Abstract
Objectives
Cell migration is necessary for numerous physiological cell processes. Although either inflammatory or hypoxic stimuli of certain dose and duration have positive influence on cell migration, their combination has not been shown to result in a synergistic effect.
Materials and methods
In this study, we investigated combined effects of hypoxia and low‐dose inflammatory stimulus (one‐tenth of that of a previously used concentration) on migration of human bone marrow‐derived mesenchymal stem cells (BMMSCs).
Results
Our results from real‐time PCR, Western blot analysis and an immunofluorescence assay, showed that dual stimulation up‐regulated CXCR4 expression. Based on tablet scratch experimentation and transwell assay, the dual stimuli exhibited greater positive effects on cell migration than a single inflammatory or hypoxic stimulus. When effects of various pre‐treatments on cell proliferation, differentiation and immunosuppression were screened, cells subjected to the hypoxic stimulus or dual stimuli had increased cell proliferation, while short‐term inflammatory stimulus and/or hypoxic stimulus had no negative effect on cell differentiation and immunosuppression.
Conclusions
These findings suggest that the combination of hypoxia and low‐dose inflammatory stimuli enhances the potential of BMMSCs to migrate, thus identifying cell pre‐treatment conditions that could enhance future stem cell‐based therapeutics.
1. Introduction
The resident pools of mesenchymal stem cells (MSCs) in many tissues are responsible for wound healing and immunomodulation. Therefore, the goal of stem cell‐based therapies is to exploit these cells for the management of diseases associated with tissue dysfunction and immunologic deficiency, either through the pharmacological mobilization of host stem cells in vivo or the transplantation of ex vivo‐manipulated MSCs from an exogenous source.1, 2, 3 In the latter case, ex vivo culture conditions play important roles in determining the fate of the transplanted cells. Notably, most, if not all, currently well‐established in vitro culture systems cannot effectively recapitulate the complex architecture and properties of the native in vivo cell milieu.4, 5 Hence, cellular characteristics, including proliferation, differentiation and migration abilities, tend to be altered, particularly during long‐term cell expansion under large‐scale cell manufacturing conditions.6, 7 In this context, maintaining the migration and homing capacities of the cells during ex vivo culture and subsequently ensuring the ability of transplanted MSCs to traffic to and reach the site of injury are prerequisites for utilizing their regeneration potential.8 Unfortunately, maintaining proper stem cell migration across expanded cell cultures and during in vivo transplantation remains a challenge, and studies have reported that culture‐expanded MSCs almost completely lose their engraftment potential in in vitro cell culture systems.9, 10
In recent years, preconditioning of MSCs before infusion using various stimuli, such as inflammation11, 12 and hypoxia,13, 14 has been employed for cell pretreatment before transplantation. Although mounting evidence has demonstrated that a single inflammatory stimulus or hypoxia alone is able to improve cell migration, an optimized pretreatment for cell manipulation could potentially consist of a combination of several inflammatory and/or hypoxic pretreatments. With this hypothesis in mind, various combinations of chemokines/cytokines11, 15, 16 or stimulus strategies17, 18 have been tested. Unfortunately, these efforts have not led to a predictable, or ideally a synergistic, outcome. An analysis of the data published thus far suggests that the dose and time used for cell preconditioning under inflammatory and/or hypoxic conditions must be optimized for translation into clinical use.11, 17 Previous data have shown that pretreating cells with inflammatory mediators, such as tumour necrosis factor‐α (TNF‐α), results in a concentration‐dependent effect on cell migration.19 Furthermore, the migration capacity of MSCs is improved at a very low oxygen concentration (1%).20 However, very high concentrations of chemicals or very low concentrations of oxygen can lead to harmful changes in cell properties.12, 21 It has been hypothesized that the combination of a hypoxic stimulus and an inflammatory stimulus could be used to avoid the need for high chemical concentrations and very low oxygen concentrations to reach a satisfactory level of cell migration.
In our previous studies examining cell pretreatment, cell medium containing TNF‐α (10 ng/mL) and interleukin‐1β (IL‐1β) (5 ng/mL) was used to establish the inflammatory stimulus, while the hypoxic condition was established using a humidified atmosphere containing 2% O2.17 However, at this particular inflammatory dose, the dual stimuli did not have any additional effects on cell migration. Given that 2% O2 has been demonstrated to be safe for a standard duration (eg, for 24 hour) in numerous studies,22, 23 in the present study, we chose to decrease the concentration of inflammatory cytokines by 10‐fold (based on our prescreening) and sought to identify safe but effective conditions involving both a hypoxic stimulus and a low‐dose inflammatory stimulus for cell conditioning.
2. Methods
2.1. BMMSCs and group design
Human bone marrow (BM) samples to be used for cell isolation were obtained from three systemically healthy donors. All donors signed informed consents for contributing their BM samples for research purposes, and the experimental procedure was approved by the Institutional Review Board of the School of Stomatology, Fourth Military Medical University (FMMU), Xi'an, China. Human bone marrow‐derived mesenchymal stem cells (BMMSCs) were isolated and characterized using the methods described in our previous work.17 Briefly, primary cells were harvested from the BM samples and then purified using a limiting dilution technique. The obtained cells at passage 3 (P3) were subjected to colony‐forming and multiplication assays, flow cytometry analysis and evaluations of osteogenic/adipogenic differentiation. Cells at P3‐P5 were used in subsequent experiments. We treated the cell cultures with an inflammatory stimulus, a hypoxic stimulus and both inflammatory and hypoxic stimuli for 24 hours; these cells constituted the inflammation group, the hypoxia group and the dual‐stimulus group, respectively. Cultures without an inflammatory or hypoxic stimulus under a parallel 24 hours incubation served as controls (no‐stimulus group). Instead of using TNF‐α and IL‐1β at concentrations of 10 and 5 ng/mL, respectively,17, 18 the inflammatory stimulus medium used in this study was produced by adding TNF‐α at 1 ng/mL and IL‐1β at 0.5 ng/mL (both from Sigma‐Aldrich, St. Louis, MO, USA) to complete medium. The cultures in the hypoxia group and the dual‐stimulus group were incubated in a humidified atmosphere containing 2% O2, while a standard oxygen concentration (20% O2) was used for the inflammation group and the no‐stimulus group.
2.2. Real‐time PCR analysis
Relative CXCR4 mRNA expression in the incubated cells (P4) was evaluated through real‐time PCR analysis. According to our group design, the cells were incubated for 24 hours, and total RNA was isolated using the TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA, USA). The RNA was subsequently reverse transcribed to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Takara, Bio, Otsu, Japan). The sequences of the primers for CXCR4 and GAPDH were previously described.17 Relative CXCR4 expression was evaluated using the CFX Connect™ Real‐Time PCR Detection System (Bio‐Rad, Hercules, CA, USA).
2.3. Western blot analysis
Relative CXCR4 protein expression in the incubated cells (P4) was evaluated through Western blot analysis. The cells were incubated as described above (for the CXCR4 gene analysis), and total protein was extracted using lysis buffer (Sigma‐Aldrich). The CXCR4 and β‐tubulin proteins were analysed as described previously.17
2.4. Immunofluorescence analysis
CXCR4 protein expression in the incubated BMMSCs (P4) was further detected via immunofluorescent staining. The methods employed for this analysis were described in detail previously.17
2.5. Tablet scratch assay
The migratory ability of the incubated cells was first determined using a tablet scratch assay. Briefly, BMMSCs (P4) were cultured in six‐well culture dishes (2 × 105 cells per well) and pre‐incubated for 24 hours according to the group design. Then, mitomycin C (10 μg/mL) was added to each culture system to inhibit proliferation. After another 2 hour of culture, a cell‐free strip was generated by scratching the cellular monolayer with a pipette tip (1000 μL), and the resultant baseline was then recorded. Following incubation for another 20 hour in complete medium under a humidified atmosphere containing 20% O2, the cells that had migrated with respect to the baseline were photographed, and an inverted microscope (Olympus, Tokyo, Japan) was used for cell counting.
2.6. Transwell assay
The migratory ability of incubated cells along a stromal‐derived factor‐1α (SDF‐1α) gradient was determined using a Transwell membrane system,17 which contained an 8 μm pore polycarbonate membrane in 24‐well culture plates. Briefly, incubated BMMSCs (P4) were transferred to the upper chamber of a Transwell membrane system (1 × 105 cells per well). To assess the migration of the cells, the same amount of incubated cells were pretreated for another 30 minutes with 5 μg/mL AMD3100 (a CXCR4 antagonist)24 and then transferred to the upper chamber. Then, 600 μL of complete medium containing 100 ng/mL SDF‐1α was added to each lower chamber. Following a 6 hour incubation, the cells on the membranes of the Transwell insert were fixed with 4% paraformaldehyde for 30 minutes, and the non‐migrating cells on the upper surface of the well were detached from the membrane. Next, 0.2% crystal violet (Sigma‐Aldrich) was used to stain the migrated cells on the lower surface of the membrane. Finally, the cells were counted in five random fields (200×), and statistical analysis was performed.
2.7. Cell proliferation analysis
The effects of different pretreatments on cell proliferation were assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; Sigma‐Aldrich) assay. The methods for this assay were described in detail previously.12
2.8. Cell differentiation analysis
The effects of different pretreatments on the expression of osteogenic and adipogenic genes/proteins were evaluated using real‐time PCR and Western blot assays. Briefly, incubated cells from each group were seeded into six‐well culture dishes (2 × 105 cells per well) and then induced with osteogenic medium (complete medium containing 50 μg/mL vitamin C, 5 mm β‐glycerophosphate and 0.1 mm dexamethasone) and adipogenic medium (complete medium containing 200 μm indomethacin, 10 μm insulin, 0.5 mm 1‐methyl‐3‐isobutylxanthine and 1 μm dexamethasone). To detect the expression levels of osteogenic (RUNX‐2, ALP and OCN) and adipogenic (PPAR‐γ) genes and proteins, total RNA and protein were collected at day 7 and then subjected to analysis via real‐time PCR and Western blotting, respectively. The sequences of the primers used for real‐time PCR analysis were described previously.18 Furthermore, the osteogenic differentiation of the cells in each group was assessed through Alizarin Red staining, while the adipogenic potential was analysed using Oil Red O staining. In parallel, the cells in all groups were incubated with osteogenic medium and adipogenic medium. After 4 weeks of osteogenic induction, the cells were fixed with 4% paraformaldehyde for 30 minutes, followed by Alizarin Red (Sigma‐Aldrich) staining. After 3 weeks of adipogenic induction, the cells were fixed and then stained with Oil Red O for 15 minutes. Finally, the mineral nodules and lipid droplets were imaged, and dissolved solutions of the samples were quantitatively measured at 560 nm.
2.9. Immunosuppression analyses
The effects of different pretreatments on cell immunomodulatory activity were evaluated by detecting the proliferation/apoptosis rate of peripheral blood mononuclear cells (PBMNCs). After co‐culture with pretreated cells, PBMNCs were subjected to analysis of the proliferation/apoptosis rate; the methods for this analysis were described in detail previously.25, 26 Briefly, density‐gradient centrifugation was used to extracted PBMNCs from fresh blood derived from three systemically healthy donors. Prior to co‐culture, PBMNCs were activated with 10 μg/mL phytohemagglutinin (PHA, Sigma‐Aldrich) for 24 hours in Roswell Park Memorial Institute (RPMI)‐1640 medium. Then, mitomycin C (10 μg/mL) was added to the culture medium to inhibit proliferation. Subsequently, the PBMNCs were co‐cultured with the pretreated BMMSCs at a 1:24 ratio (BMMSC:PBMNC) for the designated times, as described previously.25 PHA‐stimulated PBMNCs (without BMMSCs) served as the positive control. After 12, 24, and 36 hours of co‐culture, a Cell Counting Kit‐8 (CCK‐8) assay (7 Sea Pharmtech, Shanghai, China) was used to detect the proliferation rate of the PBMNCs, and the data were analysed according to the following formula: % changes in PBMNC proliferation = 100 × [(cells + PBMNC + PHA)]/(PBMNC + PA).26 After 72 hours of co‐culture, the apoptosis rate of the PBMNCs was detected using an Annexin V/propidium iodide (Annexin V/PI) kit (7 Sea Pharmtech) and analysed with a flow cytometer.
2.10. Statistical analyses
Each experiment was repeated at least three times for three cell lines. The numerical data are expressed as the mean ± standard deviation (SD). One‐way analysis of variance (ANOVA) followed by Tukey's post hoc test or two‐way ANOVA was performed using GraphPad Prism 5 software to analyse the obtained data. P<.05 was considered statistically significant.
3. Results
3.1. Isolation and identification of BMMSCs
Primary cells were successfully isolated from the BM samples from three donors (Figure 1A). After purification via a limited dilution method, all cells derived from the three individuals exhibited the ability to form cell colonies (Figure 1B), proliferate during culture (Figure 1C) and transdifferentiate into osteogenic and adipogenic lineages in vitro (Figure 1D). In addition, the isolated BMMSCs were positive for the expression of MSC markers, such as CD29, CD44, CD90, CD105, CD146 and STRO‐1, while they did not express hematopoietic markers, such as CD31 and CD34 (Figure 1E).
Figure 1.
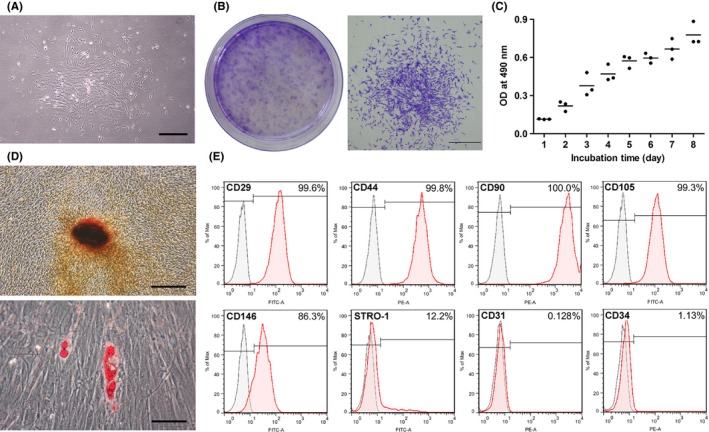
Isolation and identification of human BMMSCs. A, Representative images of BM‐derived primary cells (day 6). Scale bar: 600 μm. B, Representative images of the colony‐forming units formed on the plate (general view) and a random single‐cell clone (day 12). Scale bar: 1 mm. C, The cell proliferation of BMMSCs (P3) measured via the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay during an 8‐d incubation. D, Representative images of mineralized cell nodules (histochemically stained with Alizarin Red) following 4 wk of osteogenic induction and representative images of lipid droplets (stained with Oil Red O) following 3 wk of adipogenic induction. Scale bar: 400 μm. E, Cell surface markers were identified through flow cytometric analysis
3.2. An inflammatory stimulus and a hypoxic stimulus synergistically increase CXCR4 expression in BMMSCs
Real‐time PCR analysis showed that the combination of an inflammatory stimulus and a hypoxic stimulus significantly increased CXCR4 gene expression compared with the no‐stimulus and inflammatory stimulus groups (P<.01) (Figure 2A). Although the difference between the hypoxia group and the dual‐stimulus group was not statistically significant (P>.05), the combination of an inflammatory stimulus and hypoxic stimulus led to a more obvious increase in CXCR4 gene expression (Figure 2A). No significant differences in CXCR4 gene expression were found between the no‐stimulus, inflammation and hypoxia groups (P>.05). In the Western blot assay, cells from the dual‐stimulus group displayed the highest CXCR4 protein expression level among the four groups (Figure 2B,C). CXCR4 protein expression was higher in cells from the hypoxic group than in cells from the no‐stimulus group (P<.01) and the inflammation group (P<.05), while no significant difference in expression was found between the inflammation group and the no‐stimulus group (P>.05). In agreement with the data from the Western blot assay, immunofluorescence analysis showed that the inflammatory stimulus and hypoxic stimulus synergistically increased CXCR4 protein expression in BMMSCs (Figure 2D,E).
Figure 2.
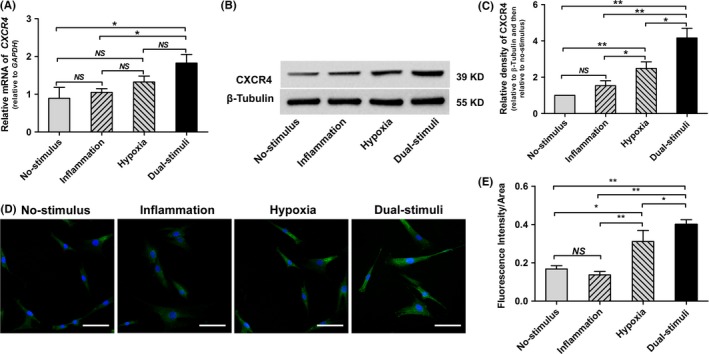
Short‐term pretreatment with hypoxic and low‐dose inflammatory stimuli synergistically up‐regulates CXCR4 expression in BMMSCs (NS>.05; *P<.05; **P<.01). A, The relative CXCR4 gene expression level determined via real‐time PCR. B, C, Representative images (B) and quantitative analysis (C) of CXCR4 protein expression, as determined via Western blotting. D, Representative immunofluorescence images of CXCR4 protein. Scale bar: 30 μm. E, Quantitative analysis of CXCR4 fluorescence intensity
3.3. The combination of inflammatory and hypoxic stimuli synergistically enhances BMMSC migration
In a tablet scratch experiment, the combination of inflammatory and hypoxic stimuli resulted in greater cell migration into the cell‐free area than a single stimulus or no stimulus (P<.05 or P<.01) (Figure 3). Significantly more cells migrated from the upper chamber to the lower surface of the membrane in the hypoxia group than in the no‐stimulus group or the inflammation group (P<.01). However, there was no significant difference with regard to migrated cells between the no‐stimulus group and the inflammation group (P>.05). Cell migration towards the SDF‐1α gradient was further analysed using a Transwell migration system. Consistent with the data obtained in the tablet scratch experiment, more cells migrated from the upper chamber to the lower chamber (supplemented with 100 ng/mL SDF‐1α) in response to the combination of inflammatory and hypoxic stimuli compared with treatment with a single stimulus or no stimulus (P<.01) (Figure 4). Again, significantly more migrated cells were observed in the hypoxia group compared with the number of migrated cells observed in the no‐stimulus group (P<.05), while no significant differences were found between the inflammation, hypoxia and no‐stimulus groups (P>.05). As expected, the migratory ability of the incubated cells towards the SDF‐1α gradient could be inhibited with the CXCR4 antagonist AMD3100. Following pretreatment with AMD3100 for 0.5 hour, cells in all four groups exhibited an impaired migration ability (Figure 4).
Figure 3.
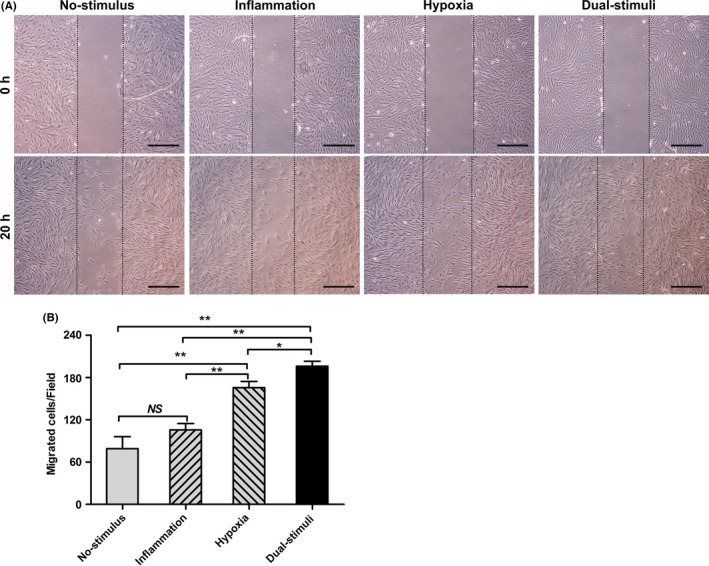
Short‐term pretreatment with hypoxic and low‐dose inflammatory stimuli synergistically enhances cell migration, as determined by a tablet scratch assay. A, Representative images of cell migration at 0 and 20 h after scratching. Scale bar: 1 mm. B, Statistical analysis of the number of migrated cells in the tablet scratch experiment (NS>.05; *P<.05; **P<.01)
Figure 4.
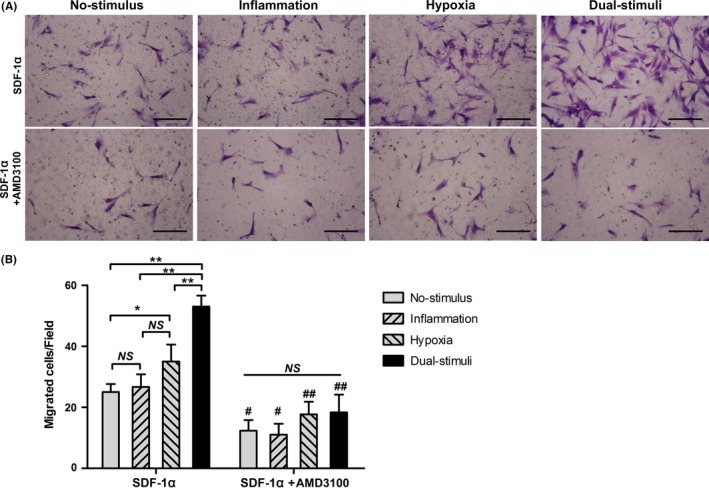
Short‐term pretreatment with hypoxic and low‐dose inflammatory stimuli synergistically enhances cell migration in a Transwell system (NS>.05; *P<.05; **P<.01). A, Representative images of cell migration in response to SDF‐1α (100 ng/mL) in the presence or absence of AMD3100. Scale bar: 200 μm. B, Statistical analysis of the number of migrated cells in the Transwell system (NS>.05, *P<.05 and **P<.01, comparison between the indicated columns; # P<.05 and ## P<.01, comparison between the corresponding columns in the presence or absence of AMD3100)
3.4. Effects of different stimuli on cell proliferation and differentiation
When the effect of the inflammatory and/or hypoxic stimuli on BMMSC proliferation was evaluated via the MTT assay, both the inflammatory and hypoxic stimuli and the hypoxic stimulus alone exhibited a significant positive influence on cell proliferation compared with either no stimulus or the inflammatory stimulus (P<.05) (Figure 5A). However, no synergistic effect was found in cells from the dual‐stimulus group compared with cells pretreated with hypoxia alone. In addition, the inflammatory stimulus had no obvious effect on cell proliferation compared with the control (P>.05). Real‐time PCR analysis revealed that the inflammatory stimulus and/or the hypoxia stimulus had no significant negative influence on the gene expression of RUNX‐2, ALP, OCN or PPAR‐γ (P>.05) (Figure 5B). Western blot analysis further showed that there was no significant difference in the protein levels of RUNX‐2, ALP or PPAR‐γ between the four designated groups (P>.05), while the protein expression level of OCN was significantly decreased in the dual‐stimulus group (P<.05) (Figure 5C,D). In addition, all cells from the four groups exhibited a similar potential to form mineralized cell nodules following 4 weeks of osteogenic induction and to form lipid droplets following 3 weeks of adipogenic induction, as demonstrated by Oil Red O staining and Alizarin Red staining, respectively (Figure 5E,F).
Figure 5.
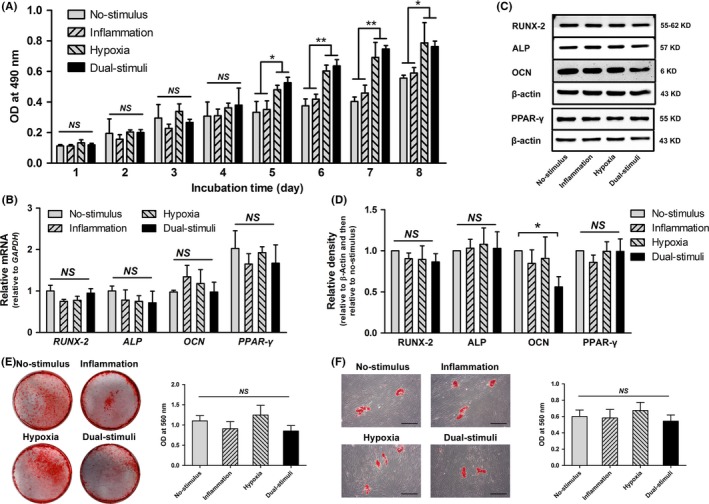
The effects of 24 h inflammatory and/or hypoxic pretreatment on cell proliferation cell differentiation (NS>.05; *P<.05; **P<.01). A, Cell proliferation evaluated via the MTT assay during an 8‐d incubation. B, Quantitative analysis of the expression levels of osteogenic genes (RUNX‐2, ALP and OCN) and an adipogenic gene (PPAR‐γ) through real‐time PCR. C, D, Representative images (C) and quantitative analysis (D) of the expression levels of osteogenic proteins (RUNX‐2, ALP and OCN) and an adipogenic protein (PPAR‐γ) via Western blot assays. E, Representative general views and corresponding quantitative analysis of the mineral nodules formed by cells in the four groups. F, Representative images and corresponding quantitative analysis of the lipid droplets formed by cells in the four groups. Scale bar: 600 μm
3.5. Effects of different stimuli on cell immunomodulation
When the effect of the inflammatory and/or hypoxic stimuli on BMMSC immunomodulation was first evaluated via PBMNC proliferation assays, all of the evaluated cells exhibited significant immunosuppression of PBMNC proliferation compared with that of the positive control at all time points (Figure 6A). Statistical analysis showed that there was no significant difference in PBMNC proliferation between the four designated groups (P>.05). The percentages of PBMNC apoptosis were also detected following 72 hours of co‐culture. All cell types appeared to promote PBMNC apoptosis compared with the control (Figure 6B,C); similarly, no significant difference in PBMNC apoptosis was found between the four designated groups (P>.05).
Figure 6.
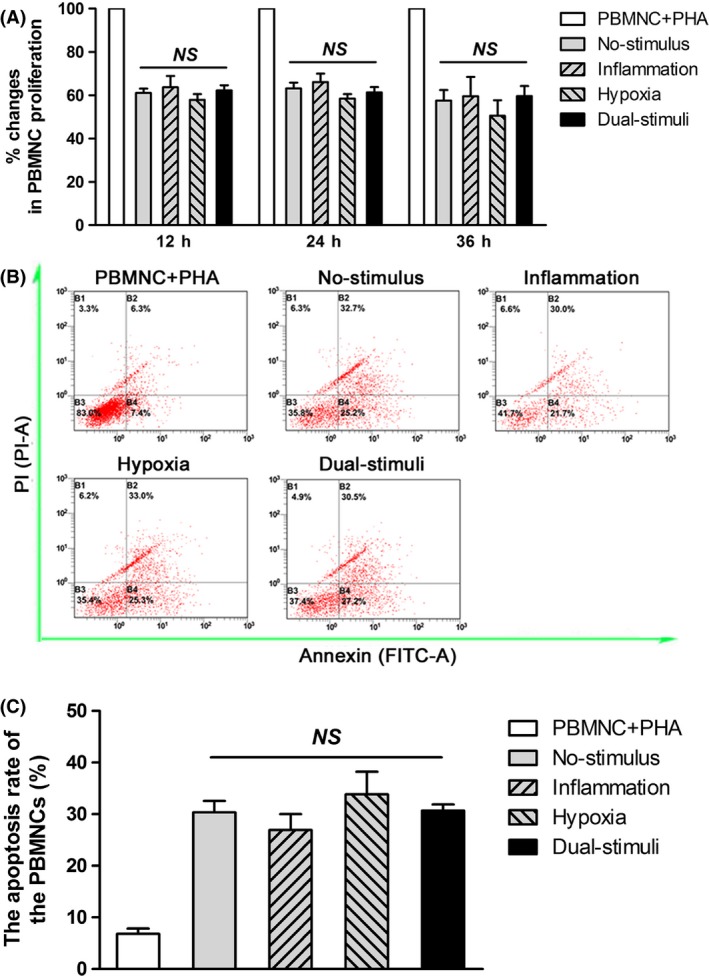
The effects of 24 h inflammatory and/or hypoxic pretreatment on cell immunomodulation (NS>.05). A, The percentages of proliferation in PBMNCs co‐cultured with the tested cells for 12, 24 and 36 h was detected via the Cell Counting Kit‐8 (CCK‐8) assay. B, Representative images of the PBMNC apoptosis rate following 72 h of co‐culture. C, Quantitative analysis of the PBMNC apoptosis rate following 72 h of co‐culture
4. Discussion
Compelling evidence suggests that impaired cell migration during the cell expansion process is associated with loss of the cell surface expression of C‐X‐C chemokine receptor type 4 (CXCR4).16, 27 The interaction of CXCR4 with SDF‐1α was found to mediate migration in ex vivo‐expanded MSCs28 and to regulate the trafficking of transplanted cells in vivo,29 suggesting that loss of cell migration potential can be rescued by targeting the SDF‐1α/CXCR4 axis in culture, for example, through up‐regulation of the expression of CXCR4 in MSCs. Compared with using viral delivery vectors, preconditioning of MSCs under particular conditions has been shown to be simpler, more practical and safer.19, 30
In this study, we established a low‐dose inflammatory stimulus medium by adding TNF‐α (1 ng/mL) and IL‐1β (0.5 ng/mL) to complete culture medium. The concentrations of TNF‐α and IL‐1β used in the medium were 10‐fold lower than those employed in our previous work (10 and 5 ng/mL, respectively).17 In this study, human BMMSCs were treated with no stimulus, an inflammatory stimulus, hypoxia or dual stimulation to determine whether a synergistic effect could be achieved using a combination of hypoxic and low‐dose inflammatory stimuli. Based on real‐time PCR, Western blot and immunofluorescence analyses, we found that the hypoxic stimulus and low‐dose inflammatory stimulus synergistically increased both the gene and protein expression of CXCR4 in cultured cells (Figure 2). The expression of CXCR4 in MSCs at the protein and mRNA levels is a commonly used indicator of cell migration potential.28 However, low‐dose inflammatory pretreatment alone failed to increase CXCR4 expression, in contrast to what was demonstrated previously under relatively strong inflammatory conditions.17 Furthermore, the effect of the dual stimuli on cell migration was evaluated using both a tablet scratch experiment and a Transwell system, as they are the most commonly used methods for investigating in vitro cell migration. In the tablet scratch assay, the combination of inflammatory and hypoxic stimuli was more effective in enhancing migration than either stimulus alone (Figure 3), but no significant difference in cell migration was observed between the no‐stimulus group and the low‐dose inflammatory treatment group. Similar findings were obtained in the Transwell experiments (Figure 4). Taken together, these data suggest that although pretreatment of BMMSCs with TNF‐α (1 ng/mL) and IL‐1β (0.5 ng/mL) does not modify the cell migration potential, a low‐dose inflammatory stimulus can enhance the effect of hypoxic preconditioning. The synergistic enhancement of BMMSC migration under the combination of a hypoxic stimulus and a low‐dose inflammatory stimulus could be blocked by AMD3100, a CXCR4 antagonist, indicating that such positive influences on cell migration are achieved via the up‐regulation of cell surface CXCR4 expression. Our data indicate that an inflammatory stimulus and a hypoxic stimulus synergistically increase CXCR4 expression in BMMSCs over 24 hours; hence, the strategy presented in this study would be particularly useful for cell preconditioning when sufficient cells are ready to be used, prior to cell transplantation.
Given the evidence that cell preconditioning with inflammatory mediators impairs cell function, at least to a certain degree,12, 21 we further investigated the influences of inflammatory and/or hypoxic stimuli on cell proliferation and differentiation in this particular experimental design. Our results revealed that hypoxia, either alone or in combination with inflammation, could lead to increased cell proliferation, while a single low‐dose inflammatory stimulus had no obvious effect on cell growth (Figure 5A). Although in our previous studies12, 17, 25 as well as many others,15, 31, 32 stem cells have been shown to grow faster in an inflammatory environment than in normal conditions; in the present study, pretreatment of BMMSCs in medium containing TNF‐α (1 ng/mL) and IL‐1β (0.5 ng/mL) did not lead to such an increase. Additionally, a short‐term inflammatory stimulus and/or hypoxic stimulus did not have a significant negative impact on cell osteogenic/adipogenic differentiation (Figure 5B‐F). These data suggest that a low‐dose inflammatory stimulus serves as an adjuvant that could work synergistically with hypoxic stimuli to increase cell migration, while exerting no negative effects on cell proliferation and differentiation. Although inflammation has been widely demonstrated to impair the osteogenic potential of MSCs,25, 32 our results show that media containing low doses of TNF‐α and IL‐1β are safe for cell incubation, at least within a period of 24 hours. Additionally, the immunomodulatory activity of BMMSCs is as important as their proliferation/differentiation potential when these cells are used for tissue engineering and stem cell therapy.3 Hence, we co‐cultured the pretreated cells with PBMNCs to evaluate the effects of different pretreatments on cell immunomodulation, based on the theory that ex vivo‐expanded MSCs can secrete soluble factors conferring them with immunosuppressive properties and causing them to mediate the suppression of proliferating PBMNCs.33 Our data indicated that all of the pretreated cells subjected to 24 hours of preconditioning maintained their immunomodulatory activity, ie, their ability to suppress the proliferation and promote the apoptosis of activated PBMNCs (Figure 5G‐I). That is, a 24 hours inflammatory stimulus and/or hypoxic stimulus did not have a negative impact on cell immunomodulation. Taken together, the findings of this study demonstrate an effective cell pretreatment strategy that can be used to rescue the cell migration potential during in vitro expansion. However, understanding the in vitro control associated with both the dose and time of either the inflammatory or hypoxic stimulus will require further in‐depth investigation.
5. Conclusion
In this study, we report that the combination of a hypoxic stimulus and a low‐dose inflammatory stimulus can be used to rescue BMMSC migration during ex vivo expansion. A low dose of inflammatory cytokines (ie, TNF‐α at 1 ng/mL and IL‐1β at 0.5 ng/mL) was not found to impair cell proliferation, differentiation or immunomodulation and functioned synergistically with a hypoxic stimulus to increase surface CXCR4 expression and in vitro migration in BMMSCs. These findings suggest that the combination of a hypoxic stimulus and a low‐dose inflammatory stimulus may represent a reliable cell pretreatment system for future stem cell‐based therapeutics.
Authors' contributions
YY and RXW designed the study, coordinated the experiments, executed most of the experiments, analysed the data, and wrote and revised the manuscript. YY and XTH conducted some of the experiments and aided in data analysis. XYZ conducted some of the experiments and aided in manuscript preparation and revision. FMC coordinated the experiments and aided in study design, data analysis and manuscript preparation and revision.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (nos. 81530050, 81471791 and 81500853).
Disclosure of interest
The authors declare no conflicts of interest.
Funding information
This work was supported by the National Natural Science Foundation of China (nos. 81530050, 81471791 and 81500853).
References
- 1. Chen FM, Zhao YM, Jin Y, Shi S. Prospects for translational regenerative medicine. Biotechnol Adv. 2012;30:658. [DOI] [PubMed] [Google Scholar]
- 2. Daley GQ, Scadden DT. Prospects for stem cell‐based therapy. Cell. 2008;132:544. [DOI] [PubMed] [Google Scholar]
- 3. Knaän‐Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014;32:603. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Kilian KA. Bridging the gap: from 2D cell culture to 3D microengineered extracellular matrices. Adv Healthc Mater. 2015;4:2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16. [DOI] [PubMed] [Google Scholar]
- 6. Ren G, Chen X, Dong F, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350. [DOI] [PubMed] [Google Scholar]
- 8. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206. [DOI] [PubMed] [Google Scholar]
- 9. Maijenburg MW, van der Schoot CE, Voermans C. Mesenchymal stromal cell migration: possibilities to improve cellular therapy. Stem Cells Dev. 2012;21:19. [DOI] [PubMed] [Google Scholar]
- 10. Pineault N, Abu‐Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43:498. [DOI] [PubMed] [Google Scholar]
- 11. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737. [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Gao LN, An Y, et al. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34:7033. [DOI] [PubMed] [Google Scholar]
- 13. Annabi B, Lee YT, Turcotte S, et al. Hypoxia promotes murine bone‐marrow‐derived stromal cell migration and tube formation. Stem Cells. 2003;21:337. [DOI] [PubMed] [Google Scholar]
- 14. Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202. [DOI] [PubMed] [Google Scholar]
- 15. Fu X, Xiao J, Wei Y, et al. Combination of inflammation‐related cytokines promotes long‐term muscle stem cell expansion. Cell Res. 2015;25:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue‐derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Y, Wu RX, Gao LN, Xia Y, Tang HN, Chen FM. Stromal cell‐derived factor‐1‐directed bone marrow mesenchymal stem cell migration in response to inflammatory and/or hypoxic stimuli. Cell Adh Migr. 2016;10:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu Y, Bi CS, Wu RX, et al. Effects of short‐term inflammatory and/or hypoxic pretreatments on periodontal ligament stem cells: in vitro and in vivo studies. Cell Tissue Res. 2016; doi: 10.1007/s00441-016-2437-3. [DOI] [PubMed] [Google Scholar]
- 19. Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N. Involvement of TNF‐α in differential gene expression pattern of CXCR4 on human marrow‐derived mesenchymal stem cells. Mol Biol Rep. 2014;41:1059. [DOI] [PubMed] [Google Scholar]
- 20. Liu L, Yu Q, Lin J, et al. Hypoxia‐inducible factor‐1α is essential for hypoxia‐induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev. 2011;20:1961. [DOI] [PubMed] [Google Scholar]
- 21. Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS. Human bone marrow‐derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med. 2014;3:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flann KL, Rathbone CR, Cole LC, Liu X, Allen RE, Rhoads RP. Hypoxia simultaneously alters satellite cell‐mediated angiogenesis and hepatocyte growth factor expression. J Cell Physiol. 2014;229:572. [DOI] [PubMed] [Google Scholar]
- 23. Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF‐1 alpha in skin tissue and induces migration of circulating bone marrow‐derived stem cells into the expanded skin. Stem Cells. 2013;31:2703. [DOI] [PubMed] [Google Scholar]
- 25. Tang HN, Xia Y, Yu Y, Wu RX, Gao LN, Chen FM. Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities: a patient‐matched comparison. J Clin Periodontol. 2016;43:72. [DOI] [PubMed] [Google Scholar]
- 26. Yazid FB, Gnanasegaran N, Kunasekaran W, Govindasamy V, Musa S. Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin Oral Investig. 2014;18:2103. [DOI] [PubMed] [Google Scholar]
- 27. Foguenne J, Di Stefano I, Giet O, Beguin Y, Gothot A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha4 integrin‐ and CXCR4‐mediated engraftment in NOD/SCID beta2‐microglobulin‐null mice. Haematologica. 2009;94:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Son BR, Marquez‐Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal‐derived factor‐1‐CXCR4 and hepatocyte growth factor‐c‐met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254. [DOI] [PubMed] [Google Scholar]
- 29. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415. [DOI] [PubMed] [Google Scholar]
- 30. Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012;2:148. [PMC free article] [PubMed] [Google Scholar]
- 31. Park DH, Eve DJ, Musso J 3rd, et al. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009;18:693. [DOI] [PubMed] [Google Scholar]
- 32. Kong X, Liu Y, Ye R, et al. GSK3β is a checkpoint for TNF‐α‐mediated impaired osteogenic differentiation of mesenchymal stem cells in inflammatory microenvironments. Biochim Biophys Acta. 2013;1830:5119. [DOI] [PubMed] [Google Scholar]
- 33. Wada N, Gronthos S, Bartold PM. Immunomodulatory effects of stem cells. Periodontology 2000. 2013;63:198. [DOI] [PubMed] [Google Scholar]


