERENCES
- 1. Hughes‐Fulford M. Altered cell function in microgravity. Exp Gerontol. 1991;26:247‐256. [DOI] [PubMed] [Google Scholar]
- 2. Zhang C, Li L, Chen J, Wang J. Behavior of stem cells under outer‐space microgravity and ground‐based microgravity simulation. Cell Biol Int. 2015;39:647‐656. [DOI] [PubMed] [Google Scholar]
- 3. Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901‐907. [DOI] [PubMed] [Google Scholar]
- 4. Zhang S, Zhang Y, Chen L, et al. Efficient large‐scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones. Stem Cell Res Ther. 2013;4:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuge L, Kajiume T, Tahara H, et al. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 2006;15:921‐929. [DOI] [PubMed] [Google Scholar]
- 6. Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K. Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A. 2010;16:3285‐3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang Y, Zhang S, et al. Rotating microgravity‐bioreactor cultivation enhances the hepatic differentiation of mouse embryonic stem cells on biodegradable polymer scaffolds. Tissue Eng Part A. 2012;18:2376‐2385. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, An L, Jiang Y, Hang H. Effects of simulated microgravity on embryonic stem cells. PLoS ONE. 2011;6:e29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinde V, Brungs S, Henry M, et al. Simulated microgravity modulates differentiation processes of embryonic stem cells. Cell Physiol Biochem. 2016;38:1483‐1499. [DOI] [PubMed] [Google Scholar]
- 10. Monticone M, Liu Y, Pujic N, Cancedda R. Activation of nervous system development genes in bone marrow derived mesenchymal stem cells following spaceflight exposure. J Cell Biochem. 2010;111:442‐452. [DOI] [PubMed] [Google Scholar]
- 11. Davis TA, Wiesmann W, Kidwell W, et al. Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J Leukoc Biol. 1996;60:69‐76. [DOI] [PubMed] [Google Scholar]
- 12. Blaber EA, Finkelstein H, Dvorochkin N, et al. Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem Cells Dev. 2015;24:2605‐2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lei X, Deng Z, Zhang H, et al. Rotary suspension culture enhances mesendoderm differentiation of embryonic stem cells through modulation of Wnt/beta‐catenin pathway. Stem Cell Rev. 2014;10:526‐538. [DOI] [PubMed] [Google Scholar]
- 14. Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545‐548. [DOI] [PubMed] [Google Scholar]
- 15. Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF‐beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806‐16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lei X, Deng Z, Duan E. Uniform embryoid body production and enhanced mesendoderm differentiation with murine embryonic stem cells in a rotary suspension bioreactor. Methods Mol Biol. 2016;1502:63‐75. [DOI] [PubMed] [Google Scholar]
- 17. Feng C, Wan H, Zhao X, Wang L, Zhou Q. Generation of tetraploid complementation mice from embryonic stem cells cultured with chemical defined medium. Chin Sci Bull. 2014;59:2743‐2748. [Google Scholar]
- 18. Tamm C, Pijuan GS, Anneren C. A comparative study of protocols for mouse embryonic stem cell culturing. PLoS ONE. 2013;8:e81156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lü D, Luo C, Zhang C, Li Z, Long M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials. 2014;35:3945‐3955. [DOI] [PubMed] [Google Scholar]
- 20. Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B‐Cdc2/PP2A balance. Proc Natl Acad Sci U S A. 2010;107:12564‐12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken). 2013;296:378‐381. [DOI] [PubMed] [Google Scholar]
- 22. Verhaar AP, Peppelenbosch MP. Correspondence on “microgravity reduces the differentiation and regenerative potential of embryonic stem cells”. Stem Cells Dev. 2016;25:572‐573. [DOI] [PubMed] [Google Scholar]
- 23. Blaber EA, Sato K, Almeida EA. Stem cell health and tissue regeneration in microgravity. Stem Cells Dev. 2014;23(Suppl 1):73‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blaber EA, Dvorochkin N, Torres ML, et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell‐mediated tissue regeneration. Stem Cell Res. 2014;13:181‐201. [DOI] [PubMed] [Google Scholar]
- 25. Kawahara Y, Manabe T, Matsumoto M, Kajiume T, Matsumoto M, Yuge L. LIF‐free embryonic stem cell culture in simulated microgravity. PLoS ONE. 2009;4:e6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doolin EJ, Geldziler B, Strande L, Kain M, Hewitt C. Effects of microgravity on growing cultured skin constructs. Tissue Eng. 1999;5:573‐582. [DOI] [PubMed] [Google Scholar]
- 27. Hauslage J, Cevik V, Hemmersbach R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground‐based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity. 2017;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuge L, Hide I, Kumagai T, et al. Cell differentiation and p38(MAPK) cascade are inhibited in human osteoblasts cultured in a three‐dimensional clinostat. In Vitro Cell Dev Biol Anim. 2003;39:89‐97. [DOI] [PubMed] [Google Scholar]
- 29. Lei XH, Ning LN, Cao YJ, et al. NASA‐approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis‐like structure. PLoS ONE. 2011;6:e26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pietsch J, Ma X, Wehland M, et al. Spheroid formation of human thyroid cancer cells in an automated culturing system during the Shenzhou‐8 Space mission. Biomaterials. 2013;34:7694‐7705. [DOI] [PubMed] [Google Scholar]
- 31. Jha R, Wu Q, Singh M, et al. Simulated microgravity and 3D culture enhance induction, viability, proliferation and differentiation of cardiac progenitors from human pluripotent stem cells. Sci Rep. 2016;6:30956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potter CM, Lao KH, Zeng L, Xu Q. Role of biomechanical forces in stem cell vascular lineage differentiation. Arterioscler Thromb Vasc Biol. 2014;34:2184‐2190. [DOI] [PubMed] [Google Scholar]
- 33. Mao X, Chen Z, Luo Q, Zhang B, Song G. Simulated microgravity inhibits the migration of mesenchymal stem cells by remodeling actin cytoskeleton and increasing cell stiffness. Cytotechnology. 2016;68:2235‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen SS, Fitzgerald W, Zimmerberg J, Kleinman HK, Margolis L. Cell‐cell and cell‐extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553‐561. [DOI] [PubMed] [Google Scholar]


