Abstract
Objectives
Long non‐coding RNA cancer susceptibility candidate 2 (CASC2) is a novel lncRNA and has been indicated as playing tumour suppressor gene in several tumours. However, the role of CASC2 in osteosarcoma is still uncovered.
Materials and methods
The CASC2 and miR‐181a expressions were measured via qRT‐PCR. CCK‐8 assay and colony formation assay were performed to determine the cell growth, and transwell assay was performed to assess the cell invasion.
Results
We showed that CASC2 expression was downregulated in osteosarcoma samples and cell lines. Moreover, we showed that downregulated expression of CASC2 was correlated with advanced TNM stage. Furthermore, overexpression of CASC2 inhibited osteosarcoma cell proliferation, colony formation, and invasion. In addition, we indicated that ectopic expression of CASC2 suppressed miR‐181a expression and enhanced the expression of Ras association domain family member 6 (RASSF6), PTEN and ATM in osteosarcoma cell, which were the direct target gene of miR‐181a. Moreover, we indicated that RASSF6 expression was downregulated in osteosarcoma samples and cell lines and downregulated expression of RASSF6 was correlated with advanced TNM stage. We found that the expression of RASSF6 was positively correlated with the expression of CASC2 in osteosarcoma tissues. Ectopic expression of CASC2 suppressed the osteosarcoma cell proliferation, colony formation and invasion through regulating RASSF6 expression.
Conclusions
Our data illuminated that CASC2 acted as a tumour suppressor in osteosarcoma progression.
1. INTRODUCTION
Osteosarcoma is one of the most common primary bone malignancies in adolescents and children that is mainly localized in the metaphysis of the long bones.1, 2, 3, 4, 5 With the improvements of osteosarcoma therapeutic strategies including adjuvant chemotherapy, radiotherapy and wide tumour excision, the 5‐year survival rate of osteosarcoma cases without metastatic disease is improved to 65%.6, 7, 8, 9 However, a half of patients will develop metastases and few of these patients could be cured.10, 11, 12 Therefore, it is urgent to find new biomarkers and therapeutic targets for osteosarcoma patients.
Long non‐coding RNAs (lncRNAs) are one major members of ncRNA family that are >200 bases in length with limited protein coding capacity.13, 14, 15, 16 Recent researches have demonstrated that the expression of lncRNAs was deregulated in many types of tumours.17, 18, 19, 20 Increasing evidences have showed that lncRNAs play essential roles in a variety of cell biological processes such as cell development, proliferation, apoptosis, invasion, metastasis and stem cell pluripotency.18, 21, 22, 23 lncRNA cancer susceptibility candidate 2 (CASC2) is a novel lncRNA that is located in human 10q26 and acts as a tumour suppressor gene in several tumours such as endometrial cancer, bladder cancer and gastric cancer.24, 25, 26, 27 However, the expression and functional role of CASC2 in the development of osteosarcoma are still uncovered.
In this study, we try to analyse the expression of CASC2 in the osteosarcoma cell lines and tissues. We also investigated the role of CASC2 in osteosarcoma cell.
2. MATERIALS AND METHODS
2.1. Human tissue samples
The 40 osteosarcoma tissues and the matched non‐cancerous samples utilized in our study were obtained from The Fifth Hospital of Harbin. This study was approved by The Fifth Hospital of Harbin Institutional Review Board. All patients were provided the informed consent for using of their tissues in our research.
2.2. Cell culture and transfect
The human osteosarcoma cell lines MG‐63, SW1353, Saos‐2, SOSP‐9607 and U2OS and human osteoblasts hFOB were obtained from the cell bank of Chinese Academy of Sciences. These cells were cultured in the Roswell Park Memorial Institute (RPMI)‐1640 medium supplemented with the foetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and streptomycin and penicillin. pcDNA‐CASC2 and empty pcDNA3.1 vector and miR‐181a mimic and scramble were synthesized from GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, Wyman Street, Waltham, MA, USA) according to the manufacturer's instructions.
2.3. Quantitative real‐time RT‐PCR (qRT‐PCR) analysis
Total RNA was isolated from cells or tissues using the TRIzol kit (Invitrogen, Carlsbad, CA, USA) following to the manufacture's guide. Quantitative real‐time RT‐PCR (qRT‐PCR) analysis was performed to detect the miRNA and mRNA expressions through using SYBR green kit (TaKaRa, Dalian, China) on the Light Cycler 480 (Roche, Switzerland) in accordance with the instructions. The expression of mRNA and miRNA was normalized to GAPDH or U6 expression level, respectively. The PCR primers were shown as follows: CASC2 forward primers: 5′‐GCACATTGGACGGTGTTTCC‐3′, reverse primers: 5′‐CCCAGTCCTTCACAGGTCAC‐3′; U6 forward primers: 5′‐CTCGCTTCGGCAGCACATATACT‐3′, reverse primers: 5′‐ACGCTTCACGAATTTGCGTGTC‐3′; GAPDH forward primers: 5′‐ AATGGGCAGCCGTTAGGAAA‐3′, reverse primers: 5′‐TGAAGGGGTCATTGATGGCA‐3′.
2.4. Cell proliferation, invasion and colony formation assay
The cell proliferation was analysed by CCK‐8 assay (DOJINDO, Tokyo, Japan) in accordance with the manufacturer's instructions. Cells were cultured in the 96‐well plate and continued to culture for 0, 24, 48 and 72 hours. The optical density (OD) value at 450 nm was read in a microplate reader. For cell invasion, transwell assay was performed using transwell chamber with 8 μm pores which coated with Matrigel (Sigma‐Aldrich, Oakville, ON, Canada). Cells were cultured in the upper chamber with no FBS, and 10% FBS was added in the lower chamber. After 48 hours, cells on the upper chamber were removed and cells from the lower surface were stained with crystal violet and counted using a microscope (Olympus, Tokyo, Japan). For cell colony formation assay, cells at 4000 cells/cm2 in 6‐well plates were cultured and incubated in RPMI‐1640 medium with 10% FBS. Two weeks later, the cell colonies were stained with crystal violet and counted.
2.5. Western blot analysis
Protein from cells or tissues was extracted by a lysis buffer, and the concentration of protein was determined using the Bicinchoninic Acid (BCA) protein assay (Santa Cruz, CA, USA) according to the manufacturer's instructions. Equal protein was separated by 10% SDS‐PAGE and transferred onto the nitrocellulose membranes (Millipore Corporation, Boston, MA, USA). Membrane was blocked 5% non‐fat milk and then incubated with primary antibodies (RASSF6 and GAPDH, Abcam, Cambridge, UK) at 1:5000 dilutions. The membrane was incubated with secondary antibody for 1 hour at the room temperature. Protein band was determined by the enhanced chemiluminescence (ECL) detection system.
2.6. Statistical analysis
The data were shown as the mean ± standard deviation (SD) and measured using SPSS18.0 statistical software (IBM, Chicago, IL, USA). Differences were determined by the two‐tailed Student's t test. The relationship between CASC2 and RASSF6 expressions was assessed with two‐tailed Pearson's correlation. P < .05 was defined as statistically significant.
3. RESULTS
3.1. CASC2 expression was downregulated in osteosarcoma tissues
We firstly explored CASC2 expression in osteosarcoma samples (n = 35) compared with non‐tumour tissues (n = 35) using qRT‐PCR. As shown in the Figure 1A,B, CASC2 expression was significantly decreased in osteosarcoma samples compared with non‐tumorous samples. Moreover, we showed that downregulated expression of CASC2 was correlated with advanced TNM stage (Figure 1C).
Figure 1.
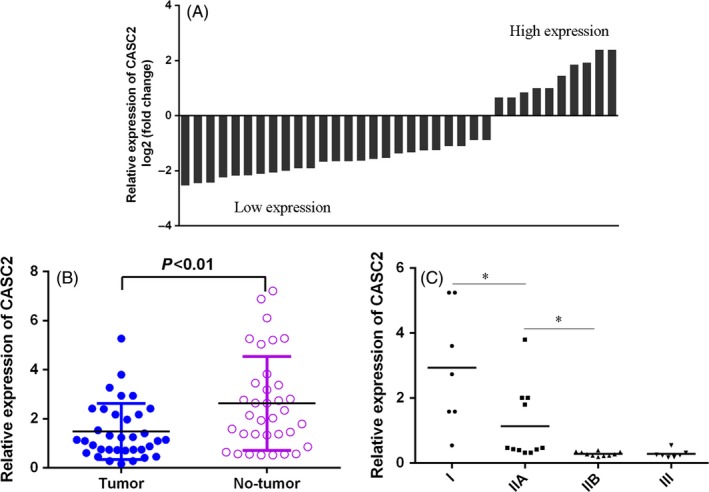
Cancer susceptibility candidate 2 (CASC2) expression was downregulated in the osteosarcoma tissues. A, The expression of CASC2 in the osteosarcoma samples and non‐tumour tissues was determined by qRT‐PCR. U6 was used as the internal control. B, The CASC2 expression level in the osteosarcoma samples was lower than in the non‐tumorous samples. C, The lower expression of CASC2 was associated with the advanced TNM stage. *P < .05
3.2. CASC2 suppressed osteosarcoma cell proliferation
We determined the expression of CASC2 in osteosarcoma cell lines. As shown in the Figure 2A, the CASC2 expression was downregulated in osteosarcoma cell lines (MG‐63, U2OS, SOSP‐9607 and Saos‐2) compared with human osteoblasts cell line (hFOB). To further study the role of CASC2 in osteosarcoma cell, the pcDNA‐CASC2 was designed and transfected into MG‐63 and U2OS cells. As shown in Figure 2B,C, cells transfected with pcDNA‐CASC2 had a remarkably increased CASC2 expression compared with the control vector group in both cell lines by qRT‐PCR. In addition, overexpression of CASC2 suppressed MG‐63 (Figure 2D) and U2OS (Figure 2E) cell proliferation.
Figure 2.
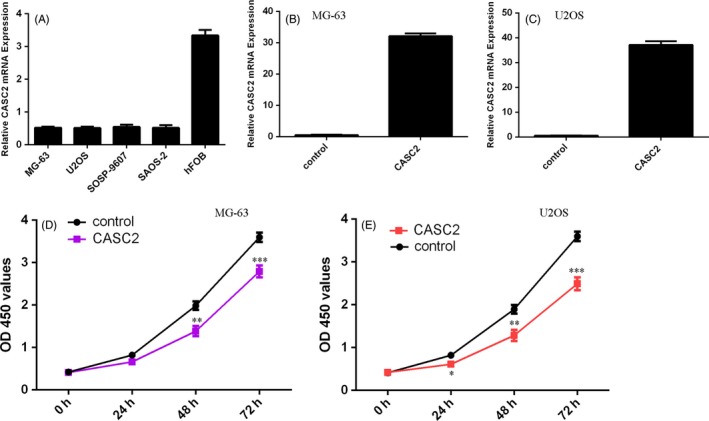
Cancer susceptibility candidate 2 (CASC2) suppressed the osteosarcoma cell proliferation. A, The expression of CASC2 in the osteosarcoma cell lines (MG‐63, U2OS, SOSP‐9607 and Saos‐2) and human osteoblasts cell line (hFOB) was measured by qRT‐PCR. U6 was used as the internal control. B, The expression level of CASC2 in the MG‐63 cell with transfecting pcDNA‐CASC2 vector was determined using qRT‐PCR. C, The expression level of CASC2 in the U2OS cell was measured by qRT‐PCR. D, Ectopic expression of CASC2 decreased the MG‐63 proliferation. The cell proliferation was determined by CCK‐8 assay. E, CCK‐8 assay was performed to measure the U2OS cell proliferation. *P < .05, **P < .01 and ***P < .001
3.3. CASC2 inhibited osteosarcoma cell colony formation and invasion
To further examine the influence of CASC2 on osteosarcoma cell invasion, we performed transwell assays in MG‐63 and U2OS cells. Elevated expression of CASC2 suppressed MG‐63 and U2OS cell invasion (Figure 3A). In addition, we performed a colony formation assay to further study the role of CASC2 on osteosarcoma cell growth. Consistently, we found that ectopic expression of CASC2 decreased the MG‐63 and U2OS cell colony formation (Figure 3B).
Figure 3.
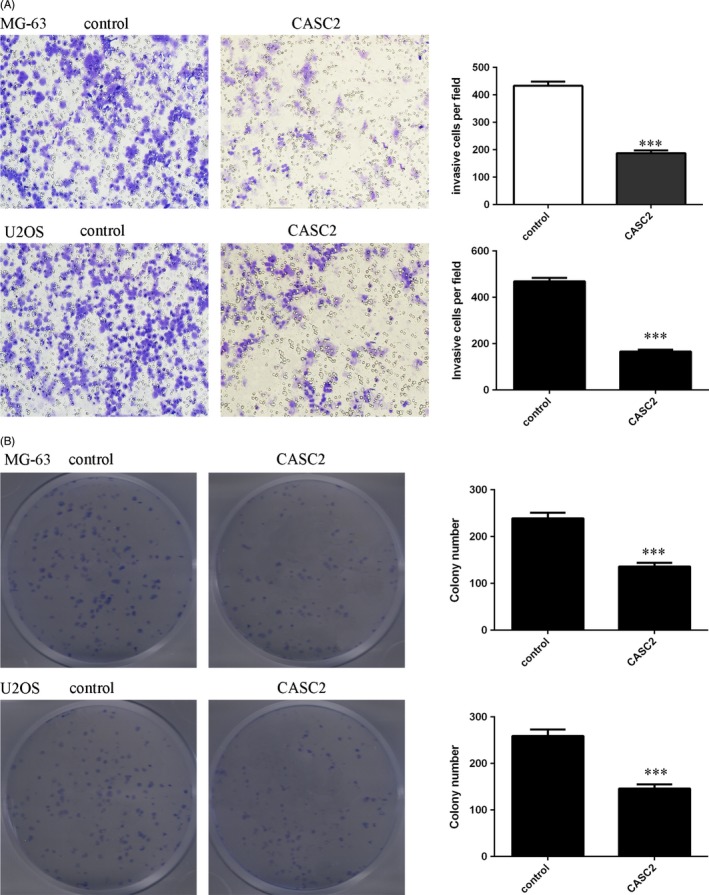
Cancer susceptibility candidate 2 (CASC2) inhibited the osteosarcoma cell colony formation and invasion. A, Elevated expression of CASC2 decreased the MG‐63 and U2OS cell invasion. Relative ratio of invasive cells per field is shown. B, Ectopic expression of CASC2 suppressed the MG‐63 and U2OS cell colony formation. The relative colony numbers were shown. ***P < .001
3.4. CASC2 suppressed miR‐181a expression in osteosarcoma cell
Next, we demonstrated that ectopic expression of CASC2 decreased miR‐181a expression in the MG‐63 cell (Figure 4A). In addition, we showed that overexpression of CASC2 enhanced RASSF6 expression in the MG‐63 cell (Figure 4B). Moreover, we demonstrated that elevated expression of CASC2 promoted the expression of PTEN (Figure 4C) and ATM (Figure 4D) in the MG‐63 cell.
Figure 4.
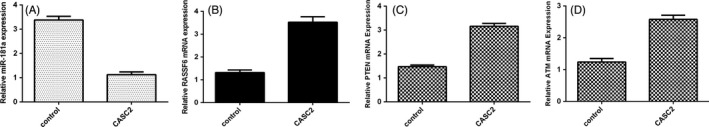
Cancer susceptibility candidate 2 (CASC2) suppressed the miR‐181a expression in the osteosarcoma cell. A, Ectopic expression of CASC2 suppressed the expression of miR‐181a in the MG‐63 cell. B, Overexpression of CASC2 promoted the Ras association domain family member 6 (RASSF6) expression in the MG‐63 cell. C, Ectopic CASC2 expression elevated the PTEN expression in the MG‐63 cell. D, CASC2 overexpression enhanced the ATM expression in the MG‐63 cell
3.5. RASSF6 expression was downregulated in osteosarcoma tissues
We then explored the RASSF6 expression in osteosarcoma samples (n = 35) compared with non‐tumour tissues (n = 35) using qRT‐PCR. The RASSF6 expression was significantly decreased in osteosarcoma samples compared with non‐tumorous samples (Figure 5A,B). Moreover, we showed that downregulated expression of RASSF6 was correlated with advanced TNM stage (Figure 5C). In addition, we found that the expression of RASSF6 was positively correlated with the expression of CASC2 in osteosarcoma tissues (Figure 5D). As shown in Figure 5E, the RASSF6 expression was downregulated in osteosarcoma cell lines (MG‐63, U2OS, SOSP‐9607 and Saos‐2) compared with human osteoblasts cell line (hFOB).
Figure 5.
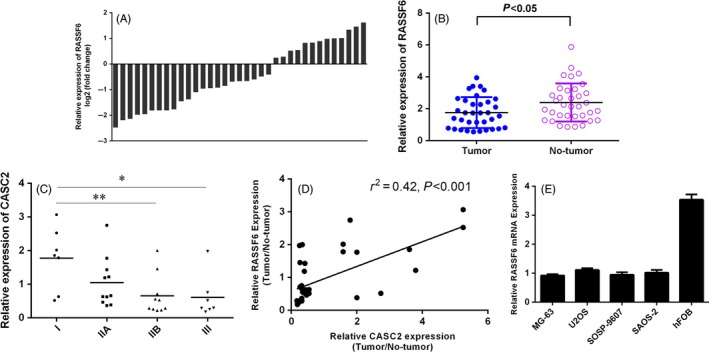
Ras association domain family member 6 (RASSF6) expression was downregulated in the osteosarcoma tissues. A, The RASSF6 expression in the osteosarcoma samples and non‐tumour tissues using qRT‐PCR. GAPDH was used as the control. B, The RASSF6 expression was significantly downregulated in the osteosarcoma tissues compared with non‐tumorous samples. C, The lower expression of RASSF6 was associated with the advanced TNM stage. D, The expression of RASSF6 was positively correlated with the expression of CASC2 in the osteosarcoma tissues. E, The expression level of RASSF6 in the osteosarcoma cell lines (MG‐63, U2OS, SOSP‐9607 and Saos‐2) and human osteoblasts cell line (hFOB) was determined by qRT‐PCR. *P < .05 and **P < .01
3.6. CASC2 suppressed osteosarcoma cell proliferation, colony formation and invasion through regulating RASSF6 expression
To further study the role of RASSF6 in osteosarcoma cell, si‐RASSF6 was designed and transfected into MG‐63 cells. As shown in Figure 6A, cells transfected with si‐RASSF6 had a remarkably decreased RASSF6 expression compared with the control vector group in the MG‐63 cell. The protein expression of RASSF6 was also decreased in the si‐RASSF6 group (Figure 6B). Next, si‐RASSF6 or control vector was transfected into the CASC2‐overexpressing MG‐63 cell. We found that knockdown of RASSF6 promoted the CASC2‐overexpressing MG‐63 cell proliferation, colony formation and invasion (Figure 6C,D,E).
Figure 6.
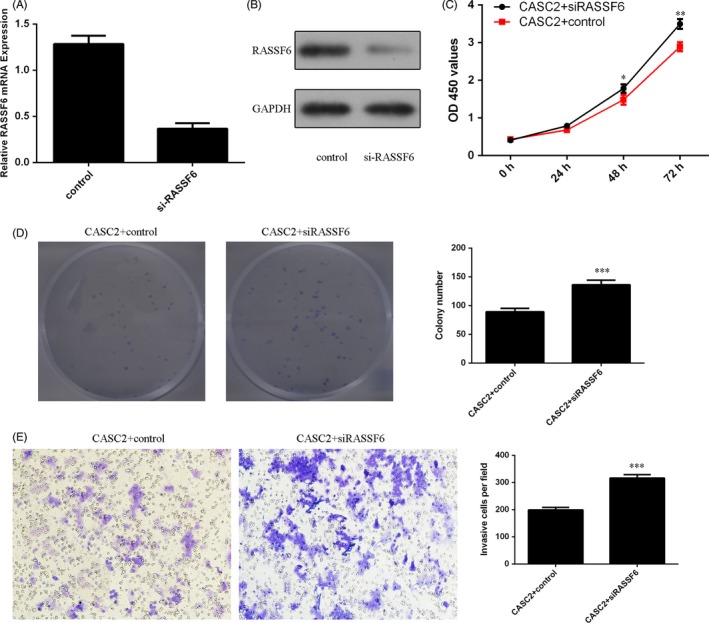
Cancer susceptibility candidate 2 (CASC2) suppressed the osteosarcoma cell proliferation, colony formation and invasion through regulating Ras association domain family member 6 (RASSF6) expression. A, The expression of RASSF6 in the MG‐63 cell transfected with si‐RASSF6 was determined by qRT‐PCR. B, The protein expression of RASSF6 was measured by Western blot. C, Knockdown of RASSF6 promoted the CASC2 overexpressing MG‐63 cell proliferation. D, Downregulation of RASSF6 enhanced the CASC2‐overexpressing MG‐63 cell colony formation. The relative colony numbers were shown. E, Downregulation of RASSF6 enhanced the CASC2‐overexpressing MG‐63 cell invasion. Relative ratio of invasive cells per field is shown. *P < .05, **P < .01 and ***P < .001
4. DISCUSSION
Increasing evidence has showed that lncRNAs act vital roles in more biological processes.28, 29 Many studies demonstrated that lncRNAs were deregulated in several tumours and lncRNAs played as cancer suppressors or oncogenes, contributing to tumour pathogenesis and progression.30, 31, 32, 33 In our study, we showed that CASC2 expression was significantly decreased in osteosarcoma samples compared with non‐tumorous samples. Moreover, we showed that downregulated expression of CASC2 was correlated with advanced TNM stage. We also demonstrated that the expression of CASC2 was downregulated in osteosarcoma cell lines (MG‐63, U2OS, SOSP‐9607 and Saos‐2) compared with human osteoblasts cell line (hFOB). Furthermore, we showed that overexpression of CASC2 inhibited osteosarcoma cell proliferation, colony formation and invasion. In addition, we indicated that ectopic expression of CASC2 suppressed miR‐181a expression in osteosarcoma cell and enhanced the expression of RASSF6, PTEN and ATM, which were the direct target gene of miR‐181a. Moreover, we indicated that RASSF6 expression was significantly downregulated in osteosarcoma samples compared with non‐tumorous tissues and the downregulated expression of RASSF6 was correlated with advanced TNM stage. In addition, we found that the expression of RASSF6 was positively correlated with the expression of CASC2 in osteosarcoma tissues. The expression of RASSF6 was downregulated in the osteosarcoma cell lines compared with hFOB. CASC2 suppressed osteosarcoma cell proliferation, colony formation and invasion through regulating RASSF6 expression. Taken together, our data illuminated that CASC2 acted as a tumour suppressor in osteosarcoma progression.
CASC2 is a new lncRNA that has been proved to be associated with tumour progression.34 For example, Wang et al35 demonstrated that CASC2 expression was downregulated in glioma tissues and cell lines. CASC2 overexpression suppressed glioma cell proliferation, invasion and migration and enhanced the cell apoptosis through regulating the miR‐21 expression. He et al36 showed that CASC2 expression was decreased in non‐small‐cell lung cancer tissues and lower CASC2 expression was associated with tumour size and advanced TNM stage. Elevated expression of CASC2 suppressed non‐small‐cell lung cancer cell proliferation. Huang et al37 indicated that the expression of CASC2 was downregulated in the colorectal cancer samples and cell lines. Overexpression of CASC2 inhibited colorectal cancer cell proliferation and tumour growth through regulating the miR‐18a/PIAS3 expression. However, the expression and role of CASC2 in osteosarcoma are still unclear. In this study, we manifested that CASC2 expression was downregulated in osteosarcoma tissues and cell lines. Overexpression of CASC2 inhibited osteosarcoma cell proliferation, colony formation and invasion.
However, the mechanism about how CASC2 affects the function of osteosarcoma cell remains to be studied. It has been shown that lncRNAs play as a “Sponge” of miRNA to decrease their effect on mRNAs. For example, Liao et al38 indicated that overexpression of CASC2 inhibited glioma cell proliferation and sensitize temozolomide (TMZ)‐resistant glioma cells to TMZ through regulating miR‐181a. In line with this result, we showed that ectopic expression of CASC2 decreased miR‐181a expression and enhanced the expression of RASSF6, PTEN and ATM in the MG‐63 cell, which were the direct target genes of miR‐181a.39, 40, 41 Previous studies demonstrated that miR‐181a played important roles in the development and progression of tumours including osteosarcoma.42, 43, 44 Zhu et al44 also showed that miR‐181a expression was increased in osteosarcoma, and knockdown of miR‐181a suppressed osteosarcoma cell proliferation and enhanced the cell apoptosis through targeting CFIm25 expression. Moreover, overexpression of miR‐181a enhanced gastric cancer cell proliferation, colony formation and cell invasion, and cell cycle by targeting RASSF6.39 RASSF6 is one member of C‐terminal RASSF protein such as RASSF3 and RASSF1A.45, 46 The expression of RASSF6 was downregulated in several tumours such as gastric cancer, hepatocellular carcinoma, colorectal cancer and pancreatic ductal adenocarcinoma.47, 48, 49 RASSF6 played as a tumour suppressor gene and suppressed tumour cell growth, invasion and migration and promoted the cell cycle and apoptosis.48, 50 In this study, we demonstrated that RASSF6 expression was downregulated in osteosarcoma tissues and cell lines and downregulated expression of RASSF6 was correlated with advanced TNM stage. Moreover, the expression of RASSF6 was positively correlated with the expression of CASC2 in osteosarcoma tissues. Furthermore, we showed that ectopic expression of CASC2 decreased osteosarcoma cell proliferation, colony formation and invasion through regulating RASSF6 expression.
In conclusion, our data revealed that CASC2 expression was downregulated in osteosarcoma tissues and cell lines and overexpression of CASC2 suppressed osteosarcoma cell growth, colony formation and invasion through regulating miR‐181a/RASSF6 expression. This study suggested that CASC2 might serve as a tumour suppressor lncRNA in the development of osteosarcoma.
Ba Z, Gu L, Hao S, Wang X, Cheng Z, Nie G. Downregulation of lncRNA CASC2 facilitates osteosarcoma growth and invasion through miR‐181a. Cell Prolif. 2018;51:e12409 10.1111/cpr.12409
REFERENCES
- 1. Li Z, Yu X, Shen JX. Long non‐coding RNAs: emerging players in osteosarcoma. Tumor Biol. 2016;37:2811‐2816. [DOI] [PubMed] [Google Scholar]
- 2. Xu M, Jin H, Xu CX, Bi WZ, Wang Y. MiR‐34c inhibits osteosarcoma metastasis and chemoresistance. Med Oncol. 2014;31:972. [DOI] [PubMed] [Google Scholar]
- 3. Geng S, Zhang X, Chen J, et al. The tumor suppressor role of miR‐124 in osteosarcoma. PLoS ONE. 2014;9:e91566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Sun XH, Geng XL, Zhang J, Zhang C. miRNA‐646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2). Tumour Biol. 2015;36:2127‐2134. [DOI] [PubMed] [Google Scholar]
- 5. Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR‐135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol Cell Biochem. 2015;400:245‐252. [DOI] [PubMed] [Google Scholar]
- 6. Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B. MicroRNA‐101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol Rep. 2014; 10.3892/or.2014.3459. [DOI] [PubMed] [Google Scholar]
- 7. Cai H, Zhao H, Tang J, Wu H. Serum miR‐195 is a diagnostic and prognostic marker for osteosarcoma. J Surg Res. 2015;194:505‐510. [DOI] [PubMed] [Google Scholar]
- 8. Zhang C, Yao C, Li H, Wang G, He X. Serum levels of microRNA‐133b and microRNA‐206 expression predict prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2014;7:4194‐4203. [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang C, Yao C, Li H, Wang G, He X. Combined elevation of microRNA‐196a and microRNA‐196b in sera predicts unfavorable prognosis in patients with osteosarcomas. nt J Mol Sci. 2014;15:6544‐6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones KB, Salah Z, Del Mare S, et al. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865‐1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mori F, Sacconi A, Canu V, et al. miR‐181c associates with tumor relapse of high grade osteosarcoma. Oncotarget. 2015;6:13946‐13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Cai H, Lin L, Tang M. Upregulated expression of microRNA‐214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatr Blood Cancer. 2014;61:206‐210. [DOI] [PubMed] [Google Scholar]
- 13. Cai X, Liu Y, Yang W, et al. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2015;. 10.1002/jor.23105. [DOI] [PubMed] [Google Scholar]
- 14. Sun L, Sun P, Zhou QY, Gao XC, Han Q. Long noncoding RNA MALAT1 promotes uveal melanoma cell growth and invasion by silencing of miR‐140. Am J Transl Res. 2016;8:3939‐3946. [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang YH, Fu J, Zhang ZJ, Ge CC, Yi Y. LncRNA‐LINC00152 down‐regulated by miR‐376c‐3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res. 2016;8:5286‐5297. [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu HQ, Zhou X, Chang H, et al. Aberrant expression of CCAT1 regulated by c‐Myc predicts the prognosis of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16:5181‐5185. [DOI] [PubMed] [Google Scholar]
- 17. Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let‐7 sponge. J Exp Clin Cancer Res. 2015;34:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu H, Li X, Song Y, Zhang P, Xiao Y, Xing Y. Long non‐coding RNA ANRIL is up‐regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem Biophys Res Commun. 2015;467:223‐228. [DOI] [PubMed] [Google Scholar]
- 19. Naemura M, Murasaki C, Inoue Y, Okamoto H, Kotake Y. Long noncoding RNA ANRIL regulates proliferation of non‐small cell lung cancer and cervical cancer cells. Anticancer Res. 2015;35:5377‐5382. [PubMed] [Google Scholar]
- 20. Wang C, Yan G, Zhang Y, Jia X, Bu P. Long non‐coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma. 2015;62:541‐549. [DOI] [PubMed] [Google Scholar]
- 21. He X, Tan X, Wang X, et al. C‐Myc‐activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol. 2014;35:12181‐12188. [DOI] [PubMed] [Google Scholar]
- 22. Mourtada‐Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non‐protein‐coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195‐208. [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa T, Endo H, Yokoyama M, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease‐free survival in human non‐small cell lung cancer. Mol Cell Biol Res Commun. 2013;436:319‐324. [DOI] [PubMed] [Google Scholar]
- 24. Li P, Xue WJ, Feng Y, Mao QS. Long non‐coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8:3522‐3529. [PMC free article] [PubMed] [Google Scholar]
- 25. Baldinu P, Cossu A, Manca A, et al. CASC2a gene is down‐regulated in endometrial cancer. Anticancer Res. 2007;27:235‐243. [PubMed] [Google Scholar]
- 26. Baldinu P, Cossu A, Manca A, et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat. 2004;23:318‐326. [DOI] [PubMed] [Google Scholar]
- 27. Pei Z, Du X, Song Y, et al. Down‐regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/beta‐catenin signaling pathway. Oncotarget. 2017;8:18145‐18153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu X, Li Z. Long non‐coding RNA HOTAIR: a novel oncogene (Review). Mol Med Rep. 2015;12:5611‐5618. [DOI] [PubMed] [Google Scholar]
- 29. Yu X, Li Z. Long non‐coding RNA growth arrest‐specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang L, Bai HS, Deng Y, Fan L. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015;19:3187‐3193. [PubMed] [Google Scholar]
- 31. Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non‐coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non‐small cell lung cancer. BMC Cancer. 2013;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He X, Bao W, Li X, et al. The long non‐coding RNA HOTAIR is upregulated in endometrial carcinoma and correlates with poor prognosis. Int J Mol Cell Med. 2014;33:325‐332. [DOI] [PubMed] [Google Scholar]
- 33. Yang F, Xue X, Bi J, et al. Long noncoding RNA CCAT1, which could be activated by c‐Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437‐445. [DOI] [PubMed] [Google Scholar]
- 34. Palmieri G, Paliogiannis P, Sini MC, et al. Long non‐coding RNA CASC2 in human cancer. Crit Rev Oncol Hemat. 2017;111:31‐38. [DOI] [PubMed] [Google Scholar]
- 35. Wang P, Liu YH, Yao YL, et al. Long non‐coding RNA CASC2 suppresses malignancy in human gliomas by miR‐21. Cell Signal. 2015;27:275‐282. [DOI] [PubMed] [Google Scholar]
- 36. He XZ, Liu ZL, Su J, et al. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non‐small cell lung cancer. Tumor Biol. 2016;37:9503‐9510. [DOI] [PubMed] [Google Scholar]
- 37. Huang GL, Wu XL, Li S, Xu XQ, Zhu H, Chen XJ. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR‐18a in colorectal cancer. Sci Rep. 2016;6. doi: Artn 2652410.1038/Srep26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liao Y, Shen L, Zhao H, et al. LncRNA CASC2 interacts with miR‐181a to modulate glioma growth and resistance to TMZ through PTEN pathway. J Cell Biochem. 2017;118:1889‐1899. [DOI] [PubMed] [Google Scholar]
- 39. Mi YS, Zhang DY, Jiang WL, et al. miR‐181a‐5p promotes the progression of gastric cancer via RASSF6‐mediated MAPK signalling activation. Cancer Lett. 2017;389:11‐22. [DOI] [PubMed] [Google Scholar]
- 40. Ping PH, Bo TF, Li L, Hui YN, Hong Z. IL‐1 beta/NF‐kb signaling promotes colorectal cancer cell growth through miR‐181a/PTEN axis. Arch Biochem Biophys. 2016;604:20‐26. [DOI] [PubMed] [Google Scholar]
- 41. Liu XD, Liao W, Peng HX, et al. miR‐181a promotes G1/S transition and cell proliferation in pediatric acute myeloid leukemia by targeting ATM. J Cancer Res Clin Oncol. 2016;142:77‐87. [DOI] [PubMed] [Google Scholar]
- 42. Chorzalska A, Kim JF, Roder K, Olszewski AJ, Terentyev D, Dubielecka PM. Downregulation of Mir‐181a restores chemosensitivity to imatinib mesylate in chronic myeloid leukemia cells resistant to tyrosine kinase inhibitors. Blood. 2015;126:23. [Google Scholar]
- 43. Peng J, Thakur A, Zhang S, et al. Expressions of miR‐181a and miR‐20a in RPMI8226 cell line and their potential as biomarkers for multiple myeloma. Tumor Biol. 2015;36:8545‐8552. [DOI] [PubMed] [Google Scholar]
- 44. Zhu ZJ, Huang P, Chong YX, et al. MicroRNA‐181a promotes proliferation and inhibits apoptosis by suppressing CFIm25 in osteosarcoma. Mol Med Rep. 2016;14:4271‐4278. [DOI] [PubMed] [Google Scholar]
- 45. Allen NPC, Donninger H, Vos MD, et al. RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene. 2007;26:6203‐6211. [DOI] [PubMed] [Google Scholar]
- 46. Dunwell T, Hesson L, Catchpoole D, Maher E, Latif F. The novel RASSF6 and RASSF10 candidate tumor suppressor genes are frequently epigenetically inactivated in childhood leukemias. Cancer Res. 2009;69:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo W, Dong ZM, Guo YL, et al. Decreased expression and frequent promoter hypermethylation of RASSF2 and RASSF6 correlate with malignant progression and poor prognosis of gastric cardia adenocarcinoma. Mol Carcinog. 2016;55:1655‐1666. [DOI] [PubMed] [Google Scholar]
- 48. Chen EF, Yang FF, He HJ, et al. Decreased level of RASSF6 in sporadic colorectal cancer and its anti‐tumor effects both in vitro and in vivo. Oncotarget. 2016;7:19813‐19823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye HL, Li DD, Lin Q, et al. Low RASSF6 expression in pancreatic ductal adenocarcinoma is associated with poor survival. World J Gastroentero. 2015;21:6621‐6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iwasa H, Kudo T, Maimaiti S, et al. The RASSF6 tumor suppressor protein regulates apoptosis and the cell cycle via MDM2 protein and p53 protein. J Biol Chem. 2013;288:30320‐30329. [DOI] [PMC free article] [PubMed] [Google Scholar]


