Abstract
Objective
Over 5% of the world's population suffers from disabling hearing loss. Stem cell homing in target tissue is an important aspect of cell‐based therapy, which its augmentation increases cell therapy efficiency. Deferoxamine (DFO) can induce the Akt activation, and phosphorylation status of AKT (p‐AKT) upregulates CXC chemokine receptor‐4 (CXCR4) expression. We examined whether DFO can enhance mesenchymal stem cells (MSCs) homing in noise‐induced damaged cochlea by PI3K/AKT dependent mechanism.
Materials and Methods
Mesenchymal stem cells were treated with DFO. AKT, p‐AKT protein and hypoxia inducible factor 1‐ α (HIF‐1α) and CXCR4 gene and protein expression was evaluated by RT‐ PCR and Western blot analysis. For in vivo assay, rats were assigned to control, sham, noise exposure groups without any treatment or receiving normal, DFO‐treated and DFO +LY294002 (The PI3K inhibitor)‐treated MSCs. Following chronic exposure to 115 dB white noise, MSCs were injected into the rat cochlea through the round window. Number of Hoechst‐ labelled cells was determined in the endolymph after 24 hours.
Results
Deferoxamine increased P‐AKT, HIF‐1α and CXCR4 expression in MSCs compared to non‐treated cells. DFO pre‐conditioning significantly increased the homing ability of MSCs into injured ear compared to normal MSCs. These effects of DFO were blocked by LY294002.
Conclusions
Pre‐conditioning of MSCs by DFO before transplantation can improve stem cell homing in the damaged cochlea through PI3K/AKT pathway activation.
Keywords: CXCR4, deferoxamine, NIHL, PI3K/AKT pathway, stem cell homing
1. INTRODUCTION
Several factors such as complications at birth, genetic disorders, drug, acoustic trauma, chronic ear infections and ageing may be risk factors for hearing loss.1, 2, 3, 4 Noise‐induced hearing loss (NIHL) is the most common form of hearing impairment, which occurs due to prolonged or high intensity level of noise exposure. Loud sound can cause damage to the hair cells in the cochlea.5 In mammalians, hair cells are not replaced or repaired. At present, there is not any definite cure for reconstruction of damaged hair cells resulting from acoustic stimuli. In recent years, stem cell transplantation has been investigated to repair damaged tissues of the inner ear. Arriving of enough injected cells into the target area is necessary for the treatment of irreversible damage to hair cells and neurons. Stem cell homing is one of the essential mechanisms for effective delivery of injected cells to the cochlear tissue.6 The most known molecular mechanism that directs migration and homing of mesenchymal stem cells (MSCs) into target tissue is the activation of some chemokine receptors such as CXCR4.7, 8 Recent evidence has revealed that chemical agents such as Deferoxamine (DFO) which mimic hypoxic pre‐conditioning can increase cell therapeutic efficacy through increment of stem cells homing factors.9 DFO is an iron‐chelator drug and a family of prolyl‐4 hydroxylase inhibitor that stabilizes hypoxia inducible factor 1‐ α (HIF‐1α) through the inhibition of prolyl hydroxylases enzyme which target HIF‐1 protein through degradation.10, 11 A previous study has been shown that hypoxia stimulates the activation of the phosphatidylinositol‐3 kinase (PI3‐K) /AKT survival pathway in PC12 cells. The same effect was also reported following DFO treatment.12 AKT activation can induce the expression of CXCR4 in stem cells. PI3K/AKT pathway contributes in hypoxia pre‐conditioning‐induced HIF‐1ɑ expression and CXCR4 upregulation in BMSCs.13 Stromal cell‐derived factor 1 (SDF‐1) (potent chemoattractant for BMSCs) ‐CXCR4 signalling pathway has a key role in the regulation of stem cell trafficking towards the injured area.14, 15, 16, 17 In this regard, we examined the mechanism of DFO effect on MSCs chemokine receptor and homing ability in noise‐induced injured cochlea through PI3K/AKT pathway.
2. MATERIALS AND METHODS
2.1. Experimental animals
Male Wistar rats were purchased from the Experimental Animal Center of Shahid Beheshti Medical University and housed under a steady condition. All animal procedures were approved by the Ethical Committee of Shahid Beheshti University of Medical Sciences based on National Institutes of Health Principles of Laboratory Animal Care (NIH publication no. 85‐23, revised1985). Noise exposure and DPOAEs assay were performed in Animal Research Lab, Department of Audiology, School of Rehabilitation Sciences, Iran University of Medical Sciences.
Rats with 4‐6 weeks of age and average weight of 120‐140 g were used for MSCs isolation. For in vivo study, rats (200‐250 g, 3 months) were randomly assigned into six groups: control, sham, noise exposure groups without any treatment or transplanted with normal, DFO (Sigma‐Aldrich, St. Louis, MO, USA)‐treated and DFO+LY294002 (Abcam, Cambridge, UK)‐treated MSCs (n = 4/ each group).
2.2. Isolation, culturing and characterization of rat MSCs
Following rats anaesthetizing by Xylazine and Ketamine administration, MSCs were extracted by flushing marrow from the femur and tibia cavity with a 22 gauge needle. Cells were harvested in Dulbecco's medium (Gibco BRL, Grand Island, NY, USA) culture media with 10% foetal bovine serum (FBS, Gibco BRL, Grand Island, NY, USA) and 1% penicillin—streptomycin (Pen/ Strep). Cultures (25‐cm2 flasks) were maintained in a humidified incubator at 37°C with 5% CO2. Culture medium was replaced every 3 days. For assessment of adipogenic differentiation potential, fourth‐passage cells were incubated for 21 days with 5 μg/mL insulin (Sigma‐Aldrich, St. Louis, MO, USA) and 10−9 M of dexamethasone. Lipid droplets accumulation in vacuoles was visualized by Oil Red O (Sigma‐Aldrich, St. Louis, MO, USA) staining and determined by phase‐contrast microscopy.18, 19 For osteogenesis assay, cultures of cells (passage 4) were incubated with osteogenesis medium (50 μg/mL ascorbic acid 2‐phosphates, 10 mM β‐glycerophosphate and 109 M dexamethasone) for 21 days.20, 21 Next, the cells were fixed with 10% formalin for 20 minutes, and then cells were treated with 2% (weight/volume) solution of Alizarin Red S (Sigma‐Aldrich, St. Louis, MO, USA) for 20 minutes, pH was adjusted to 4.1 with ammonium hydroxide.22 Flow cytometry was performed using caliber cytometer FACS (Becton Dickinson, San Diego, CA, USA) to analyse fourth‐passage MSCs surface markers including CD105, CD73, CD90, CD34, CD45 and CD184 (CXCR4) (Becton Dickinson, San Diego, CA, USA).
2.3. Cytotoxicity assay
Cells (5 × 103) were plated into individual wells of a 96 well culture plates and were then treated with medium containing 0, 10, 50, 100 and 200 μM of DFO. Cultures without DFO served as control. After 24, 48 and 72 hours of incubation, 100 μL MTT 3‐ (4, 5‐dimethylthiazol‐2‐yl) ‐2, 5‐diphenyltetrazolium bromide) reagent was added into each well, and the cells were incubated for 3 hours at 37°C. After incubation, medium was removed (carefully) and dissolved in 100 μL DMSO (Carl Roth GmbH & Co., Karlsruhe, Germany) to avoid any interference with the absorbance readings. Absorbance was measured at 570 nm with a microplate reader.
2.4. In vitro migration assay
The migration ability of BMSCs was quantified using the QCM ™ chemotaxis 8 μm 96‐well cell fluorometric migration assay (Millipore, Billerica, MA, USA). Cell cultures (4 × 104 cells/100 μL) were treated with DFO (100 μM), DMSO (solvent for AMD3100 and LY294002), DFO+AMD3100 (a specific inhibitor of CXCR4, 5 μg/mL, Sigma‐Aldrich, St. Louis, MO, USA) and DFO+LY294002 (30 μmol/L) in the upper chamber and then their migration ability towards SDF‐1 (100 ng/ml, Enzo Life Sciences, Farmingdale, NY, USA) in the lower chamber was assayed. MSCs without any treatment were considered as control. Plates were then incubated for 24 hours at 37°C. Migratory cells were lysed and detected by CyQuant GR dye. Optical density (OD) value of migrated cells was determined using 480/520 nm filter set.
2.5. rt‐pcr
Total RNA of BMSCs was isolated using TRIZOL reagent, according to the manufacturer's instructions. The RNA concentrations were quantified by a spectrophotometer. cDNA was synthesized by reverse transcriptase with a cDNA synthesis kit (Fermentas, Thermo Fisher Scientific Inc., Leicestershire, UK).23 The cDNA was amplified by PCR for 30 cycles for HIF‐1α and CXCR4 or 35 cycles for β‐Actin using the following primers: HIF‐1α; forward (5′_GGATTACCACAGCTGACCA‐3′) and reverse (5′_ GATCCAAAGCTCTGAGTAAT ‐3′), CXCR4; forward (5′_ ACACCGTCAACCTTTACAG ‐3′) and reverse (5′_ CTGTTGGTGGCGTGGACAA ‐3′) and β‐actin (as a loading control); forward (5′_TGTCCACCTTCCAGCAGATGT‐3′) and reverse (5′_AGCTCAGTAACAGTCCGCCTAGA‐3′). Each cycle consisted of denaturation at 94°C for 30 s, annealing at 55°C (HIF‐1α) or 57°C (CXCR4 and β‐actin) for 1 min, extension at 72°C for 30 s.
2.6. Western blot
For protein expression assay in the cochlear tissue, following anaesthesia and decapitation of the animal, the temporal bone was quickly removed. Cochlea sample included the organ of corti, and modiolus was dissected and then homogenized in a RIPA lysis (Santa Cruz Biotechnology, Dallas, TX, USA) buffer. For in vitro assay, DFO untreated and treated cultured cells were trypsinized and washed with PBS and lysed in a RIPA lysis buffer. The homogenates were centrifuged at 4°C for 10 minutes at 15 000 g. Measurement of protein concentration was done using the Bradford assay.24 Samples containing equal amounts of protein were loaded and separated using SDS‐PAGE electrophoresis and transferred onto a nitrocellulose membrane. The membrane was incubated with the specific antibodies: anti‐ p‐AKT (1: 1000), anti‐ AKT (1: 1000), anti‐ HIF‐1α (1: 1000), anti‐ CXCR4 (1: 1000) anti‐ SDF‐1 (1: 1000) and anti‐ β Actin (1: 2000) antibody (Abcam, Cambridge, UK), (as an internal control). A peroxidase‐conjugated goat anti‐rabbit IgG (Abcam, Cambridge, UK, 1:2000) was used as a secondary antibody. Protein bands were visualized by ECL detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and then analysed using TotalLab software.25, 26
2.7. Noise exposure
Noise exposures were conducted in a glass chamber and lasted 6 hours for 5 consecutive days. Male rats were exposed to 115 dB sound pressure level (SPL). Sound intensity was measured using a sound level meter (TES‐1358 Sound Analyse, Taiwan).
2.8. DPOAEs
DPOAEs are generated in the response to the simultaneous presentation of two sounds pure tones with different frequencies F1 and F2 in the inner ear.27 DPOAEs present active mobility of the outer hair cells (OHC) in the cochlea.28 Briefly, animals were anaesthetized using 90/10 mg/kg i.p. injection of ketamine/xylazine mixture. DPOAEs were recorded from the left ear. DPOAEs measurement was performed in a sound‐attenuated chamber by Neuro‐Audio (NEUROSOFT, Russia) system pre‐ and post‐noise exposure.
2.9. In vivo migration assay
P4 cells were seeded in 6‐well plates at an initial density of 500 000 cells/well and cultured to reach 60%‐70% confluence. For in vivo chemotaxis analysis, 1 × 105 BMSCs were treated with or without 30 μmol/L LY294002 for 2 hours before the treatment with DFO. Then, 20 μL of MSC suspension was perfused for 10 minutes at a rate of 2 μL/min into the inner ear (left) through the round window niche.
2.10. Histological assay
Twenty‐four hours after transplantation, rats were sacrificed by decapitation, the temporal bones were quickly removed and cochleae were fixed by intrascalar perfusion of 4% paraformaldehyde. After overnight immersion in fixative, cochleae were decalcified by immersion in 5% sucrose, 5% ethylenediaminetetraacetic acid (pH 7.4) with stirring at 4°C for 15 days. Paraffin‐embedded 5‐μm sections were prepared. Hochest (Hoechst 33342; Thermo Fisher Scientific, Waltham, MA, USA)—labelled MSCs was observed by using fluorescence microscope. The number of labelled MSCs in the cochlea was analysed by Image J software.
2.11. Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was done by Student's t test and one‐way analysis of variance (ANOVA). P value less than 0.05 was statistically significant.
3. RESULTS
3.1. Characterization of MSCs
Differentiation capacity of BMSCs (passage 4) (Figure 1A) was detected with alizarin red staining of matrix calcium deposition on the 21th day of osteogenic differentiation (Figure 1B) and oil Red O staining of lipid droplets on the 21th day of adipocyte differentiation (Figure 1C). In addition, we evaluated the expression of cell surface markers by flow cytometry. Flow cytometry analysis indicated the expression of MSCs surface markers such as CD73 (Figure 1F), CD105 (Figure 1G) and CD90 (Figure 1H) and the absence of haematopoietic lineage markers such as CD45 (Figure 1I) and CD34 (Figure 1J). Flow cytometric analysis was also performed to determine the percentages of CXCR4 (CD184)‐positive cells (Figure 1L).
Figure 1.
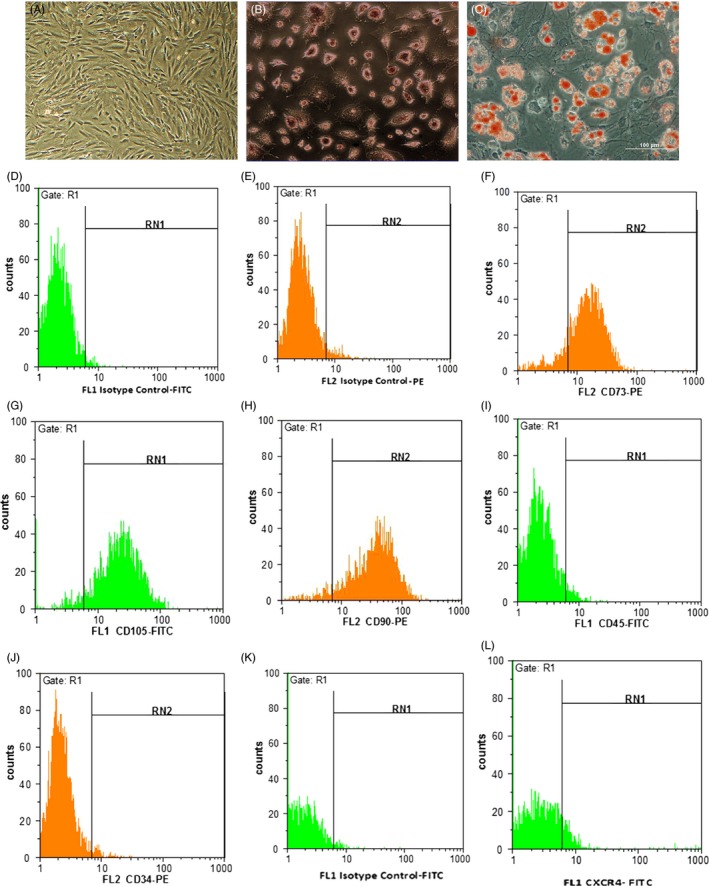
Morphological features and flow cytometry analysis of cultured BMSCs. Figures of morphology (A), osteogenic (B) and adipogenic (C) differentiation of BMSCs. Flow cytometry assay showed that BMSCs were positive for CD markers such as CD73 (74.3%) (F), CD105 (92.12%) (G), and CD90 (91.63%) (H), and negative for CD45 (4.6%) (I) and CD34 (2.9%) (J). Isotype control‐FITC (D), Isotype control‐PE (E). CXCR4 expression (8.5%) was also determined in MSCs by flow cytometry assay (L). Isotype control ‐FITC (K).
3.2. Cell viability
After incubation of cells with DFO at a concentration of 0, 10, 50, 100 and 200 μM for 24, 48 and 72 hours periods, the viability of cells was examined by MTT assay. Our result showed that 24 hours treatment with 100 μM of DFO significantly enhanced MSCs viability, and was used for all experiments in this study (Figure 2).
Figure 2.
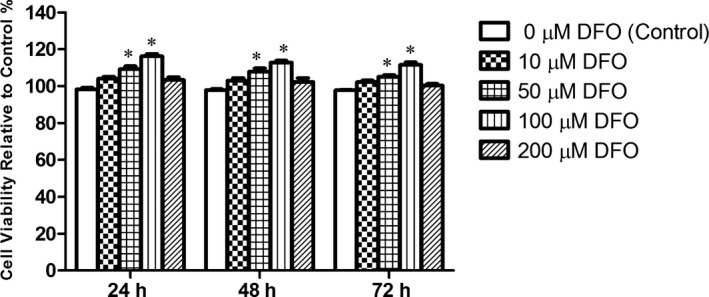
Viability assay of MSCs treated with various concentrations of DFO for 24, 48 and 72 h by MTT. DFO‐treated MSCs at 50 and 100 μM concentration showed significantly more viability in comparison with control groups. *P < .001 compared to the control group (n = 4), (One‐way ANOVA)
3.3. In vitro migration assay
In vitro migration assay was performed with a QCM chemotaxis 96‐well cell migration kit. DFO treatment for 24 hours increased MSCs migration to SDF‐1 (P < .001) compared to the non‐treated MSCs group. This effect was inhibited after treatment with AMD3100 (P < .001) or LY294002 (P < .001) (Figure 3).
Figure 3.
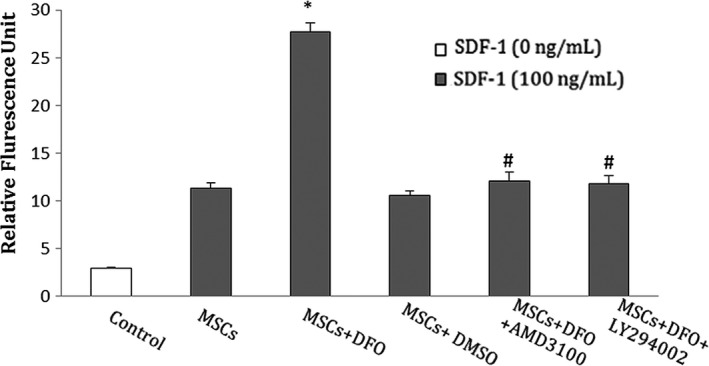
DFO improved in vitro migration of BMSCs to SDF‐1. This treatment effect was blocked by AMD3100 or LY294002. In control group, no SDF‐1 was applied to the MSCs. SDF‐1, stromal cell‐derived factor‐1; BMSCs, bone marrow mesenchymal stem cells. *P < .001 compared to non‐treated MSCs group; # P < .001 compared to the DFO group (One‐way ANOVA)
3.4. AKT and p‐AKT expression analysis
Cells were treated with 100 μM of DFO for 0, 12 and 24 hours. Densitometric analysis revealed that the p‐AKT protein expression was significantly enhanced in the DFO‐incubated MSCs for 24 hours in comparison with untreated controls. The overexpression of p‐AKT was reversed by LY294002. AKT protein expression was not changed in DFO‐treated cells compared to control group (Figure 4).
Figure 4.
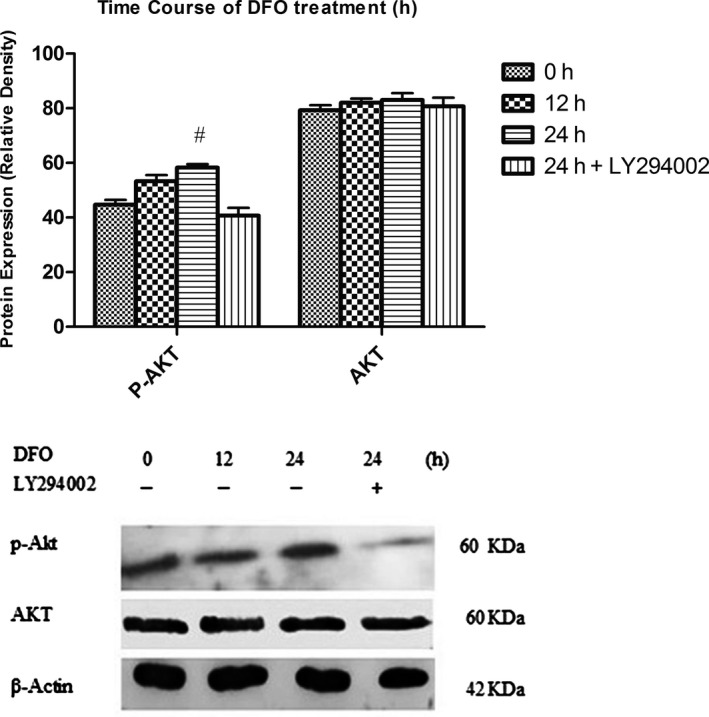
Effect of DFO treatment on AKT and p‐AKT protein expression. DFO enhanced p‐AKT protein expression in BMSCs compared to untreated cells. This effect was reversed when treated with LY294002. # P < .01 compared to the control group (n = 3), (One‐way ANOVA)
3.5. HIF‐1α and CXCR4 Gene and protein expression analysis
The effect of DFO on expression of HIF‐1α and CXCR4 was determined by PCR and Western blot analysis. The time‐course statistical analysis revealed DFO treatment increased genes expression in a time‐dependent manner (0, 12 and 24 hours), reaching the highest levels at 24 hours, and this effect was diminished by pre‐treatment of cells with LY294002 (Figure 5A). As well, HIF‐1α and CXCR4 protein expression was significantly elevated after incubation with DFO for 24 hours (Figure 5B).
Figure 5.
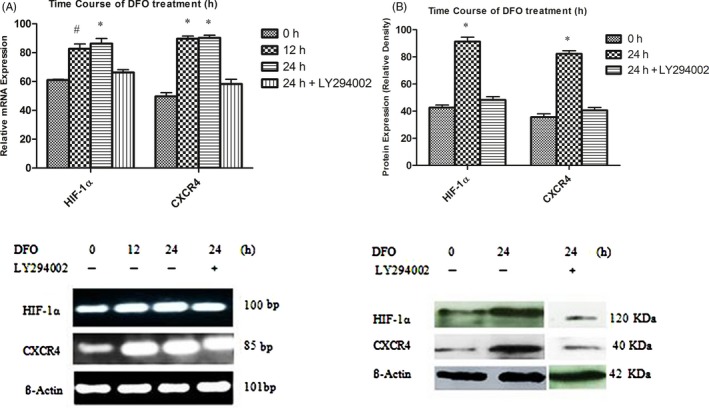
(A) Cells were treated with 100 μM DFO at different times (0, 12 and 24 h). DFO markedly enhanced HIF‐1α and CXCR4 mRNA level at 12 and 24 h in BMSCs compared to untreated stem cells. Effect of DFO treatment was decreased by LY294002. # P < .01, *P < .001 compared to the control group (n = 3), (One‐way ANOVA). (B) DFO significantly potentiated HIF‐1α and CXCR4 protein expression which These effects were blocked by LY294002 treatment. *P < .001 compared to untreated cells (n = 3), (One‐way ANOVA)
3.6. DPOAE assay
The hearing function of the control (n = 4), sham (n = 4), noise‐induced groups (n = 16) (including a group with no treatment [n = 4] and 3 noise experimental groups which were then transplanted with normal [n = 4], DFO‐treated [n = 4] and DFO+LY294002‐treated MSCs [n = 4]) were evaluated using the DPOAE. DPOAE (in dB SPL) were measured in stimulus frequencies of 2, 3, 4, 5, 6, 7, 8, 9 and 10 kHz. Baseline audiometry measure was normal in all groups (Figure 6A& B). Six days after surgery, the mean DPOAE values in the sham‐operated group were not significantly different across the measured frequency range compared to day 0 (Figure 6A). Following 6 days noise exposure, noise‐induced rat showed a decrease in DPOAE responses compared to day 0 (P < .001, Figure 6B).
Figure 6.
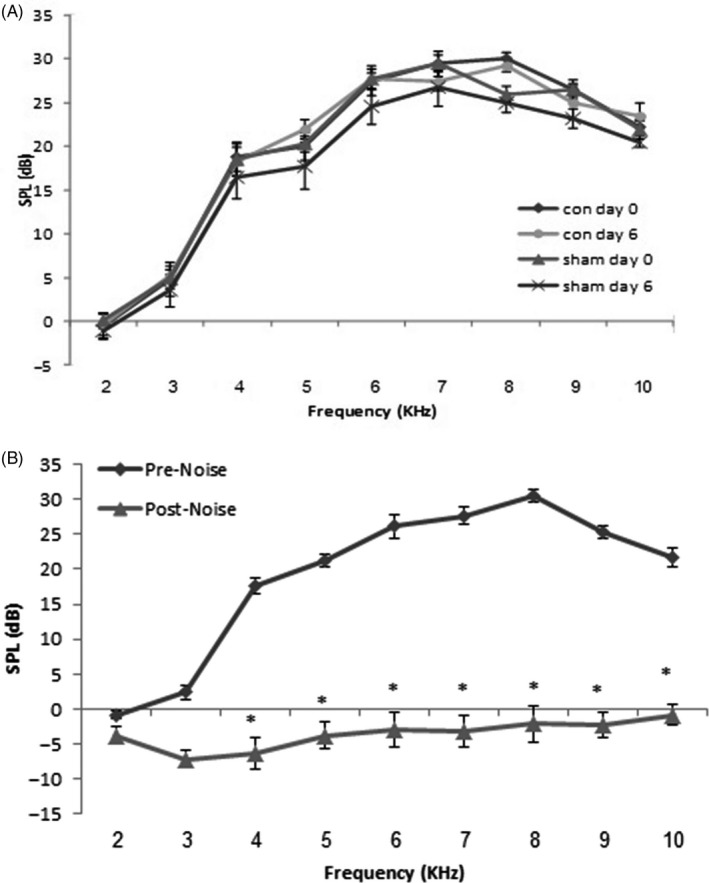
Mean ± SEM of DPOAE responses in the control and sham groups at day 0 and day 6 (A) and in the noise group before (day0) and after (day 6) noise exposure (B). SPL; sound pressure level. *P < .001 compared to day 0 (one‐way ANOVA)
3.7. SDF‐1 protein expression analysis
Protein expression of SDF‐1 was assayed in the cochlea tissue of control (n = 4), sham‐operated (n = 4) and noise exposure (n = 4) groups by Western blotting test. SDF‐1 band was detected on the blot around 11 kDa. Analysis of western blotting data showed that protein expression of SDF‐1 was significantly enhanced in the cochlea tissue of rats in the noise‐induced group compared to the control group (P < .001) (Figure 7).
Figure 7.
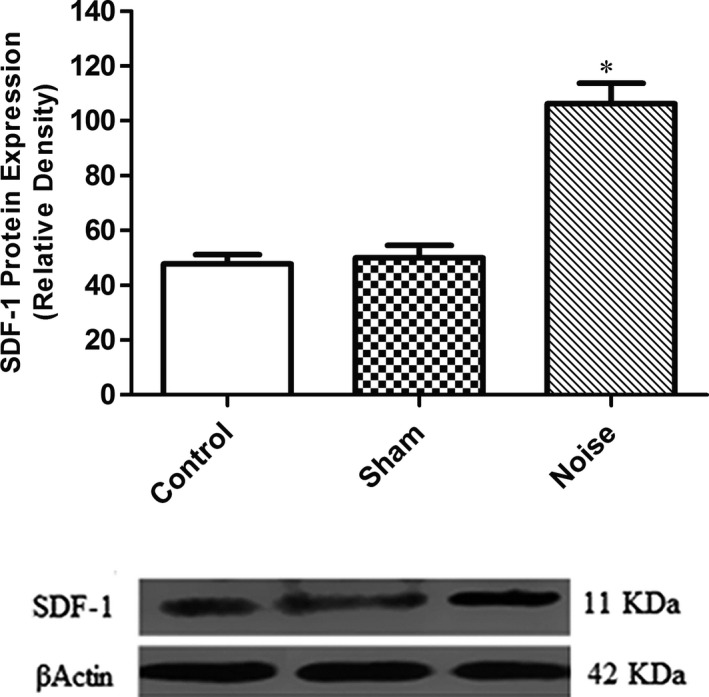
Protein expression of SDF‐1 in the cochlea of rat. After noise exposure, SDF‐1 protein expression was increased in the injured cochlea (mean ± SEM). SDF‐1, stromal cell‐derived factor‐1. *P < .001 compared to the control group (one‐way ANOVA, post hoc). β‐Actin was used as an internal control
3.8. MSCs migration assay
The Hochest‐labelled MSCs were injected through the round window into the perilymph of the inner ear of deaf rat. The animals were sacrificed by decapitation 24 hours after MSCs transplantation. The number of Hoechst‐stained cells was counted under fluorescence microscopy. Our results showed that the number of DFO‐treated MSCs that were detected in the endolymph of cochlea was significantly higher than non‐treated MSCs (n = 4), (*P < .001) (Figure 8).
Figure 8.
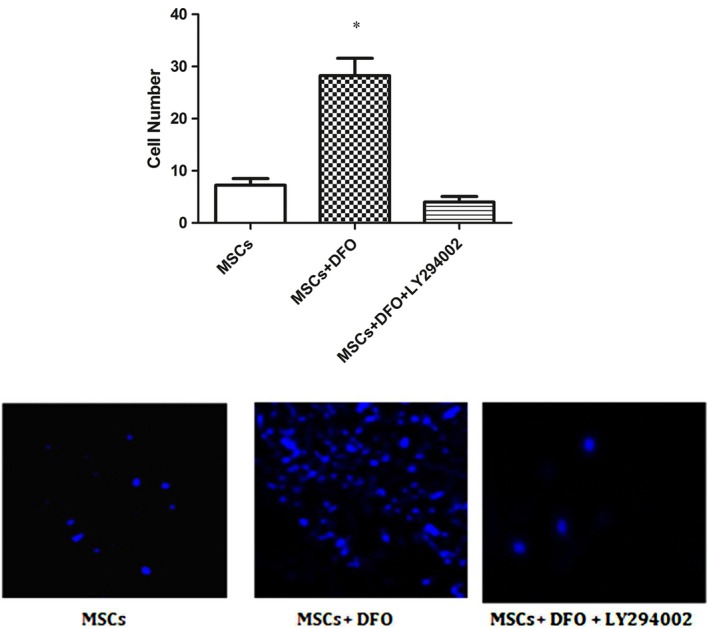
In vivo migration assay: DFO enhances MSCs homing in deaf rat injured cochlea. *P < .001 compared to average number of untreated MSCs was counted in cochlear tissue of deaf rats, (scale bar = 100 μm) (n = 4, one‐way ANOVA)
4. DISCUSSION
There are two commonly used methods of cell delivery into the perilymphatic duct of cochlea—cochleostomy and the surgical intervention through the round window membrane. In this study, MSCs were transplanted into the cochlea via round window niche, which is easy and safe surgical technique.29, 30 Although the endolymphatic compartment is encapsulated by tight junctions, studies have shown that number of cells receiving to the scala media through intra‐perilymphatic transplantations modest which confirmed that injected cells can migrate from perilymphatic space to endolymphatic compartment.31, 32, 33 The query of how cells could pass through this tightly sealed compartment is under investigation. A report by Kamiya et al. revealed that upregulation of homing receptors such as chemokine receptor 2 (CCR2) and CXCR4 in cultured MSCs which are implanted via the intra‐perilymphatic route can potentiate cell homing capacity to injured target of cochlea. They have found that monocyte chemotactic protein‐1, SDF‐1 and their receptors are main regulators for stem cell homing into target site of cochlea in the hereditary deafness.6
In this study, we examined whether DFO pre‐treatment can promote BMSCs homing through the PI3K/AKT signalling pathway in the NIHL rat model. For this purpose, the pre‐treated cells were transplanted into the perilymphatic space via the round window and then injected cells which received to endolymph were evaluated. Our data demonstrated that DFO enhances BMSCs homing in noise‐induced cochlear injury through PI3K/AKT‐dependent mechanism. Under in vitro condition, DFO increased phosphorylation status of AKT and expression of HIF‐1α and CXCR4 in BMSCs. In addition, in vivo homing of DFO‐treated MSCs in the injured rat cochlea increased compared to non‐treated MSCs. These effects of DFO were blocked by treatment with the PI3K inhibitor, which indicates that DFO effect was mediated by the PI3K/AKT‐ HIF‐1α ‐CXCR4 pathway. The PI3K/AKT signalling pathway has an outstanding role in cell biology. This cascade is involved in cell survival, cell cycle, cellular growth and proliferation.34 Activation of the PI3K/AKT pathway has recently been reported under hypoxic conditions in PC12 cells. DFO (as hypoxia mimicking compound) treatment effects have been also observed in the same direction.12 In addition, hypoxia induces AKT activation in cell‐derived pten2/2 tumours.35 A previous study has been shown that exposure of MSCs to hypoxic condition results in Akt phosphorylation, which induces HIF1‐α stabilization.36 Furthermore, Xia et al.37 has been reported that AKT activation can upregulate CXCR4 expression. Results of Liu et al's study13 have also been demonstrated that in response to hypoxia, expression of CXCR4 enhances by activating HIF‐1α within MSCs through the PI3K/Akt pathway. CXCR4, the receptor of SDF‐1, is crucial for cell survival, migration and homing of MSCs. Stem cell delivering in the target tissue is one of the most important aspects of the cell‐based therapy. First of all, any treatment with these cells depends on their reaction with target tissue that leads to their homing. Numerous previous studies have reported that SDF‐1‐CXCR4 pathway has a critical role in BMSCs mobilization and homing towards the injured area.38, 39, 40, 41, 42, 43, 44 A previous study has been shown that DFO pre‐conditioning induces HIF‐1α and chemokine receptor expression that resulted in enhancement of MSCs migration and homing in diabetic rats.45 Kamiya et al6 have been shown that chemokine signalling can be participated in stem cell homing in cochlear tissue in hereditary deafness.
Our data revealed the influences of DFO on biological characteristics of BMSCs through activation of the PI3K/AKT signalling pathway. This cascade involved in hypoxic pre‐conditioning‐induced HIF‐1ɑ and upregulation of CXCR4 expression in stem cells.13
The PI3K/AKT signalling pathway also increases CXCR4 expression in several kinds of tumours cells, including colorectal cancer,46 hepatoma47 and prostate tumour cells.48 In addition, inhibition of DFO‐activated AKT and overexpression of CXCR4 and HIF‐1ɑ by LY294002 treatment revealed that the PI3K/AKT signalling pathway contributes in DFO‐induced homing of MSCs via affecting some chemokine receptors such as CXCR4.
5. CONCLUSION
To conclude, our finding revealed that DFO pre‐conditioning prior to transplantation enhances MSCs homing in noise‐induced injured cochlea through the PI3K/AKT‐ HIF1‐α‐CXCR4 pathway. These results may also have clinical implications in that DFO pre‐conditioning of MSCs can potentiate therapeutic efficiency of cell‐based therapy in hearing loss.
AUTHOR CONTRIBUTIONS
All authors were contributed to study design, analysis and interpretation of data, drafting the article and approved the final version for publication.
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by grant of the Hearing Disorders Research Center of Shahid Beheshti University of Medical Sciences. The authors would like to thank the Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their cooperation and assistance throughout the period of study
Peyvandi AA, Abbaszadeh H‐A, Roozbahany NA, et al. Deferoxamine promotes mesenchymal stem cell homing in noise‐induced injured cochlea through PI3K/AKT pathway. Cell Prolif. 2018;51:e12434 10.1111/cpr.12434
REFERENCES
- 1. Willems PJ. Genetic causes of hearing loss. N Engl J Med. 2000;342:1101‐1109. [DOI] [PubMed] [Google Scholar]
- 2. Gordon‐Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42:9. [DOI] [PubMed] [Google Scholar]
- 3. Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10‐year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84‐88. [DOI] [PubMed] [Google Scholar]
- 4. Rabinowitz PM. Noise‐induced hearing loss. Am Fam Phys. 2000;61:2759‐2760. [PubMed] [Google Scholar]
- 5. Clark WW, Bohne BA. Effects of noise on hearing. JAMA. 1999;281:1658‐1659. [DOI] [PubMed] [Google Scholar]
- 6. Kamiya K. Inner ear cell therapy targeting hereditary deafness by activation of stem cell homing factors. Front Pharmacol. 2015;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kucia M, Jankowski K, Reca R, et al. CXCR4–SDF‐1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233‐245. [DOI] [PubMed] [Google Scholar]
- 9. Esfahani M, Karimi F, Afshar S, Niknazar S, Sohrabi S, Najafi R. Prolyl hydroxylase inhibitors act as agents to enhance the efficiency of cell therapy. Expert Opin Biol Ther. 2015;15:1739‐1755. [DOI] [PubMed] [Google Scholar]
- 10. Chu K, Jung K‐H, Kim S‐J, et al. Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia‐inducible factor‐1α stabilization in the host brain. Brain Res. 2008;1207:182‐192. [DOI] [PubMed] [Google Scholar]
- 11. Cioffi CL, Liu XQ, Kosinski PA, Garay M, Bowen BR. Differential regulation of HIF‐1α prolyl‐4‐hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Comm. 2003;303:947‐953. [DOI] [PubMed] [Google Scholar]
- 12. Alvarez‐Tejado M, Naranjo‐Suárez S, Jiménez C, Carrera AC, Landázuri MO, del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3‐Kinase/Akt cell survival pathway in PC12 cells protective role in apoptosis. J Biol Chem. 2001;276:22368‐22374. [DOI] [PubMed] [Google Scholar]
- 13. Liu H, Xue W, Ge G, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF‐1α in MSCs. Biochem Biophys Res Comm. 2010;401:509‐515. [DOI] [PubMed] [Google Scholar]
- 14. Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF‐1 signals mobilization and homing of CXCR4‐positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772‐1784. [DOI] [PubMed] [Google Scholar]
- 15. Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF‐1α/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339‐351. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Deng Y, Zhou G‐Q. SDF‐1α/CXCR4‐mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104‐112. [DOI] [PubMed] [Google Scholar]
- 17. Marquez‐Curtis LA, Janowska‐Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF‐1/CXCR4 axis. Biomed Res Int. 2013;2013:561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rombouts W, Ploemacher R. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160‐170. [DOI] [PubMed] [Google Scholar]
- 19. Darabi S, Tiraihi T, Ruintan A, Abbaszadeh HA, Delshad A, Taheri T. Polarized neural stem cells derived from adult bone marrow stromal cells develop a rosette‐like structure. In Vitro Cell Dev Biol Anim. 2013;49:638‐652. [DOI] [PubMed] [Google Scholar]
- 20. Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570‐585. [PubMed] [Google Scholar]
- 21. Abbaszadeh H‐A, Tiraihi T, Delshad A, et al. Differentiation of neurosphere‐derived rat neural stem cells into oligodendrocyte‐like cells by repressing PDGF‐α and Olig2 with triiodothyronine. Tissue Cell. 2014;46:462‐469. [DOI] [PubMed] [Google Scholar]
- 22. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662‐1668. [DOI] [PubMed] [Google Scholar]
- 23. Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Roozbahany NA, Abbaszadeh H‐A. Hippocampal NR3C1 DNA methylation can mediate part of preconception paternal stress effects in rat offspring. Behav Brain Res. 2017;324:71‐76. [DOI] [PubMed] [Google Scholar]
- 24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 1976;72:248‐254. [DOI] [PubMed] [Google Scholar]
- 25. Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Akhtari AS, Karimi M. Comparison of the adulthood chronic stress effect on hippocampal BDNF signaling in male and female rats. Mol Neurobiol. 2016;53:4026‐4033. [DOI] [PubMed] [Google Scholar]
- 26. Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Mehrjerdi FZ, Karimi M. Effect of maternal stress prior to conception on hippocampal BDNF signaling in rat offspring. Mol Neurobiol. 2017;54:6436‐6445. [DOI] [PubMed] [Google Scholar]
- 27. Pouyatos B, Campo P, Lataye R. Use of DPOAEs for assessing hearing loss caused by styrene in the rat. Hear Res. 2002;165:156‐164. [DOI] [PubMed] [Google Scholar]
- 28. Dallos P. The active cochlea. J Neurosci. 1992;12:4575‐4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stöver T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124‐130. [DOI] [PubMed] [Google Scholar]
- 30. Lalwani A, Walsh B, Reilly P, et al. Long‐term in vivo cochlear transgene expression mediated by recombinant adeno‐associated virus. Gene Ther. 1998;5:277‐281. [DOI] [PubMed] [Google Scholar]
- 31. Hu Z, Wei D, Johansson CB, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302:40‐47. [DOI] [PubMed] [Google Scholar]
- 32. Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol. 2004;185:7‐14. [DOI] [PubMed] [Google Scholar]
- 33. Tateya I, Nakagawa T, Iguchi F, et al. Fate of neural stem cells grafted into injured inner ears of mice. NeuroReport. 2003;14:1677‐1681. [DOI] [PubMed] [Google Scholar]
- 34. Jain MV, Jangamreddy JR, Grabarek J, et al. Nuclear localized Akt enhances breast cancer stem‐like cells through counter‐regulation of p21Waf1/Cip1 and p27kip1. Cell Cycle. 2015;14:2109‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang J, Ding M, Yang L, Liu L‐Z, Jiang B‐H. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487‐2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia‐inducible factor (HIF)‐1α. J Cell Physiol. 2008;216:708‐715. [DOI] [PubMed] [Google Scholar]
- 37. Xia M, Tong J, Ji N, Duan M, Tan Y, Xu J. Tramadol regulates proliferation, migration and invasion via PTEN/PI3K/AKT signaling in lung adenocarcinoma cells. Eur Rev Med Pharmacol Sci. 2016;20:2573‐2580. [PubMed] [Google Scholar]
- 38. De Becker A, Van Riet I. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem cells. 2016;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF‐1/CXCR4‐mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55:496‐505. [DOI] [PubMed] [Google Scholar]
- 40. Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF‐1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138‐147. [DOI] [PubMed] [Google Scholar]
- 41. Mazzinghi B, Ronconi E, Lazzeri E, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homingof human renal progenitor cells. J Exp Med. 2008;205:479‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene‐modified bone marrow‐derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114:2677‐2689. [DOI] [PubMed] [Google Scholar]
- 43. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheumatol. 2009;60:813‐823. [DOI] [PubMed] [Google Scholar]
- 44. Ding J, Hori K, Zhang R, et al. Stromal cell‐derived factor 1 (SDF‐1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair and Regeneration. 2011;19:568‐578. [DOI] [PubMed] [Google Scholar]
- 45. Najafi R, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin‐diabetic rats. Expert Opin Biol Ther. 2013;13:959‐972. [DOI] [PubMed] [Google Scholar]
- 46. Zhou Zh, Rao J, Yang J, et al. SEMA3F prevents metastasis of colorectal cancer by PI3K–AKT‐dependent down‐regulation of the ASCL2–CXCR4 axis. J Pathol. 2015;236:467‐478. [DOI] [PubMed] [Google Scholar]
- 47. Zhu M, Guo J, Xia H, et al. Alpha‐fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singareddy R, Semaan L, Conley‐LaComb MK, et al. Transcriptional regulation of CXCR4 in prostate cancer: significance of TMPRSS2‐ERG fusions. Mol Cancer Res. 2013;11:1349‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]


