Abstract
Objectives
Ginsenoside Rh2 (GRh2) has demonstrative therapeutic effects on a variety of diseases, including some tumours. However, the effects of GRh2 on prostate cancer (PC) cell growth remain unknown, and were, thus, addressed in the present study.
Materials and methods
PC3 and DU145 PC cell lines were exposed to GRh2. Cell proliferation was assessed in an MTT assay and by BrdU incorporation. Apoptosis of the cells were assessed by TUNEL staining. Total RNA was assessed by RT‐qPCR. Protein levels were assessed by Western blotting. Bioinformatics and dual luciferase reporter assay were applied to determine the functional binding of miRNA to mRNA of target gene.
Results
GRh2 dose‐dependently decreased PC cell proliferation, but did not alter cell apoptosis. Mechanistically, GRh2 dose‐dependently increased the protein, but not mRNA of a cell‐cycle suppressor CDKN1A in PC cells, suggesting the presence of microRNA (miRNA)‐mediated protein translation control of CDKN1A by GRh2. In all candidate miRNAs that bind to 3′‐UTR of CDKN1A, miR‐4295 was specifically found to be suppressed dose‐dependently by GRh2 in PC cells. Moreover, miR‐4295 bound CDKN1A to suppress its protein translation. Furthermore, cell proliferation in PC cells that overexpressed miR‐4295 did not alter in response to GRh2.
Conclusions
GRh2 may inhibit PC cell growth through suppression of microRNA‐4295 that activates CDKN1A.
1. INTRODUCTION
Prostate cancer (PC) is the most prevalent cancer occurred in aged male.1 In most cases, PC consists of small knots of abnormal cells growing slowly within the prostate gland.2 In infrequent occasions, PC cells may increase growth rate, break out of the gland to spread to distant sites, and cause lethal threat to the body.1 Since most PCs are now diagnosed very early due to the growing use of prostate‐specific antigen (PSA), proper control of growth of PC in these patients allows them to be exempt from aggressive treatments but still to live well without being affected by this PC problems in their lifetime.2 Hence, it is of great importance to identify agents that are non‐toxic but effective in controlling the growth of PC.
Ginsenoside Rh2 (GRh2) is a well‐characterized component detected in red ginseng with demonstrative therapeutic effects on many human diseases, including inflammatory disorders3, 4, 5 and malignant tumours.6, 7, 8 Moreover, GRh2 has been reported to suppress the outgrowth of PC in vivo and in vitro, likely through activation of transforming growth factor β receptor signalling.9
MicroRNAs (miRNAs) are non‐coding RNAs with typical length of 20~22 nucleotides.10 Most of miRNAs combine to 3′‐UTR of genes by imprecisely binding, although there may be binding sites for miRNAs at 5′‐UTR or extron region of mRNA.11 After binding, genes are silenced due to spatial structural alternation. MiRNAs made significant effects on various biological processes, including cell differentiation regulation, cell identity determination, apoptotic cell death, cell migration and cell cycles, etc. MiRNAs play an important role in a number of different biological processes.12, 13, 14, 15 Specifically, miR‐4295 has been rarely studied, and only a few target genes for miR‐4295 have been reported, for example, BTG1,16 p63alpha,17 and RUNX3.18
CDKN1A is also known as p21, which is a 164 amino‐acid cell‐cycle suppressor expressed in both developing embryo and adult tissue and organs.19 CDKN1A plays a key role in the control of cell‐cycle arrest.20, 21, 22 Of note, very recently, CDKN1A has been shown to be a miR‐4295‐targeting gene in anaplastic thyroid carcinoma.23 However, the role of miR‐4295 in PC cell growth has not been reported.
We have studied the tumour biology of PC for years.24, 25 Here, we studied the effects of GRh2 on the growth of PC cells. Using PC3 and DU145 PC cell lines, we found that GRh2 dose‐dependently inhibited PC cell proliferation, but did not alter cell apoptosis, seemingly through downregulation of miR‐4295, which inhibits protein translation of CDKN1A.
2. MATERIALS AND METHODS
2.1. Human PC cell lines and GRh2
PC3 and DU145 were human prostatic cancer cell lines purchased from American Type Culture Collection (ATCC). PC3 and DU145 cells were maintained in RPMI 1640 medium (Thermo Scientific, Rockford, IL, USA) supplied with 5% foetal bovine serum (Thermo Scientific). GRh2 was purchased from Weikeqi Bioscience, Chengdu, China and was exposed to the cultured PC cells at a dose of 0.01, 0.1 and 1 mg/mL. Control non‐GRh2 solution was shown as 0 mg/mL GRh2.
2.2. Cell transfection, bioinformatics, and dual luciferase‐reporter assay
PC3 and DU145 cells were transfected with plasmids carrying miR‐4295 or an antisense (as) of miR‐4295 (as‐miR‐4295), or a scramble sequence (scr) as a control, under the control of a CMV promoter (RiboBio Co. Ltd, Shanghai, China). The plasmid construct also processes a green fluorescence protein reporter co‐expressed with the transgene, to allow the transfected cells being visualized by green fluorescence. MiR‐4295 sequence: 5′‐UCCCUCCCCAGUUCAUUGCACUU‐3′, as‐miR‐4295 sequence: 5′‐AAGUGCAAUGAACUGGGGAGGGA‐3′, scrambled (scr) sequence: 5′‐AAUUACUACACAAAAGUCCGC‐3′. A lipofectamine 3000 kit (Invitrogen, Carlsbad, CA, USA) was used to transfect the cells, as instructed by the manufacturer. The target miRNAs for CDKN1A in mice and humans were predicted using online tools from TargetScan, based on a context++ score system, as has been described.26 The dual‐luciferase 3′‐UTR reporter plasmids include a p3′‐UTR‐CDKN1A that contains the wild‐type CDKN1A 3′‐UTR‐binding site in luciferase reporter plasmid and a p3′‐UTR‐CDKN1A‐mut that contains the mutant CDKN1A 3′‐UTR. Either 3′‐UTR plasmid was co‐transfected into PC3 cells with a plasmid carrying either miR‐4295 or as‐miR‐4295. The luciferase activity was assessed 48 hours after transfection.
2.3. MTT assay
Cell viability was assessed in cultured cells using an MTT Kit (Roche, Indianapolis, IN, USA), in which the quantification was done at 540 nm absorbance value (OD) from 5 repeats.
2.4. Quantitative PCR (RT‐qPCR)
A miRNeasy mini kit (Qiagen, Burlingame, CA, USA) was used to isolate total RNA including miRNAs from cultured cells. Synthesis of cDNA and RT‐qPCR were performed using QuantiTect Reverse Transcription Kit and QuantiTect SYBR Green PCR Kit, respectively (Qiagen). Specific primers were all purchased from Qiagen. Data were assessed using 2−△△Ct method. Values of genes were obtained after sequential normalization against housekeeping control gene β‐actin and then the experimental control condition.
2.5. Western blotting
Proteins were isolated from cultured cells with RIPA lysis buffer (Bio‐Rad, Shanghai, China). Protein concentration was assessed with a BCA protein assay kit (Bio‐Rad). Cell lysates were mixed with 4× SDS loading buffer at 3:1 (Bio‐Rad) and separated on SDS‐polyacrylamide gels after heating at 95°C. Transferred PVDF membrane was first probed with a primary antibody, and then with a horseradish peroxidase‐conjugated second antibody, after which photographing was done using enhanced chemiluminescent system. Primary antibodies were rabbit anti‐CDKN1A and rabbit anti‐β‐actin (Cell Signaling, Carpinteria, CA, USA). Secondary antibody is HRP‐conjugated anti‐rabbit (Dako, Carpinteria, CA, USA). Protein levels were assessed using NIH ImageJ software (Bethesda, MA, USA).
2.6. BrdU and TUNEL staining
BrdU (10 mg/ml; Sigma‐Aldrich, St. Louis, MO, USA) was given to cultured cells 20 minutes before harvesting and assessment. Primary antibody is rat anti‐BrdU (Dako). Indirect fluorescent staining for the cells was done with Cy3‐conjugated donkey anti‐rat antibody (Dako). TUNEL staining was performed with an In Situ Apoptosis Detection Kit (Thermo Scientific). DNA was stained with DAPI (Abcam, Cambridge, MA, USA). A total of 1500 cells was analysed in each individual.
2.7. Statistical analysis
The statistical studies were done with GraphPad prism software (GraphPad Software, Inc., La Jolla, CA, USA), in which a one‐way ANOVA with a Bonferroni correction was applied for comparison among several groups. Mean ± SD was used to represent values, and the differences were considered significant if P < .05.
3. RESULTS
3.1. GRh2 dose‐dependently suppresses PC cell proliferation
Here, we aimed to examine the effects of GRh2 on PC cell growth. In 2 commonly used PC cell lines PC3 and DU145, we found that GRh2 (from 0.01 to 1 mg/dl) dose dependently inhibited the growth of PC3 cells (Figure 1A) and DU145 cells (Figure 1B). Moreover, GRh2 dose‐dependently inhibited the BrdU incorporation into PC cells in both lines, shown by quantification (Figure 1C), and by representative images (Figure 1D). Since BrdU is a cell proliferation marker that incorporates into S‐phase proliferating cells, these data suggest that GRh2 may dose‐dependently suppress PC cell proliferation. Moreover, different doses of GRh2 did not alter apoptosis of the cells in an TUNEL assay (Figure 1E). Thus, the effects of GRh2 on proliferation arrest of PC cells do not involve significant changes in cell apoptosis.
Figure 1.
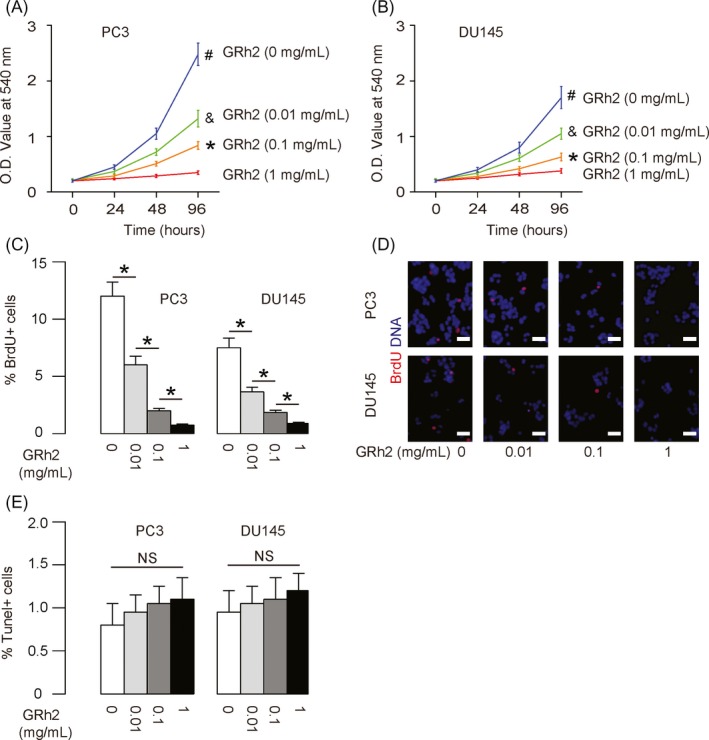
GRh2 dose‐dependently suppresses PC cell proliferation. A, B GRh2 inhibited the growth of PC3 (A) and DU145 (B) cells in a dose‐dependent manner in an MTT assay. C, D GRh2 decreased the percentage of BrdU+ PC3 and DU145 cells in a dose‐dependent manner, shown by quantification (C) and by representative images (D). (E) TUNEL assay for PC3 and DU145 cells treated with GRh2. *P < .05. NS: non‐significant. N = 5. Scale bars are 20 μm
3.2. GRh2 inhibits PC cell proliferation through upregulation of CDKN1A
In order to understand the underlying mechanisms, we examined protein levels of cell‐cycle regulators, and specifically found that the levels of a cell‐cycle suppressor CDKN1A were dose‐dependently altered by GRh2 in PC3 cells (Figure 2A), and in DU145 cells (Figure 2B), concomitant with the changes in cell proliferation. However, the CDKN1A mRNA levels were not altered by GRh2 in either line (Figure 2C,D), suggesting that GRh2 may modulate the protein translation of CDKN1A, possibly through miRNAs.
Figure 2.
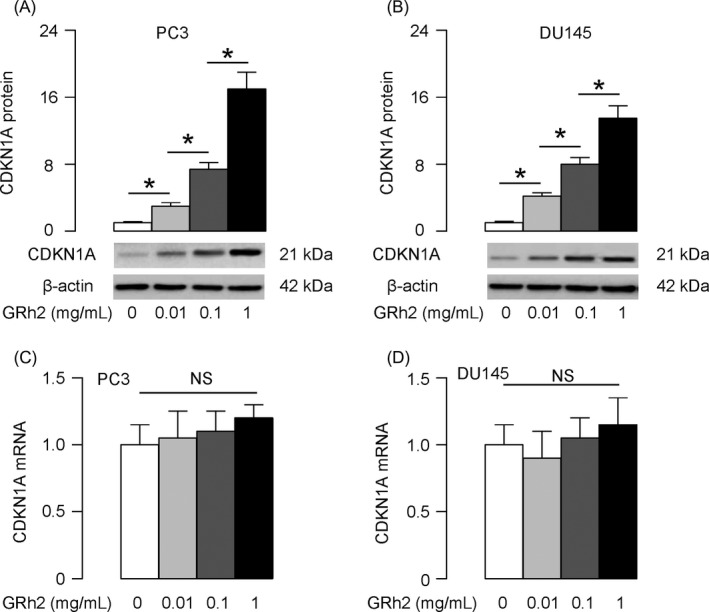
GRh2 inhibits PC cell proliferation through upregulation of CDKN1A. A, B, Western blotting for CDKN1A protein in PC3 cells (A) and DU145 cells (B) treated with GRh2. C, D, RT‐qPCR for CDKN1A mRNA in PC3 cells (C) and DU145 cells (D) treated with GRh2. *P < .05. NS: non‐significant. N = 5
3.3. MiR‐4295 targets 3′‐UTR of CDKN1A mRNA to suppress its protein translation
We used bioinformatics tools to screen miRNAs that bind to 3′‐UTR of CDKN1A and found miR‐4295 as the one candidate that was specifically and dose‐dependently downregulated by GRh2 (Figure 3A). Indeed, miR‐4295 has a binding site at the 1146th‐1152th base pair of the 3′‐UTR of CDKN1A mRNA (Figure 3B). Thus, we examined whether the binding of miR‐4295 to 3′‐UTR of CDKN1A is functional. Hence, PC3 cells were transfected with plasmids carrying miR‐4295, or as‐miR‐4295, or a scramble sequence (scr), under the control of a CMV promoter. RT‐qPCR for miR‐4295 was performed, which confirmed the changes in miR‐4295 levels in miR‐4295 or as‐miR‐4295‐transfected cells (Figure 3C). Next, the intact 3′‐UTR of wild‐type CDKN1A mRNA (wt CDKN1A 3′‐UTR) or the 3′‐UTR of CDKN1A mRNA with a mutant at miR‐4295‐binding site (mut CDKN1A 3′‐UTR) were, respectively, cloned into luciferase reporter plasmids. PC3 cells were then co‐transfected with 1 miR‐4295‐modified plasmid and 1 plasmid from either wt CDKN1A 3′‐UTR or mut CDKN1A 3′‐UTR, and then luciferase activity was analysed in a dual luciferase reporter assay. We found that miR‐4295 depletion increased luciferase activity of wt CDKN1A 3′‐UTR, while re‐expression of miR‐4295 reduced luciferase activity of wt CDKN1A 3′‐UTR but not those of mut CDKN1A 3′‐UTR (Figure 3D). These results suggest that miR‐4295 specifically binds to the 3′‐UTR of CDKN1A mRNA to suppress its protein translation in PC cells.
Figure 3.
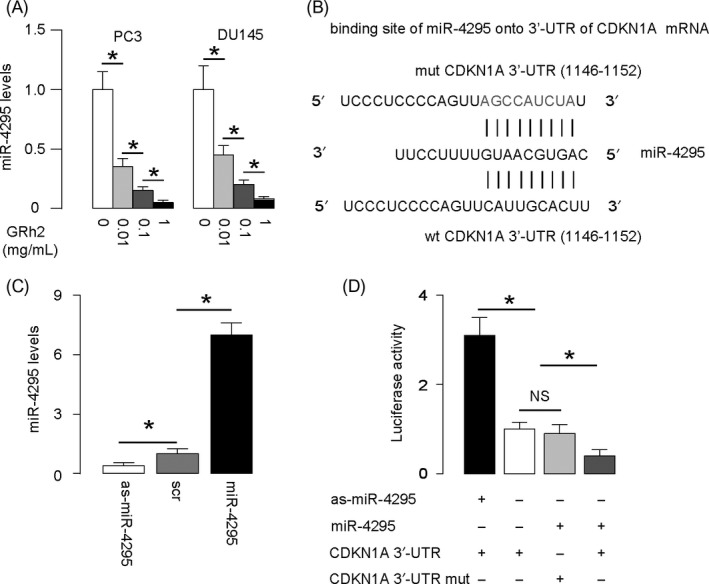
MiR‐4295 binds to 3′‐UTR of CDKN1A to inhibit its protein translation. (A) RT‐qPCR for miR‐4295 levels in PC3 and DU145 cells treated with GRh2. (B) Bioinformatics evidence of binding of miR‐4295 onto 3′‐UTR of CDKN1A mRNA. (C) Transfection of PC3 cells. RT‐qPCR for miR‐4295 was done in these cells. (D) Then, the intact 3′‐UTR of wild‐type CDKN1A mRNA (wt CDKN1A 3′‐UTR) and the 3′‐UTR of CDKN1A mRNA with a mutant at miR‐4295‐binding site (mut CDKN1A 3′‐UTR) were respectively cloned into luciferase reporter plasmids. PC3 cells were then co‐transfected with 2 certain plasmids, and luciferase activity was assessed a dual luciferase reporter assay. *P < .05. NS: non‐significant. N = 5
3.4. Re‐expression of miR‐4295 abolishes the suppression of GRh2 on PC cell growth
To understand if the suppression of PC cell growth by GRh2 is mediated by alteration in miR‐4295‐suppressed CDKN1A expression, we exposed PC3 cells that re‐expressed miR‐4295 with GRh2. First, PC3 cells were transfected with scr or miR‐4295 to obtain PC3‐scr or PC3‐miR‐4295 cells (Figure 4A). PC3‐miR‐4295 cells expressed significantly higher levels of miR‐4295, compared with the control PC3‐scr cells (Figure 4B). Next, we treated PC3‐miR‐4295 and PC3‐scr cells with different doses of GRh2. We found that re‐expression of miR‐4295 in PC cells abolished the suppression of GRh2 on cell growth (Figure 5A,B). Moreover, the abolishment of the GRh2 effects on cell growth appeared to result from the abolishment of the GRh2 effects on cell proliferation, shown by quantification (Figure 5C), and by representative images (Figure 5D). Together, these data suggest that GRh2 inhibits PC cell growth through suppression of miRNA‐4295 that activates CDKN1A (Figure 6).
Figure 4.
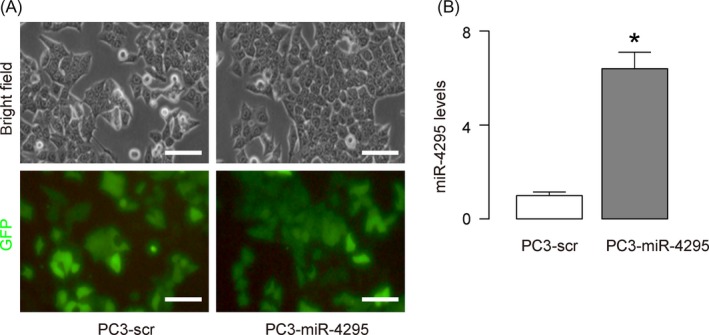
Re‐expression of miR‐4295 in PC cells. (A) Cultured PC3 transfected with scr or miR‐4295. (B) RT‐qPCR for miR‐4295 was done in PC3‐scr and PC3‐miR‐4295 cells. *P < .05. N = 5. Scale bars are 20 μm
Figure 5.
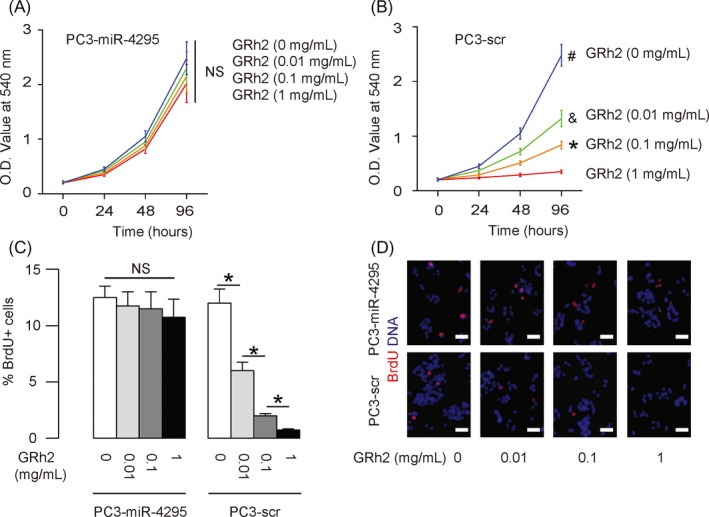
Re‐expression of miR‐4295 in PC cells abolishes the suppression of GRh2 on PC cell growth. A, B, In an MTT assay, cell growth was determined for GRh2‐treated PC3‐miR‐4295 cells (A) and PC3‐scr cells (B). C, D, BrdU+ cells were determined for GRh2‐treated PC3‐miR‐4295 and PC3‐scr cells, shown by quantification (C) and by representative images (D). *P < .05. NS: non‐significant. N = 5. Scale bars are 20 μm
Figure 6.
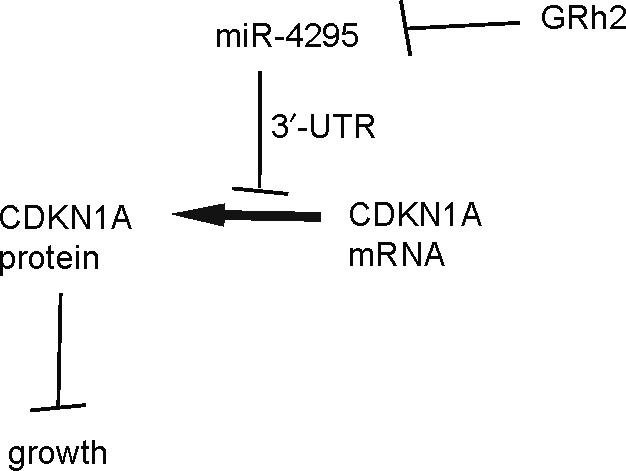
Schematic of the model. GRh2 inhibits PC cell growth through suppression of microRNA‐4295 that activates CDKN1A
4. DISCUSSION
GRh2 is the major bioactive component in ginseng, and its antitumour activity had been reported in quite a few of previous studies. For example, Yang et al27 showed that GRh2 enhanced colorectal cancer cell death, seemingly through regulation of the phosphorylation of ERK1/2 and histone H3. In several other studies, GRh2 was shown to suppress cancer cell proliferation in human breast carcinoma cells,7, 28 human ovarian cancer cells,29 through a variety of targets related to cell‐cycle control, for example, p15, p21, p27, Cdk2, cyclin E‐dependent kinase. Interestingly, GRh2 was also shown to dose‐dependently reduce Lgr5‐positive squamous cancer stem‐like cells.6 Moreover, GRh2 was shown to alter miRNAs to regulate the non–small‐cell lung cancer cell proliferation.30 However, tumour growth in these cancers that had been treated with GRh2 was not the first reason that contributes to the tumour malignancy, since these cancers were quite invasive which renders their invasion and metastasis more important than tumour cell proliferation. In the case of PC, since most PCs are now diagnosed very early and since most PCs grow very slowly to allow proper control of PC growth an applied treatment to most patients,2 the anti‐proliferation effects of GRh2 against PC appear to be a very attractive strategy in the PC treatment.
Here, we used PC3 and DU145, 2 classical PC lines, to assess the role of GRh2 in control of PC cell growth. DU145 cells do not express PSA, are not androgen‐responsive, and have moderate metastatic potential. On the other hand, PC3 cells express PSA, are androgen‐responsive, and have high metastatic potential.31 The different characteristics of the 2 lines make our results more useful and applicable to general PC study and treatment. We found that the inhibitory effects of GRh2 on PC growth mainly stemmed from the suppression of cell proliferation, rather than from the activation of cell apoptosis. This finding is consistent with most previous reports, as an anti‐proliferative role of GRh2 has been demonstrated in several cancers.7, 29, 30, 32, 33
The fact that GRh2 dose‐dependently decreased CDKN1A protein, but not mRNA, in PC cells suggests that GRh2 may regulate the levels of CDKN1A at the post‐transcriptional level, rather than at the transcriptional level through a direct interaction with CDKN1A promoter either by itself or through a DNA‐binding complex. By bioinformatics analyses, we found a number of miRNAs that target CDKN1A. However, we found that miR‐4295 was the only one, the expression of which was dose‐dependently regulated by GRh2. This concomitant change in expression levels was further confirmed as a regulatory axis by luciferase reporter assay and a loss‐of‐function experiment.
The exact molecular regulation of GRh2 on miR‐4295 expression is not known. GRh2 may alter the expression of miR‐4295 at transcriptional level, for example, through promoter hypermethylation, or at post‐transcriptional level, for example, through changing miRNA processing, or even indirectly through hormones or cytokines. This interesting question may be addressed in future studies. Of note, miR‐4295 may also have target genes other than CDKN1A. Apparently, these targets did not affect the role of miR‐4295 in controlling cell proliferation in PC cells. Interestingly, a very recent study has shown an inhibitory effect of GRh2 on PC growth, seemingly through regulation of both cell‐cycle regulators and MMPs.9 However, the exact underlying molecular mechanisms were not determined in this study.9 In another study on anaplastic thyroid carcinoma, control of CDKN1A by miR‐4295 has been reported.23 However, to the best of our knowledge, our study is the first one to show a role of miR‐4295/CDKN1A in PC cell growth which could be regulated by application of GRh2. Taking into account of the importance of control of PC growth, our study should provide an important basis for further investigation of the possible application of GRh2 in PC therapy.
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
Gao Q, Zheng J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA‐4295 that activates CDKN1A. Cell Prolif. 2018;51:e12438 10.1111/cpr.12438
REFERENCES
- 1. Kruslin B, Ulamec M, Tomas D. Prostate cancer stroma: an important factor in cancer growth and progression. Bosn J Basic Med Sci. 2015;15:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donkena KV, Yuan H, Young CY. Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets. 2010;10:402‐410. [DOI] [PubMed] [Google Scholar]
- 3. Vinoth Kumar R, Oh TW, Park YK. Anti‐inflammatory effects of ginsenoside‐Rh2 inhibits LPS‐induced activation of microglia and overproduction of inflammatory mediators via modulation of TGF‐beta1/Smad pathway. Neurochem Res. 2016;41:951‐957. [DOI] [PubMed] [Google Scholar]
- 4. Zhou J, Gao Y, Yi X, Ding Y. Ginsenoside Rh2 suppresses neovascularization in xenograft psoriasis model. Cell Physiol Biochem. 2015;36:980‐987. [DOI] [PubMed] [Google Scholar]
- 5. Bi WY, Fu BD, Shen HQ, et al. Sulfated derivative of 20(S)‐ginsenoside Rh2 inhibits inflammatory cytokines through MAPKs and NF‐kappa B pathways in LPS‐induced RAW264.7 macrophages. Inflammation. 2012;35:1659‐1668. [DOI] [PubMed] [Google Scholar]
- 6. Liu S, Chen M, Li P, et al. Ginsenoside rh2 inhibits cancer stem‐like cells in skin squamous cell carcinoma. Cell Physiol Biochem. 2015;36:499‐508. [DOI] [PubMed] [Google Scholar]
- 7. Oh M, Choi YH, Choi S, et al. Anti‐proliferating effects of ginsenoside Rh2 on MCF‐7 human breast cancer cells. Int J Oncol. 1999;14:869‐875. [DOI] [PubMed] [Google Scholar]
- 8. Kim MJ, Yun H, Kim DH, et al. AMP‐activated protein kinase determines apoptotic sensitivity of cancer cells to ginsenoside‐Rh2. J Ginseng Res. 2014;38:16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Q, Hong B, Wu S, Niu T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour Biol. 2015;36:2377‐2381. [DOI] [PubMed] [Google Scholar]
- 10. Seok H, Ham J, Jang ES, Chi SW. MicroRNA target recognition: insights from transcriptome‐wide non‐canonical interactions. Mol Cells. 2016;39:375‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forman JJ, Legesse‐Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let‐7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA. 2008;105:14879‐14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial‐mesenchymal plasticity. Oncotarget. 2017;8:12472‐12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papagiannakopoulos T, Kosik KS. MicroRNA‐124: micromanager of neurogenesis. Cell Stem Cell. 2009;4:375‐376. [DOI] [PubMed] [Google Scholar]
- 14. Ivey KN, Muth A, Arnold J, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Gregory RI. MicroRNA regulation of stem cell fate. Cell Stem Cell. 2008;2:195‐196. [DOI] [PubMed] [Google Scholar]
- 16. Nan YH, Wang J, Wang Y, et al. MiR‐4295 promotes cell growth in bladder cancer by targeting BTG1. Am J Transl Res. 2016;8:4892‐4901. [PMC free article] [PubMed] [Google Scholar]
- 17. Jin H, Xu J, Guo X, et al. XIAP RING domain mediates miR‐4295 expression and subsequently inhibiting p63alpha protein translation and promoting transformation of bladder epithelial cells. Oncotarget. 2016;7:56540‐56557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Zheng J, Diao H, Liu Y. RUNX3 is down‐regulated in glioma by Myc‐regulated miR‐4295. J Cell Mol Med. 2016;20:518‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E. Biology of the cell cycle inhibitor p21(CDKN1A): molecular mechanisms and relevance in chemical toxicology. Arch Toxicol. 2015;89:155‐178. [DOI] [PubMed] [Google Scholar]
- 20. Kreis NN, Friemel A, Zimmer B, et al. Mitotic p21Cip1/CDKN1A is regulated by cyclin‐dependent kinase 1 phosphorylation. Oncotarget. 2016;7:50215‐50228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu BH, Chen H, Cai CM, et al. Epigenetic silencing of JMJD5 promotes the proliferation of hepatocellular carcinoma cells by down‐regulating the transcription of CDKN1A 686. Oncotarget. 2016;7:6847‐6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brock M, Haider TJ, Vogel J, et al. The hypoxia‐induced microRNA‐130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol. 2015;61:129‐137. [DOI] [PubMed] [Google Scholar]
- 23. Shao M, Geng Y, Lu P, et al. miR‐4295 promotes cell proliferation and invasion in anaplastic thyroid carcinoma via CDKN1A. Biochem Biophys Res Commun. 2015;464:1309‐1313. [DOI] [PubMed] [Google Scholar]
- 24. Gao N, Jiang BH, Leonard SS, et al. p38 Signaling‐mediated hypoxia‐inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J Biol Chem. 2002;277:45041‐45048. [DOI] [PubMed] [Google Scholar]
- 25. Gao Q, Zheng J, Yao X, Peng B. Adiponectin inhibits VEGF‐A in prostate cancer cells. Tumour Biol. 2015;36:4287‐4292. [DOI] [PubMed] [Google Scholar]
- 26. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:1‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang J, Yuan D, Xing T, et al. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ‐binding kinase/T‐LAK cell‐originated protein kinase. J Ginseng Res. 2016;40:400‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choi S, Kim TW, Singh SV. Ginsenoside Rh2‐mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1‐dependent inhibition of cyclin‐dependent kinases. Pharm Res. 2009;26:2280‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakata H, Kikuchi Y, Tode T, et al. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998;89:733‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An IS, An S, Kwon KJ, Kim YJ, Bae S. Ginsenoside Rh2 mediates changes in the microRNA expression profile of human non‐small cell lung cancer A549 cells. Oncol Rep. 2013;29:523‐528. [DOI] [PubMed] [Google Scholar]
- 31. Marchiani S, Tamburrino L, Nesi G, et al. Androgen‐responsive and ‐unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl. 2010;33:784‐793. [DOI] [PubMed] [Google Scholar]
- 32. Qian J, Li J, Jia JG, et al. Ginsenoside‐Rh2 inhibits proliferation and induces apoptosis of human gastric cancer SGC‐7901 side population cells. Asian Pac J Cancer Prev. 2016;17:1817‐1821. [DOI] [PubMed] [Google Scholar]
- 33. Tode T, Kikuchi Y, Kita T, Hirata J, Imaizumi E, Nagata I. Inhibitory effects by oral administration of ginsenoside Rh2 on the growth of human ovarian cancer cells in nude mice. J Cancer Res Clin Oncol. 1993;120:24‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]


