Abstract
Objectives
B7 family has been identified as co‐stimulatory or co‐inhibitory molecules on T‐cell response and plays an important role in tumour mortality and malignancy. In this study, the expression pattern of B7 family in gastrointestinal (GI) cancer was examined. Its upstream regulating mechanism, downstream targets and association with clinical parameters were also studied.
Materials and methods
The expression level of B7 members was analysed by FIREHOUSE. The gene mutation, DNA methylation, association with clinical parameters and downstream network of B7 members were analysed in cBioportal. The mutation frequency was analysed by Catalogue of Somatic Mutations in Cancer (COSMIC) analysis. The phylogenetic tree was constructed in MEGA7. The interaction protein domain analysis was performed by Pfam 31.0.
Results
Differential expression of B7 family molecules was detected in different kinds of GI cancer. High‐frequency gene alteration was found in tumour samples. There was negative correlation of promoter methylation and mRNA expression of B7 family members in tumour samples, suggesting the epigenetic basis of B7 family gene deregulation in GI cancer. The overexpression of B7‐H1 in pancreatic cancer, B7‐H5 in oesophageal cancer and B7‐H6 in liver cancer were significantly associated with worse overall survival. Finally, by network analysis, we identified some potential interacting proteins for B7‐1/2 and B7‐H1/DC.
Conclusions
Overall, our study suggested that B7 member deregulation was strongly involved in GI cancer tumorigenesis.
1. INTRODUCTION
Gastrointestinal (GI) cancer has high tumour incidence and mortality rate in the world and has poor prognosis, which is affected by geographical environment, diet habits and sex.1 GI cancer has complex and multifactorial nosogenesis, and the mechanism is still unclear. Factors of genetic, cigarette smoking, diet habits, geographical environment were implicated.2 Oesophageal cancer is the sixth cause of death (386 000 death, 5.7% of total 2002) from cancer and the risk is associated with age. Black men are more likely to have disease compared with other races.1 Among oesophageal cancer, 51.6% squamous‐cell carcinoma and 41.9% adenocarcinoma have been identified.3 Gastric cancer (GC) is the third cause of death (723 100 death, 2012) from cancer to the incidence is twice higher in male compared with female. The 5‐year survival rate from 2004 to 2010 is nearly 30% in all races of United States.4 Helicobactor pylori (H. pylori) infection has been identified as a risk factor for GC by International Agency for Research on Cancer (IARC) in 1994.5 Colorectal cancer (CRC) is the second cause of death (529 000 deaths in 2002) from cancer.1 The survival from 2004 to 2010 is 65%‐68% in all races of United States.4 Chronic inflammation is deemed as the major contributor to this malignant disease, such as Crohn’s disease (CD) and ulcerative colitis (UC), which could induce colitis‐associated CRC with poor prognosis.6 Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer.1 About 110 000 persons died each year from HCC in China, which accounts for 45% of the deaths from HCC worldwide. China is also a hyperepidemic area for hepatitis B virus (HBV) infection (with an estimated carrier rate exceeding 10% in the general population) and hepatitis C virus (HCV) infection (with a prevalence of anti‐HCV 3.1% of in the general population).7 The infection of HBV and HCV are regarded as important risk factor for liver cancer. Other environmental risk factors also contribute to malignant liver tumour like aflatoxin, pickle, etc.8 Pancreatic cancer represents the fourth most common cause of cancer mortality worldwide9 and will probably be the second or third deadly cancer by 2030.10 The 5‐year survival rate was still lower than 6% in the United States and only 20% patients were able to receive resection.11 The serious local invasion, distant metastasis and chemoresistance make it the most devastating and lethal malignancy worldwide.12
The B7/CD28 family regulate T‐cell activation, tolerance and autoimmunity and provide crucial positive or negative second signals on T‐cell co‐stimulatory or co‐inhibitory functions.13 The B7/CD28 family regulates T‐cell response to influence proliferation and cytokine production and may become attractive immunotherapeutic target.14 B7 family genes are essential elements for the adaptive immune system regulation based on emergence of the Ig/TCR/MHC.15 In the past decade, B7 family have been proved to have 9 members (Figure 1A) which are listed in NCBI gene database. Each member of B7 family contains at least 15% conjunct amino acid sequences. They are expressed by antigen‐presenting cells (APCs) and tumour cells and have high expression in lung, ovarian cancer and breast cancer (more than half of patients having at least one B7 family gene amplification).16 The B7 family belongs to immunoglobulins superfamily and can be divided into 3 groups: group I including B7‐1/B7‐2/CD28/CTLA4 and B7‐H2/ICOS; group II containing B7‐H1 (PD‐L1)/B7‐DC (PD‐L2)/PD‐1 and group III consisting of B7‐H3 (CD276), B7x (B7‐H4) and HHLA2 (B7‐H7/B7‐H5). Their ligands and receptors play important roles in T‐cell co‐stimulation or co‐inhibition to affect the immune system function.17 Research have shown that many inhibitory B7 members were massively expressed in tumour and contribute to tumour cell immunosuppression through the negative second signals, in which the abundance of inhibitory signals may attenuate T‐cell responses.13 Experimental evidence has shown that manipulation of B7 family could affect antitumour immunity and has great clinical implications.18, 19, 20, 21 The involvement of B7 family members in GI carcinogenesis is manifested by many investigations.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Previously, we have analysed the various aspects of B7 family members in breast cancer using existing, publically available data.16 In the current study, we tried to get an overview of B7 family deregulation in GI cancer by analysing the mRNA expression level, genetic mutation, prognostic value and interaction network of the B7 family members using large quantity patient data.
Figure 1.
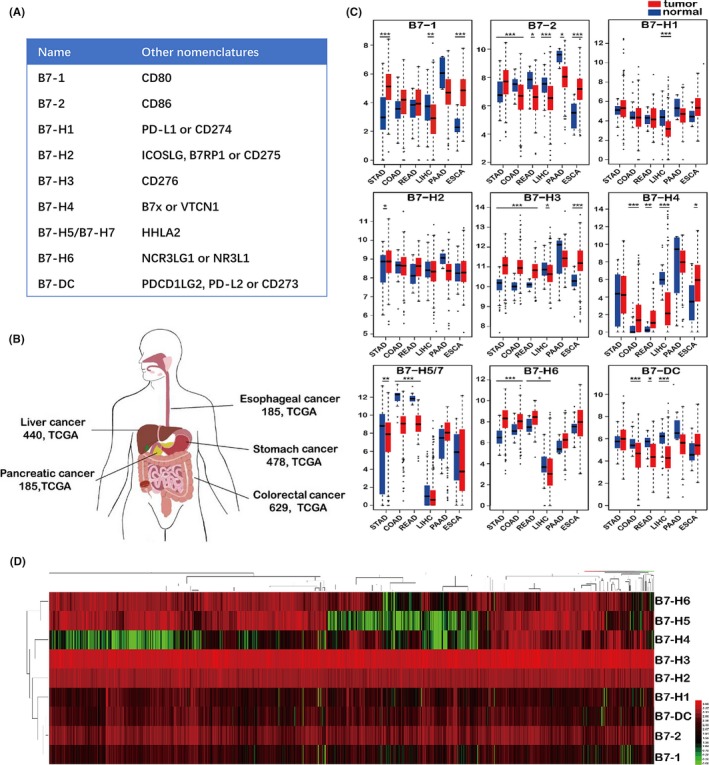
The mRNA expression level of B7 family members in GI cancer and matched normal tissue. (A) Nomenclatures of each B7 family member. (B) Number of patients analysed for each type of cancer. Colorectal cancer includes colon cancer and rectum cancer. (C) Expression level of B7 family members in tumour tissue and adjacent normal tissue from different cancer types. Data were analysed by FIREHOUSE using TCGA RNA‐Seq data. STAD, stomach carcinoma; COAD, colon adenocarcinoma; READ, rectum adenocarcinoma; LIHC, liver hepatocellular carcinoma; PAAD, pancreatic adenocarcinoma; ESCA, oesophageal carcinoma. Overall, B7‐1, B7‐2, B7‐H3, B7‐H4 and B7‐H6 were significantly upregulated. B7‐2 was downregulated in colon and rectum adenocarcinoma and pancreatic cancer. B7‐H5/7 and B7‐DC were downregulated in colon and rectum adenocarcinoma and GC. (D) Heatmap showing the expression level and clustering of B7 family members in CI cancer. *P < .05, **P < .01 and ***P < .001
2. MATERIALS AND METHODS
2.1. FIREHOUSE analysis
The expression profiles of B7 family genes in human GI cancer were analysed by Broad Institute FireBrowse portal using TCGA data (http://firebrowse.org/). We reconstructed the histogram through computational biological methods.
2.2. Bioinformatics analysis
The Heatmap plot was based on Log2(RSEM+1) analysis38 using data from 1698 GI tumour samples in TCGA. The phylogenetic tree was constructed based on the full‐length amino acid sequences of B7 family proteins and their corresponding targets using the neighbour‐joining algorithm in MEGA7. The interaction protein domain analysis was carried out by Pfam 31.0 (http://pfam.xfam.org/).
2.3. cBioPortal database analysis
The cBioPortal for Cancer Genomics (http://www.cbioportal.org/) provides large‐scale cancer genomic data and GI cancer clinical sample information. The 9 B7 family members were analysed in cBioportal for Cancer Genomics database in GI cancer (1917 samples). The role of B7 family in GI cancer were analysed following the online instructions of cBioPortal database for Genetic Alteration, Mutation level, Interaction Network, Clinical Attribute, mRNA expression and overall survival.
2.4. COSMIC analysis
The mutation frequency of B7 family genes in GI cancer was analysed by COSMIC database (http://www.sanger.ac.uk/cosmic/) (233 samples).
2.5. Statistical analysis
GraphPad Prism 6 was used for statistical analysis. T test was used to compare the discrepancy of 2 groups and one‐way ANOVA was used to compare multiple groups. The correlation of DNA methylation and mRNA expression was analysed by Pearson test and Spearman test. Overall survival was showed as Kaplan‐Meier curve with P values calculated using the log‐rank test. P value<.05 was considered statistically significant.
3. RESULTS
3.1. B7 family expression level in GI cancer
We analysed the mRNA expression level of B7 family members (Figure 1A) in GI cancer by FIREHOUSE using TCGA database (case number shown in Figure 1B, gender and age information available in Table S1). The comparison of B7 family member expression level in different GI tumour tissue and adjacent normal tissue is shown in Figure 1C. Overall, most of B7 family members were downregulated in HCC including B7‐1, B7‐2, B7‐H1, B7‐H3, B7‐H4, B7‐H6 and B7‐DC. For other cancer, B7‐1, B7‐2, B7‐H3, B7‐H4 and B7‐H6 were significantly upregulated in 2‐4 different cancer types and B7‐H2 is upregulated in GC, whereas B7‐2, B7‐H5/7 and B7‐DC were downregulated in colon and rectum adenocarcinoma. B7‐2 was also downregulated in pancreatic cancer, and B7‐H5/7 was also downregulated in GC. Overall, the B7 family gene expression level was higher in GI cancer compared with normal tissues. Heatmap clustering of B7 family was shown in Figure 1D. The expression level of B7‐H2 and B7‐H3 was especially high in different kinds of GI cancer. B7 family members were clustered in a manner very similar to their phylogenetic grouping.17 B7‐1 and B7‐2 were closely linked which belongs to the first group of B7 family. B7‐H1 and B7‐DC were closely linked, and they belong to the second group. B7‐H3 and B7‐H4 were clustered together and very closely related to B7‐H5/7 and they form the third group. Principal component analysis (PCA) demonstrated that the expression pattern of B7 family was similar across different GI cancers (Figure S1).
3.2. Gene alteration of B7 family in GI cancer
We studied the gene alteration of B7 family members through cBioPortal in oesophageal cancer, stomach cancer, colorectal cancer, liver cancer and pancreatic cancer using data from TCGA and an Oncoprint figure was generated (Figure 2). The overall alteration rate was the highest in oesophageal cancer and the second highest in pancreatic cancer. mRNA upregulation was frequently found in oesophageal cancer, colorectal cancer, liver cancer and pancreatic cancer. Moreover, gene amplification was relatively higher in oesophageal cancer and stomach cancer than in other cancer types.
Figure 2.
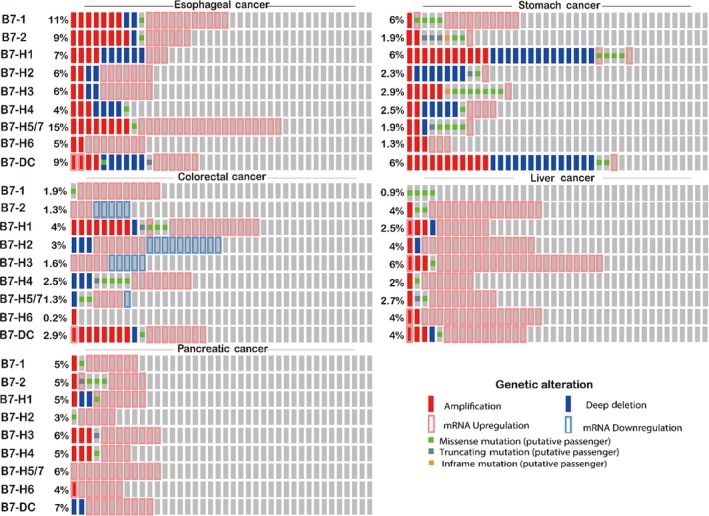
The genetic alteration of B7 family members were analysed by cBioportal. The alteration percentage was present proportionally. mRNA upregulation was frequently found in oesophageal cancer, colorectal cancer, liver cancer and pancreatic cancer. Gene amplification was relatively higher in oesophageal cancer and stomach cancer than in other cancer types
The genomic alteration frequency of B7 family in GI cancer was shown in Figure 3A. The frequency of amplification, mutation, deletion and multiple alteration was shown in Figure 3A. Results showed that about 1%‐6% of oesophageal cancer, 0.4%‐6.4% of stomach cancer, 0.4%‐4.1% of colorectal cancer, 0.3%‐6.4% of liver cancer and 0.5%‐3.4% of pancreatic cancer clinical samples contained B7 family genomic alteration. Gene amplification was found for all B7 members in oesophageal cancer patients, and the frequency was higher than in other cancer types. B7‐H5/H7 had the highest amplification ratio (4.3%, 8/184) in oesophageal cancer. Notably, B7‐DC was amplified in oesophageal (2.2%, 4/184), stomach (2.3%, 9/393), colorectal (2.3%, 5/220) and liver cancer (0.8%, 3/366). Overall, more than 4.8% of samples (64/1312) had B7 gene amplifications in this analysis. Two patients were without B7 genomic alteration.
Figure 3.
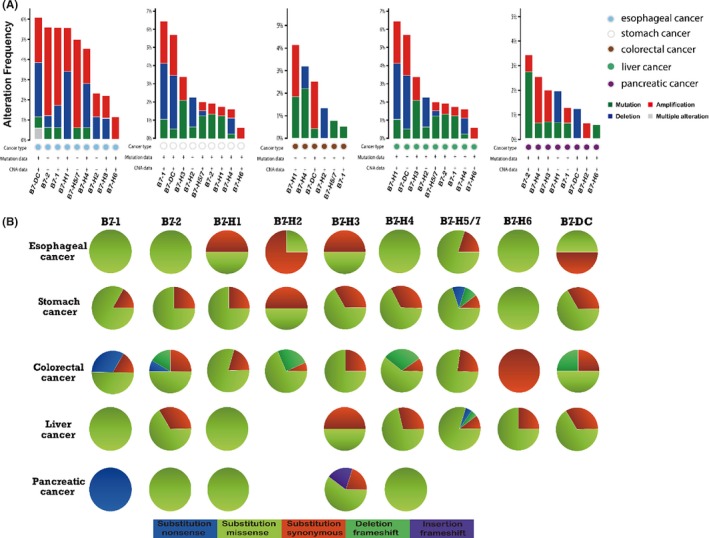
The frequency of genetic alteration of B7 family members in GI cancer. (A) Cases with mutation and copy number alterations were selected from TCGA database and analysed by cBioportal. About 1%‐6% of oesophageal cancer, 0.4%‐6.4% of stomach cancer, 0.4%‐4.1% of colorectal cancer, 0.3%‐6.4% of liver cancer and 0.5%‐3.4% of pancreatic cancer clinical samples contained B7 family genomic alteration. Overall, more than 4.8% of samples (64/1312) had B7 gene amplifications. In total, 9 studies of oesophageal cancer, liver cancer and stomach cancer; 6 studies of colorectal cancer; and 8 studies of pancreatic cancer were included. (B) COSMIC analysis of B7 family mutation through COSMIC database. Nearly 68% missense substitution was found in all GI cancers (158 from 233 samples) for B7 family genes. Missense substitution was most frequently found in stomach cancer and liver cancer. Synonymous substitution ranks the second in frequency. Frameshift deletion and frameshift insertion were less frequently found in GI cancer
We also investigated the genomic alteration of B7 family by COSMIC analysis. The frequency of nonsense, missense and synonymous substitution, frameshift deletion and frameshift insertion were summarized in pie graph (Figure 3B). Nearly, 68% missense substitution was found in all GI cancers (158 from 233 samples) for B7 family genes. Missense substitution was most frequently found in stomach cancer and liver cancer, with the frequency of 74% (32 in 43 samples) and 72% (36 in 50 samples), respectively. Its frequency was 64% (16 in 25 samples) in oesophageal cancer, 63% (65 in 103 samples) in colorectal cancer and 75% (9 in 12 samples) in pancreatic cancer. Synonymous substitution ranks the second in frequency with nearly 19% (20 in 103 samples) in colorectal cancer, 32% (8 in 25 samples) in oesophageal cancer, 28% (12 in 43 samples) in stomach cancer and 18% (10 in 50 samples) in liver cancer. Frameshift deletion (only 6%, 14 in 233 samples) and frameshift insertion (only 0.4%, 1 in 233 samples) were less frequently found in GI cancer.
3.3. Promoter DNA methylation was involved in regulating B7 family expression
In order to investigate the underlying mechanism in the regulation of B7 family expression in GI cancer, we further explored the correlation between B7 family member promoter DNA methylation and mRNA expression using Genetic Profile analysis in cBioportal with data from TCGA (Figure 4). Intriguingly, there was significant negative correlation between mRNA expression and promoter DNA methylation for B7‐H4 and B7‐H5/7 in oesophageal cancer, stomach cancer and pancreatic cancer. The negative correlation was also significant for B7‐H3 in stomach cancer, pancreatic cancer and colorectal cancer, B7‐H6 in oesophageal cancer and liver cancer, and B7‐H1 in oesophageal cancer and B7‐2 in pancreatic cancer. The results indicated that promoter DNA methylation might contribute to the dysregulation of B7 family expression in GI cancer.
Figure 4.
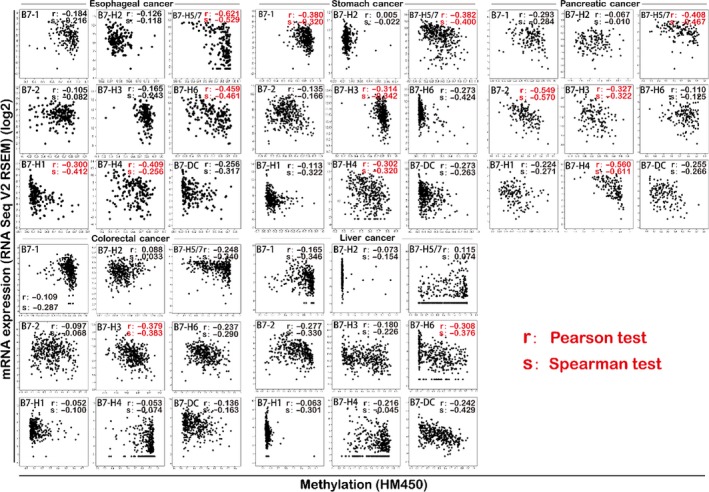
The association between promoter DNA methylation and mRNA expression of B7 family in GI cancer. There was significant negative correlation between mRNA expression and promoter DNA methylation for B7‐H4 and B7‐H5/7 in oesophageal cancer, stomach cancer and pancreatic cancer. The negative correlation was also significant for B7‐H3 in stomach cancer, pancreatic cancer and colorectal cancer; B7‐H6 in oesophageal cancer and liver cancer; B7‐H1 in oesophageal cancer; and B7‐2 in pancreatic cancer. The correlation was verified by the Pearson test and Spearman test. The red label indicated significant negative correlation by the Pearson test (r < −.3)
3.4. Impact of B7 family on patient survival in GI cancer
Overall survival was compared between cases with and without gene alteration for every B7 family member in GI cancer by cBioPortal using data from TCGA (Figure 5). Kaplan‐Meier analysis revealed that gene alteration in B7‐H5/H7 was significantly associated with poor survival in oesophageal cancer (P < .01). Similar results were found for B7‐H1 in pancreatic cancer (P < .01) and B7‐H6 in liver cancer (P < .05). From the above‐mentioned genetic alteration analysis (Figure 2), we observed that the frequency of mRNA upregulation was high for B7‐H5/H7 in oesophageal cancer, B7‐H1 in pancreatic cancer and B7‐H6 in liver cancer among all B7 family members.
Figure 5.
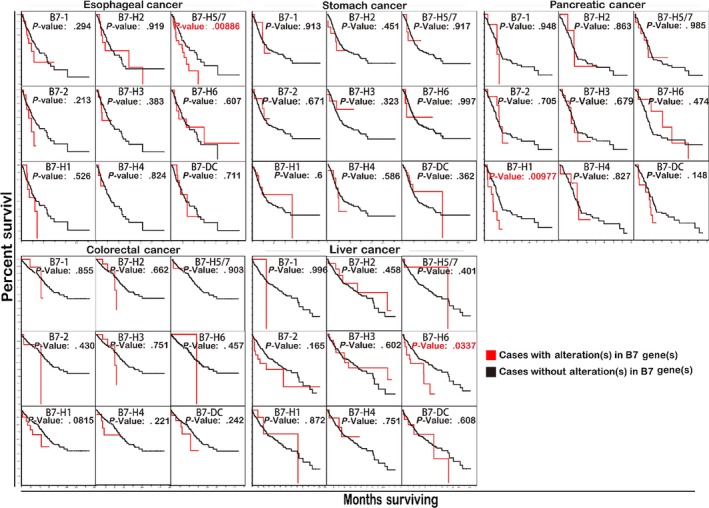
The association between overall survival and B7 family gene expression analysed by cBioportal. The overexpression of B7‐H1 in pancreatic cancer, B7‐H5 in oesophageal cancer and B7‐H6 in liver cancer were significantly associated with worse overall survival. Statistical analysis was carried out by Kaplan‐Meier analysis
3.5. Association of B7 family with clinical parameters
Since we have shown that gene alteration in several B7 members predict poor patient survival, we further analysed the association of these B7 member expression with clinical parameters (Figure 6). Results demonstrated that in oesophageal cancer, patients with Barrett’s oesophagus (145 samples, P < .0001), reflux history (154 samples, P < .0001), history of oesophageal cancer (146 samples, P = .0022), primary lymph node presentation (167 samples, P = .0002), columnar metaplasia (109 samples, P < .0001), tumour neoplasm(172 samples, P = .0004) and tumour lymph node metastasis (144 samples, P = .0197) have significantly higher level of B7‐H5/H7 mRNA expression. Moreover, B7‐H5/H7 expression level was negatively correlated with tumour differentiation grade (184 samples, P = .0012) (Figure 6A). In liver cancer, patients with higher neoplasm stage (323 samples, P = .0019) and poor disease‐free survival (372 samples, P = .0154) have higher level of B7‐H6 (Figure 6B). Although in pancreatic cancer B7‐H1 expression was not significantly associated with clinical attributes, patients with recurred/progressed disease, tumour lymph node metastasis and higher tumour histological grade tend to have higher level of B7‐H1 (Figure S2).
Figure 6.
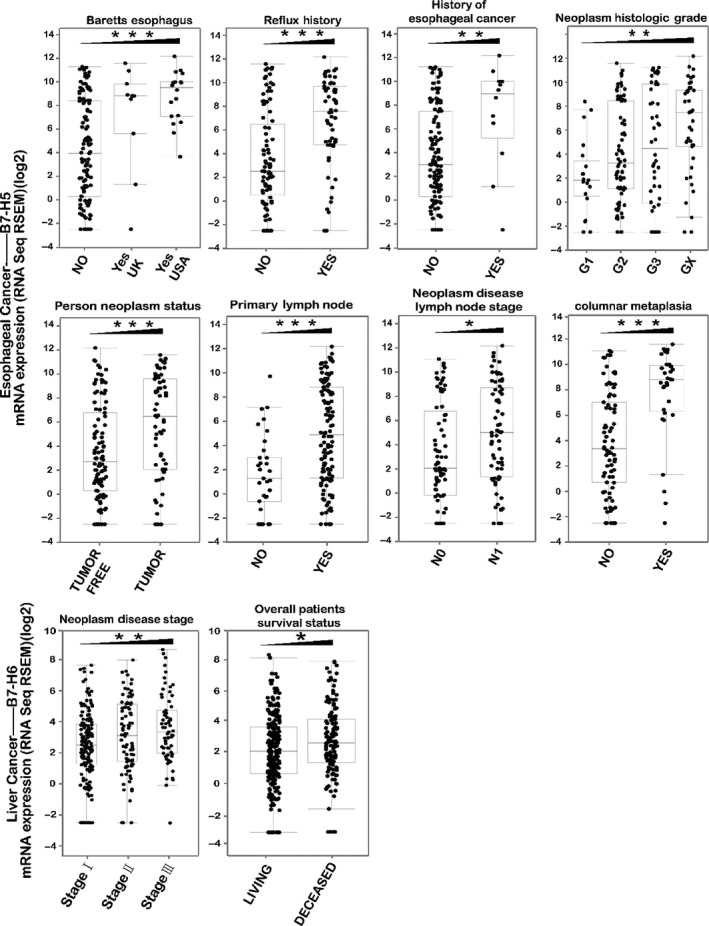
Association of B7‐H5/7 and B7‐H6 expression with clinical parameters. (A) In oesophageal cancer, patients with Barrett’s oesophagus, reflux history, history of oesophageal cancer, primary lymph node presentation, columnar metaplasia, tumour neoplasm and tumour lymph node metastasis have significantly higher level of B7‐H5/H7 mRNA expression. Moreover, B7‐H5/H7 expression level was negatively correlated with tumour differentiation grade. (B) In liver cancer, patients with higher neoplasm stage and poor disease‐free survival have higher level of B7‐H6. *P < .05, **P < .01 and ***P < .001
3.6. Network analysis of B7 family proteins in GI cancer
The interaction network of B7 family signalling in GI cancer was studied. From the cBioportal analysis, B7‐1 and B7‐2 showed high combined interaction to other gene signalling, while B7‐H1 and B7‐DC demonstrated limited combined interaction to other gene singling (Figure 7A). Gene amplification and/or mRNA upregulation were frequently found for B7‐1/2 potential targets in all kinds of GI cancer and also some potential targets for B7‐H1/DC, including FGF4, PIK3CA, ERBB2, PLCG1, SRC, PPP2R5A, SHIC1, AKT2, LCK, PTPN11, CSK and CD4. We also noticed that FGF4 and PIK3CA as potential targets of B7‐1/2 manifested significant gene amplification and mRNA upregulation. We postulate that PTPN11 might be a novel B7 receptor since it demonstrated similar gene alterations with B7‐H1 and B7‐DC in oesophageal, stomach, liver and pancreatic cancer. From the phylogenetic tree (Figure 7B), we discovered that many potential B7 targets from the network analysis were very closely related to known B7 receptors, including FGF4, SRC, PLCG1, PPP2R5A, ERBB2 and CD3G. These may possibly be novel B7 receptors or targets and require further validation. Furthermore, protein domain analysis revealed that the SH2, SH3 and Pkinase domain existed in most potential B7 interacting proteins (Figure 7C). The B7‐H1/DC potential target CD4 has the V‐set domain which is similar to the known CD28 family,16 indicating it might be a novel B7 receptor. Other newly identified potential targets do not have the V‐set domain, suggesting that they may possibly be associated with T‐cell signalling under the regulation of B7 family members.
Figure 7.
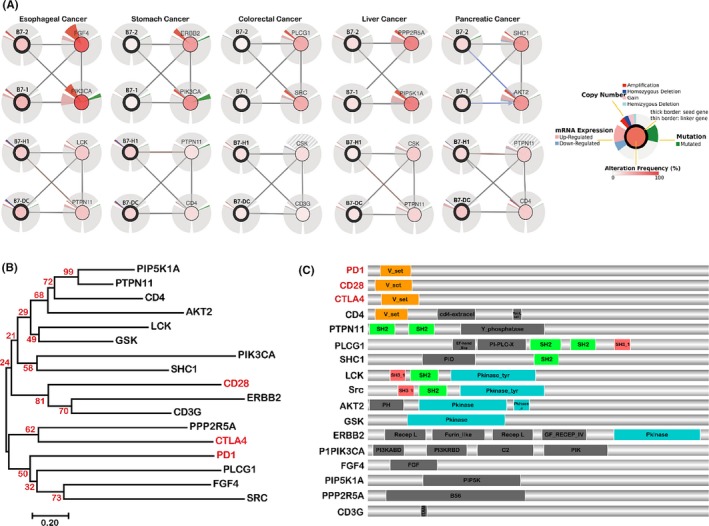
The potential targets of B7 family members predicted by cBioportal. (A) B7‐1 and B7‐2 showed highly combined interaction to other gene signalling, while B7‐H1 and B7‐DC demonstrated limited combined interaction to other gene singling. (B) Phylogenetic analysis of B7 family members and their potential targets. Neighbour‐joining tree was constructed with Mega7 program. Red colour represents known B7 receptors. Many potential B7 targets from the network analysis were very closely related to known B7 receptors. (C) Domain analysis of B7 family reacting proteins. The SH2, SH3 and Pkinase domain existed in most potential B7 interacting proteins. The B7‐H1/DC potential target CD4 has the V‐set domain which is similar to the known CD28 family, indicating it might be a novel B7 receptor
4. DISCUSSION
The immunological response is an important protective mechanism against cancer. The escape of tumours from immune monitoring always attributes to immune system dysfunction and contributes to tumour progression, metastasis and recurrence.39 T‐cell‐meditated immune response is a crucial step in cancer immunosurveillance and immunotherapy.40 Super Ig‐immunoglobulin B7‐CD28 family comprises of a complex network of co‐stimulatory and co‐inhibitory molecules for T‐cell response and is overexpressed on cell surface of some kinds of cancer. Moreover, they have a positive relationship with mutation, poor overall survival, gene amplification and/or DNA methylation.16 Therefore, we were prompt to investigate the relationship between B7 family members and GI cancer. The expression level of the currently known 9 members of B7 family from TCGA data in different GI cancer was summarized (Figure 1). Overall, B7‐1, B7‐2, B7‐H3, B7‐H4 and B7‐H6 were significantly overexpressed in 2‐4 cancer types, whereas B7‐2, B7‐H5/7 and B7‐DC were downregulated in a few cancer types. Our previous study in breast cancer also demonstrated the upregulation of B7‐H3, B7‐H4, B7‐H6 and downregulation of B7‐2, B7‐H5/7.16 B7‐H3 and B7‐H4 negatively regulate T‐cell activation and their overexpression has been reported in many cancers and predicts poor patient survival.22, 37, 41, 42, 43, 44 Moreover, overexpression of B7‐H3 and B7‐H4 was found to suppress antitumour immunity.21, 45 Thus, the upregulation of B7‐H3 and B7‐H4 might contribute to GI carcinogenesis through inhibiting antitumour immunity. B7‐H6 has been reported to be upregulated in several types of cancer, and its expression is deemed as prognostic indicator.46, 47, 48 However, research evidence shows that downregulation of B7‐H6 impairs tumour cell recognition by NK cells.20 Whether B7‐H6 functions as co‐stimulator or co‐inhibitor in GI cancer needs further investigation. B7‐DC is highly homologous to co‐inhibitory B7‐H1. However, it has been demonstrated that B7‐DC functions as a co‐stimulator in HCC,49 which is also consistent with our finding that B7‐DC was mainly downregulated in GI cancer. Together, the findings support a co‐stimulatory role for T‐cell response for B7‐DC in the pathogenesis of GI cancer.
Another analysis from cBioportal revealed the alteration frequency of B7 family members in different kinds of GI cancer (Figure 2). The alteration rate was higher in oesophageal cancer (average 7.9%) and pancreatic cancer (average 5.1%). Gene amplification and mRNA upregulation were frequently found especially in oesophageal cancer. We further checked the B7 family genomic alteration (Figure 3). Similar to previous observation, gene amplification was found for all B7 members in oesophageal cancer patients and the frequency was higher than in other cancer types. Overall, more than 40% of samples had B7 gene amplifications. By COSMIC analysis, we found that missense substitution was most frequently found in GI cancer and accounts for nearly 68% of all samples (158 from 233 samples) especially in stomach cancer and liver cancer. Substitution synonymous accounts for nearly 22% of all samples (51 from 230 samples).
Promoter DNA methylation may represent a key epigenetic mechanism in regulating gene expression.50 Herein, we further analysed the B7 family DNA methylation in GI cancer (Figure 4). The mRNA expression was negatively correlated with B7 family DNA methylation in most cancers, including B7‐H1, B7‐H4, B7‐H5/H7, B7‐H6 in oesophageal cancer, B7‐1, B7‐H3, B7‐H4, B7‐H5/H7 in stomach cancer, B7‐2, B7‐H3, B7‐H4, B7‐H5/H7 in pancreatic cancer, B7‐H3 in colorectal cancer and B7‐H6 in liver cancer. Taking results of gene amplification into consideration, our results suggested the expression of B7 family gene B7‐1, B7‐2, B7‐H1, B7‐H3, B7‐H4, B7‐H5/H7 and B7‐H6 might be regulated by DNA methylation and gene amplification in GI cancer.
Of all B7 family members, we observed that alterations of B7‐H5/H7 in oesophageal cancer, B7‐H6 in liver cancer and B7‐H1 in pancreatic cancer were significantly associated with worse overall survival (Figure 5). Patients with Barrett’s oesophagus and reflux history have significantly higher level of B7‐H5/H7 mRNA expression. Reflux disease may lead to dysplasia, which has been regarded as an important factor in forming Barrett’ oesophagus and damaging the distal oesophagus51 (Figure 6). Barrett’s oesophagus is a critical risk factor in oesophageal cancer.52 Our results suggested B7‐H5/H7 was overexpressed in oesophageal premalignant condition emphasizing the key influence of B7‐H5/H7 on oesophageal cancer progression and it may be a potential target for immunotherapy. Moreover, B7‐H5/H7 high expression was associated with higher tumour histological grade and lymph node presentation and stage suggesting its involvement in tumour metastasis. Research evidence revealed that B7‐H5/H7 was a significant risk factor for poor overall survival in osteosarcoma.53 Moreover, consistent with our finding, study has found that B7‐H5/H7 protein was widely expressed in oesophageal cancer and predicted poor patient survival through inhibiting anti‐cancer immunity.54
Our result also demonstrated that in liver cancer increased B7‐H6 mRNA expression was associated with higher tumour stage and increased risk of death for patients (Figure 6). The infection of HBV has been proved to be a risk factor for liver cancer.8 The NK cells’ cytotoxicity receptor NKp30 was upregulated in hepatitis B virus‐related acute‐on‐chronic liver failure (HBVACLF) patients55 and NKp30 has also been regarded as the receptor of B7‐H6. Thus, we speculate that B7‐H6 was involved in the pathogenesis of liver cancer. We have previously reported the notorious role of B7‐H6 in breast cancer.16 Study in ovarian cancer also revealed that B7‐H6 expression was significantly correlated with distant metastasis and tumour stage and high expression of B7‐H6 predicts poor overall patient survival.46 Although the mechanism has not been investigated in GI cancer, it may well illustrate the clinical significance of increased expression of B7‐H6 in liver cancer and B7‐H6 might function as a prognostic marker for liver cancer.
Although B7‐H1 was not significantly associated with overall survival in pancreatic cancer, there was tendency that patients with recurred/progressed disease, higher lymph node and tumour histological grade had higher levels of B7‐H1. The significance of B7‐H1 in pancreatic cancer needs further illustration by increasing the clinical sample size. Other study has reported that higher B7‐H1 (PD‐L1) expression indicated a poor prognosis for patients’ survival in oesophageal, stomach and ovarian cancer tissues.56 Combination of anti‐B7‐H1 (PD‐L1) and anti‐CTLA‐4 (T‐lymphocyte‐associated antigen 4) can have a good clinical response in melanoma patients regardless of tumour B7‐H1 (PD‐L1) status.57
The crosstalk of B7 family with other signalling pathway was analysed by network analysis in cBioportal (Figure 7). Limitedly, only 4 B7 family members were found associated with other genes by cBioportal, that is, B7‐1, B7‐2, B7‐H1, B7‐DC. In this study, we observed that B7‐1/2 was significantly associated with FGF4 in oesophageal cancer and with PIK3CA in oesophageal and stomach cancer. FGF4 acts to stimulate epithelial‐mesenchymal transition (EMT) in lung adenocarcinoma.58 The upregulation of FGF4 also promotes the migration of breast cancer cells.59 PIK3CA is regarded as a predictor for malignance in colorectal cancer.60 Mutation of PIK3CA leads to higher expression level of p‐AKT/AKT in primary tumours and metastases in endometrial cancer, which also predicts poor overall survival.61 Moreover, PIK3CA stands second on the list of various genes which have already been reported to cause breast cancer.62 However, the impact of B7‐1/2 on these 2 genes needs further investigation. Meanwhile, we identified B7‐H1 and B7‐DC was significantly associated with PTPN11 in oesophageal cancer, stomach cancer, liver cancer and pancreatic cancer. PTPN11 is reported as a gastric cancer risk.63 PTPN11 also promotes HCC growth and metastasis through Ras/Raf/Erk pathway and PI3‐K/AKT/MTOR cascade and acts as a predictor of poor prognosis supporting an oncogenic role of PTPN11.64 Our protein domain analysis showed that PTPN11 is similar to some of the other potential B7 interacting proteins in possessing the Src‐homology 2 (SH2) domain. The SH‐2 domain was reported to regulate tumorigenesis and immune function in several proteins.65, 66, 67, 68 Therefore, we postulate that PTPN11 might be a novel B7 receptor or target. Moreover, the Pkinase domain shared by some B7 interacting proteins may have important influence on tumour progression and malignancy.69, 70 The result suggests that the newly identified B7 interacting proteins may be strongly involved in tumorigenesis.
In summary, our research findings revealed overexpression of B7 family members in GI cancer at different frequencies, and it was possibly associated with gene amplification and/or DNA methylation. Our results also provided insight into the association of B7 family with patient survival and clinical parameters and the interaction network with B7 family, underlining the importance of B7 family members in GI cancer.
CONFLICT OF INTEREST
The authors declare that the fundings mentioned in the Acknowledgements section do not lead to any conflict of interest. In addition, the authors declare that there is no conflict of interest regarding the publication of this manuscript.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant nos. 81503093, 81602166 and 81672444), the Joint Funds of the Southwest Medical University & Luzhou (2016LZXNYD‐T01, 2017LZXNYD‐Z05 and 2017LZXNYD‐J09).
Zhao Q, Hu F, Xiao Z, et al. Comprehensive molecular profiling of the B7 family in gastrointestinal cancer. Cell Prolif. 2018;51:e12468 10.1111/cpr.12468
Qijie Zhao and Fuyan Hu contributed equally to this manuscript.
Contributor Information
Zhangang Xiao, Email: xzg555898@hotmail.com.
Chi Hin Cho, Email: chcho@cuhk.edu.hk.
Jing Shen, Email: crystal_stray@126.com.
REFERENCES
- 1. Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74‐108. [DOI] [PubMed] [Google Scholar]
- 2. Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302‐308. [PubMed] [Google Scholar]
- 3. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562‐572; discussion 572‐563. [DOI] [PubMed] [Google Scholar]
- 4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 5. Moller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori . Int J Cancer. 1995;60:587‐589. [DOI] [PubMed] [Google Scholar]
- 6. Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297‐305. [DOI] [PubMed] [Google Scholar]
- 7. Lei X, Naitoh S, Deng X, et al. Prevalence of hepatitis C virus infection in the general population and patients with liver disease in China. Hepatol Res. 1999;14:135‐143. [Google Scholar]
- 8. Shi J, Zhu L, Liu S, et al. A meta‐analysis of case‐control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9‐29. [DOI] [PubMed] [Google Scholar]
- 10. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 11. Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta‐analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu H, Li L, Chen H, et al. Silencing IGFBP‐2 decreases pancreatic cancer metastasis and enhances chemotherapeutic sensitivity. Oncotarget. 2017;8:61674‐61686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515‐548. [DOI] [PubMed] [Google Scholar]
- 14. Zhao R, Chinai J, Buhl S, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T‐cell function. Proc Natl Acad Sci USA. 2013;110:9879‐9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flajnik MF, Tlapakova T, Criscitiello MF, et al. Evolution of the B7 family: co‐evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7’s historical relationship with the MHC. Immunogenetics. 2012;64:571‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Z, Shen J, Wang MH, et al. Comprehensive molecular profiling of the B7 family of immune‐regulatory ligands in breast cancer. Oncoimmunology. 2016;5:e1207841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janakiram M, Shah UA, Liu W, et al. The third group of the B7‐CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7‐H3. Immunol Rev. 2017;276:26‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jung D, Hilmes C, Knuth A, et al. Gene transfer of the Co‐stimulatory molecules B7‐1 and B7‐2 enhances the immunogenicity of human renal cell carcinoma to a different extent. Scand J Immunol. 1999;50:242‐249. [DOI] [PubMed] [Google Scholar]
- 19. Li G, Wu X, Zhang F, et al. Triple expression of B7‐1, B7‐2 and 4‐1BBL enhanced antitumor immune response against mouse H22 hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011;137:695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiegler N, Textor S, Arnold A, et al. Downregulation of the activating NKp30 ligand B7‐H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684‐693. [DOI] [PubMed] [Google Scholar]
- 21. Chen C, Shen Y, Qu QX, et al. Induced expression of B7‐H3 on the lung cancer cells and macrophages suppresses T‐cell mediating anti‐tumor immune response. Exp Cell Res. 2013;319:96‐102. [DOI] [PubMed] [Google Scholar]
- 22. Chen L, Xie Q, Wang Z, et al. Assessment of combined expression of B7‐H3 and B7‐H4 as prognostic marker in esophageal cancer patients. Oncotarget. 2016;7:77237‐77243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Chen J, Xu B, et al. B7‐H3 expression associates with tumor invasion and patient’s poor survival in human esophageal cancer. Am J Transl Res. 2015;7:2646‐2660. [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Deng H, Lu M, et al. B7‐H1 expression associates with tumor invasion and predicts patient’s survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:6015‐6023. [PMC free article] [PubMed] [Google Scholar]
- 25. Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7‐H1, B7‐H4 and Foxp3 + Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2015;20:273‐281. [DOI] [PubMed] [Google Scholar]
- 26. Cui Y, Li Z. B7‐H4 is predictive of poor prognosis in patients with gastric cancer. Med Sci Monit. 2016;22:4233‐4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koyama S, Maruyama T, Adachi S, et al. Expression of costimulatory molecules, B7‐1 and B7‐2 on human gastric carcinoma. J Cancer Res Clin Oncol. 1998;124:383‐388. [DOI] [PubMed] [Google Scholar]
- 28. Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD‐L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaux P, Moutet M, Faivre J, et al. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7‐1 and B7‐2 costimulatory molecules of the T‐cell activation. Lab Invest. 1996;74:975‐983. [PubMed] [Google Scholar]
- 30. Habicht A, Lindauer M, Galmbacher P, et al. Development of immunogenic colorectal cancer cell lines for vaccination: expression of CD80 (B7.1) is not sufficient to restore impaired primary T cell activation in vitro. Eur J Cancer. 1995;31a:2396‐2402. [DOI] [PubMed] [Google Scholar]
- 31. Nooren‐Staal WH, Frederiks CM, te Wierik MJ. [Validation: its effect in residents and staff in a home for the aged]. Tijdschr Gerontol Geriatr. 1995;26:117‐121. [PubMed] [Google Scholar]
- 32. Yu MC, Chen CH, Liang X, et al. Inhibition of T‐cell responses by hepatic stellate cells via B7‐H1‐mediated T‐cell apoptosis in mice. Hepatology. 2004;40:1312‐1321. [DOI] [PubMed] [Google Scholar]
- 33. Sun TW, Gao Q, Qiu SJ, et al. B7‐H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Sun J, Zhao H, et al. The coexpression and clinical significance of costimulatory molecules B7‐H1, B7‐H3, and B7‐H4 in human pancreatic cancer. Onco Targets Ther. 2014;7:1465‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Song X, Liu J, Lu Y, et al. Overexpression of B7‐H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol Rep. 2014;31:1191‐1198. [DOI] [PubMed] [Google Scholar]
- 36. Okudaira K, Hokari R, Tsuzuki Y, et al. Blockade of B7‐H1 or B7‐DC induces an anti‐tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741‐749. [DOI] [PubMed] [Google Scholar]
- 37. Xu H, Chen X, Tao M, et al. B7‐H3 and B7‐H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett. 2016;11:1841‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B, Dewey C. RSEM: accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao X, Guo F, Li Z, et al. Aberrant expression of B7‐H4 correlates with poor prognosis and suppresses tumor‐infiltration of CD8 + T lymphocytes in human cholangiocarcinoma. Oncol Rep. 2016;36:419‐427. [DOI] [PubMed] [Google Scholar]
- 40. Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu CL, Zang XX, Huang H, et al. The expression of B7‐H3 and B7‐H4 in human gallbladder carcinoma and their clinical implications. Eur Rev Med Pharmacol Sci. 2016;20:4466‐4473. [PubMed] [Google Scholar]
- 42. Fauci JM, Straughn JM Jr, Ferrone S, et al. A review of B7‐H3 and B7‐H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127:420‐425. [DOI] [PubMed] [Google Scholar]
- 43. Sun Y, Wang Y, Zhao J, et al. B7‐H3 and B7‐H4 expression in non‐small‐cell lung cancer. Lung Cancer. 2006;53:143‐151. [DOI] [PubMed] [Google Scholar]
- 44. Wang L, Cao NN, Wang S, et al. Roles of coinhibitory molecules B7‐H3 and B7‐H4 in esophageal squamous cell carcinoma. Tumour Biol. 2016;37:2961‐2971. [DOI] [PubMed] [Google Scholar]
- 45. Yi KH, Chen L. Fine tuning the immune response through B7‐H3 and B7‐H4. Immunol Rev. 2009;229:145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou Y, Xu Y, Chen L, et al. B7‐H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. Int J Clin Exp Pathol. 2015;8:9428‐9433. [PMC free article] [PubMed] [Google Scholar]
- 47. Sun J, Tao H, Li X, et al. Clinical significance of novel costimulatory molecule B7‐H6 in human breast cancer. Oncol Lett. 2017;14:2405‐2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang J, Jin X, Liu J, et al. The prognostic value of B7‐H6 protein expression in human oral squamous cell carcinoma. J Oral Pathol Med. 2017;46:766‐772. [DOI] [PubMed] [Google Scholar]
- 49. Shin T, Yoshimura K, Shin T, et al. In vivo costimulatory role of B7‐DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aznar M, Labiano S, Diaz‐Lagares A, et al. CD137 (4‐1BB) costimulation modifies DNA methylation in CD8 + T‐cell relevant genes. Cancer Immunol Res. 2017;6:69‐78. [DOI] [PubMed] [Google Scholar]
- 51. Quante M, Graham TA, Jansen M. Insights into the pathophysiology of esophageal adenocarcinoma. Gastroenterology. 2017;158:406‐420. [DOI] [PubMed] [Google Scholar]
- 52. Hvid‐Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375‐1383. [DOI] [PubMed] [Google Scholar]
- 53. Koirala P, Roth ME, Gill J, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janakiram M, Chinai JM, Fineberg S, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21:2359‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zou Y, Chen T, Han M, et al. Increased killing of liver NK cells by Fas/Fas ligand and NKG2D/NKG2D ligand contributes to hepatocyte necrosis in virus‐induced liver failure. J Immunol. 2010;184:466‐475. [DOI] [PubMed] [Google Scholar]
- 56. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qi L, Song W, Li L, et al. FGF4 induces epithelial‐mesenchymal transition by inducing store‐operated calcium entry in lung adenocarcinoma. Oncotarget. 2016;7:74015‐74030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shi H, Li Y, Feng G, et al. The oncoprotein HBXIP up‐regulates FGF4 through activating transcriptional factor Sp1 to promote the migration of breast cancer cells. Biochem Biophys Res Commun. 2016;471:89‐94. [DOI] [PubMed] [Google Scholar]
- 60. Chang PY, Chen JS, Chang SC, et al. Acquired somatic TP53 or PIK3CA mutations are potential predictors of when polyps evolve into colorectal cancer. Oncotarget. 2017;8:72352‐72362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mjos S, Werner HMJ, Birkeland E, et al. PIK3CA exon9 mutations associate with reduced survival, and are highly concordant between matching primary tumors and metastases in endometrial cancer. Sci Rep. 2017;7:10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thirumal Kumar D, George Priya Doss C. Role of E542 and E545 missense mutations of PIK3CA in breast cancer: a comparative computational approach. J Biomol Struct Dyn. 2017;35:2745‐2757. [DOI] [PubMed] [Google Scholar]
- 63. Zhuo C, Shao M, Chen C, et al. Chemotherapy effectiveness and prognosis of gastric cancer influenced by PTPN11 polymorphisms. Cell Physiol Biochem. 2016;39:1537‐1552. [DOI] [PubMed] [Google Scholar]
- 64. Han T, Xiang DM, Sun W, et al. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol. 2015;63:651‐660. [DOI] [PubMed] [Google Scholar]
- 65. Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906‐7909. [DOI] [PubMed] [Google Scholar]
- 66. Son DJ, Zheng J, Jung YY, et al. MMPP attenuates non‐small cell lung cancer growth by inhibiting the STAT3 DNA‐binding activity via direct binding to the STAT3 DNA‐binding domain. Theranostics. 2017;7:4632‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Woo SJ, Jo HI, Lee HH, et al. Molecular characterization and expression analysis of olive flounder (Paralichthys olivaceus) phospholipase C gamma 1 and gamma 2. Fish Shellfish Immunol. 2017;63:353‐366. [DOI] [PubMed] [Google Scholar]
- 68. Ahn R, Sabourin V, Ha JR, et al. The ShcA PTB domain functions as a biological sensor of phosphotyrosine signaling during breast cancer progression. Cancer Res. 2013;73:4521‐4532. [DOI] [PubMed] [Google Scholar]
- 69. Roskoski R Jr. The ErbB/HER family of protein‐tyrosine kinases and cancer. Pharmacol Res. 2014;79:34‐74. [DOI] [PubMed] [Google Scholar]
- 70. Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116‐132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


