Abstract
Background and objectives: Ovarian cancer is the most fatal primary malignancy among gynecological cancers. Circular RNAs (circRNAs) play an important role in the development of various cancers, but the functions of circRNAs in ovarian cancer development have not been studied. We aim to explore the function and mechanism of CDR1as in the progression of ovarian cancer and to provide a new target for the diagnosis and treatment of ovarian cancer.
Methods: Ovarian cancer cell proliferation was assessed according to proliferating cell nuclear antigen (PCNA) and Ki67 protein expression levels and MTT and CCK8 assays. The migration ability of cells was detected by scratch-wound assays, and the invasion ability of the cells was determined by Transwell® assays. qRT-PCR and Western blotting were used to verify miRNA and protein expression.
Results: CDR1as expression in ovarian tissues was significantly lower in ovarian cancer patients than in patients without ovarian cancer. CDR1as overexpression inhibited the proliferation, invasion and migration of ovarian cancer cells. Silencing CDR1as increased the expression of miR-135b-5p and decreased the expression of hypoxia-inducible factor 1-alpha inhibitor (HIF1AN), thus increasing the proliferation capacity of ovarian cancer cells.
Conclusions: CDR1as, acting as a sponge of miR-135b-5p, promotes the expression of HIF1AN and therefore plays a role in tumor inhibition.
Keywords: circular RNAs, CDR1as, ovarian cancer, miR-135b-5p, HIF1AN
Introduction
The incidence of ovarian cancer ranks second among gynecologic tumors, but its mortality rate ranks first. Worldwide, approximately 240,000 people are diagnosed with ovarian cancer each year, and 150,000 people die of ovarian cancer annually.1 The development of ovarian cancer often goes unnoticed, and most ovarian cancers are diagnosed at an advanced stage.2 The 5-year survival rate has been reported to be greater than 90% in patients diagnosed with stage I ovarian cancer, while the 5-year survival rate is approximately 17–39% in most women diagnosed with stage III or IV ovarian cancer.3 Hence, finding new targets for the early diagnosis and treatment of ovarian cancer is essential.
Circular RNAs (circRNAs) are noncoding RNAs characterized by a lack of 5’ and 3’ polarities and poly(adenylate) tails, and their existence was first proposed in 1976.4 CircRNAs have long been considered transcriptional byproducts with no biological function. In recent years, with the continuous progress of gene sequencing technology and bioinformatics, an increasing number of circRNAs and their biological functions have been discovered. Studies have shown that circRNAs can be widely, stably and conservatively expressed in a variety of organisms, and their expression is specific to tissues and stages of development.5 Numerous studies have also demonstrated that circRNAs play an important regulatory role in the progression of various types of human tumors.6,7 Therefore, the study of the role of circRNAs in cancer can provide new targets for the diagnosis and treatment of cancers and improve the overall survival of cancer patients. CircRNA CDR1as (CDR1as) is an antisense transcription product of cerebellar degenerative-related protein-1. A large number of studies have found that CDR1as can act as a ceRNA of miR-7 to regulate the expression and function of miR-7 target genes, thus participating in the regulation of the progression of human liver cancer, lung cancer, esophageal cancer and other types of tumors.8–10
MiRNAs and hypoxia-inducible factor 1-alpha inhibitor (HIF1AN) are closely correlated with cancer. MiRNAs are a class of small noncoding RNAs between 19 and 25 nucleotides in length that can inhibit protein synthesis by binding directly to the 3’ untranslated region (UTR) of the target mRNA.11 MiR-135b has been shown to play a role in promoting tumor progression through targeting different suppressive genes in a variety of human cancers, including colorectal cancer, gastric cancer, cutaneous melanoma and other types of tumors.12,13 HIF1AN is an asparagine hydroxylase, and previous research has found that HIF1AN can inhibit the activities of G9a and GLP by hydroxylating them at their asparagine residues, thereby inhibiting the malignant behavior of ovarian cancer cells.14 However, the relationships among CDR1as, MiR-135b, and HIF1AN in the progression of ovarian cancer are still unclear. Therefore, in the present study, we explored the function and mechanism of CDR1as through determining the expression of CDR1as and its relationships with miR-135b-5p and HIF1AN and the proliferation of ovarian cancer cells.
Materials and methods
Cell lines and tissue samples
The HO8910 and A2780 ovarian cancer cell lines were purchased from Bioleaf Biotech (China) and Chuanbo Biotechnology (China), respectively. Both cell lines were cultured in 10% fetal bovine serum (FBS)-1640 (Corning, USA) in a humidified incubator (37 °C, 5% CO2). Ovarian tissues (65 samples) from ovarian cancer patients and ovarian epithelial tissues (37 samples) from patients without ovarian cancer were collected from the Second Affiliated Hospital of Harbin Medical University. Written informed consents were obtained from all patients. The study protocol was conducted in accordance with the Declaration of Helsinki, and was approved by Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (KY2018-041).
Transfection
Specific siRNAs were constructed by GenePharma Co., Ltd., of China. Their sequences were as follows: hsa-miR-135b-5p inhibitor: UCACAUAGGAAUGAAAAGCCAUA (antisense 5’-3’), negative control (NC) inhibitor: UUGUACUACACAAAAGUACUG (antisense 5’-3’), Si-CDR1as: GCAAUAUCCAGGGUUUCCGTT (sense 5’-3’) and CGGAAACCCUGGAUAUUGCTT (antisense 5’-3’), Si-NC: UUCUCCGAACGUGUCACGUTT (sense 5’-3’) and ACGUGACACGUUCGGAGAATT (antisense 5’-3’). These siRNAs were transfected into HO8910 and A2780 cells using Lipofectamine 2000 siRNA transfection reagent according to the manufacturer’s instructions. Ad-CDR1as and Ad-NC were designed and synthesized by Hanbio (China). Adenovirus particles were used according to the manufacturer’s instructions. To measure the overexpression efficiency of adenovirus-targeted CDR1as, quantitative polymerase chain reaction (qPCR) was used.
Western blot analysis
Proteins were incubated with cold lysis buffer (Tris 50 mm, pH 7.4, NaCl 150 mm, Triton x-100%, EDTA 1 mm, PMSF 2 mm) for 5 mins to dissolve the proteins. These lysates were centrifuged at 13,500 RPM for 15 mins to obtain the supernatant. The protein concentration was determined using a bicinchoninic acid protein assay kit. The protein samples were separated by dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. The membranes were then blocked with 5% skim milk powder in TBST (NaCl: 150 mM, Tris: 20 mM, Tween 20: 0.1%, pH 7.6) for 1 h and incubated with specific protein antibodies at 4 °C overnight. On the second day, the membranes were treated with horseradish peroxidase-conjugated secondary antibodies. The proteins were measured using enhanced chemiluminescence reagents. Antibodies against proliferating cell nuclear antigen (PCNA) and β-actin were purchased from Santa Cruz (CA, USA). The HIF1AN antibody was purchased from ABclonal (China).
qRT-PCR
Total RNA was extracted by using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Subsequently, the total RNA was reverse-transcribed into cDNA using a First Strand cDNA Synthesis Kit (TOYOBO, China). The cDNA was then amplified and measured with a Roche 480 apparatus and SYBR® Green Real-time PCR Master Mix (TOYOBO, China). The specific PCR primers were as follows: CDR1as-forward: 5′-CGTCTTCCAGCATCTCTGTGT-3′ and reverse: 5′-GCCATCGGAAACCCTGGATA-3′; actin-forward: 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse: 5′- GCTGTCACCTTCACCGTTCC −3′; GAPDH-forward: 5′-AATCCCATCACCATCTTCC-3′ and reverse: 5′-CATCACGCCACAGTTTCC-3′; HIF1AN-forward: 5′-GTAATGACTCTATGTGATGCC-3′ and reverse: 5′-GATAAAAGGTTACAAACGATG-3′; hsa-miR-135b-5p-forward: 5′-CGGGCTATGGCTTTTTATTCC-3′, and reverse: 5′-CAGCCACAAAAGAGCACAAT-3′; and Stem-loop, 5′-CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGTCACATA-3′.
MTT assay
To assess proliferation, HO8910 and A2780 cells were cultured at a concentration of approximately 1×104 cells per well in 96-well plates. These cells were then treated in different ways. First, 0.5% 3-4,5-dimethylthiazol-2-yl-2,5-diphenyl-tetrazolium bromide (MTT) was added to each well and incubated for 4 h at 37 °C. Subsequently, 150 µl of dimethyl sulfoxide (DMSO) was added to each well. The absorbance was detected at 490 nm using a spectrophotometer.
Scratch-wound assay
The HO8910 and A2780 cells cultured in 6-well plates were scratched with pipette tips, and the ablated cells were washed away with phosphate-buffered saline (PBS). The cells were photographed in the same area of the culture plate at 0 h and 24 h after wounding. The mobility of the cells was determined by measuring the wound width. Migration width (%)=(W0–W24)/W0×100, where W0 is the width of the initial wound, and W24 is the width of the remaining wound after 24 h.
Transwell® assay
Cell invasion was determined using a Matrigel-coated modified Boyden chamber with a polycarbonate filter with a pore size of 8 μM. After coating the upper chamber filter of the transwell with 50 mg/ml of Matrigel solution, the cells were cultured with serum-free medium in the upper chamber of the transwell, which was inserted into a 24-well plate. Cell culture medium (10% FBS+1640) was then added to the lower chamber. After incubation for 24 h, the cells were fixed with a 4% formaldehyde solution for 30 mins and then stained with a 0.4% crystal violet solution for 15 mins. Cells from five fields were selected randomly in each chamber and photographed with a microscope. The invasion capacity of the cells was quantified by ImagePro +6.0.
CCK8 assay
To assess the proliferation rate, HO8910 and A2780 cells were cultured at a concentration of approximately 1×104 cells per well in 96-well plates. The cells were treated with different conditions and then incubated with CCK8 solution according to the manufacturer’s instructions. The absorbance was measured at 450 nm with a spectrophotometer.
Immunofluorescence assay
Immunofluorescence assays were performed using previously described techniques.15 Cells were incubated with a Ki67-specific antibody overnight at 4 °C and with a FITC-conjugated secondary antibody for 1 h in the dark at 37 °C. Fluorescence was measured by confocal laser scanning microscopy (CLSM). The antibody against Ki67 was obtained from Abcam (Cambridge, UK).
Statistical analysis
Statistical analysis was carried out using SPSS software (version 21, SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± S.E.M. Statistical analysis was performed with Student’s t-test or one-way ANOVA, followed by Dunnett’s test. All statistical tests were two-sided, and differences were considered to be significant at P≤0.05.
Results
CDR1as expression is decreased in ovarian cancer
To investigate whether CDR1as is expressed in ovarian cancer tissues, we measured CDR1as expression levels in the ovarian tissues of 65 ovarian cancer patients and 37 patients without ovarian cancer. The qRT-PCR results showed that CDR1as expression was significantly lower in the ovarian cancer tissues than in the normal ovarian tissues (Figure 1A). Subsequently, we examined CDR1as expression in ovarian cancer cell lines and normal ovarian epithelial cell lines by qRT-PCR. The results were consistent with those of previous experiments. CDR1as expression was lower in the ovarian cancer cell lines (HO8910 and A2780) than in the normal ovarian epithelial cell line (IOSE80) (Figure 1B and C). To explore the potential functions of CDR1as in ovarian cancer, we constructed an adenovirus (Ad-CDR1as) that specifically overexpressed CDR1as and a small interfering RNA (Si-CDR1as) that specifically silenced CDR1as. Ad-CDR1as and Si-CDR1as were transfected into ovarian cancer cell lines. Then, the efficiency of transfection was detected by qRT-PCR (Figure 1D and E).
Figure 1.
CDR1as expression in ovarian cancer tissues and cells. (A) The relative expression of CDR1as in tissues from 65 ovarian cancer patients and 37 patients without ovarian cancer was measured by qRT-PCR. (B) and (C) The relative expression of CDR1as in ovarian cancer cells (HO8910 and A2780) was analyzed by qRT-PCR. (D) CDR1as was overexpressed in ovarian cancer cells via a CDR1as-specific adenovirus (Ad-CDR1as), and its expression was detected by qRT-PCR. (E) CDR1as expression was silenced in ovarian cancer cells with a CDR1as-specific siRNA (Si-CDR1as) and then measured by qRT-PCR. All values are expressed as the mean ± SEM (*P<0.05 and ***P<0.001).
CDR1as inhibits the proliferation of ovarian cancer cells
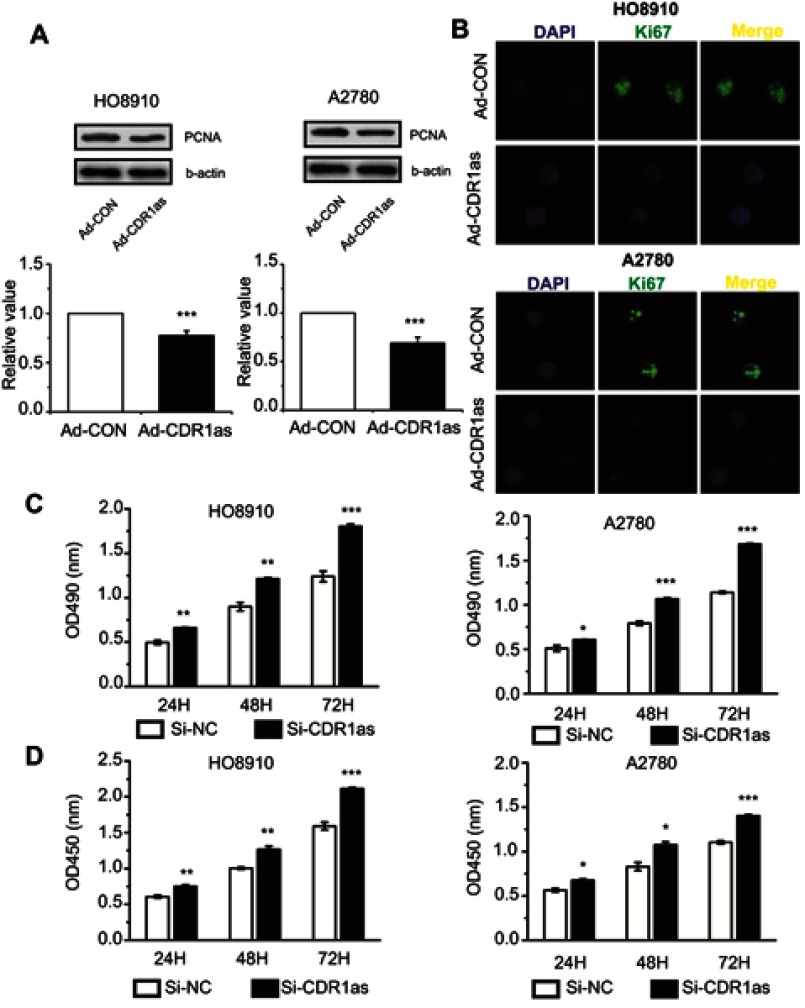
To explore the role of CDR1as in ovarian cancer, we determined the proliferation, invasion and migration of ovarian cancer cells after silencing or overexpressing CDR1as. The Western blot assay results showed that PCNA expression was reduced after CDR1as overexpression (Figure 2A). Consistent results were also found in the Ki67 detection (Figure 2B). The MTT and CCK8 assay results showed that the proliferation capacity of ovarian cancer cells was promoted after CDR1as silencing (Figure 2C and D). These data suggest that CDR1as may inhibit the proliferation of ovarian cancer cells.
Figure 2.
CDR1as inhibits the proliferation of ovarian cancer cells. (A) PCNA protein expression levels were analyzed by Western blot. (B) Ki67 expression was estimated in ovarian cancer cells after staining for Ki67; the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Magnification, ×400. (C) The proliferation of ovarian cancer cells was assessed by MTT assay. (D) The proliferation rate of ovarian cancer cells was measured by CCK8 assay. All values are expressed as the mean ± SEM (*P<0.05, **P<0.01, and ***P<0.001).
CDR1as inhibits the invasion and migration of ovarian cancer cells
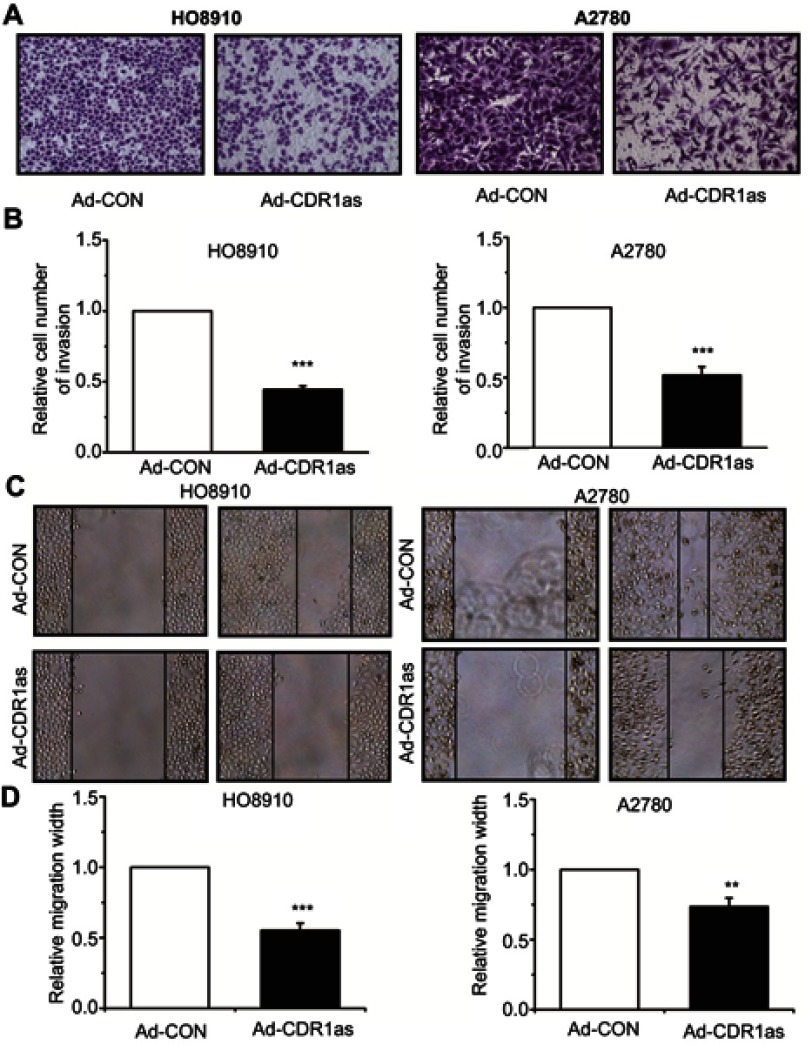
To explore whether CDR1as is involved in regulating the invasion and migration of ovarian cancer cells, we performed Transwell® and scratch-wound assays. Transwell® assay results showed that the invasion ability of ovarian cancer cells was inhibited after CDR1as overexpression (Figure 3A and B). Consistent results were also found in the scratch-wound assay. The scratch-wound assay results showed that the migration ability of ovarian cancer cells was inhibited after CDR1as overexpression (Figure 3C and D).
Figure 3.
CDR1as inhibits the invasion and migration of ovarian cancer cells. (A) and (B) The invasion ability of ovarian cancer cells was measured by Transwell® assay. Magnification, ×100. (C) and (D) The migration ability of ovarian cancer cells was detected by scratch-wound assay. Magnification, ×100. All values are expressed as the mean ± SEM (**P<0.01 and ***P<0.001).
MiR-135b-5p promotes the proliferation of ovarian cancer cells
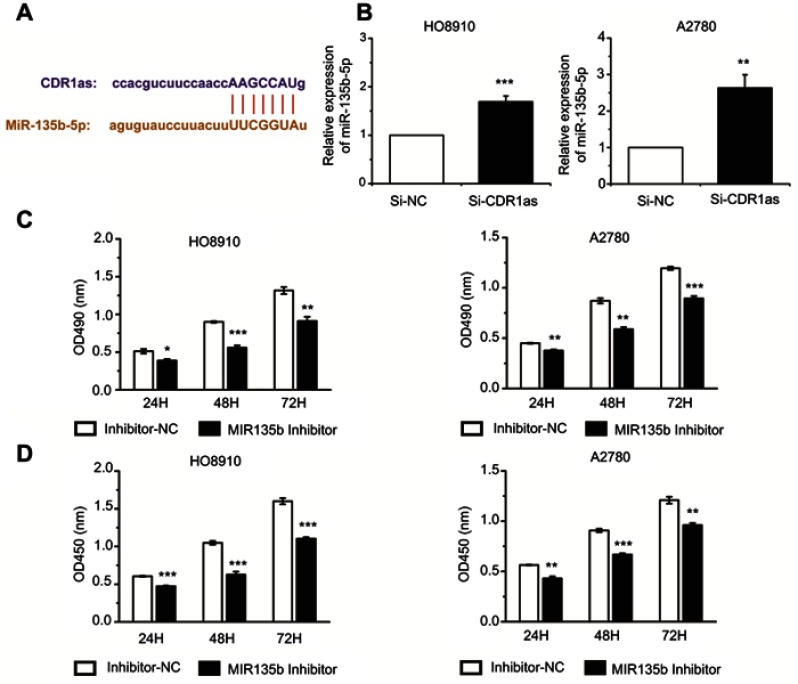
To investigate the role of CDR1as in ovarian cancer, we predicted which miRNA was associated with CDR1as using a bioinformatics database (http://starbase.sysu.edu.cn). We found that CDR1as may interact with miR-135b-5p (Figure 4A). To further confirm the correlation between CDR1as and miR-135b-5p, we inhibited the expression of CDR1as in ovarian cancer cells. The qRT-PCR assay results showed that silencing CDR1as can increase miR-135b-5p expression (Figure 4B). To study the role of miR-135b-5p in ovarian cancer cells, we inhibited miR-135b-5p expression in ovarian cancer cells using a miR-135b-5p inhibitor. Using MTT and CCK8 assays, we found that the proliferation ability of ovarian cancer cells was suppressed when miR-135b-5p expression was inhibited (Figure 4C and D).
Figure 4.
MiR-135b-5p promotes the proliferation of ovarian cancer cells. (A) The interaction between CDR1as and miR-135b-5p was predicted by a bioinformatics database. (B) The miR-135b-5p expression level was analyzed by qRT-PCR. (C) The proliferation of ovarian cancer cells was measured by MTT assay. (D) The proliferation rate of ovarian cancer cells was detected by CCK8 assay. All values are expressed as the mean ± SEM (*P<0.05, **P<0.01, and ***P<0.001).
HIF1AN expression was positively correlated with CDR1as and negatively correlated with miR-135b-5p
Through the bioinformatics database (www.targetscan.org), we found that HIF1AN may be a target gene of miR-135b-5p (Figure 5A). HIF1AN is an inhibitor of HIF-1α, which plays a role in inhibiting tumors in the progression of cancer. The qRT-PCR assay results showed that the miR-135b-5p inhibitor increased the mRNA expression of HIF1AN (Figure 5B) and that silencing CDR1as reduced the mRNA expression of HIF1AN (Figure 5C). The Western blot and qPCR results were similar. The protein expression level of HIF1AN was positively correlated with that of CDR1as and negatively correlated with that of miR-135b-5p (Figure 5D and E).
Figure 5.
HIF1AN expression was positively correlated with CDR1as and negatively correlated with miR-135b-5p. (A) HIF1AN was identified as a potential target gene of miR-135b-5p by bioinformatics analysis (TargetScanHuman database). (B) and (C) HIF1AN mRNA expression levels were analyzed by qRT-PCR. (D) and (E) HIF1AN protein expression levels were analyzed by Western blotting. All values are expressed as the mean ± SEM (*P<0.05, **P<0.01, and ***P<0.001).
CDR1as functions by targeting miR-135b-5p and promoting HIF1AN in ovarian cancer cells
To validate that CDR1as plays a role in ovarian cancer cells by inhibiting miR-135b-5p and promoting the expression of HIF1AN, we inhibited CDR1as and miR-135b-5p expression in ovarian cancer cells. The qRT-PCR and Western blot results revealed that a miR-135b-5p inhibitor blocked the downregulation of HIF1AN mRNA and protein after CDR1as silencing in ovarian cancer cells (Figure 6A and B). The MTT and CCK8 assay results showed that miR-135b-5p knockdown could significantly decrease the CDR1as silencing-mediated promotion of HO8910 and A2780 cell proliferation (Figure 6C and D). In summary, CDR1as, acting as a sponge of miR-135b-5p, inhibited miR-135b-5p and increased the expression of HIF1AN, thus playing a role in tumor inhibition in ovarian cancer.
Figure 6.
CDR1as targeted miR-135b-5p and promoted HIF1AN in ovarian cancer cells. (A) HIF1AN mRNA expression levels were analyzed by qRT-PCR assay. (B) HIF1AN protein expression levels were analyzed by Western blotting. (C) The proliferation of ovarian cancer cells was measured by MTT assay. (D) The proliferation ratio of ovarian cancer cells was measured by CCK8 assay. All values are expressed as the mean ± SEM (*P<0.05, **P<0.01, and ***P<0.001).
Discussion
Previous studies have shown that circRNAs have many important biological functions. First, circRNAs act as sponges of miRNAs to regulate the function of miRNA target genes.16,17 Second, they directly regulate the level of other RNAs through complementary base-pairing.18 Third, circRNAs can regulate the activity of proteins by binding to them directly.19 Finally, a small number of circRNAs can also be translated into a template to guide protein synthesis.20,21 Therefore, circRNAs play an important regulatory role in organisms by regulating the expression of different genes, and how this function relates to the progression of human cancer has attracted extensive attention. In the present study, we assessed the function and mechanism of CDR1as, a circRNA, in the progression of ovarian cancer.
Previous studies have shown that CDR1as can exert cancer-promoting effects in the progression of various types of cancer in humans. The expression level of CDR1as is increased in esophageal cancer and is closely related to the overall survival (OS) of patients. CDR1as can increase the expression of MMP2, KLF-4 and HOXB13 by inhibiting miR-7, thus leading to increased proliferation, invasion and migration in esophageal cancer cells.9,22 CDR1as expression in the tissues and cells of lung cancer is also increased. Numerous clinical studies have shown that the expression level of CDR1as is positively correlated with tumor size, lymph node metastasis, TNM stage and poor clinical outcome in patients, and it is an independent prognostic factor for patients with lung cancer. CDR1as acts as a ceRNA of miR-7 to up-regulate EGFR, CCNE1, PIK3CD and RELA, thus promoting proliferation, migration and invasion and inhibiting apoptosis in lung cancer cells.23–25 However, the expression and function of CDR1as in the development of ovarian cancer remain unclear. Our study revealed that the expression level of CDR1as is decreased in ovarian cancer tissues. Moreover, CDR1as overexpression can inhibit the proliferation, invasion and migration of ovarian cancer cells. We demonstrated for the first time that CDR1as functions as a tumor suppressor.
MiRNAs have been identified to interact with different genes to regulate the progression of various cancers.26,27 Valeri et al revealed that miR-135b is a downstream effector of the PTEN/PI3K pathway that leads to increased proliferation and invasion and decreased apoptosis in colorectal cancer (CRC) mouse models.28 In nonsmall cell lung cancer, miR-135b targets multiple key components of the Hippo pathway and LZTS1, thereby enhancing cancer cell invasion and migration in vitro and promoting cancer cell metastasis in vivo.29 In our study, miR-135b-5p promoted the proliferation of ovarian cancer cells by inhibiting the expression of HIF1AN.
A previous study showed that HIF1AN hydroxylates HIF-1α at its asparagine residues, thereby inhibiting its activity by blocking the recruitment of p300/CBP coactivators.30 Thus, HIF1AN can play a tumor suppressive role in human prostate cancer, colon cancer, breast cancer and other types of cancer.31–33 Our study found that miR-135b-5p can promote ovarian cancer cell proliferation by inhibiting the expression of HIF1AN. Our study suggests that CDR1as inhibits the function of ovarian cancer cells by acting as a ceRNA of miR-135b-5p. However, we cannot rule out that CDR1as can also regulate ovarian cancer progression through other mechanisms. Other mechanisms involving CDR1as in ovarian cancer need further study, and we will continue to investigate this possibility in future studies.
Conclusion
In summary, we detected that CDR1as expression is significantly decreased in ovarian tissues and cells and that CDR1as overexpression can inhibit the proliferation, invasion and migration of ovarian cancer cells. CDR1as functions as a tumor suppressor by sponging miR-135b-5p, which exerts a cancer-promoting effect by targeting HIF1AN in ovarian cancer. These findings provide new information for the diagnosis and treatment of ovarian cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/S0140-6736(13)62146-7 [DOI] [PubMed] [Google Scholar]
- 3.Lu KH. Screening for ovarian cancer in asymptomatic women. JAMA. 2018;319(6):557–558. doi: 10.1001/jama.2017.21894 [DOI] [PubMed] [Google Scholar]
- 4.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular rna molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo L, Salzman J. Detecting circular rnas: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17(11):679–692. doi: 10.1038/nrg.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo JN, Cieslik M, Zhang Y, et al. The landscape of circular rna in cancer. Cell. 2019;176(4):869–881 e13. doi: 10.1016/j.cell.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Huang V, Xu X, et al. Widespread and functional rna circularization in localized prostate cancer. Cell. 2019;176(4):831–843e822. doi: 10.1016/j.cell.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M. The circular rna cirs-7 (cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(1):17–27. doi: 10.1007/s00432-016-2256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RC, Ke S, Meng FK, et al. Cirs-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of mir-7/hoxb13. Cell Death Dis. 2018;9(8):838. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Hu H, Zhao Y, Zhao Y. Cdr1as is overexpressed in laryngeal squamous cell carcinoma to promote the tumour‘s progression via mir-7 signals. Cell Prolif. 2018;51(6):e12521. doi: 10.1111/cpr.2018.51.issue-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. Micrornaome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311–336. doi: 10.3322/caac.21244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L, Luo S, Ren X, et al. Mir-182 and mir-135b mediate the tumorigenesis and invasiveness of colorectal cancer cells via targeting st6galnac2 and pi3k/akt pathway. Dig Dis Sci. 2017;62(12):3447–3459. doi: 10.1007/s10620-017-4755-z [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Wang Q, Zhu XH. Mir-135b is a novel oncogenic factor in cutaneous melanoma by targeting lats2. Melanoma Res. 2019;29(2):119–125. doi: 10.1097/CMR.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Shin SH, Yoon H, et al. Fih is an oxygen sensor in ovarian cancer for g9a/glp-driven epigenetic regulation of metastasis-related genes. Cancer Res. 2018;78(5):1184–1199. doi: 10.1158/0008-5472.CAN-17-2506 [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Liu Y, Yan L, et al. Increased levels of the long noncoding rna, hoxa-as3, promote proliferation of a549 cells. Cell Death Dis. 2018;9(6):707. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen TB, Jensen TI, Clausen BH, et al. Natural rna circles function as efficient microrna sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- 17.Han D, Li J, Wang H, et al. Circular rna circmto1 acts as the sponge of microrna-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164. doi: 10.1002/hep.29270 [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Zhao K, Xu X, et al. A peptide encoded by circular form of linc-pint suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9(1):4475. doi: 10.1038/s41467-018-06862-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holdt LM, Stahringer A, Sass K, et al. Circular non-coding rna anril modulates ribosomal rna maturation and atherosclerosis in humans. Nature Communications. 2016;7:12429. doi: 10.1038/ncomms12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Fan X, Mao M, et al. Extensive translation of circular rnas driven by n(6)-methyladenosine. Cell Res. 2017;27(5):626–641. doi: 10.1038/cr.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legnini I, Di Timoteo G, Rossi F, et al. Circ-znf609 is a circular rna that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37 e29. doi: 10.1016/j.molcel.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Wei L, Qin T, Yang N, Li Z, Xu Z. Circular rna cirs-7 triggers the migration and invasion of esophageal squamous cell carcinoma via mir-7/klf4 and nf-kappab signals. Cancer Biol Ther. 2019;20(1):73–80. doi: 10.1080/15384047.2018.1507254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su C, Han Y, Zhang H, et al. Cirs-7 targeting mir-7 modulates the progression of non-small cell lung cancer in a manner dependent on nf-kappab signalling. J Cell Mol Med. 2018;22(6):3097–3107. doi: 10.1111/jcmm.13587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Yang D, Wei Y. Overexpressed cdr1as functions as an oncogene to promote the tumor progression via mir-7 in non-small-cell lung cancer. Onco Targets Ther. 2018;11:3979–3987. doi: 10.2147/OTT.S158316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan B, Zhang W, Mao XW, Jiang LY. Circular rna cirs-7 correlates with advance disease and poor prognosis, and its down-regulation inhibits cells proliferation while induces cells apoptosis in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2018;22(24):8712–8721. doi: 10.26355/eurrev_201812_16636 [DOI] [PubMed] [Google Scholar]
- 26.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60(6):376–392. doi: 10.3322/caac.20085 [DOI] [PubMed] [Google Scholar]
- 27.Rupaimoole R, Slack FJ. Microrna therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- 28.Valeri N, Braconi C, Gasparini P, et al. Microrna-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 2014;25(4):469–483. doi: 10.1016/j.ccr.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CW, Chang YL, Chang YC, et al. Microrna-135b promotes lung cancer metastasis by regulating multiple targets in the hippo pathway and lzts1. Nat Commun. 2013;4:1877. doi: 10.1038/ncomms2876 [DOI] [PubMed] [Google Scholar]
- 30.Mahon PC, Hirota K, Semenza GL. Fih-1: a novel protein that interacts with hif-1alpha and vhl to mediate repression of hif-1 transcriptional activity. Genes Dev. 2001;15(20):2675–2686. doi: 10.1101/gad.924501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Zhang D, Wang X, et al. Hypoxia-inducible mir-182 enhances hif1alpha signaling via targeting phd2 and fih1 in prostate cancer. Sci Rep. 2015;5:12495. doi: 10.1038/srep12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roscigno G, Puoti I, Giordano I, et al. Mir-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget. 2017;8(12):19507–19521. doi: 10.18632/oncotarget.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Yao LQ, Shi Q, et al. Microrna-31 contributes to colorectal cancer development by targeting factor inhibiting hif-1alpha (fih-1). Cancer Biol Ther. 2014;15(5):516–523. doi: 10.4161/cbt.28017 [DOI] [PMC free article] [PubMed] [Google Scholar]