Abstract
Objectives
The purpose of this study was to explore the effectiveness of concurrent GRP78 overexpression combined with Cripto on hMSC proliferation and migration both in vitro and in vivo. Specifically, we explored whether the treatment enhances effectiveness of hMSC transplantation in ischaemic tissue.
Materials and methods
Human MSCs obtained from human adipose tissue were cultured in α‐minimum essential medium (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) foetal bovine serum (Hyclone), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. Murine hindlimb ischaemic model was generated with 8‐week‐old male nude BALB/c mice (Biogenomics, Seoul, Korea) maintained under a 12‐h light/dark cycle following the established protocol with minor modification. Cellular injection was performed no later than 3 hour after surgery. Lipofectamine transfection, single‐cell cultivation assay, transwell assay, scratch wound‐healing migration assay, immunohistochemistry and western blotting assays were performed.
Results
Overexpression of GRP78 along with Cripto enhanced hMSC proliferation, migration and invasion. It increased interaction of surface GRP78 receptor with Cripto via JAK2/STAT3 pathway. We confirmed our proposed mechanism by showing that treatment with GRP78 antibody blocks the enhancement in vitro. In vivo, we observed that Cripto induced by the hypoxic environment in hindlimb ischaemic model interacts with the overexpressed GRP78 and increases hMSC proliferation, migration and invasion potentials as well as angiogenesis around transplanted ischaemic site via cytokine secretions.
Conclusions
These results demonstrate supporting evidences that GRP78‐Cripto combination technique offers novel strategy to enhance MSC proliferation, migration and invasion potentials as well as angiogenesis around ischaemic site, ultimately facilitating MSC‐based transplantation therapy in ischaemic conditions.
Keywords: angiogenesis, cell proliferation, Cripto, GRP78, invasion, mesenchymal stem cells
1. INTRODUCTION
Mesenchymal stem cells (MSCs) are widely used as regenerative therapy for their special self‐renewal ability and multidirectional differentiation capacity to differentiate into tissues such as osteoblasts, chondrocytes, adipocyte and tenocytes. Such versatility of MSCs therapy has been proven to be effective for targeting hard‐to‐treat diseases such as cardiovascular disease and myocardial infarction (MI), stroke, diabetes, cartilage and bone injury, Crohn's disease and graft versus host disease (GvHD) via incorporation of MSCs into the respective tissues.1, 2, 3 In addition to the direct therapeutic effects via differentiation and incorporation, MSCs indirectly accelerates tissue repair through enhanced secretion of the angiogenic cytokines such as VEGF, FGF and HGF, inducing migration and proliferation of the endothelial cells nearby the ischaemic site and increasing the survival of the transplanted MSCs within the transplantation site by providing better stem cell niche environment.1, 2, 3, 4 However, one of the major drawbacks in clinical applicability of MSC therapy is allogenic rejection via hypoxia, oxidative stress and inflammation around the injury site.5, 6 For such reasons, more researches to increase cell viability of MSCs are urgent in developing more effective and applicable approaches to MSCs transplantation therapy.
Cripto is a small glycosylphosphatidylinositol‐anchored signalling protein that is known to regulate cell proliferation, survival, migration and differentiation7 A previous study discovered that Cripto, a cytokine overexpressed via interaction between HIF‐1α and hypoxia responsive element (HRE), is induced by hypoxic environment and helps with self‐renewal and differentiation of the embryonic stem cells via Nodal/Smad2 signalling pathway.8, 9 In addition, studies show that Cripto expression is directly associated with proliferation and differentiation of stem cells into various cells: differentiation of embryonic stem cells into cardiomyocytes and the repairing of myocardium in acute or chronic skeleton muscle damage via increased migration of satellite cells and differentiation into myosin.10, 11 Granted its benefits on stem cell viability, survival, migration and differentiation, Cripto is a protein of interest of in tackling the difficulty involved in MSC transplantation therapy withstanding hypoxic conditions. Given that hypoxic conditions induce Cripto protein expression, Cripto is especially apt, promoting further studies on its effectiveness, efficacy and safety. Thus, more studies are needed to testify Cripto secreted in ischaemic site strengthens MSC viability in the host.
GRP78 is part of the heat shock protein 70 (HSP70) family that functions as a receptor for Cripto.12 GRP78, commonly known as endoplasmic reticulum (ER) chaperone, is found in various locations in the cell such as ER, mitochondria, cytosol and cell membranes. Specifically, GRP78 located on the ER membrane are known to be overexpressed by ER stress, and when overexpressed, the proteins translocate to surface membrane of the cell; surface GRP78 are then made available to bind to Cripto proteins, regulating induction of stem cell differentiation and survival.12, 13, 14 Various Cripto‐GRP78 proliferation pathways were discovered by scientists. For example, previous studies have shown that binding of Cripto with the surface GRP78 receptor enhances both MSC growth by activation of c‐Src and MAPK/PI3K pathway and migration/invasion potentials by suppression of E‐cadherin expression.15, 16 In addition, JAK2‐STAT3 cell proliferation pathway were involved in Cripto and GRP78 receptor binding.17.
In response to accumulating evidences suggesting the potentials of both Cripto and GRP78 in proliferation, migration and invasion of hMSCs, our study aimed to test whether administration of Cripto with transient GRP78 overexpression enhances viability, proliferation and migration and invasion potentials of hMSCs. We tested to see whether GRP78+Cripto better potentiates the hMSC in vitro via JAK2‐STAT3 signal and CKD4/cyclin D1 activation. Then, we tested in vivo whether transient overexpression of GRP78 enhances hMSCs migration and viability as well as angiogenic cytokines such as VEGF, FGF and HGF by binding to Cripto secreted in ischaemic site of murine hindlimb ischaemia model.
2. MATERIAL AND METHODS
2.1. Cell culture
Human MSCs (hMSC) derived from human adipose tissue were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). hMSC was confirmed to be pathogen‐ and mycoplasma‐free; they expressed cell surface markers, such as cluster of differentiation (CD) 73 and CD 105, but not CD31 and exhibited adipogenic and osteogenic differentiation potential when cultured in specific differentiation media. hMSC was cultured in α‐minimum essential medium (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) foetal bovine serum (Hyclone), 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin. hMSC cultures were grown in a humidified incubator in the atmosphere of 95% air and 5% CO2 at 37°C.
2.2. Lipofectamine transfection
hMSC cultured in 60 mm plates were overexpressed with 3 μg of pcDNA3.1 GRP78 vector (Invitrogen, Carlsbad, CA, USA), using lipofectamine 2000 (Invitrogen). The cells were transfected according to the manufacturer's specifications. Overexpression of pcDNA3.1 GRP78 vector was confirmed via western blot analysis in all experiments.
2.3. Chemical treatment of human MSCs
Human MSC (hMSC) was washed twice with phosphate buffer saline (PBS), and fresh α‐MEM supplemented with 10% FBS was added. To investigate phosphorylated Janus kinase 2 (JAK2)—signal transducer and activator of transcription 3 (STAT3), pre‐treated hMSC with or without antibody GRP78 (Santa Cruz Biotechnology, Dallas, TX, USA) and transiently GRP78 overexpression hMSC were at 37°C for 24 hour and then treated with 1 μM recombinant human Cripto (abcam, Cambridge, UK) for various times (0, 5, 10 or 20 minutes). To assess another cell analysis, the hMSC groups were treated with Cripto (1 μM) for 24 hour at 37°C.
2.4. Human MSC differentiation
For hMSC differentiation, cells were grown in StemPro adipogenic, osteogenic or chondrogenic culture medium (Thermo Fisher Scientific, Waltham, MA, USA). Cells were grown in the adipogenic medium for 1 week, those grown in osteogenic medium for 1 week, and those grown in chondrogenic medium for 2 weeks. Adipocytes were stained with oil red O (Sigma‐Aldrich, St. Luis, MO, USA) for 10 minutes, osteoblasts were stained with alkaline phosphatase stain kit (Sigma‐Aldrich) for 10 minutes and chondrocytes were stained with Safranin O (Sigma‐Aldrich) for 5 minutes. The samples were visualized by inverted microscopy (Nikon, Tokyo, Japan).
2.5. RNA isolation and reverse transcription polymerase chain reaction
To isolate RNA, hMSCs was extracted in TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). RNA quality was assessed using the 260/280 nm absorbance ratio. The concentration of RNA was quantified using a microplate reader (Tecan Group AG, Mannedorf, Switzerland) and reverse transcription polymerase chain reaction (RT‐PCR) was performed with 100 ng total RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). PCR amplification was performed with synthetic gene‐specific primer for Osteopontin (OPN) forward, 5′‐CTCCATTGACTCGAACGACTC‐3′; OPN reverse, 5′‐CAGGTCTGCGAAACTTCTTAGAT‐3′ FABP4 forward, 5′‐CGTGGAAGTGACGCCTTTCATG‐3′; FABP4 reverse, 5′‐ACTGGGCCAGGAATTTGACGAA‐3′; SOX9 forward, 5′‐AGCGAACGCACATCAAGAC‐3′; SOX9 reverse, 5′‐CTGTAGGCGATCTGTTGGGG‐3′; β‐actin forward, 5′‐AACCGCGAGAAGATGACC‐3′; β‐actin reverse, 5′‐AGCAGCCGTGGCCATCTC‐3′.
2.6. Quantitative real‐time PCR
Quantitative real‐time PCR (qRT‐PCR) analysis was performed using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific). qRT‐PCR reaction using the PIKOREAL 96 (Thermo Fisher Scientific) was performed under cycling conditions: 95°C for 15 seconds (denaturation), 55°C for 30 seconds (annealing) and 72°C for 60 seconds (extension) for 45 cycles. The gene expression level, normalized to β‐actin, was then calculated using the 2−∆∆Ct formula with reference to the hMSC. Primer sequences were as follows: OPN forward, 5′‐CTCCATTGACTCGAACGACTC‐3′; OPN reverse, 5′‐CAGGTCTGCGAAACTTCTTAGAT‐3′ FABP4 forward, 5′‐CGTGGAAGTGACGCCTTTCATG‐3′; FABP4 reverse, 5′‐ACTGGGCCAGGAATTTGACGAA‐3′; SOX9 forward, 5′‐AGCGAACGCACATCAAGAC‐3′; SOX9 reverse, 5′‐CTGTAGGCGATCTGTTGGGG‐3′; β‐actin forward, 5′‐AACCGCGAGAAGATGACC‐3′; β‐actin reverse, 5′‐AGCAGCCGTGGCCATCTC‐3′.
2.7. Kinase assays
The cells were lysed using RIPA lysis buffer (Thermo Fisher Scientific). Cyclin‐dependent kinase 4 (CDK 4) kinase assays were performed using a CDK 4 Kinase Assay Kit (Cusabio, Baltimore, USA). Briefly, 10 mM ATP was added to 1.25 mL of 6 mM substrate peptide. The mixture was diluted with dH2O to 2.5 mL to yield a 2× ATP/substrate cocktail. Then 1 mL of 10× kinase buffer was added to 1.5 mL of dH2O to yield a 2.5 mL 4× reaction buffer and the enzyme was diluted in reaction buffer to give the reaction cocktail. The reaction cocktail was added to 12.5 mL/well of pre‐diluted compound of interest (usually approximately 10 mM) and incubated for 5 min at room temperature. ATP/substrate cocktail was added to 25 mL/well pre‐incubated reaction cocktail/compound. The final assay conditions for a 50 mL reaction were therefore 25 mM Tris‐HCl (pH 7.5), 10 mM MgCl2, 5 mM b‐glycerophosphate, 0.1 mM Na3 VO4, 2 mM DTT, 200 mM ATP, 1.5 mM peptide and 50 ng CDK4 kinase. The reaction was incubated at room temperature for 30 minutes, then the stop buffer was added. From each reaction, 25 mL was transferred to a 96 well streptavidin‐coated plate and incubated at room temperature for 1 hour. Plates were washed 3 times with PBS. Phospho‐Rb (Ser780) antibody was added at 100 mL/well and incubated at room temperature for 2 hour. Plates were washed 3 times with PBS. HRP‐avidin was added to each well, and incubated at 37°C for 1 hour. Plates were washed 5 times with PBS. TMB substrate and stop solution were added. The absorbance was read at 450 nm with a microtiter plate reader (Tecan Group AG).
2.8. Cell prolifreation assay
Subconfluent, expotentially growing hMSC were incubated in a 96 well plate with Crpto. Cell proliferation were determined using a modified 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay, which is based on the conversion of tetrazolium salt to formazan by mitochondrial NAD(P)H‐dependent oxidoreductase enzymes. Formazan levels were quantified by measuring the absorbance at 575 nm using a microplate reader (Tecan Group AG).
2.9. Single‐cell cultivation assay
hMSC were treated with trypsin to prepare single cell suspensions in growth media. A limiting dilution assay was used to aliquot single hMSC into individual wells of 96 well culture plates. Briefly, cell suspensions containing 1 × 103 cells in 10 mL of growth media were diluted 10‐folds, and 100 μL of the diluted sample (approximately 1 cell/100 μL) was seeded into 96 well plates. We seeded hMSC, GRP78 overexpressed hMSC, hMSC treated with GRP78 antibody (Santa Cruz Biotechnology) in the presence or absence of 100 ng mL−1 Cripto were then cultured in a humidified incubator for 10 days.
2.10. Flow cytometry
hMSC and transiently overexpression GRP78 hMSC were incubated with primary antibody to GRP78 (Santa Cruz Biotechnology) for 1 hour on ice to confirm the expression of GRP78. After carefully washing with PBS twice, the cells were incubated on ice with fluorescent dye‐conjugated Alexa‐488 secondary antibody (Thermo Fisher Scientific) for 30 minutes. Next, the cells were washed with PBS. Transiently overexpressed GRP78 hMSC and control hMSC were stained with RNase and propidium iodide (PI) for 30 minutes to calculate the proportion of cells in each phase of the cell cycle. We analysed each sample numerically via CyFlow Cube 8 (Sysmex Partec, Münster, Germany) and FSC Express (De Novo Software, Los Angeles, CA, USA).
2.11. Transwell assays
hMSC migration and invasion assays were performed in transwell chambers (Millipore, Burlington, MA, USA) with Matrigel (Becton‐Dickinson Pharmingen, San Diego, CA, USA), which was dissolved at 4°C overnight, diluted with serum‐free medium (1:3), added to the upper chambers with 50 μL in each well and balanced in an incubator for 30 minutes. The cell suspensions (1 × 106 cells/mL) were seeded into the upper chambers containing serum‐free medium, and in the lower chambers medium containing 10% foetal bovine serum with Cripto or without Cripto. The cell invasion potential was evaluated using numbers of cells in the Matrigel using microscope.
2.12. Scratch wound‐healing migration assay
hMSC were cultured to 90% confluence in 6 well cell culture plates in 4 mL of growth medium per well. To inhibit cell proliferation, cells were treated with mytomycin C (10 μg mL−1; Sigma‐Aldrich) for 3 hour before the assay. The cell layer was scratched using a 2 mm wide tip to make a line‐shaped wound. The cells were then treated with Cripto (100 ng mL−1) or Cripto with pre‐treated antibody GRP78 (100 ng mL−1, Santa Cruz Biotechnology) at 37°C, and treated with Cripto (100 ng mL−1) for 24 hour. The cells including the control cells were allowed to migrate, and the results were acquired under an inverted microscope (Nikon).
2.13. Western blot analysis
Total protein and tissue homogenates were collected using RIPA lysis buffer (Thermo Fisher Scientific). Cell lysates (20 μg protein) in a sample buffer were separated by electrophoresis in 8%‐12% sodium dodecyl sulphate polyacrylamide gel and transferred to the nitrocellulose membranes for probing with antibodies. After washing with Tris‐buffered saline/Tween‐20 buffer (0.05% Tween‐20, 150 mM NaCl, 10 mM Tris‐HCl; pH 7.6), membranes were blocked with 5% bovine serum albumin for 1 h at room temperature and then incubated with primary antibodies against 78 kDa glucose‐regulated protein (GRP78), SRY (sex determining region Y)‐box 2 (SOX2), Nanog, octamer‐binding transcription factor 4 (OCT4), phosphorylated Janus kinase 2 (p‐JAK2), phosphorylated Signal transducer and activator of transcription 3 (p‐STAT3), Cyclin‐dependent kinase 2 (CDK 2), Cyclin E, Cyclin‐dependent kinase 4 (CDK 4), Cyclin D1, Cripto, B‐cell lymphoma 2 (BCL‐2), Bcl‐2‐associated X protein (BAX), cleaved caspase‐3, cleaved Poly ADP‐ribose polymerase 1 (c‐PARP‐1), β‐actin and α‐tubulin (all from Santa Cruz Biotechnology). After incubation of the membranes with peroxidase‐conjugated secondary antibodies (Santa Cruz Biotechnology), bands were detected using enhanced chemiluminescence regents (Amersham Biosciences, Little Chalfont, UK) in a dark room.
2.14. Murine hindlimb ischaemia model and cell transplantation
Eight‐week‐old male nude BALB/c mice (Biogenomics, Seoul, Korea) maintained under a 12‐hour light/dark cycle were used. All animal experiments were approved by the Institutional Animal Care and Use Committee of Soonchunhyang University, Seoul Hospital, Korea (project number: IACUC2013‐5; 6 February 2014). The murine hindlimb ischaemia model was established as previously described, with minor modifications. At no later than 3 hour after surgery, PBS, hMSC, overexpression GRP78 on hMSC and treated hMSC with antibody GRP78 were injected intramuscularly into the ischaemic thigh (5 × 105 cells/100 μL PBS per mouse; 5 mice per treatment group). Cells were injected into 5 ischaemic sites.
2.15. Immunohistochemistry
At post‐operative 3 days, ischaemia injury sites were removed and fixed with 4% paraformaldehyde (Sigma‐Aldrich). Each sample was embedded in paraffin. After de‐paraffinized in xylene and alcohol, we boiled each sample in sodium citrate buffer (pH 6.5). Immunohistochemistry was performed using primary antibodies Cripto (Santa Cruz Biotechnology), human nuclear antigen (HNA; Millipre, Billerica, MA, USA), proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology) and cleaved caspase‐3 (Santa Cruz Biotechnology) and secondary antibodies Alexa‐488 and Alexa‐594 (Thermo Fisher Scientific). Cell nuclei were stained with 4′,6′‐diaminido‐2‐phemylindol (DAPI; Sigma‐Aldrich), and immunohistochemistry samples were observed using confocal microscopy (Olympus, Tokyo, Japan).
2.16. Determination of human angiogenic cytokine
Human angiogenic cytokines released from transplanted hMSC in ischaemic‐injured sites were assessed by ELISA at post‐operative 3 days. After quantification of the protein in ischaemic‐injured site homogenates by bicinchoninic acid (BCA) protein assay (100 μg protein), the levels of human VEGF, human FGF and human HGF were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. The levels of cytokines were quantified by measuring the absorbance at 450 nm using a microplate reader (Tecan Group AG).
2.17. Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). All experiments were analysed by one‐way analysis of variance (ANOVA). If a significant effect of treatment was revealed by one way ANOVA in comparisons involving ≥3 groups, Bonferroni‐Dunn tests were used to reveal inter‐group differences. Differences were considered statistically significant if P < .05.
3. RESULTS
3.1. Increasing binding GRP78 with Cripto enhanced cell proliferation via JAK2‐STAT3 pathway
To assure that GRP78‐hMSC increases the expression of GRP78, we measured the overexpression of GRP78 in GRP78‐hMSC by western blot and immunohistochemistry (Figure 1A,B). In accordance with our prediction, GRP78‐hMSC had a significantly higher expression of GRP78 than the control. We confirmed that differentiation/pluripotency capabilities of adipogenesis, chondrogenesis and osteogenesis of GRP78‐hMSC were similar to those of control via differentiation media, and RT‐PCR (Figure 1C,D). GRP78‐hMSC also expressed stem cell surface marker (SOX2, Nanog, and OCT4) similar to the control, as determined by using western blot analysis. JAK2‐STAT3 signalling has been known to be the pathway responsible for cellular survival in transplanted hMSCs when surface GRP78 receptor binds with Cripto.17 To reassure whether GRP78‐hMSC increases the activation of the JAK2‐STAT3 signal pathway in our study, we treated GRP78‐hMSC and the control hMSCs with Cripto (0, 5, 10 and 20 minutes) and measured protein levels of JAK2 and STAT3 by western blot assay (Figure 2A). Phosphorylation of JAK2 and STAT3 were significantly increased in GRP78‐hMSC compared to those of the control. Treatment with GRP78 antibody inhibited activation of JAK2‐STAT3 pathway via neutralizing the surface GRP78 hMSCs (Figure 2A). Finally, to measure the differences in the magnitudes of cell proliferation, we assessed the MTT assay, single cell assay, expression of cell cycle protein (CDK2, Cyclin E, CDK4 and Cyclin D1), CDK4/cyclin D1 kinase activation and FACS cell cycle analysis, in control hMSCs, hMSCs with Cripto treatment (Cripto), GRP78‐hMSC with Cripto treatment (Cripto+GRP78‐hMSC) and hMSCs pre‐treated with both Cripto and GRP78 antibody (Ab‐GRP78) (Figure 2B‐G). Such results determined by via ELISA, single cell assay, western blot assay and FACS suggest that GRP78‐Cripto most enhances hMSCs proliferation the most via activation of the JAK2‐STAT3 signal pathway.
Figure 1.
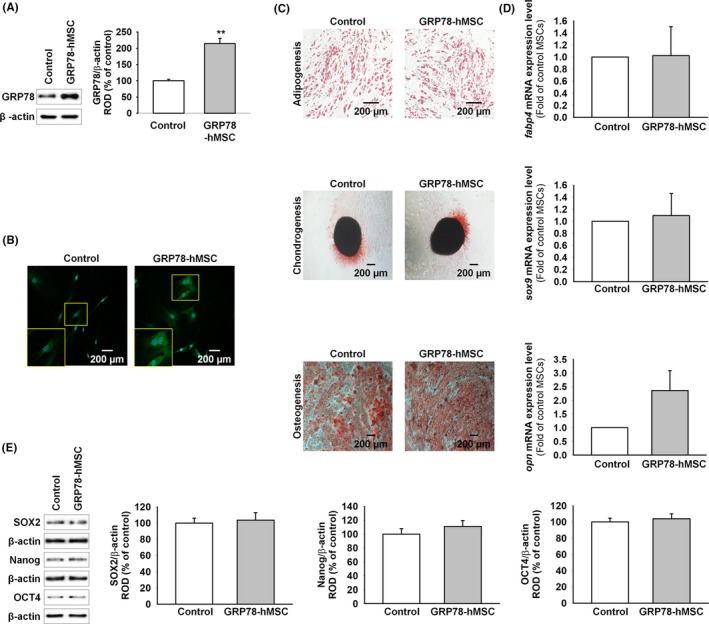
Overexpression of GRP78 on human MSCs retains differentiation capabilities of stem cells. (A) Western blot analysis of GRP78 expression in hMSCs compared to that in overexpression GRP78‐hMSCs (GRP78‐hMSC). The right panel represents the expression levels of GRP78 that were normalized to β‐actin. Values represent mean ± SEM. **P < .01 vs. Control. (B) GRP78‐hMSC was investigated by immunohistochemistry for GRP78 (Green). Scale bar = 200 μM. (C) hMSCs and GRP78‐hMSC were differentiated into adipocytes, osteocytes and chondrocytes, and examined by Oli red O, Safranin O, and alkaline phosphatase staining respectively. Scale bar = 200 μM. (D) mRNA expression of fabp4, sox9 and opn in hMSC and GRP78‐hMSC. (E) Western blot analysis of SOX2, Nanog and OCT4 expressions in GRP78‐hMSC and the control hMSC
Figure 2.
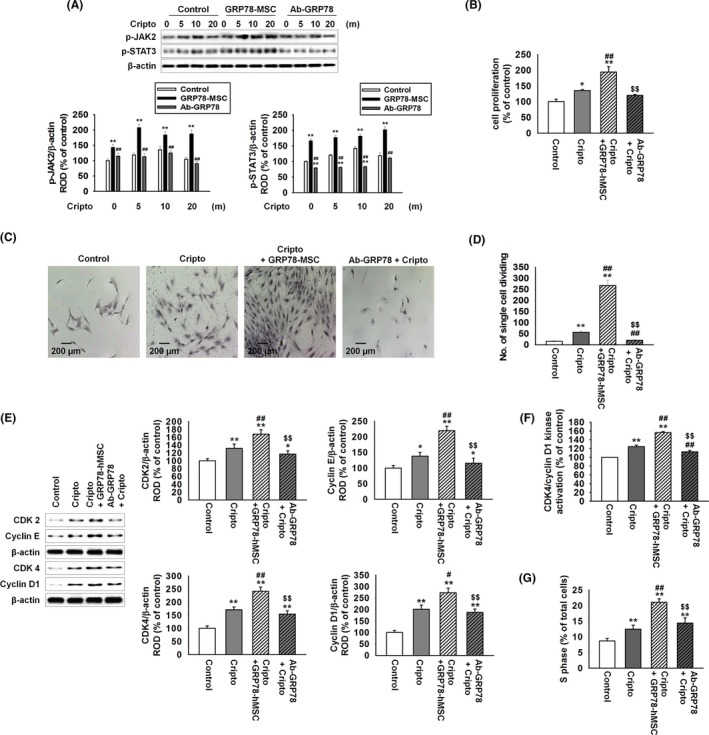
Binding of GRP78 with Cripto enhances cellular proliferation via JAK2‐STAT3 pathway. (A) Western blot analysis of p‐JAK2 and p‐STAT3 expressions in hMSCs, GRP78‐hMSC and hMSC pre‐treated with antibody GRP78 (Ab‐GRP78) exposed to Cripto for 0, 5, 10 and 20 minutes. The down panel represents the phosphorylation levels of JAK2 and STAT‐3 in control to β‐actin. Values represent the mean ± SEM. **P < .01 vs. control, ##P < .01 vs. GRP78‐hMSC. (B) hMSC, GRP78‐hMSC, and Ab‐GRP78 were treated with or without Cripto for 24 hour. Images of cellular proliferation measurement were shown using MTT assay. Values represent the mean ± SEM. *P < .05 vs control, **P < .01 vs. control, ##P < .01 vs treated Cripto, and $$P < .01 vs Cripto+GRP78‐hMSCs. (C) Image of single cell assay after 10 days in 96 well plate, stained with Giemsa stain. Scale bar = 100 μM. (D) The number of cells per field of view in each well of a 96 well plates is plotted (n = 3). Values represent the mean ± SEM. **P < .01 vs control, ##P < .01 vs treated Cripto, and $$P < .01 vs Cripto+GRP78‐hMSCs. (E) Western blot analysis of CDK 2, Cyclin E, CDK 4, and Cyclin D1 in hMSC, GRP78‐hMSC and Ab‐GRP78 were treated with or without Cripto for 24 hour. Values represent the mean ± SEM. *P < .05 vs control, **P < .01 vs control, #P < .05 vs treated Cripto, ##P < .01 vs treated Cripto and $$P < .01 vs Cripto+GRP78‐hMSCs. (F) hMSC, GRP78‐hMSC and Ab‐GRP78 were treated with or without Cripto for 24 hour. Measurement of CDK4/cyclin D1 kinase activation concentration in hMSCs using ELISA (n = 3). Values represent the mean ± SEM. **P < .01 vs untreated hMSCs. ##P < .01 vs treated Cripto, and $$P < .01 vs Cripto+GRP78‐hMSCs. (G) Images of flow cytometric analysis for PI staining to assess S phase populations in hMSC, GRP78‐hMSC and Ab‐GRP78 treated with Cripto. **P < .01 vs untreated hMSCs. ##P < 0.01 vs treated Cripto and $$P < .01 vs Cripto+GRP78‐hMSCs
3.2. Overexpression GRP78 on human MSCs increased migration and invasion through susceptibility of Cripto
To determine whether Cripto binding with GRP78 enhances mobility and invasion of the transplanted hMSCs, we measured stem cell migration and invasion of hMSCs (Figure 3A‐D). As expected, treatment with Cripto increased migration and invasion of both hMSCs (Cripto) and hMSCs with overexpressed GRP78 (Cripto+GRP78‐hMSC). As Cripto+GRP78‐hMSC most significantly increased the migration and invasion cell number of hMSCs compare with other groups, we identified whether surface GRP78 related with cell migration and invasion. The level of migration and intrusion of MSCs was significantly reduced by neutralization of GRP78. These results indicated overexpression GRP78 on hMSCs further enhanced susceptibility of Cripto that increased cell migration and invasion compared to the control without GRP78 overexpression.
Figure 3.
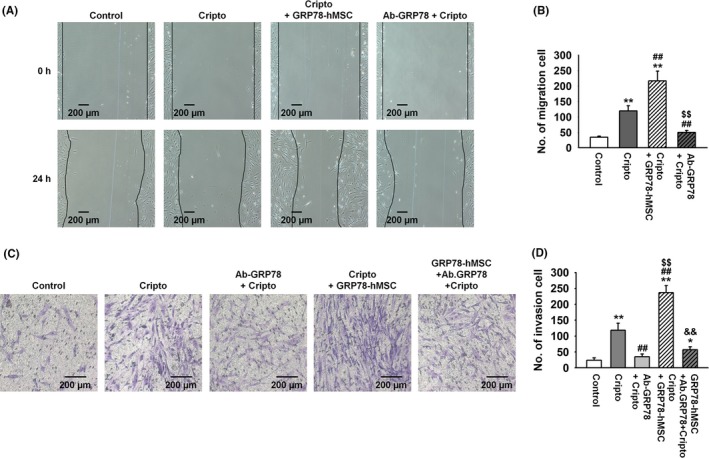
Overexpression of GRP78 on human MSCs increased migration and invasion through binding of surface GRP78 with Cripto. (A) Images of scratch wound‐healing migration assay of hMSCs, hMSCs with Cripto, GRP78+hMSC with Cripto and hMSCs with Cripto pre‐treated with Ab‐GRP78 for 0, and 24 hour. Scale bar = 100 μM. (B) The number of migrating cells is presented as the number of migrated cells per filed. Values represent mean ± SEM. **P < .01 vs control, ##P < .01 vs treated Cripto, and $$P < .01 vs Cripto+GRP78‐hMSC. (C) Invasion assay images of hMSCs, hMSCs with Cripto, hMSCs with Cripto pre‐treated with Ab‐GRP78, GRP78+hMSC with Cripto and GRP78+hMSCs with Cripto pre‐treated with Ab‐GRP78 for 24 hour. Scale bar = 100 μM. (D) Values represent the mean ± SEM. *P < .05 vs control, **P < .01 vs control, ##P < .01 vs treated Cripto, and $$P < .01 vs Ab‐GRP78‐hMSCs, &&P < .01 vs Cripto+GRP78‐hMSCs
3.3. In ischaemia injury sites, over‐expression GRP78 on human MSCs increased survival and neovascularization through binding GRP78 with Cripto
We showed that binding surface GRP78 with Cripto increased cell cycle, migration and invasion via JAK2‐STAT3 signalling in vitro. Previous study suggests that as ischaemic injury region renders hypoxic condition, and binding of Hif‐1α in HSE promoter site in the hypoxic site encourages the expression of Cripto.10 In accordance with the previous study, our immunohistochemistry assay and western blot results indicated that in vivo, expression of Cripto is similarly increased in the region of ischaemic hypoxia‐induced region (Figure 4A,B). To confirm that GRP78 binds with Cripto in ischaemia site, immunohistochemistry for HNA and Cripto was performed at post‐operative 3 days (Figure 4C). The results indicated that HNA and Cripto are bound together in all of the experimental groups: transplanted hMSCs (hMSC), overexpression of GRP78 on hMSCs (GRP78‐hMSC), and treatment hMSCs with Ab‐GRP78 (Ab‐GRP78+hMSC). In particular, GRP78‐hMSC had the highest HNA/Cripto positive cells compared to the other groups, indicating highest binding between the GRP78 containing cells and Cripto (Figure 4D). In addition, immunohistochemistry for PCNA and caspase 3 performed at post‐operative 3 days indicates that transplanted GRP78‐hMSC experiences highest cell proliferation and survival in comparison with the other groups. (Figure 5A‐D). Transplanted GRP78‐hMSC in ischaemia tissue experienced most decreased levels of cell apoptosis‐associated proteins BAX, cleaved caspase‐3 and cleaved PARP‐1 as indicated by western blot analysis (Figure 5E). In addition to detect the increased cell survival of transplanted GRP78‐hMSC, our ELISA results determined that GRP78‐hMSC group experienced the highest expression of VEGF, HGF and FGF in ischaemia site compared to the other groups (Figure 6A‐C). Our results from the Ab‐GRP78+hMSC group with decreased expression of neovascularization associated factor VEGF, HGF and FGF, as decreased cell proliferation and survival indicates that GRP78 plays a crucial role in hMSC cell survival pathway trough Cripto treatment; these results ultimately suggest that concurrent overexpression of GRP78 and Cripto enhances not only the cell proliferation of survival in ischaemic injury site, but also enhances neovascularization.
Figure 4.
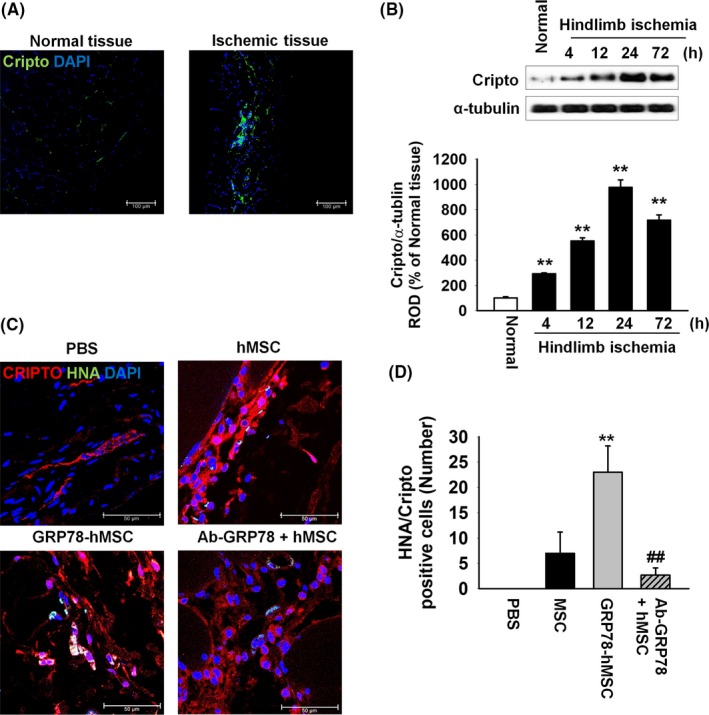
In vivo, Cripto is overexpressed in hindlimb ischaemia and overexpression of GRP78 on human MSCs increased recruitment of Cripto to MSCs via GRP78 receptor of ischaemia injury site. (A) Images of immunohistochemistry results for Cripto (green) in the ischaemia injury tissue 3 days after the murine hindlimb ischaemia operation. Scale bar = 100 μm. (B) Western blot analysis showing the expression of Cripto in ischaemia injury tissue at post‐operative for 0, 4, 12, 24 and 72 hour. The down panel represents the expression levels of Cripto which were normalized to α‐tubulin. Values represent the mean ± SEM. **P < .01 vs normal tissues. (C) At post‐operative of 3 days, immunohistochemistry assay for HNA (green) and Cripto (red) was performed in the ischaemia injury tissue of each group. Scale bar = 100 μm. (D) Existence of transplanted hMSCs were quantified based on the number of HNA and Cripto double positive cells. Values represent the mean ± SEM. **P < .01 vs. transplanted hMSCs, ##P < .01 vs transplanted overexpression GRP78 on hMSCs
Figure 5.
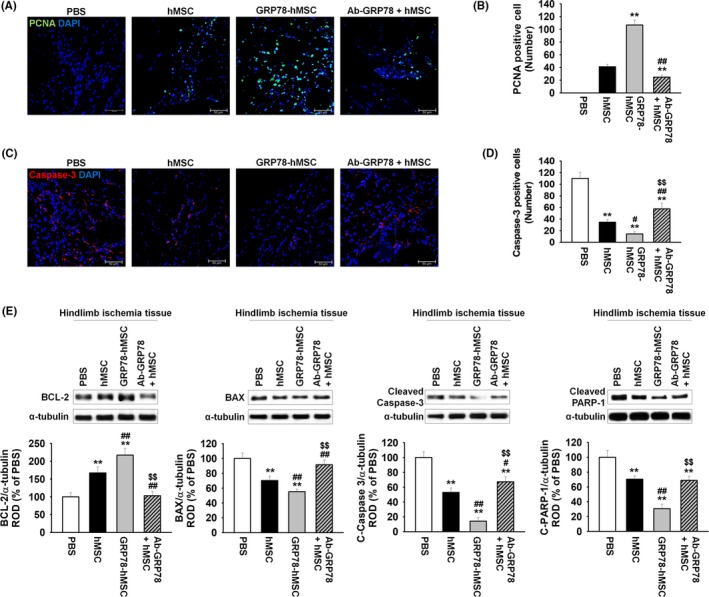
Overexpression GRP78 on human MSCs decreased cell apoptosis in murine hindlimb ischaemia. (A) At post‐operative of 3 days, immunohistochemistry assay for PCNA (green) was performed in the ischaemia injury tissue of each group. Scale bar = 100 μm. (B) Cell proliferation was quantified as the number of PCNA positive cells. Values represent the mean ± SEM. **P < .01 vs transplanted hMSCs, ##P < .01 vs transplanted overexpression GRP78 on hMSCs. (C) At post‐operative of 3 days, immunohistochemistry assay for caspase‐3 (red) was performed in the ischaemia injury tissue of each group. Scale bar = 100 μm. (D) Cell apoptosis was quantified as the number of caspase‐3 positive cells. Values represent the mean ± SEM. **P < .01 vs injection of PBS, #P < .05, ##P < .01 vs transplanted hMSCs, and $$P < .01 vs transplanted overexpression GRP78 on hMSCs. (E) Western blot analysis showing the expression of BCL‐2, BAX, cleaved caspase‐3 and cleaved PARP‐1 in ischaemia injury tissue of each group at post‐operative for 3 days. The down panel represents the expression levels of GRP78 were normalized to α‐tubulin. Values represent the mean ± SEM. **P < .01 vs injection of PBS, #P < .05, ##P < .01 vs transplanted hMSCs, and $$P < .01 vs transplanted overexpression GRP78 on hMSCs
Figure 6.
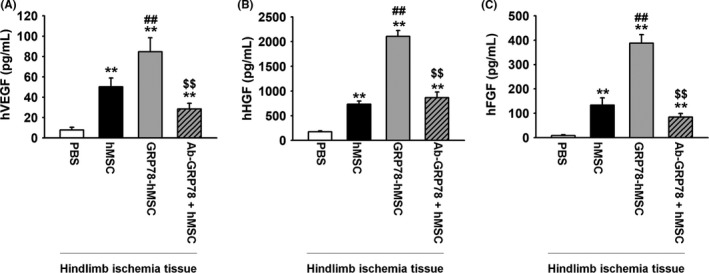
Overexpression GRP78 on human MSCs increased secretion of angiogenic cytokines in murine hindlimb ischaemia. (A) Measurements of human VEGF, HGF and FGF concentrations in ischaemia injury tissue of each group at post‐operative for 3 days using ELISA. Ischaemia injury tissue of each group at post‐operative for 3 days. Values represent the mean ± SEM. **P < .01 vs injection of PBS, ##P < .01 vs transplanted hMSCs, and $$P < .01 vs transplanted overexpression GRP78 on hMSCs
4. DISCUSSION
A recent study suggested that Cripto‐GRP78 signalling positively affects hMSC function via autocrine signals.18 In this study, we showed that concurrent overexpression of GRP78 in hMSCs (GRP78‐hMSC) augmented with Cripto accelerates and enhances the therapeutic effect of hMSCs both in vitro and in vivo. Specifically, we sought out to see whether our therapy enhances effectiveness of hMSC transplantation in murine hindlimb ischaemic model. First, we demonstrated that hMSC with transiently overexpressed GRP78 (GRP78‐hMSC) treated with Cripto enhances increases cellular proliferation, invasion and migration in vitro by activation of JAK2‐STAT3 and CDK4/cyclin signalling. Our in vivo results suggested that Cripto is overexpressed in the ischaemic injury site of murine ischaemic model. The upregulated Cripto then attached to the overexpressed GRP78 on hMSC near the transplantation site, enhancing the hMSC proliferation, viability, migration and invasion potentials. In addition, GRP78‐Cripto interaction near the transplantation site increased the levels of angiogenic cytokines near the implantation site. Ultimately, these results suggest that augmentation of Cripto in GRP78 overexpressed hMSCs may be a novel therapeutic approach to enhance effectiveness of hMSC transplantation therapy.
GRP78 is an ER chaperone synthesized in response to environmental and physiological stress conditions such as oxidative stress, hypoxia and inflammation.19 GRP78 is known to regulate various ER‐resident transmembrane proteins to act as a transducer for ER stress signalling and proper protein folding, maintaining protein in folded state and preventing abnormal protein aggregation.20, 21 In cancer cells, surface GRP78 expression enhanced cytoprotection and chemoresistance of cells, promoting cell proliferation, survival and resistance to apoptosis.22, 23, 24 Although GRP78 is a focal point of interest for regulation of MSC apoptosis and proliferation, we know very little of GRP78's effectiveness on stemness of the transplanted hMSCs.25, 26 Thus, we tested to verify that transiently overexpressed GRP78 in hMSC (GRP78‐hMSC) maintained pluripotency to differentiate into various cell types by measuring the adipogenesis, chondrogenesis and osteogenesis of both control and GRP78‐hMSC (Figure 1C,D). The results show that GRP78 overexpression not only enhances hMSC viability and survival but also maintains its stemness.19
Cripto is a small glycosylphosphatidylinosital‐anchored signalling protein that regulates MSC survival, proliferation differentiation and migration by binding to the surface GRP78 protein.17 Cripto, commonly found to be expressed in cancer cells and embryonic cells,7, 27, 28, 29, 30 is known to facilitate epithelial‐mesenchymal stem cell transition, promote cell proliferation, enhance reconstitution capacity and is essential in maintenance of stem cell pluripotency.7, 12 However, because Cripto's expression is repressed and lower in adult tissues and cells compared to embryonic stem cells, there is a gap in literature in Cripto's positive roles in adult stem cells including the MSCs.27 Thus, we aimed to discover whether concurrent overexpression of GRP78 in hMSCs (GRP78‐hMSC) augmented with the administration of Cripto better enhance hMSC proliferation, migration and invasion. Our in vitro results demonstrated that concurrent overexpression of GRP78 in hMSCs (GRP78‐hMSC) augmented with the administration of Cripto increased binding of GRP78 with Cripto, enhanced activation of JAK2‐STAT3 and CDK4/cyclin D1 kinase signals and increased activation of the signal compared to control hMSC (Figure 2A,B). Ultimately, enhanced signalling of GRP78 with Cripto resulted in highest cellular survival of hMSCs determined by single‐cell assay, proving our proposed concurrent treatment of GRP78‐hMSC with Cripto to be an effective mode of enhancing hMSCs proliferation and survival (Figure 2C,D). Pre‐treatment of hMSCs with a GRP78 antibody (Ab‐GRP78) nullified all the significant proliferation effects of GRP78+Cripto, suggesting that the binding of GRP78 receptor in the hMSCs with Cripto is crucial in our proposed cell signalling pathway.
Cell migration and invasion, in addition to cell proliferation, play integral roles in survival and safe transplantation of allogeneic stem cells in an injection site. Similarly, previous studies have succeeded in elucidating several pathways in which migration and invasion are facilitated.18 STAT3 has been known to promote cell migration and invasion and overexpression of GRP78 cell is known to progress colon cancer metastasis.31, 32, 33 In accordance with the literature, we aimed to verify that GRP78‐hMSC activated by Cripto increases migration and invasion, and thus the proliferation of the cell. Our migration and invasion assay indicated that increased signalling of GRP78 and STAT3 due to binding of GRP78‐hMSC and Cripto resulted in increased number of migration and invasion cells (Figure 3A‐D). As expected, the dual supplementation of overexpressed GRP78 and Cripto resulted in the highest number of invasion and migration cells, whereas pre‐treatment of hMSCs with GRP78 antibody blocked the binding of surface GRP78 with Cripto, thus nullifying the positive effects. With our accumulated in vitro results that suggest that concurrent GRP78‐hMSC with Cripto increases not only cell proliferation but also cell migration and invasion, we tested whether GRP‐Cripto exerts similar positive effects on viability and neovascularization of hMSCs transplanted, in vivo.
To verify our results in vitro to in vivo models, we used murine hindlimb ischaemia model for transplantation of GRP78‐hMSCs. According to a previous study, Cripto levels were significantly elevated de novo in hypoxic cardiac tissues.10 In addition, another study suggested that hypoxic precondition of MSC promotes MSC survival via HIF‐1 ‐GRP78‐Akt signal pathway.34 Thus, we assumed that hypoxic conditions in ischaemic application site would show an increased level of Cripto and hypoxia‐mediated GRP78 expression in hMSCs would facilitate co‐localization of Cripto‐GRP78. Similarly, our immunohistochemistry results determined that the expression of Cripto is significantly increased and that Cripto is co‐localized within the tissue in ischaemic tissue compared to non‐ischaemic tissues (Figure 4A). More specifically, our western blot analysis indicated that presence of Cripto in hindlimb ischaemic tissue gradually increased in a time‐dependent manner and peaked at the greatest level at 24 hour. However, the level of Cripto declined after the 24th hour.
As predicted in vitro, we utilized immunohistochemistry to see if cellular proliferation is enhanced through binding of Cripto‐GRP78 with 4 different models of MSCs transplantation injected on hindlimb ischaemia. (PBS, hMSC, GRP78‐hMSC, and Ab. GRP78) Through HNA and Cripto immunohistochemistry assay, we confirmed that GRP78‐hMSC group had the highest increase in the existence of HNA/Cripto positive hMSCs among the PBS, hMSC, GRP‐hMSC and Ab GRP78 (Figure 4C). Furthermore, immunohistochemistry results indicate that PCNA cells are most highly expressed and localized with hMSCs in GRP78‐hMSC cells, and Caspase‐3 is least expressed in GRP78‐hMSC (Figure 5A‐D). Accordingly, western blot analysis demonstrated that levels of pro‐survival protein BCL‐2 was highest and levels of pro‐apoptosis proteins BAX, cleaved caspase‐3, and cleaved PARP‐1 were the least in the GRP78‐hMSC group (Figure 5E). Such results suggest that overexpressed GRP78 (GRP‐hMSC) bound with Cripto most significantly increases migration, invasion and cell proliferation of hMSCs in comparison to the controls. In addition, our western blot analysis on endothelial growth factor and angiogenesis‐related proteins such as,vascular endothelial growth factor (VEGF), human growth factor (HGF) and fibroblast growth factor (FGF) suggest that GRP78‐hMSC promotes migration, invasion and adhesiveness of the hMSCs to the ischaemic transplantation site by enhancing the binding of GRP78 and Cripto. On the contrary, Ab. GRP78 group resulted in protein levels of VEGF, HGF and FGF proteins similar to regular hMSC. Such results accumulate the evidence in that GRP78‐hMSCs accelerated cellular proliferation, transplanted cell survival, neovascularization and angiogenesis in hindlimb ischaemia mice model.
Overall, our study verified for the first time that increased expression of surface GRP78 of hMSCs bound with Cripto enhances stem cell therapeutic efficacy through increasing cell migration, cell survival and secreted vascularization cytokine both in vivo and in vitro. These findings suggest that GRP78‐hMSC augmented with Cripto treatment may offer a better mode of treatment than either intervention alone, and provides a better mode of hMSC transplantation in ischaemic site.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation grant funded by the Korean government (NRF‐ 2017M3A9B4032528). The funders had no role in the study design, data collection or analysis, the decision to publish or preparation of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Kim S, Yoon YM, Han Y‐S, Lee JH, Hur J, Lee SH. Administration of Cripto in GRP78 overexpressed human MSCs enhances stem cell viability and angiogenesis during human MSC transplantation therapy. Cell Prolif. 2018;51:e12463 10.1111/cpr.12463
REFERENCES
- 1. Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue‐derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542‐2547. [DOI] [PubMed] [Google Scholar]
- 2. Vaananen HK. Mesenchymal stem cells. Ann Med. 2005;37:469‐479. [DOI] [PubMed] [Google Scholar]
- 3. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896‐2902. [DOI] [PubMed] [Google Scholar]
- 4. Potapova IA, Gaudette GR, Brink PR, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761‐1768. [DOI] [PubMed] [Google Scholar]
- 5. Andrades JA, Han B, Becerra J, Sorgente N, Hall FL, Nimni ME. A recombinant human TGF‐beta1 fusion protein with collagen‐binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp Cell Res. 1999;250:485‐498. [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Li Y, Chen J, et al. Ischemic cerebral tissue and MCP‐1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831‐836. [DOI] [PubMed] [Google Scholar]
- 7. Klauzinska M, Castro NP, Rangel MC, et al. The multifaceted role of the embryonic gene Cripto‐1 in cancer, stem cells and epithelial‐mesenchymal transition. Semin Cancer Biol. 2014;29:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minchiotti G. Nodal‐dependant Cripto signaling in ES cells: from stem cells to tumor biology. Oncogene. 2005;24:5668‐5675. [DOI] [PubMed] [Google Scholar]
- 9. Fiorenzano A, Pascale E, D'Aniello C, et al. Cripto is essential to capture mouse epiblast stem cell and human embryonic stem cell pluripotency. Nat Commun. 2016;7:12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bianco C, Cotten C, Lonardo E, et al. Cripto‐1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells. Am J Pathol. 2009;175:2146‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prezioso C, Iaconis S, Andolfi G, et al. Conditional Cripto overexpression in satellite cells promotes myogenic commitment and enhances early regeneration. Front Cell Dev Biol. 2015;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spike BT, Kelber JA, Booker E, et al. CRIPTO/GRP78 signaling maintains fetal and adult mammary stem cells ex vivo. Stem cell reports. 2014;2:427‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelber JA, Panopoulos AD, Shani G, et al. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yun S, Yun CW, Lee JH, Kim S, Lee SH. Cripto enhances proliferation and survival of mesenchymal stem cells by up‐regulating JAK2/STAT3 pathway in a GRP78‐dependent manner. Biomol Ther. 2017;99:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelagalli A, Nardelli A, Lucarelli E, Zannetti A, Brunetti A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin‐1 and CXCR4 overexpression. J Cell Physiol. 2018. [DOI] [PubMed] [Google Scholar]
- 19. Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373‐381. [DOI] [PubMed] [Google Scholar]
- 20. Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20‐28. [DOI] [PubMed] [Google Scholar]
- 21. Sommer T, Jarosch E. BiP binding keeps ATF6 at bay. Dev Cell. 2002;3:1‐2. [DOI] [PubMed] [Google Scholar]
- 22. Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45‐54. [DOI] [PubMed] [Google Scholar]
- 23. de Tornyay R. Setting limits. J Nurs Educ. 1990;29:101. [DOI] [PubMed] [Google Scholar]
- 24. Yun S, Han YS, Lee JH, Kim S, Lee SH. Enhanced susceptibility to 5‐fluorouracil in human colon cancer cells by silencing of GRP78. Anticancer Res. 2017;37:2975‐2984. [DOI] [PubMed] [Google Scholar]
- 25. Luo C, Xiong H, Chen L, et al. GRP78 promotes hepatocellular carcinoma proliferation by increasing FAT10 expression through the NF‐kappaB pathway. Exp Cell Res. 2018;365:1‐11. [DOI] [PubMed] [Google Scholar]
- 26. Lee JH, Yoon YM, Lee SH. GRP78 regulates apoptosis, cell survival and proliferation in 5‐Fluorouracil‐resistant SNUC5 colon cancer cells. Anticancer Res. 2017;37:4943‐4951. [DOI] [PubMed] [Google Scholar]
- 27. Jin JZ, Ding J. Cripto is required for mesoderm and endoderm cell allocation during mouse gastrulation. Dev Biol. 2013;381:170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park KS, Moon YW, Raffeld M, Lee DH, Wang Y, Giaccone G. High cripto‐1 and low miR‐205 expression levels as prognostic markers in early stage non‐small cell lung cancer. Lung Cancer. 2018;116:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding J, Yang L, Yan YT, et al. Cripto is required for correct orientation of the anterior‐posterior axis in the mouse embryo. Nature. 1998;395:702‐707. [DOI] [PubMed] [Google Scholar]
- 30. Park SW, Do HJ, Han MH, Choi W, Kim JH. The expression of the embryonic gene Cripto‐1 is regulated by OCT4 in human embryonal carcinoma NCCIT cells. FEBS Lett. 2018;592:24‐35. [DOI] [PubMed] [Google Scholar]
- 31. Teng TS, Lin B, Manser E, Ng DC, Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J Cell Sci. 2009;122:4150‐4159. [DOI] [PubMed] [Google Scholar]
- 32. Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL‐6 family of cytokine receptors. Oncogene. 2000;19:2548‐2556. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Li Z, Fan Y, Li H, Li Z, Li Y. Overexpressed GRP78 affects EMT and cell‐matrix adhesion via autocrine TGF‐beta/Smad2/3 signaling. Int J Biochem Cell Biol. 2015;64:202‐211. [DOI] [PubMed] [Google Scholar]
- 34. Lee JH, Yoon YM, Lee SH. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF‐1alpha‐GRP78‐Akt Axis. Int J Mol Sci. 2017;18:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]


