Abstract
Background and aims
Human mutL homologl (MLH1) works coordinately in sequential steps to initiate repair of DNA mismatches, and aberrant MLH1 expression is related to spermatogenetic malfunction. In the present study, MLH1 expression in patients with azoospermia was investigated, and moderating effects of miR‐188‐3p on MLH1 expression and spermatogenesis were identified.
Methods
Testicular tissues from 16 patients with obstructive azoospermia (OA) and non‐obstructive azoospermia (NOA), and tissues of eight healthy patients were collected. Real‐time PCR, Western blotting and immunohistochemical staining were used to detect MLH1 expression. Chromatin immunoprecipitation assay and luciferase reporter assay were performed to evaluate histone acetylation level of miR‐188‐3p and relationships between miR‐188‐3p and MLH1.
Results
Testicular MLH1 expression at mRNA and protein levels was significantly increased, while miR‐188‐3p expression was lower in patients with OA and NOA than that in controls. Reduced histone acetylation level of miR‐188‐3p promoter was observed in patients with azoospermia. Overexpression/inhibition of HDAC1, but not HDAC2, contributed to the significant reduction/increase of miR‐188‐3p expression. miR‐188‐3p targeted 3′ UTR of MLH1 and regulated MLH1 expression. miR‐188‐3p inhibitor led to elevation of apoptotic level of spermatogenic cells in mice, while this effect was reversed by si‐MLH1.
Conclusion
Down‐regulation of miR‐188‐3p by reducing histone acetylation up‐regulated MLH1 expression and contributed to promotion of apoptosis in spermatogenic cells, in patients with azoospermia.
1. Introduction
About 15–20% of couples of childbearing age in China suffer from infertility, with male‐factor infertility accounting for 50% and female‐factor infertility accounting for 50% respectively. The incidence of infertility is increasing, and it has become a global health problem. Azoospermia, the medical condition of a man not having any measurable level of spermatozoa in his semen, is a very important contributor to male infertility. In recent years, many new techniques, including spermatogonial stem cell transplantation, testis tissue transplantation, induced pluripotent stem cell and gene therapy, have emerged. Testicular sperm extraction technology and intracytoplasmic sperm injection (ICSI) have also been improved, increasing the chances for men to father a child naturally.1 However, there remains no radical solution for male infertility. Clarifying the molecular mechanisms of azoospermia and spermatogenic failure holds the strongest promise for the successful treatment of male infertility.
Meiosis drives the differentiation of spermatogonia into spermatozoa. The process of synaptonemal complex (SC) formation is very critical in meiosis and determines its success or failure.2 Studies have confirmed that the disorder of meiosis and meiotic recombination defects can lead to the male aspermia and result in infertility.3 Human mutL homolog l (MLH1) is one component of DNA mismatch repair (MMR) proteins that work coordinately in sequential steps to initiate repair of DNA mismatches. In addition, antibodies against MLH1 are used to identify recombination sites in SCs. It has been established that the location and number of MLH1 foci are important indicators for meiotic recombination.4, 5 Aberrant numbers and distribution of MLH1 foci are observed in the spermatocyte and spermatozoa of patients with azoospermia.6, 7 However, few and limited studies have established the determination of testicular MLH1 expression in male infertility. The role of MLH1 expression in spermatogenesis and its potential molecular mechanisms also remain to be fully illustrated.
MicroRNA (miRNA) is a kind of non‐coding RNA that participates in the regulation of gene expression through translational repression, mRNA cleavage and deadenylation. miRNA interacts with its mRNA targets by base pairing and down‐regulated mRNA expression.8 Emerging evidence has demonstrated that miRNAs play an important role in various human diseases and physiological processes.9, 10 More than one hundred miRNAs have been identified in mammalian testes.11, 12 It has been indicated that miRNA expression of spermatogenic cells is altered in different developmental stages, suggesting that miRNAs may mediate the development of spermatogenic cells and contribute to the spermatogenesis and to male infertility. The expression of miR‐188‐3p is enriched in human testis‐specific cells, including the spermatogonium and spermatocyte, and it can also be detected in the meiosis phase of mice cells.13 As reported in the TargetScan and MicroCosm database, MLH1 is a potential target of miR‐188‐3p. However, the role of miR‐188‐3p in MLH1 regulation and spermatogenesis is not clear. In the present study, the expression of MLH1 and miR‐188‐3p in testicular spermatogenic cells in patients with azoospermia were investigated and the relationship between these two molecules was also discussed.
2. Materials and methods
2.1. Subjects
In this study, eight healthy testicular tissues were collected from subjects with normal spermatogenic function who were undergoing surgical removal of testicular tissue for prostate cancer. A total of 16 patients with azoospermia were also included in this study. Eight patients were diagnosed as having obstructive azoospermia (OA), and eight patients were diagnosed as having non‐obstructive azoospermia (NOA). Testicular tissues were collected from azoospermia patients undergoing testicular biopsy or ICSI. Informed consent was obtained from all participants, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and carried out in accordance with the guideline of the ethical management.
2.2. Cell culture
Human malignant pluripotent embryonal carcinoma cell line NTERA‐2 (also known as NT‐2 cells) was purchased from ATCC (CRL‐1973, USA) and cultured in the Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum at 37°C with 5% atmosphere. When cells grew to 75% confluence, cells were treated with trypsin and used for the following experiments. Experiment one: inoculated cells with the histone deacetylase inhibitor Trichostatin A (TSA) at different concentrations (0, 10, 100 and 200 nmol/L) were plated in a 24‐well plate for 48 hours. The cells were collected and miR‐188‐3p expression level was detected by real‐time PCR. Experiment two: cells were plated in a 12‐well plate and cultured with DMEM containing no penicillin–streptomycin. Cells were transfected with overexpression vectors (Ad‐HDAC1 and Ad‐HDAC2) or RNA interference vectors (si‐HDAC1 and si‐HDAC2), with Ad‐GFP and siNC acting as controls, respectively, using Lipofectamine2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Overexpression vectors and RNA interference vectors were produced by Shanghai Sangon Biological Engineering Co., Ltd (Shanghai, China).
2.3. Animals and treatment
Male Kunming mice (20–25 g), purchased from Laboratory Animal Centre of Henan Province (Henan, China) at the age of 8 weeks, were used in this study. Animal experiments were conducted by the Guide for the Care and Use of Laboratory Animals and approved by the animal experimental ethics committee of First Affiliated Hospital of Zhengzhou University. Mice were randomly assigned in six groups. Group 1 received the injection of miR‐188‐3p inhibitor negative control (NC) in testis. Group 2 received the injection of miR‐188‐3p inhibitor. Group 3 received the injection of miR‐188‐3p inhibitor and siNC. Group 4 received the injection of miR‐188‐3p inhibitor and si‐MLH1. Group 5 received the injection of Ad‐GFP. Group 6 received the injection of Ad‐MLH1. For injection, the overexpressing/inhibiting sequences were constructed into lentiviral, and about 2–10 μL of PBS containing concentrated lentiviral/ adenovirus particles was introduced into the seminiferous tubules of mice. After 72 hours following the injections, the spermatogenic cells of testicular tissue were isolated and used to detect the expression of MLH1 expression and the apoptosis rate.
2.4. Immunohistochemical staining
The conventional paraffin section and immunohistochemical staining were performed to detect the expression of MLH1 in spermatogenic cells of testicular tissue from healthy subjects and from patients with azoospermia.
2.5. Real‐time PCR
TRIzol reagent (Invitrogen) was used to extract the total RNA from spermatogenic cells of testicular tissue and NT‐2 cells according to the manufacturer's instructions. Complementary DNA (cDNA) was reverse‐transcribed by a PrimeScript RT reagent Kit (TaKaRa, Otsu, Japan). Then, the real‐time PCR was performed on the ABI 7500HT Fast Real‐Time PCR System using QuantiNova SYBR Green PCR (Qiagen, Hilden, Germany) for the quantification of MLH1 and miR‐188‐3p expression. The specific primers are shown in Table 1. The relative expression of MLH1 and miR‐188‐3p were calculated with 2‐△△Ct, with GAPDH and U6 acting as internal controls.
Table 1.
The sequences of primers
| Gene | Sequence | |
|---|---|---|
| MLH1 | Forward | 5′‐TTTGGAAGTTGTTGGCAGGT‐3′ |
| Reverse | 5′‐GGTTGAGGCATTGGGTAGTG‐3′ | |
| miR‐188‐3p | Forward | 5′‐ATTATTGGCTCCCACATGCAGGG‐3′ |
| Reverse | 5′‐ATCCAGTGCAGGGTCCGAGG‐3′ | |
| GAPDH | Forward | 5′‐TGTGTCCGTCGTGGATCTGA‐3′ |
| Reverse | 5′‐CCTGCTTCACCACCTTCTTGA‐3′ | |
| U6 | Forward | 5′‐GCTTCGGCAGCACATATACTAA‐3′ |
| Reverse | 5′‐AACGCTTCACGAATTTGCGT‐3′ | |
| CLCN5 | Forward | 5′‐CAGTGAGACAGTAGCCAGTC‐3′ |
| Reverse | 5′‐CACCTGTACCTTCCATGCAT‐3′ |
2.6. Western blot
Total protein was extracted from spermatogenic cells of testicular tissue and NTERA‐2 cells using a lysis buffer containing 1% protease inhibition. After centrifugation, the supernatant was kept and used to detect the protein concentration using the bull serum albumin (BSA) method. The equal amount of protein in each sample was electrophoresed on 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to a nitrocellulose membrane. After blocking with non‐fat dry 5% milk in Tris‐HCl‐buffered saline, the membrane was incubated with anti‐MLH1 antibody (1:1000; Abcam, Cambridge, UK), anti‐cleaved‐caspase‐3 antibody (1:1000; Abcam) and anti‐cleaved‐PARP1 antibody (1:1000; Pierce, Rockford, IL, USA) overnight at 4°C, and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) (1:1000; Abcam) for 2 hours at room temperature. The bands were visualized by the ECL method. The relative protein expression of MLH1 was normalized with β‐actin.
2.7. Chromatin immunoprecipitation assay
The histone acetylation of miR‐188‐3p promoter was assessed by a chromatin immunoprecipitation (ChIP) Assay Kit (Upstate Biotechnology, Lake Placid, NY, USA) according to the manufacturer's instructions. In brief, we harvested NTERA‐2 cells and fixed them with 1% (v/v) formaldehyde at room temperature for 10 minutes to cross‐link the protein‐DNA complexes. After being washed with ice‐cold PBS containing protease inhibitors, the cells were re‐suspended in a ChIP lysis buffer containing 1% SDS. The lysates were sonicated to shear DNA fragments to lengths from 200 to 1000 bp. The chromatin was then immunoprecipitated with an equal amount of antibodies against histone H3, histone H4, or control IgG overnight at 4°C. Protein‐DNA‐antibody complexes were precipitated with protein A‐agarose beads for 2 hours at 4°C. Input or DNA in the complex was subjected to quantitative PCR. CLCN5 is the host gene of miR‐188‐3p. In this study, CLCN5 promoter (from −2000 to +1) was used, and the sequences are listed in Table 1.
2.8. Luciferase reporter assay
As reported in the TargetScan and MicroCosm database, MLH1 is a potential target of miR‐188‐3p. Luciferase reporter assay was used to confirm the relationship between MLH1 and miR‐188‐3p. The luciferase cDNA fuse with the 3′‐UTR of MLH1 in wild type (WT) and mutant type (MU) was amplified and cloned into plasmid to construct the luciferase reporter plasmid. When cell grew to 70% confluence, miR‐188‐3p mimic (or miR‐188‐3p inhibitor) and recombinant plasmid were co‐transfected into cells with Lipofectamine2000 (Invitrogen) following the manufacturer's instructions. Twenty‐four hours after transfection, Dual‐Luciferase Reporter Assay (Promega, Madison, WI, USA) was used to detect the relative luciferase activity of MLH1 3′UTR.
2.9. Flow cytometry
The apoptosis rate of spermatogenic cells was determined by flow cytometry with Annexin V/PI staining. In brief, we adjusted the cell concentration to 1×106/mL and centrifuged at 1000 g for 5 minutes. We discarded the supernatant, washed cells with PBS buffer, and centrifuged at 1000 g for 5 minutes. After repeating this step twice, we re‐suspended the cells with 100 μL binding buffer and added 20 μL Annexin V‐FITC (20 μg/mL) for 15 minutes on ice in the dark. We transferred the mixture to test tube and added with 400 μL PBS buffer. The 1 μL PI (50 μg/mL) was then incubated with the mixture, and we immediately performed the determination with flow cytometry. The apoptosis rate was calculated with the software.
2.10. Statistical analysis
All statistical analyses were conducted with statistical software spss 18.0. All data were expressed as mean ± SD. A one‐way ANOVA with Turkey's multiple comparison tests was used to analyse the mean with more than two groups. Differences between the two groups were analysed by an independent Student's t‐test. The P value <.05 was considered a significant difference.
3. Results
3.1. Increased testicular MLH1 expression in patients with azoospermia
The MLH1 expression was first investigated in the testicular spermatogenic cells of patients with azoospermia. As shown in Fig. 1a,b, the MLH1 expression in mRNA and protein levels were significantly elevated in NOA and OA samples than that in control ones (P<.05). Immunohistochemical results confirmed that testicular spermatogenic cells in NOA and OA patients had higher MLH1 expression than that in the control group (P<.05, Fig. 1c). The MLH1 expression levels in NOA and OA were also compared. These results demonstrated that there was no statistical difference in MLH1 expression between NOA group and OA group.
Figure 1.
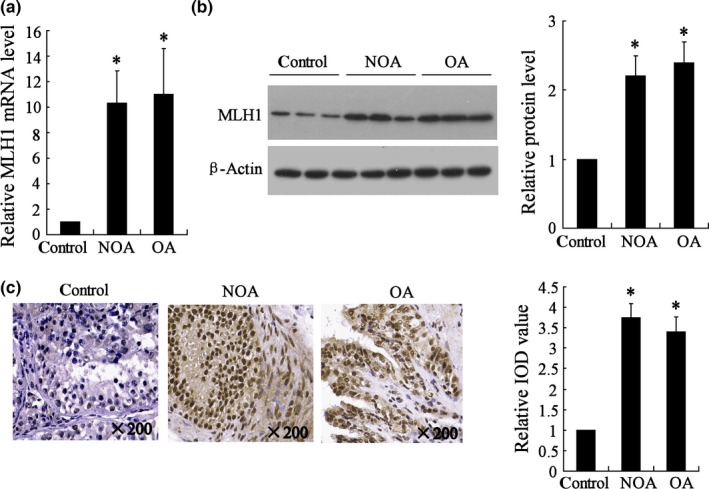
MLH1 expression in testicular spermatogenic cells of healthy subjects and patients with azoospermia. NOA (n=8), non‐obstructive azoospermia; OA (n=8), obstructive azoospermia; IOD, integrated optical density. (a) MLH1 mRNA expression detected by real‐time PCR; (b) MLH1 protein expression detected by Western blot; (c) Immunohistochemical staining measured the MLH1 expression. * vs control, P<.05
3.2. Decreased testicular miR‐188‐3p expression in patients with azoospermia
Previous study reported that miR‐188‐3p expression was enriched in spermatogonium and spermatocyte,13 and we found that MLH1 had the complementary sequences with miR‐188‐3p using bioinformatics soft. To illustrate the potential mechanism of the aberrant MLH1 in patients with azoospermia, the miR‐188‐3p expression was also evaluated. The results showed that miR‐188‐3p was markedly down‐regulated in testicular spermatogenic cells of NOA and OA patients (P<.01, Fig. 2), suggesting a potential role of miR‐188‐3p in spermatogenesis process.
Figure 2.
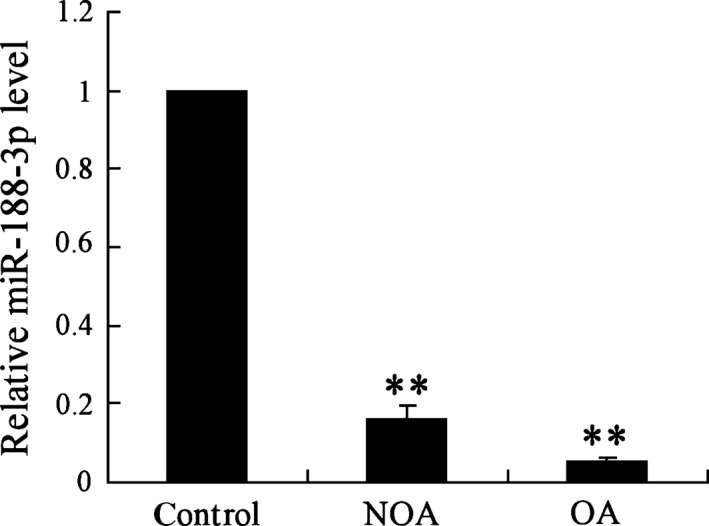
miR‐188‐3p expression in testicular spermatogenic cells of healthy subjects and patients with azoospermia. NOA (n=8), non‐obstructive azoospermia; OA (n=8), obstructive azoospermia. ** vs control, P<.01
3.3. Decreased histone acetylation level of miR‐188‐3p promoter in patients with azoospermia
Whether epigenetic modification of miR‐188‐3p promoter contributes to the decreased miR‐188‐3p expression was then investigated. It was observed that H3 and H4 histone acetylation levels of miR‐188‐3p promoter were significantly decreased in testicular spermatogenic cells of the NOA and OA groups (P<.01, Fig. 3a,b), indicating the alteration of histone acetylation might cause the decrease of miR‐188‐3p expression in azoospermia.
Figure 3.
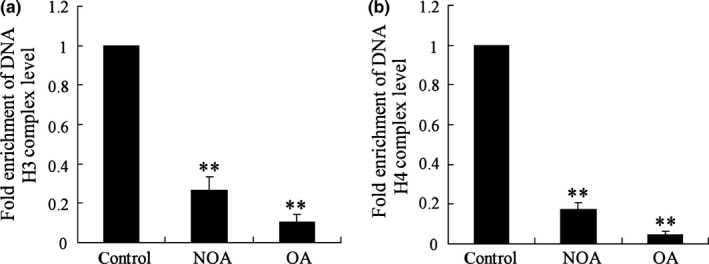
Histone acetylation level of miR‐188‐3p promoter in testicular spermatogenic cells of healthy subjects and patients with azoospermia. NOA (n=8), non‐obstructive azoospermia; OA (n=8): obstructive azoospermia. (a) H3 histone acetylation levels of miR‐188‐3p promoter was measured by ChIP assay; (b) H4 histone acetylation levels of miR‐188‐3p promoter was measured by ChIP assay. ** vs control, P<.01
3.4. HDAC1 contributes to the regulation of miR‐188‐3p expression
In vitro experiments were performed to further confirm whether histone acetylation participates in the alteration of miR‐188‐3p expression in azoospermia. It showed that the miR‐188‐3p expression level was increased more than 5‐fold by the incubation of histone deacetylase inhibitor TSA at the concentrations of 100 and 200 nmol/L (Fig. 4a). We then transfected Ad‐HDAC1/2 or si‐HDAC1/2 into NTERA‐2 cells for overexpressing or inhibiting HDAC1/2 expression (Fig. 4b). Overexpressed HDAC1, but not HDAC2, contributed to the significant decrease of H3 and H4 histone acetylation levels in miR‐188‐3p promoter, while the inhibition of HDAC1, but not HDAC2, led to their significant increase (Fig. 4c). In addition, only HDAC1 overexpression or inhibition caused the alteration of miR‐188‐3p expression (Fig. 4d).
Figure 4.
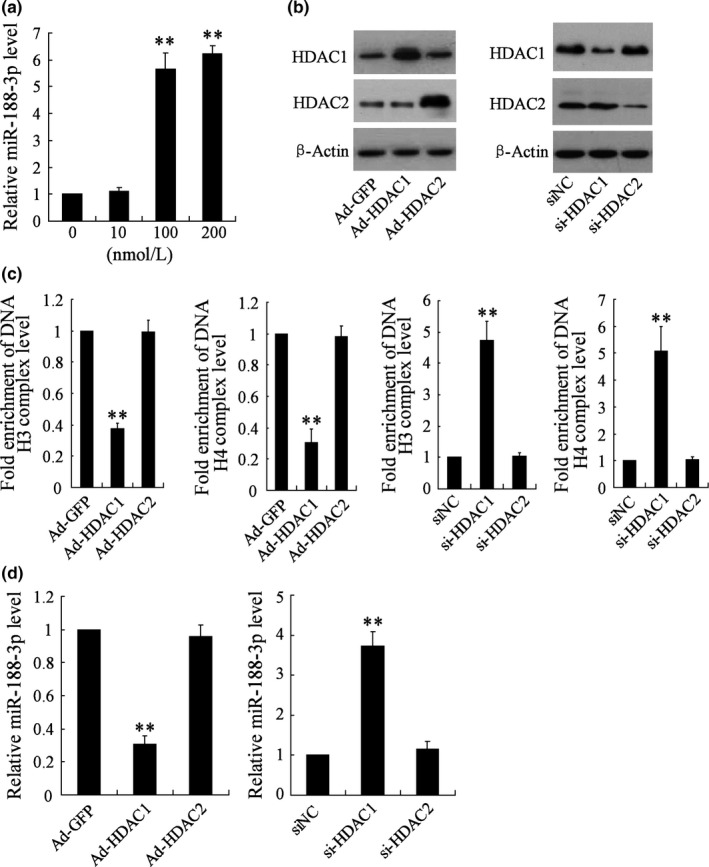
The effects of histone deacetylase inhibitor and histone deacetylases on the expression of miR‐188‐3p in NTERA‐2 cells. (a) The miR‐188‐3p expression was determined by real‐time PCR after the treatment of histone deacetylase inhibitor trichostatin A (TSA) at the different concentrations (0, 10, 100 and 200 nmol/L). NTERA‐2 cells were transfected with overexpression vectors (Ad‐HDAC1 and Ad‐HDAC2, with Ad‐GFP acting as control) or RNA interference vectors (si‐HDAC1 and si‐HDAC2, with siNC acting as control), then (b) the HDAC1 and HDAC2 protein expression was confirmed by Western blot, (c) H3 or H4 histone acetylation levels of miR‐188‐3p promoter were measured by ChIP assay, and (d) the miR‐188‐3p expression level was measured by real‐time PCR. **. vs group without the treatment of TSA, or Ad‐GFP, or siNC, P<.01
3.5. miR‐188‐3p targets MLH1 and regulates its expression
As reported in the TargetScan and MicroCosm database, MLH1 is a potential target of miR‐188‐3p (Fig. 5a). The relationship between miR‐188‐3p and MLH1 was then further discussed. The results showed that miR‐188‐3p mimic significantly decreased the 3′UTR luciferase activity of MLH1 and down‐regulated MLH1 expression in mRNA and protein levels (Fig. 5b). miR‐188‐3p inhibitor had the opposite effects (Fig. 5c). These experiments confirmed that miR‐188‐3p targets MLH1 and participates in regulating its expression.
Figure 5.
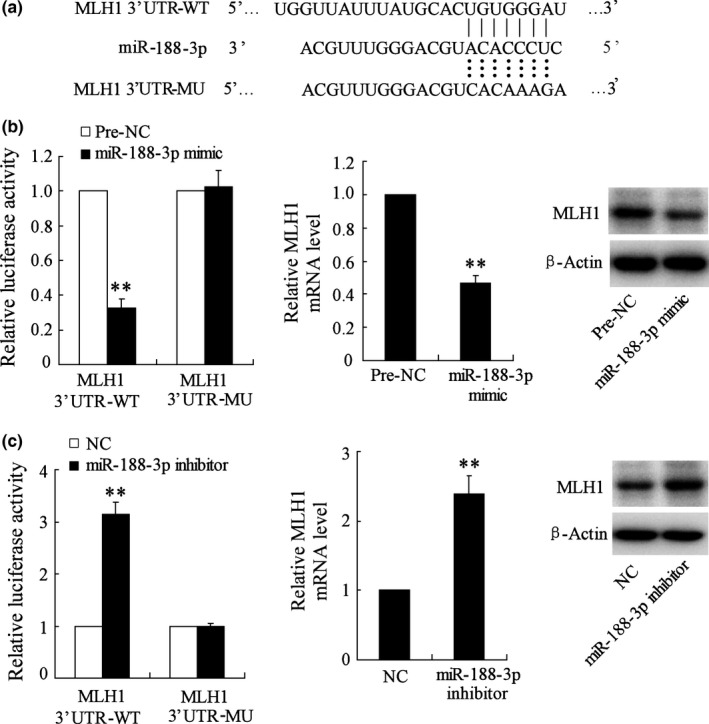
MLH1 is target gene of miR‐188‐3p. NC: negative control. (a) The binding site between MLH1 and miR‐188‐3p was reported in TargetScan and MicroCosm database. (b) The 3′UTR activity of MLH1 was detected by a luciferase reporter assay and MLH1 expression in mRNA and protein levels were determined by real‐time PCR and Western blot after the treatment of miR‐188‐3p mimic. (c) The 3′UTR activity of MLH1 and MLH1 expression in mRNA and protein levels were determined after the treatment of miR‐188‐3p inhibitor. **. vs pre‐NC or NC, P<.01
3.6. Down‐regulation of miR‐188‐3p increased MLH1 expression in vivo
To further investigate the role of miR‐188‐3p in spermatogenesis in vivo, miR‐188‐3p inhibitor was injected into testis of mice. Real‐time PCR confirmed that miR‐188‐3p expression was significantly inhibited by miR‐188‐3p inhibitor 72 hours after injection (Fig. 6a). The MLH1 protein expression was also evaluated, and the result showed that MLH1 expression was up‐regulated by miR‐188‐3p inhibitor in testicular tissue (Fig. 6b).
Figure 6.
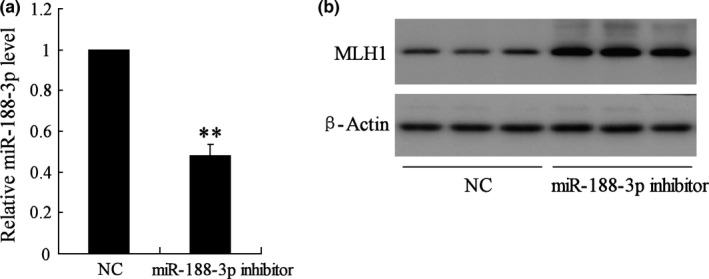
The effect of miR‐188‐3p on MLH1 expression in mice. NC, negative control. (a) The inhibitory effect on miR‐188‐3p expression induced by miR‐188‐3p inhibitor was confirmed by real‐time PCR. (b) The MLH1 protein expression was detected by Western blot. ** vs NC, P<.01
3.7. miR‐188‐3p affected spermatogenic cells apoptosis of mice through mediating MLH1
To further clarify the role of miR‐188‐3p in spermatogenic cells apoptosis in vivo, mouse experiments with the treatment of miR‐188‐3p inhibitor was then investigated. The results demonstrated that the cell apoptosis rate was significantly elevated by miR‐188‐3p inhibitor, suggesting miR‐188‐3p participates in the process of spermatogenic cells apoptosis. However, the increased apoptosis rate induced by miR‐188‐3p inhibitor was reversed by si‐MLH1 (Fig. 7a). We also observed that si‐MLH1 reversed the increased expression of apoptosis‐related proteins cleaved‐caspase‐3 and cleaved‐PARP1 induced by miR‐188‐3p inhibitor (Fig. 7b). The apoptosis of spermatogenic cells was also evaluated after the treatment of Ad‐MLH1, and the results found that Ad‐MLH1 contributed to the significant increase of apoptosis rate and cleaved‐caspase‐3 and cleaved‐PARP1 expression (Fig. 7c,d). These data indicate that miR‐188‐3p participates in modulating spermatogenic cells apoptosis, probably through regulation of MLH1 expression.
Figure 7.
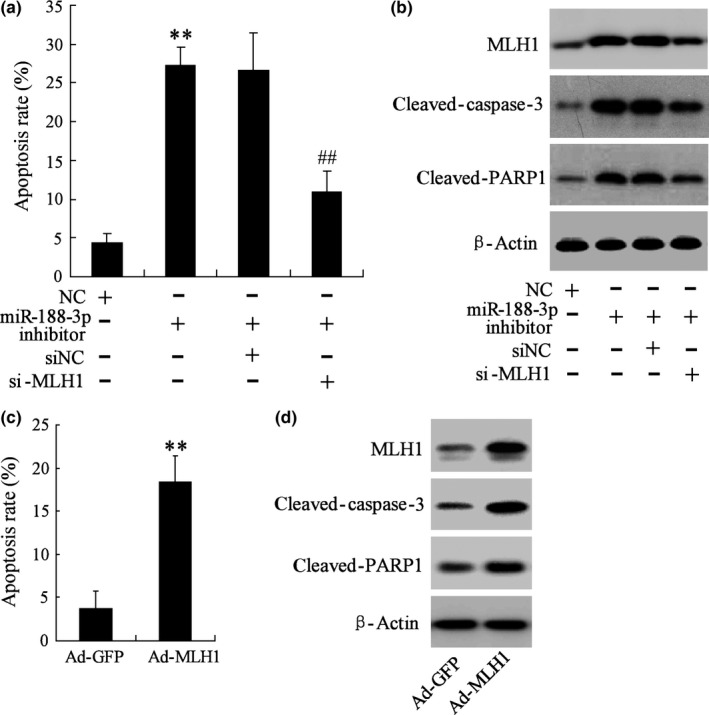
The effect of miR‐188‐3p on spermatogenic cells apoptosis in vivo. NC, negative control; siNC, siRNA‐negative control. (a, c) The apoptosis of spermatogenic cells was evaluated by flow cytometry. (b, d) The MLH1, cleaved‐caspase‐3 and cleaved‐PARP1 protein expression was detected by Western blot. ** vs NC, P<.01. ## vs miR‐188‐3p inhibitor + siNC, P<.01
4. Discussion
Azoospermia is identified in 15% of infertile men and can be classified as OA and NOA. Spermatogenesis is the process of producing spermatozoa from the primordial germ cells and occurs in the seminiferous epithelium of the testis seminiferous tubule.14 Spermatogenetic malfunction is one of the most important aetiologies of azoospermia. There are many factors that participate in the modulation of spermatogenesis, and gene regulation of spermatogenic cells plays a crucial role in this process. Several key genes related to spermatogenesis have been confirmed, including cAMP‐response element modulator (CREM), testis‐specific histone Hlt gene, heat shock protein (HSP) and MLH1.15, 16 MLH1 plays a very important role in DNA base pair mismatch during spermatogenesis. The MLH1 gene encodes MLH1 protein, which interacts with PMS2 and PMS1 proteins to form heterodimers MutLα and Mutlβ respectively. Mutlα can combine Muts from DNA and form a temporary compound, activating DNA MMR. The aberrant MLH1 may cause DNA base pair mismatch and lead to spermatogenetic malfunction.17, 18 Animal experiments with defective MLH1 shows that the process of spermatocyte meiosis is stopped and cannot enter into the pachytene stage, leading to the abnormal synapsis of homologous chromosomes and absence of spermatogenesis.19 No production of spermatozoa is observed in mice with the mutation of MLH1.20 Previous studies have shown that testicular MLH1 expression was decreased in patients with spermatogenetic malfunction.21 To our surprise, this study showed that the MLH1 expression in the mRNA level was significantly higher in patients with azoospermia than that noted in control subjects, with the NOA group having an even higher MLH1 expression level than that in the OA group. Western blot and immunohistochemical staining also demonstrated that the MLH1 protein level was higher in testicular spermatogenic cells of patients with azoospermia than in the control group, confirming our results were reliable. This study indicated that overexpression of MLH1 might be a critical factor for the spermatogenesis dysfunction. Although our result is inconsistent with prior studies, which report that MLH1 plays an important role in DNA MMR and that its expression is reduced in sterility, overexpression of MLH1 protein can form massive heterodimers MutLα with combining PMS2 and create excess temporary compounds, leading to the excessive DNA MMR during meiosis, contributing to the failure of meiosis.
miRNA has been widely considered to be a regulator of gene expression in various biological processes. In the present study, we found that testicular miR‐188‐3p expression, which is enriched in human testis‐specific cells, was lower in patients with azoospermia than in control subjects, suggesting miR‐188‐3p might be related to the process of spermatogenesis. However, no statistical difference of miR‐188‐3p expression levels was observed between NOA and OA groups. As reported in the TargetScan and MicroCosm database, miR‐188‐3p potentially targets MLH1, indicating that miR‐188‐3p participates in spermatogenesis probably through the regulation of MLH1 expression. The Luciferase reporter assay confirmed that miR‐188‐3p binds the 3′UTR of MLH1 and regulates MLH1 expression. In animal experiments, we found that MLH1 expression was up‐regulated by miR‐188‐3p inhibitor in testicular tissue. In addition, a significant elevation of spermatogenic cells apoptosis rate was also observed in mice with the treatment of miR‐188‐3p inhibitor, while this effect was reversed by down‐regulation of MLH1. Therefore, we proposed that miR‐188‐3p might play a critical role in spermatogenesis and the pathophysiology of azoospermia through mediating MLH1 expression. In a previous study, Xu et al.22 used an in vivo cross‐linking, and immunoprecipitation methods confirmed that miR‐188‐3p was a target of polypyrimidine tract‐binding protein (PTBP) 2 in mouse testis. PTBP2 is initially identified as an alternative splicing factor mainly expressed in neurons and testis. It has been found that PTBP2 stabilizes the germ cell‐specific phosphoglycerate kinase 2 mRNA by binding to a sequence in its 3′UTR.23 Therefore, PTBP2 is another potential pathway that is regulated by miR‐188‐3p and implicated in spermatogenesis.
The mechanism of miR‐188 expression alteration in azoospermia was also investigated in this study. The results showed that the decrease of H3 and H4 histone acetylation level in miR‐188‐3p promoter could explain the down‐regulation of miR‐188‐3p expression. Histone acetylation is one of the most common ways of gene regulation. In the nucleus, a dynamic balance of histone acetylation and histone deacetylation, which are typically catalysed by enzymes with histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities, respectively, participates in the regulation of gene expression. The elevation of the histone acetylation level commonly contributes to the up‐regulation of gene expression.24 It showed that the miR‐188‐3p expression level was increased by the incubation of TSA, a potent inhibitor of HDAC, at the concentrations of 100 and 200 nmol/L. Our further experiments confirmed that it was HDAC1 but not HDAC2 leading to the alteration of miR‐188‐3p expression. However, we did not investigate how HDAC1 is regulated in azoospermia. The physiological functions of miR‐188‐3p in other diseases have also been demonstrated. For example, miR‐188‐3p acts as a metastasis regulator in some cancers.25, 26 It is also involved in the processes of myocardial infarction, Alzheimer's disease, cardiac remodelling, dendritic plasticity and synaptic transmission.27, 28, 29, 30 However, the knowledge of the potential mechanisms of miR‐188‐3p in various physiological processes is limited and remains a topic requiring further studies.
In summary, the main findings of this study demonstrated that testicular MLH1 expression in mRNA and protein levels were significantly up‐regulated and miR‐188‐3p was significantly down‐regulated in patients with azoospermia. MiR‐188‐3p targeted 3′UTR of MLH1 and regulated its expression, contributing to the abnormal spermatogenic cells apoptosis and spermatogenesis. The role of miR‐188‐3p in regulating MLH1 expression during spermatogenesis helps clarify the pathology mechanism of azoospermia. More studies should be performed to identify the precise mechanisms and significance of miR‐188‐3p in spermatogenesis.
Disclosure of conflict of interest
None.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO. 31271605), the Municipal Science and Technology Bureau Project of Zhengzhou, China (NO. 340600531813) and the Tackle Key Problems in Science and Technology of Henan province (No.201503004).
References
- 1. Gassei K, Orwig KE. Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril. 2016;105:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraune J, Schramm S, Alsheimer M. The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res. 2012;318:1340–1346. [DOI] [PubMed] [Google Scholar]
- 3. Eggers S, DeBoer KD, Van den Bergen J, et al. Copy number variation associated with meiotic arrest in idiopathic male infertility. Fertil Steril. 2015;103:214–219. [DOI] [PubMed] [Google Scholar]
- 4. Gruhn JR, Nasser A, Rachael F, et al. Correlations between synaptic initiation and meiotic recombination: a study of humans and mice. Am J Human Genet. 2016;98:102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Codina‐Pascual M, Oliver‐Bonet M, Joaquima N, et al. Synapsis and meiotic recombination analyses: MLH1 focus in the XY pair as an indicator. Hum Reprod. 2005;20:2133–2139. [DOI] [PubMed] [Google Scholar]
- 6. Sun F, Mikhaail‐Philips M, Oliver‐Bonet M, et al. Reduced meiotic recombination on the XY bivalent is correlated with an increased incidence of sex chromosome aneuploidy in men with non‐obstructive azoospermia. Mol Hum Reprod. 2008;14:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vries M, Ramos L, de Boer P. Immunofluorescent characterization of meiotic recombination in human males with variable spermatogenesis. Andrology. 2013;1:262–273. [DOI] [PubMed] [Google Scholar]
- 8. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melo SA, Esteller M. Disruption of microRNA nuclear transport in human cancer. Semin Cancer Biol. 2014;27:46–51. [DOI] [PubMed] [Google Scholar]
- 10. Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11:276–288. [DOI] [PubMed] [Google Scholar]
- 11. Luo L, Lianzhi Y, Gang L, et al. Microarray‐based approach identifies differentially expressed microRNAs in porcine sexually immature and mature testes. PLoS ONE. 2010;5:e11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ro S, Chanjae P, Kenton M. Cloning and expression profiling of testis‐expressed microRNAs. Dev Biol. 2007;311:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcon E, Babak T, Chua G, Hughes T. miRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res. 2008;16:243–260. [DOI] [PubMed] [Google Scholar]
- 14. Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escalier D. Impact of genetic engineering on the understanding of spermatogenesis. Hum Reprod Update. 2001;7:191–210. [DOI] [PubMed] [Google Scholar]
- 16. Grootegoed JA, Siep M, Baarends WM. Molecular and cellular mechanisms in spermatogenesis. Best Pract Res Clin Endocrinol Metab. 2000;14:331–343. [DOI] [PubMed] [Google Scholar]
- 17. Cannavo E, Bertran G, Giancarlo M. Characterization of the interactome of the human MutL homologues MLH1, PMS1, and PMS2. J Biol Chem. 2007;282:2976–2986. [DOI] [PubMed] [Google Scholar]
- 18. Sijmons RH, Hofstra RM. Review: clinical aspects of hereditary DNA Mismatch repair gene mutations. DNA Repair. 2016;38:155–162. [DOI] [PubMed] [Google Scholar]
- 19. Cohen HR, Royce‐Tolland ME, Worringer KA. Chromatin modifications on the inactive X chromosome. Prog Mol Subcell Biol. 2005;38:91–122. [DOI] [PubMed] [Google Scholar]
- 20. Edelmann W, Paula EC, Michael K, et al. Meiotic pachytene arrest in MLH1‐deficient mice. Cell. 1996;85:1125–1134. [DOI] [PubMed] [Google Scholar]
- 21. Terribas E, Bonache S, García‐Arévalo M, et al. Changes in the expression profile of the meiosis‐involved mismatch repair genes in impaired human spermatogenesis. J Androl. 2010;31:346–357. [DOI] [PubMed] [Google Scholar]
- 22. Xu M, Hecht NB. Polypyrimidine tract‐binding protein 2 binds to selective, intronic messenger RNA and microRNA targets in the mouse testis. Biol Reprod. 2011;84:435–439. [DOI] [PubMed] [Google Scholar]
- 23. Xu M, Hecht NB. Polypyrimidine tract binding protein 2 stabilizes phosphoglycerate kinase 2 mRNA in murine male germ cells by binding to its 3'UTR. Biol Reprod. 2007;76:1025–1033. [DOI] [PubMed] [Google Scholar]
- 24. Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015;20:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zha R, Guo W, Zhang Z, et al. Genome‐wide screening identified that miR‐134 acts as a metastasis suppressor by targeting integrin β1 in hepatocellular carcinoma. PLoS ONE. 2014;9:e87665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu J, Lv Q, He J, et al. MicroRNA‐188 suppresses G1/S transition by targeting multiple cyclin/CDK complexes. Cell Commun Signal. 2014;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang K, Liu CY, Zhou LY, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR‐188‐3p. Nat Commun. 2015;6:6779. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Hu M, Teng ZQ, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2‐AG metabolism are through upregulation of microRNA‐188‐3p in a mouse model of Alzheimer's disease. J Neurosci. 2014;34:14919–14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee K, Kim JH, Kwon OB, et al. An activity‐regulated microRNA, miR‐188, controls dendritic plasticity and synaptic transmission by downregulating neuropilin‐2. J Neurosci. 2012;32:5678–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra PK, Tyagi N, Kundu S, Tyagi SC, et al. MicroRNAs are involved in homocysteine‐induced cardiac remodeling. Cell Biochem Biophys. 2009;55:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


