Abstract
Objective
To study the effect on endometrial and endometriotic cells after co‐culture with macrophages, using clonogenic, invasion and self‐renewal assays.
Materials and methods
Peripheral blood samples, endometrium and endometriotic tissues were collected. Autologous macrophages were co‐cultured with endometrial and endometriotic cells. The number of colony‐forming units (CFU), invasiveness and self‐renewal activity after co‐culture with macrophages were determined. The cytokine level of colony‐stimulating factor‐1 (CSF‐1) from macrophages with and without endometriosis was compared.
Results
Co‐culture with macrophages significantly increased the clonogenic and invasion ability of endometriotic stromal cells in vitro. Colony‐stimulating factor‐1 (CSF‐1) was up‐regulated in endometriotic macrophages conditioned medium when compared to those without the disease.
Conclusions
These data suggest that macrophages may increase the proliferation and invasion activity of stromal clonogenic cells in women with endometriosis.
1. Introduction
Endometriosis is the presence of endometrial tissue growth outside the uterine cavity and is a benign gynaecological disease affecting ~5% of women of reproductive age.1 The sex steroid‐dependent growth of ectopic endometrial tissues may result in cyclical pelvic pain and infertility. Several proposed theories have implicated the pathogenesis of endometriosis, including retrograde menstruation, peritoneal cell metaplasia, genetic predisposition and altered immunological surveillance.2 The emerging evidence of somatic stem cells in the human endometrium provides an alternate candidate cell source for the development of endometriosis.3
The physiological role of stem cells in the endometrium is to maintain the cyclical regeneration of the tissue that occurs after each menstruation. Endometrial epithelial and stromal cells with high clonogenic activity are initiated by stem/progenitor cells.4 The percentage of clonogenic cells in human endometrium does not vary significantly across the menstrual cycle.5 Occasional shedding of endometrial stem cells with colony‐forming potential can reach ectopic sites through retrograde menstruation, invading the peritoneum to generate endometriotic lesions.6 Studies examining the eutopic endometrium of women with and without endometriosis revealed striking differences in gene expression that may predispose some women to disease development.7, 8, 9 Eutopic endometrial stem cells from women with endometriosis exhibit progesterone resistance which is inherited by their progenies.10 The uncontrolled growth of ectopic endometrial tissue invades the adjacent tissues and is associated with neovascularization and local inflammatory responses. Aberrant production of cytokines and growth, adhesion and angiogenic factors are linked to the occurrence and maintenance of endometriosis.11 How the changes in the inflammatory peritoneal environment influence the behaviour of ectopic endometrial stem cells is unknown.
Pathogenesis of endometriosis is associated with dysfunctional regulation of the immune system,12 in particular, an increase in macrophages and impairment of their phagocytic activities.13, 14 Hypoxia and tissue stress recruit peripheral macrophages to the endometriotic sites and contribute to the lesion's neovasculature, sustaining the survival of endometrial cells at the ectopic locations. Chemokines produced by stromal cells have a significant role in the infiltration of macrophages into the peritoneal cavity.15, 16 Activation of macrophages is characterized by their secretion of a wide variety of cytokines and growth factors.17 Levels of peritoneal cytokines differ greatly between women with and without endometriosis,18, 19 and higher amounts of cytokines are detected in advanced stages of the disease.20
Little is known about the interactions of macrophages with endometrial colony‐forming cells. Here we described the clonal analysis of endometrial and endometriotic cells after co‐culture with macrophages and examined how it affects the cell's functional activities.
2. Materials and methods
2.1. Human tissue samples
Two types of endometrial tissues were collected: (i) endometrium from women without endometriosis (normal endometrium) and (ii) ovarian endometrioma (endometriosis). Endometrial samples (n=33) were collected from ovulating women (45.5±0.5 years) undergoing hysterectomy for leiomyoma or adenomyosis. Cyst walls of ovarian endometrioma (n=32) were collected from women (39.3±1.3 years) undergoing ovarian cystectomy. Only women who had not taken exogenous hormones for 3 months before surgery were included. Informed written consent was obtained from each patient and ethics approval was obtained from the Cluster Research Ethics Committee/Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster, Hospital Authority, Hong Kong.
The stage of the menstrual cycle was categorized into proliferative (endometrium, n=19; endometriotic, n=16) and secretory (endometrium, n=14; endometriotic, n=16). The samples were dated based on the reported day of the last menses and histology examination by histopathologists.21 Endometriosis was staged according to the 1996 revised classification of the American Society for Reproductive Medicine.22
Full thickness endometrial tissue samples or ovarian endometriotic cysts were collected in Dulbecco's modified Eagle's medium/Hams F‐12 (DMEM/F‐12; Life technologies, Calrsbad, CA, USA) containing 1% antibiotic (Gibco, Rockville, MD, USA) and 5% foetal bovine serum (FBS, Gibco). The samples were stored at 4°C and processed within 24 hours.
2.2. Isolation of endometrial and endometriotic cells
Human endometrial and endometriotic tissues were digested to single‐cell suspensions using collagenase type I (300 μg/mL; Worthington Biochemical Corp, Lakewood, NJ, USA) and deoxyribonuclease type I (40 μg/mL; Worthington Biochemical Corp) as described previously.23 Red blood cells were removed using Ficoll‐Paque (GE Healthcare, Uppsala, Sweden) density‐gradient centrifugation. Leucocytes were eliminated using anti‐CD45 antibody‐coated Dynabeads (Life Technologies). Purified epithelial cell suspensions were separated from stromal cells by using anti‐CD368 (EpCAM) antibody‐coated microbeads (Miltenyi Biotec Inc., San Diego, CA, USA).
2.3. Macrophage differentiation and collection of conditioned medium
Peripheral blood mononuclear cells from women with and without endometriosis were isolated with Ficoll‐Paque. Blood samples were collected on the same day as the endometrial or endometriotic tissue. Monocytes were enriched by the Monocyte Isolation Kit II (Miltenyi Biotec Inc.) and subsequently differentiated into macrophages in vitro according to previous method.24
Monocytes were stimulated with phorbol‐12 myristate 13‐acetate (PMA, 50 ng/mL; Sigma‐Aldrich, St. Louis, MO, USA) in RPMI 1640 medium (Life Technologies), 10% FBS and 1% penicillin. Differentiation of the monocytes to macrophages was confirmed by morphological changes such as increase in cell size, formation of pseudopodia and adhesion (Fig. S1A) and by flow cytometry detection of expression of a macrophage marker CD68 using fluorescein isothiocyanate (FITC)‐conjugated anti‐CD68 antibody (eBioscience, San Diego, CA, USA) (Fig. S1B). To determine the phenotype of macrophages, the cells were co‐stained with FITC‐conjugated anti‐CD68 (eBioscience) and classical M1 marker allophycocyanin (APC)‐conjugated anti‐CD86 antibody (BD Biosciences, San Jose, CA, USA) or alternative M2 marker APC‐conjugated anti‐CD206 antibody (eBioscience). Cells were analysed using a Fortessa flow cytometer (BD Biosciences) in the University of Hong Kong Core Facility. Macrophages were cultured in six‐well transwells (2 × 105 cells/well, EMD Millipore) and 72 hours after differentiation, the cells were washed with PBS twice and replaced with RPMI and 1% penicillin. The conditioned media (CM) from macrophages of women with or without endometriosis were collected 48 hours later, centrifuged to remove cellular debris and used for subsequent experiment or stored at −80°C until use.
2.4. Co‐culture setup and colony‐forming assay
Six different co‐cultures were set up: (i) endometrial epithelial cells co‐cultured with autologous macrophages and CM (n=4);(ii) endometrial stromal cells co‐cultured with autologous macrophages and CM (n=8); (iii) endometriotic epithelial cells co‐cultured with autologous macrophages and CM (n=8); (iv) endometriotic stromal cells co‐cultured with autologous macrophages and their CM (n=13); (v) endometriotic epithelial cells co‐cultured with macrophages (without endometriosis) and their CM (n=3) and (vi) endometriotic stromal cells co‐cultured with macrophages (without endometriosis) and their CM (n=3). In brief, cells were seeded in duplicate at a clonal density of 500 cells/cm2 in six‐well plates (BD Bioscience) and were (i) cultured in growth medium only (control); (ii) cultured in growth medium supplemented with 50 ng/mL PMA (negative control); (iii) co‐cultured indirectly with macrophages with 50 ng/mL PMA (2 × 105 cells); or (iv) treated with macrophage CM, which was diluted with growth medium at a ratio of 3:7 (v/v). We supplemented PMA to maintain macrophages differentiation in long‐term culture. The medium was changed every 7 days, and the colonies formed were regularly monitored using an Eclipse TS100 inverted microscope (Nikon, Melville, NY, USA). Endometrial cells were cultured for 15 days.4, 25 Endometriotic cells were cultured for 21 days.23 The colonies formed were fixed with 10% formalin and stained with 1% toluidine blue (Sigma‐Aldrich) (Fig. S2A). Colony‐forming units (CFUs) consisting of ≥50 cells were counted to determine the cloning efficiency (CE), which was the percentage of colonies formed per seeded cell. Large CFUs were defined as colonies with ≥4000 cells and small CFUs were those with ≤4000 cells as described previously.23
2.5. Cell invasion
Clonally derived endometrial and endometriotic cells were harvested from different conditions, and 2 × 105 cells were seeded on Matrigel‐coated transwells (24 wells, 8 μm pore size, BD Biosciences). After 48 hours, cells on the upper surface of the inset membrane were removed with cotton rods, while cells on the lower surface of the membrane were fixed in 4% paraformaldehyde and stained with 0.1% toluidine blue (Fig. S2B). The transwells were washed and the invaded cells were lysed with 10% acetic acid. Absorbance of the lysate was measured at 595 nm using a microplate reader (Tecan, Männedorf, Switzerland). Relative invasion was determined by normalization to the control group.
2.6. In vitro serial cloning
Individual large epithelial and stromal CFUs from passage 1 (P1) were trypsinized using cloning rings (Sigma‐Aldrich) to determine the self‐renewal capacity of cells from endometrial and endometriotic cells grown in growth medium and co‐cultured with autologous macrophages. A total of three individual large CFUs per patient sample (n=3) obtained from the clonogenic assays were used. The cell number of each CFU was determined and the cells were re‐seeded at a density of 20 cells/cm2.26 This process continued until the cells could no longer form CFUs (Fig. S2).
2.7. Cytokine array and ELISA
Cytokine Array C3 (RayBiotech Inc., Norcross, GA, USA) was used to determine the cytokines in the macrophage CM from women with endometriosis (n=6; proliferative n=3, secretory n=3) and without endometriosis (n=6; proliferative n=3, secretory n=3). The signal intensities of the cytokines were quantified using Quantity One software (Bio‐Rad, Hercules, CA, USA) (Fig. S5). The CSF‐1 level was determined using enzyme‐linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) from women with endometriosis (n=11; proliferative n=5, secretory n=6) and without endometriosis (n=9; proliferative n=5, secretory n=4). Each sample was measured in duplicate. Recombinant CSF‐1 (Peprotech, Rocky Hill, NJ, USA) at 30, 300, and 3000 pg/mL was added to the growth medium of endometrial and endometriotic stromal cells seeded at clonal density (500 cells/cm2) for 15 days. For neutralization assay, the anti‐human colony‐stimulating factor (CSF‐1) monoclonal antibody (10 μg/mL, Peprotech) was added to the endometrial epithelial and stromal cells co‐cultured with macrophages and CM without endometriosis.
2.8. Flow cytometry analysis
The co‐expression of CD140b and CD146 on endometrial stromal cells after 15 days of culture in different conditions (n=5) were analysed by multicolour flow cytometry as described previously.26 The cells were incubated with FITC‐conjugated anti‐CD146 (1 mg/mL, OJ79c clone, mouse IgG1; Thermo Fisher Scientific, Waltham, MA, USA) and PE‐conjugated anti‐PDGFRβ (CD140b, 2.5 μg/mL, PR7212 clone, Mouse IgG1, R&D Systems) antibodies in the dark for 45 minutes on ice. Isotype‐matched controls were included for each antibody. Following the final washing step, the labelled cells were analysed by Fortessa flow cytometer (BD Biosciences) in the University of Hong Kong Faculty Core Facility. The cells were selected with electronic gating according to the forward and side scatter profiles (Fig. S3A‐D) using the FACSDIVA software (BD Biosciences). Data were analysed using the FlowJo Software (Tree star Inc., Ashland, OR, USA).
2.9. Statistical analysis
Data were analysed using GraphPad PRISM software (version 5; GraphPad Software Inc., San Diego, CA, USA). The normal distribution of the data was determined by the D'Agostino‐Pearson test. The data were analysed by a non‐parametric one‐way ANOVA using Kruskal‐Wallis test in multiple groups or using Mann‐Whitney test in case of two groups. Differences of P<.05 were considered statistically significant.
3. Results
3.1. Clonogenicity of human endometrial and endometriotic cells in co‐culture with autologous macrophages
Autologous macrophages or their CM were co‐cultured with the endometrial and endometriotic cells. As PMA was used to induce macrophages differentiation, cells treated with PMA alone served as a negative control. To maintain macrophage differentiation in long‐term culture, PMA was also supplemented into the co‐culture treatment. The total CE (large and small colonies) was 0.33±0.17% for endometrial epithelial cells (Figure 1A). Treatment with macrophages or their CM did not change the total CE of epithelial cells. There was no difference in the CEs of large endometrial epithelial colonies between groups treated with PMA, macrophages or macrophage CM when compared to the untreated control.
Figure 1.
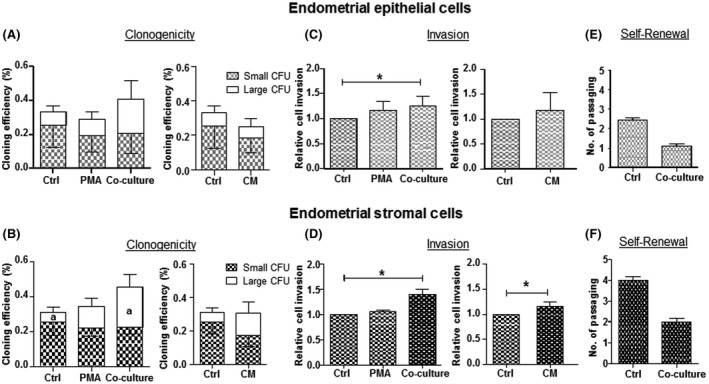
Clonogenicity, invasion ability and self‐renewal activity of endometrial epithelial and stromal cells with autologous macrophages. Cloning efficiency (CE) of (A) epithelial and (B) stromal cells after culture in PMA, co‐culture macrophages (co‐culture) and macrophage conditioned media (CM) for 15 days. Bars represent total CE (sum of small and large CFUs). White bars indicate large CFU; shaded bars indicate small CFUs. Relative cell invasion capacity of (C) epithelial and (D) stromal cells after culture in different conditions. Control was set as one. Self‐renewal activity of (E) epithelial and (F) stromal cells co‐culture with macrophages. Results reported as means±SEM; clonogenicity: epithelial n=4, stromal n=8; invasion: n=4, self‐renewal n=3. *,a,b,d,e P<.05; **,c P<.01; ***P<.001. ***; a–cSignificant differences for large CFUs, d,eSignificant differences for small CFUs. CFU, colony‐forming unit; CM, conditioned medium; PMA, phorbol‐12 myristate 13‐acetate; SEM, standard error of the mean
For endometrial stromal cells, the total CE (large and small colonies) was 0.31±0.10% (Figure 1B). Treatment with macrophages or their CM did not change the total CE of stromal cells. Interestingly, endometrial stromal cells co‐cultured with macrophages (0.23±0.08%) produced significantly more large colonies than stromal cells alone (0.06±0.03%, P<.05). Macrophage CM had no effect on the clonogenic growth of the large stromal colonies. The CEs of endometrial stromal small CFUs were similar in all conditions.
The overall clonogenicity displayed by endometriotic cells was lower. For endometriotic epithelial cells, there was significant increase in the total CE between the PMA (0.01±0.01%) and the macrophage co‐culture (0.14±0.05%, P<.05, Figure 2A) group. The proportion of large epithelial clones in the macrophage (0.13±0.05%) and the macrophage CM (0.07±0.03%)‐treated groups were significantly higher than that of the control (0.003±0.002%, P<.05). No difference was detected for the endometriotic epithelial small CFU in different conditions.
Figure 2.
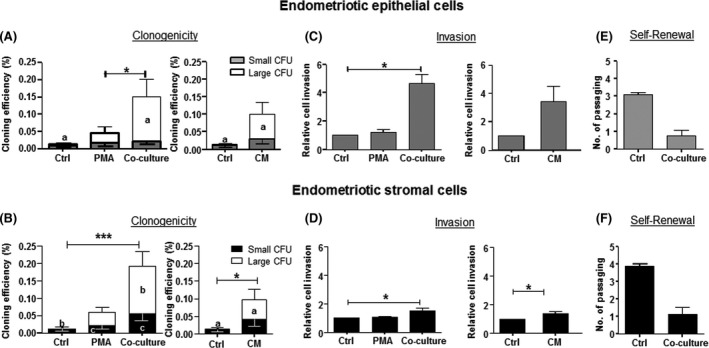
Clonogenicity, invasion ability and self‐renewal activity of endometriotic epithelial and stromal cells with autologous macrophages. Cloning efficiency (CE) of (A) epithelial and (B) stromal cells after culture in PMA, co‐culture macrophages (co‐culture) and macrophage conditioned media (CM) for 15 days. Bars represent total CE (sum of small and large CFUs). White bars indicate large CFU; shaded bars indicate small CFUs. Relative cell invasion capacity of (C) epithelial and (D) stromal cells after culture in different conditions. Control was set as one. Self‐renewal activity of (E) epithelial and (F) stromal cells co‐culture with macrophages. Results reported as means±SEM; clonogenicity: epithelial n=8, stromal n=13; invasion: epithelial n=3, stromal n=4, self‐renewal n=3. *,a,c P<.05; ***,b P<.001. a,bSignificant differences for large CFUs, cSignificant differences for small CFUs. CFU, colony‐forming unit; SEM, CM, conditioned medium; PMA, phorbol‐12 myristate 13‐acetate; standard error of the mean
For the endometriotic stromal cells, the total CE was 0.01±0.01% and significantly increased after macrophage co‐culture (0.19±0.04%, P<.001) and macrophage CM (0.10±0.04, P<.05, Figure 2B). More large endometriotic stromal CFUs were formed after co‐culture with macrophage (0.14±0.04%, P<.001) and macrophage CM (0.05±0.03%, P<.05) when compared with the control (0.0003±0.003%). Endometriotic stromal small colonies also significantly increased when co‐cultured with macrophages when compared with the control or the PMA group (P<.05).
3.2. Clonogenicity of human endometriotic cells after co‐culture with macrophages from patients without endometriosis
We performed additional co‐culture experiments to further investigate the interactions between macrophages and endometriotic cells. Endometriotic epithelial and stromal cells were co‐cultured with non‐endometriotic macrophages and their CM. The CEs for endometriotic epithelial and stromal cells were similar for all the conditions (Figure 3A,B).
Figure 3.
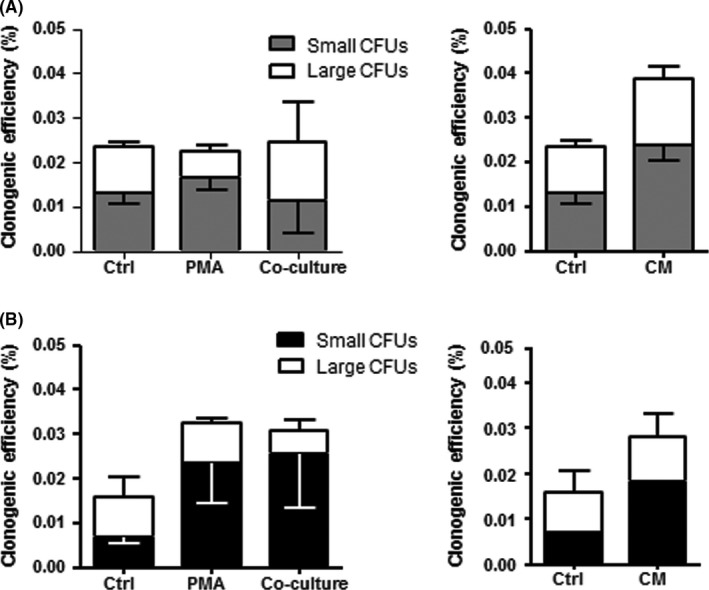
The clonogenicity of endometriotic epithelial and stromal cells after co‐culture with macrophages from patient without endometriosis. Cloning efficiency (CE) of (A) epithelial and (B) stromal cells after culture in PMA, co‐culture macrophages (without endometriosis) and macrophage conditioned media (CM) for 15 days. Bars represent total CE (sum of small and large CFUs). White bars indicate large CFU; shaded bars indicate small CFUs. Results reported as means±SEM; endometriotic epithelial and stromal cells n=3. CM, conditioned medium; PMA, phorbol‐12 myristate 13‐acetate
3.3. Invasion and self‐renewal ability of endometrial and endometriotic cells after co‐culture with autologous macrophages
There were no changes in the invasiveness of endometrial epithelial cells (Figure 1C). However, co‐culture with macrophages or macrophage CM increased the invasion of endometrial stromal cells (P<.05, Figure 1D). For endometriotic samples, the invasiveness of the epithelial cells increased after co‐culture with macrophages (P<.05, Figure 2C). This stimulatory effect was also detected on endometriotic stromal cells after co‐culture with macrophages and macrophage CM (P<.05, Figure 2D).
The self‐renewal ability of cells in the large CFU after co‐culture was assessed using a serial cloning strategy. We observed a decline in the number of self‐renewal rounds in cells after co‐culture when compared to the corresponding control (endometrial epithelial: 1.0±0.1 vs 2.4±0.1, Figure 1E; endometrial stromal: 2.0±0.2 vs 4.0±0.2, Figure 1F; endometriotic epithelial: 0.8±0.3 vs 3.1±0.1, Figure 2E; endometriotic stromal: 1.1±0.4 vs 3.9±0.1; Figure 2F) though the differences were not yet significant.
As the self‐renewal activity of stromal cells declined after macrophage co‐culture, we examined the phenotypic expression of the endometrial stromal cells using the endometrial mesenchymal‐like stem cell markers: CD140b and CD146. Flow cytometry analysis of CD140b+CD146+ cells on clonally derived stromal cells after co‐incubation with macrophages (3.12±2.50%) and their CM (6.28±5.0%) was not significantly different from the control (7.84±3.5%, Fig. S3E).
3.4. Cytokine profile of macrophages from patients with and without endometriosis
The macrophage CM from patients with and without endometriosis were compared using a cytokine array for 42 cytokines (Table S1). Densitometric analysis revealed a 4‐fold higher level of CSF‐1 in the CM of endometriosis samples (1.11±0.67) than in that of no endometriosis (0.25±0.04, P<.05, Figure 4A,B). Consistently, the amount of CSF‐1 released into the CM from endometriotic macrophages was significantly higher (597±140 pg/mL, n=11) than that from normal endometrial macrophages (159±40 pg/mL, P<.05, Figure 4B) determined by ELISA. However, CSF‐1 at concentrations of 30, 300 and 3000 pg/mL did not affect the total CEs of epithelial and stromal cells from endometrial (Figure 5A,C) and endometriotic tissues (Figure 6A,C). The different concentrations of CSF‐1 did not affect the invasion ability of endometrial (Figure 5B,D) or endometriotic cells (Figure 6B,D). Although a decline trend in the CEs of endometrial cells were observed after neutralization with CSF‐1 antibody, it did not reach statistic significance due to the small sample size (Fig. S4A,B).
Figure 4.
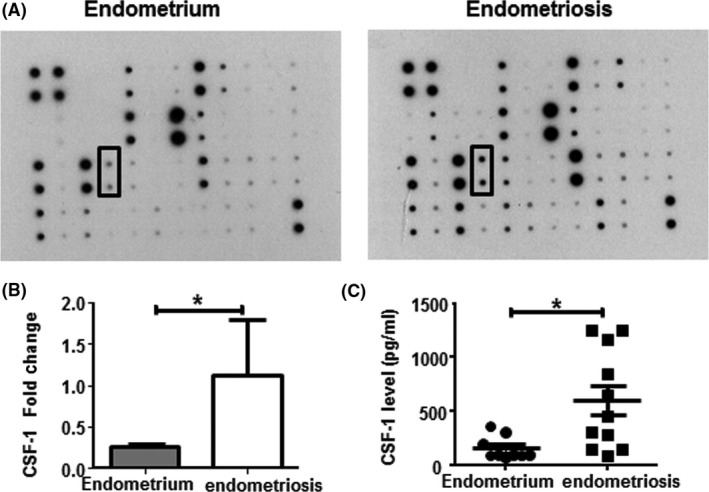
Identification of CSF‐1 released by macrophages with and without endometriosis. Cytokine arrays of the expression of 42 human cytokines in the macrophage conditioned medium from women with and without endometriosis were evaluated. (A) Representative images of the densitometry produced from the cytokine array. (B) Arrays were visualized by enhanced luminal‐based chemiluminescence and the dot intensities of CSF‐1 were quantified by densitometry using Quantity One software. Each bar consists of relative expression (%) for no endometriosis (grey bar) and endometriosis (white bar) of macrophage conditioned medium, n=6. (C) Histogram showing the amounts, in pg/mL, of the CSF‐1 as quantified by ELISA, endometrium: n=9; endometriosis: n=11. Results reported as means±SEM; *P<.05. CSF‐1, colony‐stimulating factor‐1; ELISA; enzyme‐linked immunosorbent assay, SEM, standard error of the mean
Figure 5.
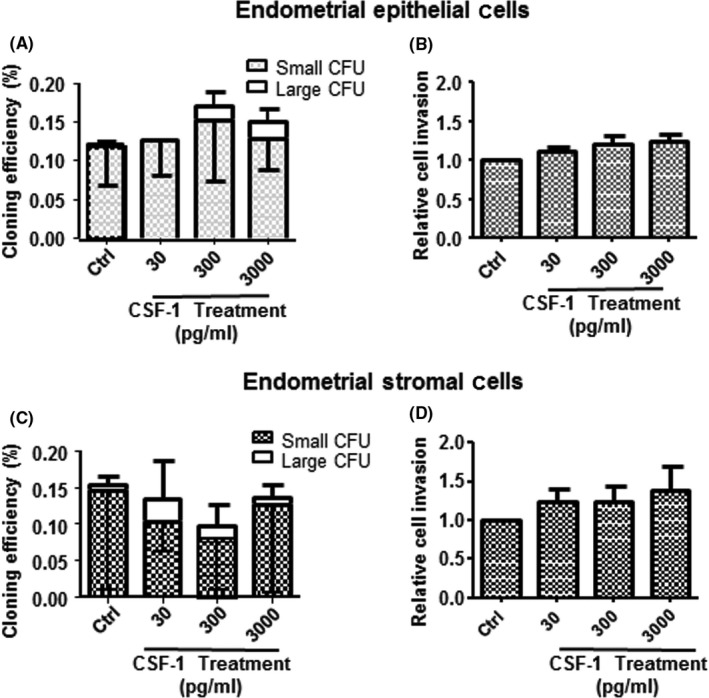
The clonogenicity and invasion activity of endometrial epithelial and stromal cells after CSF‐1 treatment. Cloning efficiency (CE) of endometrial (A) epithelial and (C) stromal cells after treatment with different concentrations of CSF‐1: 30, 300 and 3000 pg/mL for 15 days. Bars represent total CE (sum of small and large CFUs). Relative cell invasion capacity of epithelial (B) and stromal (D) cells after culture in different conditions. Control was set as one. Results reported as means±SEM; endometrial epithelial and stromal cells n=3. CFU, colony‐forming unit; CSF‐1, colony‐stimulating factor‐1; SEM, standard error of the mean
Figure 6.
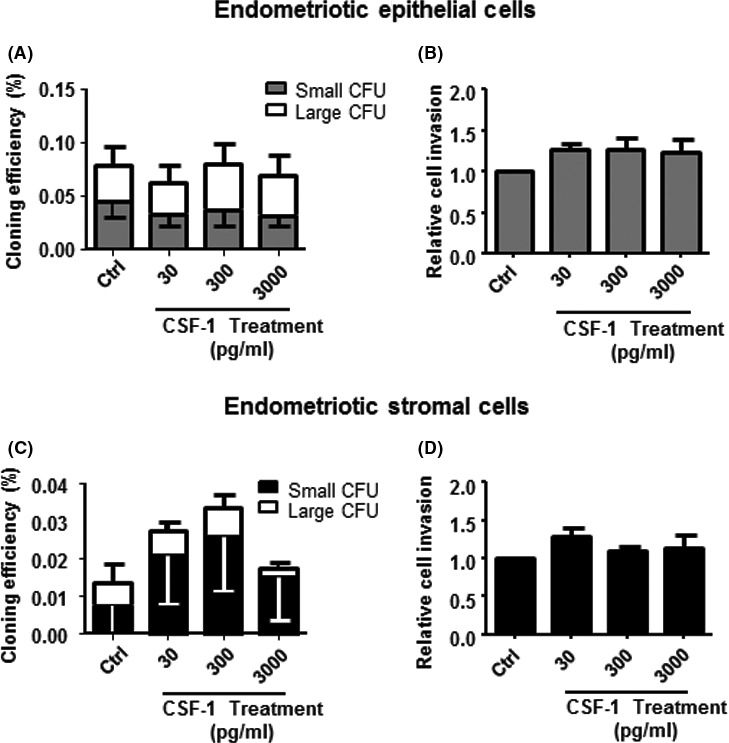
The clonogenicity and invasion activity of endometriotic epithelial and stromal cells after CSF‐1 treatment. Cloning efficiency (CE) of endometriotic (A) epithelial and (C) stromal cells after treatment with different concentrations of CSF‐1: 30, 300 and 3000 pg/mL for 15 days. Bars represent total CE (sum of small and large CFUs). Relative cell invasion capacity of epithelial (B) and stromal (D) cells after culture in different conditions. Control was set as one. Results reported as means±SEM; endometriotic epithelial cells (clonogenicity, n=7; invasion, n=3); endometriotic stromal cells (clonogenicity, n=8; invasion, n=3). CFU, colony‐forming unit; CSF‐1, colony‐stimulating factor‐1; SEM, standard error of the mean
4. Discussion
Endometriosis is a multifactorial disease, and its aetiology remains uncertain. Among the theories proposed to explain the pathogenesis of endometriosis, Sampson's theory of retrograde menstruation is most widely accepted. In reproductive‐age women, a reflux of menstrual tissue enters the peritoneal cavity and embeds into intra‐abdominal areas.27 Susceptibility to endometriosis is due to enhanced endometrial cell adhesion to the peritoneum and poor clearance of refluxed endometrial cells by the host immune response.28 Macrophage function is augmented in endometriotic lesions.14 Bacci et al. demonstrated a pro‐inflammatory role for macrophages that exacerbates growth and vascularization of endometriotic lesions.29
In this study, the clonogenicity and invasiveness of endometriotic stromal cells increased significantly after co‐cultured with autologous macrophages. Interestingly, the stimulatory effect was not observed when endometriotic stromal cells were co‐cultured with macrophages from patients without endometriosis. These observations suggest there may be a two‐way communication between macrophages and the endometriotic stromal cells in regulating the proliferation and invasion activity of colony‐forming cells. Macrophages can be stimulated by soluble factors derived from endometriotic cells and differentiate in response to the changing microenvironment. Thus, the communication between macrophages and endometriotic cells can facilitate the progression of the disease.
Previously, we demonstrated the existence of colony‐forming cells in human endometrium and endometriosis.4, 23 Endometrial and endometriotic cells from large CFUs display properties of somatic stem cells.23, 30 The cells in the large CFUs are heterogeneous, compromising stem cells and their differentiating progenies. Thus, the observed increase of large CFUs may not be due to an expansion of the number of stem cells but rather an expansion of their downstream progenitors or transit amplifying cells. This notion is supported by our finding that co‐culture with autologous macrophages lowered the self‐renewal ability of clonally derived endometrial and endometriotic cells in serial cloning assays. Furthermore, clonally derived stromal cells after co‐culture with macrophage or CM displayed lower expression of the endometrial mesenchymal stem‐like cell surface markers (CD140b and CD146). It is likely that macrophages enhanced the proliferation but readily exhausted the proliferative potential of progenitors/transit amplifying cells of large CFUs.
We also examined the differences of cytokines derived from macrophages from women with and without endometriosis. As endometrial macrophages have a role in tissue angiogenesis, tissue remodelling and immune defence, a major population of uterine tissue macrophages is alternatively activated.31 Alternatively activated macrophages are more abundant in patients with endometriosis 32 and exacerbate the growth and vascularization of endometriotic lesions.29 In this study, the macrophages from women with and without endometriosis were found to polarize towards the alternatively activated or M2 phenotype, and endometriotic macrophages released more CSF‐1, which has been associated with the early establishment of endometriotic lesions.33 The level of CSF‐1 in the peritoneal fluid of patients with endometriosis is higher than those without.34 CSF‐1 can also enhance the proliferation, attachment and invasion of endometrial cells35, 36. However, our results showed that CSF‐1 alone did not affect the clonogenicity or invasion activity of endometrial or endometriotic cells. Therefore, the stimulatory activities of macrophages co‐culture with endometrial and endometriotic cells could be mediated by one or a cocktail of regulators that were not determined in this study. In addition, it is worth noting that the endometrium would produce other factors that mediate endometrial macrophage differentiation, and our current in vitro model may therefore not fully represent the behaviour of these macrophages. A limitation of this study was the source of the macrophage used. Peritoneal macrophages would undoubtedly provide a better insight into the peritoneal phenomenon on endometrial and endometriotic cells. However, to obtain sufficient amount of peritoneal macrophages would be difficult, hence we used peripheral monocyte‐derived macrophages. Other immune cells such as T cells within the endometrial leucocyte population can also promote the growth and invasion of endometriotic stromal cells.37
Currently, direct evidence supporting the involvement of endometrial stem/progenitor cells in the aetiology of endometriosis is limited. While the existence of endometrial stem/progenitor cells in the endometrial basalis is well documented,38 some evidence supports the presence of endometrial stem/progenitor cells in endometriotic lesions.23, 39 There is also evidence that fragments of the shed endometrial basalis, likely containing endometrial stem/progenitor cells, are more often shed in the menstrual blood of women with endometriosis than in that of healthy control subjects.40, 41 Thus, when exposed to an environment conducive to the formation of endometriosis, such as the presence of dysregulated macrophages, the retrograded endometrial stem/progenitor cells differentiate and their progenies proliferate in ectopic sites, leading to the development of endometriotic lesions. However, whether the altered macrophage changes are primary or secondary occurrences remains uncertain.
In conclusion, the evidence that co‐culture of macrophages enhances the clonogenicity and invasion activity of endometriotic stromal cells suggests phagocytic cells and endometriotic cells may contribute to the committed progeny expansion of retrograde endometrial cells, giving rise to endometriosis. Further work should be undertaken to identify the kinase signals involved in the cell communication between macrophages and endometriotic stromal cells, as these pathways may represent a target for endometriosis treatment.
Conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Acknowledgements
We thank Ms. Joyce Yuen for the collection of tissue and the gynaecologists at Queen Mary Hospital for the provision of laparoscopic ovarian cystectomy and hysterectomy tissue. We acknowledge the staff at the Faculty Core Facility, the University of Hong Kong for their assistance in this study. We also thank Ms. Vicki Geall for proof reading the manuscript.
Chan RWS, Lee C‐L, Ng EHY, Yeung WSB . Co‐culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells. Cell Prolif. 2017;50:e12330 10.1111/cpr.12330
Funding information
This study was supported through a small project funding from the University of Hong Kong (201209176168) to R.W.S Chan.
References
- 1. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- 2. Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;96:21–34. [DOI] [PubMed] [Google Scholar]
- 3. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2015;22:137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. [DOI] [PubMed] [Google Scholar]
- 5. Schwab KE, Chan RW, Gargett CE. Stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84:1124–1130. [DOI] [PubMed] [Google Scholar]
- 6. Meng X, Ichim T, Zhong J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brosens I, Brosens JJ, Benagiano G. The eutopic endometrium in endometriosis: are the changes of clinical significance? Reprod Biomed Online. 2012;24:496–502. [DOI] [PubMed] [Google Scholar]
- 9. Garry R, Hart R, Karthigasu KA, Burke C. Structural changes in endometrial basal glands during menstruation. BJOG‐Int J Obstet Gy. 2010;117:1175–1185. [DOI] [PubMed] [Google Scholar]
- 10. Barragan F, Irwin JC, Balayan S, et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13:467–476. [DOI] [PubMed] [Google Scholar]
- 12. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. [DOI] [PubMed] [Google Scholar]
- 13. Chuang P‐C, Wu M‐H, Shoji Y, Tsai S‐J. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol. 2009;219:232–241. [DOI] [PubMed] [Google Scholar]
- 14. Capobianco A, Rovere‐Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. [DOI] [PubMed] [Google Scholar]
- 16. Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001;7:163–168. [DOI] [PubMed] [Google Scholar]
- 17. King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–296. [DOI] [PubMed] [Google Scholar]
- 19. Rakhila H, Al‐Akoum M, Bergeron M‐E, et al. Promotion of angiogenesis and proliferation cytokines patterns in peritoneal fluid from women with endometriosis. J Reprod Immunol. 2016;116:1–6. [DOI] [PubMed] [Google Scholar]
- 20. Olive DL. Cytokine Regulation In: David Olive, ed. Endometriosis in Clinical Practice. Taylor & Francis, United Kingdom: CRC Press;2004:121–146. [Google Scholar]
- 21. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. [DOI] [PubMed] [Google Scholar]
- 22. Canis M, Donnez JG, Guzick DS, et al. Revised american society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- 23. Chan RWS, Ng EHY, Yeung WSB. Identification of cells with colony‐forming activity, self‐renewal capacity, and multipotency in ovarian endometriosis. Am J Pathol. 2011;178:2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA‐stimulated THP‐1 cells and monocyte‐derived macrophages. PLoS ONE. 2010;5:e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwab KE, Gargett CE. Co‐expression of two perivascular cell markers isolates mesenchymal stem‐like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. [DOI] [PubMed] [Google Scholar]
- 26. Xiang L, Chan RW, Ng EH, Yeung WS. Nanoparticle labeling identifies slow cycling human endometrial stromal cells. Stem Cell Res Ther. 2014;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farquhar C. Endometriosis. BMJ. 2007;334:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. [DOI] [PubMed] [Google Scholar]
- 29. Bacci M, Capobianco A, Monno A, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen AL, Collins J, Shipman EP, Wira CR, Guyre PM, Pioli PA. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol. 2012;68:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Itoh F, Komohara Y, Takaishi K, et al. Possible involvement of signal transducer and activator of transcription‐3 in cell–cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril. 2013;99:1705–13.e1. [DOI] [PubMed] [Google Scholar]
- 33. Budrys NM, Nair HB, Liu Y‐G, et al. Increased expression of macrophage colony stimulating factor and its receptor in patients with endometriosis. Fertil Steril. 2012;97:1129–35.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinberg JB, Haney AF, Xu FJ, Ramakrishnan S. Peritoneal fluid and plasma levels of human macrophage colony‐stimulating factor in relation to peritoneal fluid macrophage content. Blood. 1991;78:513–516. [PubMed] [Google Scholar]
- 35. Jensen JR, Witz CA, Schenken RS, Tekmal RR. A potential role for colony‐stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil Steril. 2010;93:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aligeti S, Kirma NB, Binkley PA, Schenken RS, Tekmal RR. Colony‐stimulating factor‐1 exerts direct effects on the proliferation and invasiveness of endometrial epithelial cells. Fertil Steril. 2011;95:2464–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li MQ, Wang Y, Chang KK, et al. CD4(+)Foxp3(+) regulatory T cell differentiation mediated by endometrial stromal cell‐derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gargett C. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87–101. [DOI] [PubMed] [Google Scholar]
- 39. Kao AP, Wang KH, Long CY, et al. Interleukin‐1beta induces cyclooxygenase‐2 expression and promotes the invasive ability of human mesenchymal stem cells derived from ovarian endometrioma. Fertil Steril. 2011;96:678–84 e1. [DOI] [PubMed] [Google Scholar]
- 40. Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002;17:2725–2736. [DOI] [PubMed] [Google Scholar]
- 41. Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early‐onset endometriosis. Mol Hum Reprod. 2014;20:591–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


