Abstract
Objectives
Cytotoxic chemotherapy is an effective and traditional treatment of ovarian cancer. However, chemotherapy‐induced apoptosis may also trigger and ultimately accelerate the repopulation of the small number of adjacent surviving cells. This study mainly focused on the tumour cell repopulation caused by chemotherapy in ovarian cancer and the adjunctive/synergistic effect of Berberine on the prevention of tumour repopulation.
Materials and methods
The transwell system was used to mimic the co‐culture of surviving ovarian cancer cells in the microenvironment of cytotoxic chemotherapy‐treated dying cells. Tumour cell proliferation was observed by crystal violet staining. AA and PGE 2 levels were measured by ELISA, and changes of protein expression were analysed by Western blot.
Results
Chemotherapy drug VP16 treatment triggered AA pathway, leading to the elevated PGE 2 level, and ultimately enhanced the repopulation of ovarian cancer cells. Berberine can block the caspase 3‐iPLA 2‐AA‐COX‐2‐PGE 2 pathway by inhibiting the expression of iPLA 2 and COX‐2. Berberine can also reverse the increased phosphorylation of FAK caused by abnormal PGE 2 level and thus reverse the repopulation of ovarian cancer cells after VP16 treatment.
Conclusions
Our observation suggested that Berberine could inhibit the chemotherapy‐induced repopulation of ovarian cancer cells by suppressing the AA pathway and phosphorylation of FAK. And these findings implicated a novel combined use of Berberine and chemotherapeutics, which might prevent ovarian cancer recurrence by abrogating early tumour repopulation.
1. INTRODUCTION
Ovarian cancer is the most common cause of death from gynaecologic cancer worldwide.1, 2, 3 The conventional ovarian cancer treatment strategy involves debulking surgery, in which visible tumour nodules are removed before chemotherapy. Following cytoreductive surgery, ovarian cancer patients undergo platinum‐ and taxane‐based chemotherapy.4, 5 However, in over 70% of ovarian cancer cases, patients usually recur within 12‐ to 24 months after initial diagnosis and die of progressively chemotherapy‐resistant disease.6 Considering the high rate of recurrence of ovarian cancer patients, new therapeutic strategies, especially against relapse after chemotherapy, are urgently needed.7
Most common chemotherapeutics lead to the induction of apoptosis in the targeted cancer cell, including platinum‐ and taxane‐based chemotherapy in ovarian cancer.8 Although activation of the caspase 3 is a hallmark of apoptosis and usually tightly associated with response to chemotherapy, it also has been suggested a role in tissue regeneration and repopulation by stimulating signal transduction and cell proliferation in neighbouring, non‐apoptotic cells.9 The cleaved caspase 3 can activate cytosolic calcium‐independent phospholipase A2 (iPLA2)10 and consequently trigger the cascade reaction of AA metabolic pathway, leading to abnormal changes of AA and PGE2 levels in the microenvironment of tumour. And PGE2 was reported to stimulate cell proliferation and tumour growth.11, 12 PGE2 treatment was able to increase the phosphorylation of FAK in hepatocellular carcinoma cells,13 a 125‐kDa non‐receptor cytoplasmic protein tyrosine kinase, which has been implicated in the regulation of a variety of cellular signalling pathways that control cell proliferation.14, 15
Berberine, an isoquinoline alkaloid, is found in the rhizome; roots and stem bark the Berberis species, such as Hydrastis canadensis (goldenseal), Cortex phellodendri (Huangbai) and Rhizoma coptidis (Huanglian).16, 17, 18 Berberine exhibits multiple pharmacological activities, including anticancer and antidiabetes mellitus effects.19, 20, 21 In our previous studies, we found that Berberine suppressed cPLA2 and COX‐2 gene expressions and inhibited AA pathway by elevating the ratio of AA to PGE2 in hepatocellular carcinoma in vitro and in vivo.22 Additionally, Berberine was also found to inhibit phosphorylated forms of FAK of SCC‐4 and CRC cells.23, 24 Therefore, we propose to explore whether Berberine overcomes repopulation of ovarian cancer cells due to activation of caspase 3‐mediated AA pathway and phosphorylation of FAK after chemotherapy.
In our study, we utilized transwell system for co‐cultures of human ovarian cancer cells SKOV3 (upper chamber, as receptor cells) in microenvironment of the dying SKOV3 cells (lower chamber, as feeder cells) treated by chemotherapy drug VP‐16. We then showed that the VP16 treatment triggered the activation of caspase 3 and AA pathway (iPLA2‐AA‐PGE2 axis). In the mean time, the decreased AA and increased PGE2 levels in the microenvironment of tumour cells were observed and accompanied with activation of FAK. Berberine can inhibit the chemotherapy‐induced repopulation of ovarian cancer cells and suppress the AA metabolic pathway, PGE2 level as well as phosphorylation of FAK. Thus, a combination of chemotherapy and Berberine may become a promising strategy to prevent the recurrence of ovarian cancer after chemotherapy.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Human prostaglandin E2 ELISA Kit and the Human Arachidonic acid ELISA Kit were purchased from R&D Systems (Minneapolis, MN, USA). Prostaglandin E2 was purchased from Sigma (St. Louis, MO, USA). RPMI medium 1640, foetal bovine serum (FBS), penicillin, streptomycin and BCA protein assay kit were bought from Beyotime Institute of Biotechnology (Jiangsu, China). The primary antibodies were diluted 1:1000 before use, including FAK (Cat. # ab40794; Abcam), p‐FAK (Cat. # ab39967; Abcam, Pudong, Shanghai, China), iPLA2 (Cat. # sc‐25504; Santa Cruz Biotechnologies, Santa Cruz, CA, USA), COX‐2 (H‐62) (Cat. # sc‐7951; Santa Cruz Biotechnologies), caspase 3 (Cat. # 9662, Cell Signaling, Beverly, MA, USA), and β‐actin (Cat. # 1854; Epitomics, Burlingame, CA, USA), goat anti‐rabbit IgG (H+L), HRP conjugate (Cat. # SA00001‐2; Proteintech Group, Rosemont, IL, USA). VP16 (etoposide) injection was purchased from Qilu pharmaceutical Co., LTD (Jinan, China). All the chemical compounds were analytically pure reagents.
2.2. Cell culture
The human epithelial ovarian cancer cell line SKOV3 was cultured in RPMI 1640 supplemented with 10% FBS, penicillin and streptomycin at 100 U/mL and 100 μg/mL, respectively. Cells were incubated at 37°C in 5% CO2‐95% air atmosphere.
2.3. Transwell system
The transwell system (Corning, NY, USA) with 4.0‐μm pore size semipermeable membrane filter insert was used for preparing the co‐culture system. In the bottom chamber, the SKOV3 cells (105 cells per well) as feeder cell was treated with VP16, Berberine or the combination of VP16 and Berberine, and in the upper chamber the less number of SKOV3 cells (1000 cells per well) were designed as receptor cells, which were exposed to culture medium from feeder cells. For each group, feeder cells were rinsed with PBS twice after a 24‐hour culture (Control group [con] and Berberine group) or treatment with 5 μmol/L VP16 (VP16 group and VP16 + Berberine group). Then, fresh culture medium was added and upper chamber with SKOV3 receptor cells was inserted. Then, Berberine at a final concentration of 5 μmol/L was added into the culture medium of both the upper and lower chambers in Berberine group and VP16 + Berberine group on Day 1. The transwell system was sustained for 10 days. The Berberine (5 μmol/L) was supplemented every 5 days in Berberine alone group and VP16 + Berberine group. Supernatants from the upper chambers of the transwell system during the incubation period were collected daily (50 μL per day) for the analysis of the AA and PGE2 levels by ELISA. After 10‐day incubation, receptor cells in the semipermeable filter inserts were fixed by methanol for proliferation assays.
2.4. Proliferation assays
Receptor cells in the semipermeable filter inserts of the transwell system were then stained by 0.1% crystal violet for 20 minutes at room temperature. After washing the wells with PBS twice, the cells on upper surface of the membranes were photographed under the microscope. Finally, the cells were solubilized with 1 mL 33% acetic acid per well and quantified by the absorbance at 570 nm.13, 25
SKOV3 cells were seeded in 2 mL of medium at 1000 cells/well in 6‐well culture plates and grown overnight. Treatments of 5 μmol/L Berberine (as group Ber), 3.125 ng/mL prostaglandin E2 (as group PGE2) and their combination (as group Ber + PGE2) were conducted for 7 days. Prostaglandin E2 was added into relative groups daily. Afterwards, the cells were fixed by methanol for proliferation assays as previously described.
2.5. Western blot analysis
Receptor cells of the sixth‐day incubation within transwelll system and cells with prostaglandin E2 treatments were collected for protein extraction for Western blot analysis of the level of FAK and p‐FAK. Feeder cells of the third‐day incubation (as the most significant apoptosis response of SKOV‐3 cells after VP16 treatment appeared on Day 3) within transwell system were collected to analyse the level of caspase 3 and cleaved caspase 3. Feeder cells of the sixth‐day incubation (as the first detectable significant differences of AA and PGE2 level appeared since day 6) were harvested to analyse the level of iPLA2 and COX‐2. SKOV3 cells were collected by centrifugation and washed twice with cold PBS. The cell pellets were suspended in 200 μL ice‐cold RIPA lysis buffer and lysed for 15 minutes on ice, vortexed every 10 minute and then centrifuged at 15 000 g for 15 minutes. The immunoblotting analysis was performed with 20 μg sample proteins on a 12% SDS‐polyacrylamide electrophoresis gel (SDS‐PAGE). The electrophoresis was carried out first at 80 V for 20 minutes followed by 160 V for 60‐90 minutes. The proteins separated by SDS‐PAGE gel are transferred onto PVDF by wet transfer system in ice‐cold transfer buffer. The transferred membranes were blocked for 2 hour and then incubated overnight with a primary antibody (1:1000 diluted) with gentle agitation at 4°C. The membranes were then washed and incubated with a secondary antibody (1:2000 diluted) at room temperature for 1.5 hour. Bands were visualized by an ECL Western blotting detection system (Tanon 4200). Grey value of the bands in the Western blot was analysed using Quantity One software.
2.6. Measurement of AA and PGE2 levels
Supernatants collected from the transwell system during the incubation period were thawed, and prostaglandin E2 (PGE2) and arachidonic acid (AA) levels were analysed using the Human Prostaglandin E2 ELISA Kit and the Human Arachidonic acid ELISA Kit according to the manufacturer's instructions. The absorbances were read at a wavelength of 450 nm.
2.7. Statistical analysis
All experiments were completed at least three times unless otherwise indicated. All data were expressed as mean ± SD. Statistical significances among groups were analysed by one‐way analysis of variance followed by Dunnett's multiple comparison post‐test. A value with P < .05 was considered as statistically significant.
3. RESULTS
3.1. Berberine reversed the chemotherapy‐induced repopulation of ovarian cancer cells
Recurrence is considered as a cause of cancer treatment failure. Researchers have observed that radiation‐induced apoptotic tumour cells could stimulate the repopulation of tumours from a small number of surviving cells.10 In our study, we established transwell system for co‐cultures of the chemotherapy drug VP16 or vehicle treated (feeder cells at bottom floor) and ovarian cancer cell line (receptor cells at top floor) SKOV3 cells. Compare with vehicle treatment, VP16 clearly induced the feeder cell death at the bottom floor (data not shown). Consistent with previous report, the proliferation and viability of SKOV3 cells on the top chamber (receptor cells) also significantly increased (Figure 1A,B). In order to search for potential strategy to overcome the repopulation of ovarian cancer cells after chemotherapy drug VP16 treatment, we tested several small molecular drugs from the market regarding this certain type of repopulation (data not shown). The addition of 5 μmol/L Berberine in both the upper and lower chambers greatly abrogated the proliferation and viability of receptor SKOV3 cells on the top chamber in VP16 + Berberine group, while Berberine single treatment at the same concentration did not have a significant influence on either receptor or feeder cells in Berberine group (Figure 1A,B). These results demonstrated that Berberine could reverse the repopulation of ovarian cancer cells after chemotherapy drug VP16 treatment.
Figure 1.
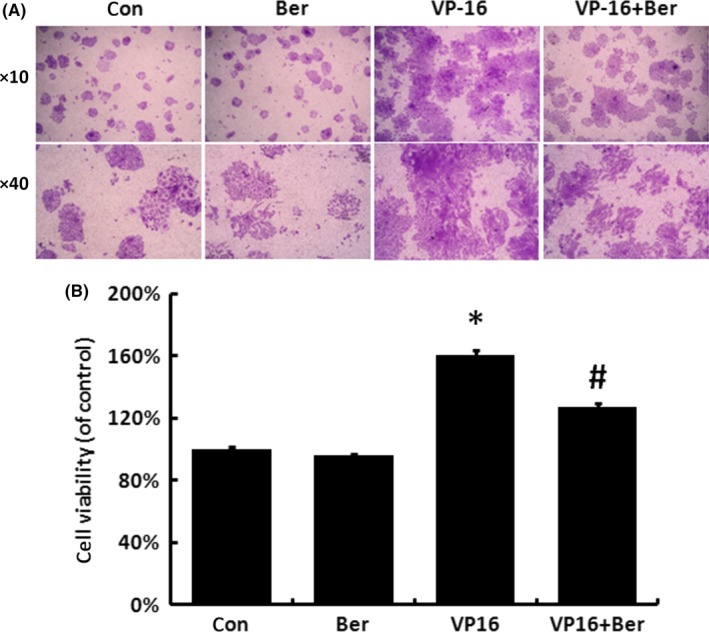
Berberine reversed the chemotherapy‐induced accelerated repopulation of ovarian cancer cells. A, After feeder cells being treated with VP16 and both feeder and receptor cells being treated with 5 μmol/L Berberine, crystal violet staining assay were applied to analyse the proliferation of the receptor cells. B, The stained cells of groups in (A) were solubilized with 1 mL of 33% acetic acid and quantified by the absorbance at 570 nm. Results were expressed as the mean ± SD of three independent experiments, *P < .05 compared with control, #P < .05 compared with VP16 group
3.2. Berberine blocked the iPLA2‐AA‐COX‐2‐PGE2 pathway induced by caspase 3 activation after chemotherapy
Activation of caspase 3 was indicated to render tissue regeneration and repopulation by stimulating signal transduction and cell proliferation in neighbouring non‐apoptotic cells.10 We found that the VP16 treatment significantly increased the cleavage of caspase 3 of feeder cells, while 5 μmol/L Berberine did not have a significant influence on caspase 3 cleavage compared with either control or VP16 group (Figure 2A,B). Receptor cells in VP16 group showed an increased iPLA2 and COX‐2 protein levels, while the addition of Berberine partly reversed these increases (Figure 2C,D).
Figure 2.
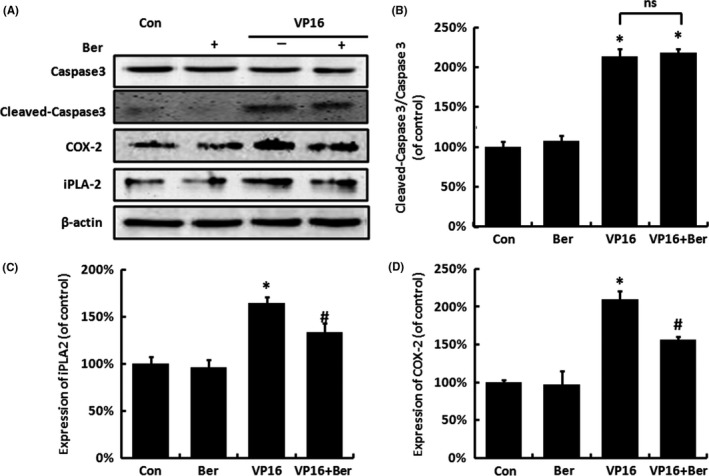
The protein expression of caspase 3, cleaved caspase 3 (of feeder cells), iPLA 2 and COX‐2 (of receptor cells) in SKOV3 cells treated by VP16 and/or Berberine within the transwell system. A, The protein expression of caspase 3, cleaved caspase 3, iPLA 2 and COX‐2 of feeder cells was measured by Western blot analysis. B, Relative quantification of cleaved caspase 3/caspase 3 levels expressed relative to control. C, Relative quantification of iPLA 2 levels expressed relative to control. D, Relative quantification of COX‐2 levels expressed relative to control. Each bar represents the mean ± SD of three independent experiments, n = 3, *P < .05 compared with control; #P < .05 compared with VP16 group; ns, no significant difference
We next measured the level of AA and PGE2 in the supernatant of the transwell system. As shown in Figure 3, 5 μmol/L Berberine did not cause significant changes in the levels of AA and PGE2 compared with control, while the VP16 treatment on feeder cells showed a decrease in AA level (Figure 3A) and an increase in PGE2 level (Figure 3B) in a time‐dependent manner, with significant differences appearing since day 6, and the addition of Berberine partly reversed these changes of AA and PGE2 production compared with VP16 group, with significant differences appearing since day 9 (Figure 3A,B). These results indicated that Berberine reverses the chemotherapy drug‐induced caspase 3‐iPLA2‐AA‐COX‐2‐PGE2 pathway in SKOV‐3 cells.
Figure 3.
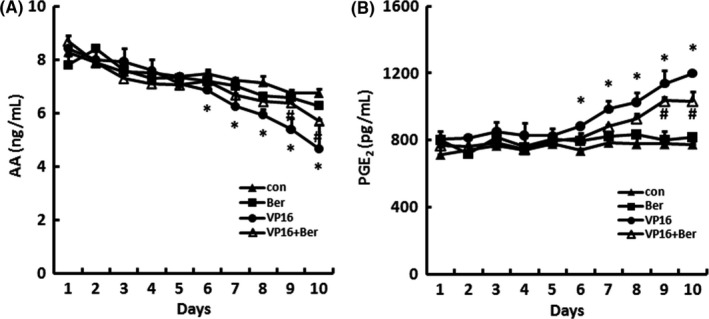
The AA and PGE 2 levels in SKOV3 cells treated by Berberine within the transwell system. The supernatants of each group were collected each day from the transwell system during the 10‐day incubation, A, AA and B, PGE 2 levels were measured by ELISA. Three independent experiments were carried out. Results were expressed as the mean ± SD, n = 3, *P < .05 compared with control; #P < .05 compared with VP16 group
3.3. Berberine inhibited PGE2‐stimulated repopulation through the inhibition of FAK phosphorylation
As it was shown in Figures 2 and 3, we found that the VP16 treatment led to the induction of iPLA2 and COX‐2, as well as the following upregulation of PGE2 level. In order to determine whether PGE2 is the key molecule for transducing the activation of caspase 3‐mediated regeneration signalling to adjacent cells, exogenous PGE2 was added into the culture media to mimic the abnormal level of PGE2 caused by VP16 treatment. SKOV‐3 cell proliferation was enhanced expectedly after exogenous PGE2 treatment compared with Control group. More importantly, the addition of Berberine was still capable to reverse exogenous PGE2‐stimulated cell proliferation (Figure 4A,B).
Figure 4.
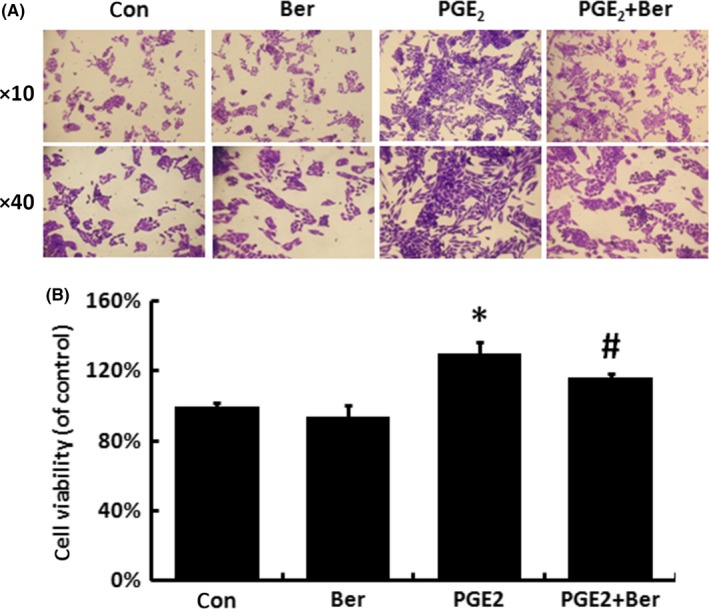
The inhibitory effect of Berberine on the exogenous PGE 2 induced proliferation of SKOV‐3 cells. A, After 7‐day incubation with exogenous PGE 2, crystal violet staining assay was applied to analyse the proliferation of SKOV‐3 cells. B, The stained cells of groups in (A) were solubilized with 1 mL of 33% acetic acid and quantified by the absorbance at 570 nm. Results were expressed as the mean ± SD of three independent experiments, *P < .05 compared with control; #P < .05 compared with PGE 2 group
Previous research found that PGE2 treatment can increase the phosphorylation of FAK.13 We then investigated the influence of Berberine on FAK and its phosphorylation. VP16 treatment significantly increased the phosphorylation of FAK, while the expression of FAK remained still, and Berberine could partly reverse the increase in FAK phosphorylation (Figure 5A,B). And as shown in Figure 6, exogenous PGE2 also caused an increase in FAK phosphorylation, while the addition of Berberine partly reversed this effect. These data suggested that the abnormal PGE2 level may accelerate the repopulation through the activation of FAK. Despite of iPLA2 and COX‐2, Berberine also inhibited the repopulation through the inhibition of the FAK phosphorylation.
Figure 5.
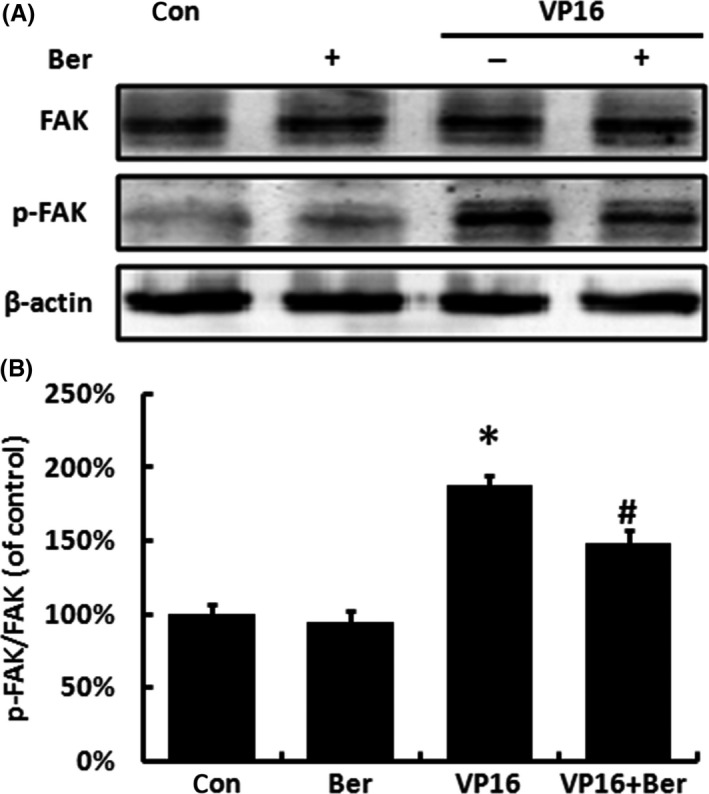
The protein expression of FAK and p‐FAK of receptor cells treated by VP16 and/or Berberine within the transwell system. A, The protein expression of FAK and p‐FAK of receptor cells was measured by Western blot analysis. B, Relative quantification of p‐FAK/FAK levels expressed relative to control. Each bar represents the mean ± SD of three independent experiments, n = 3, *P < .05 compared with control; #P < .05 compared with VP16 group
Figure 6.
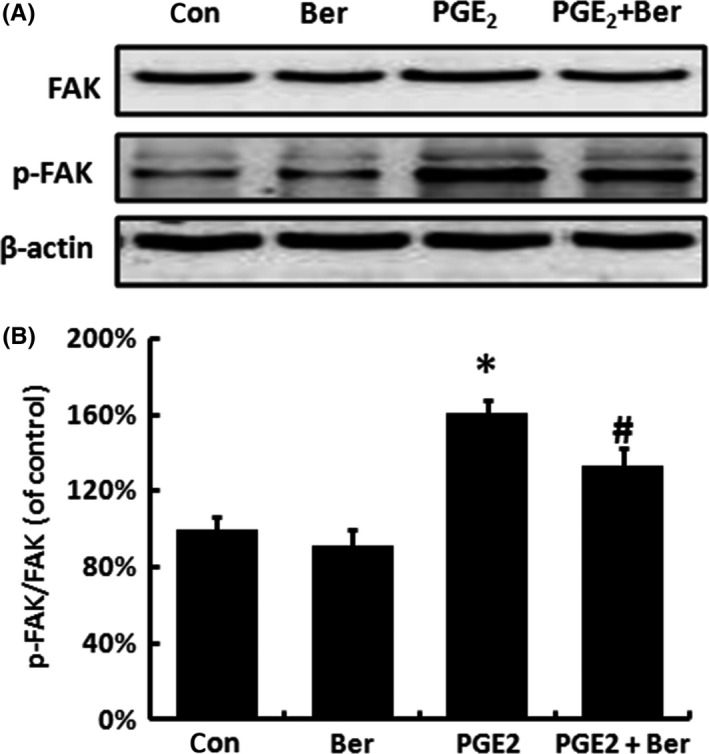
Berberine inhibited the exogenous PGE 2‐induced FAK phosphorylation. A, After treated with exogenous PGE 2 and Berberine, the protein expression FAK and p‐FAK of SKOV‐3 cells was measured by Western blot analysis. B, Relative quantification of p‐FAK/FAK levels expressed relative to control. Each bar represents the mean ± SD of three independent experiments, n = 3, *P < .05 compared with control; #P < .05 compared with PGE 2 group
4. DISCUSSION
Despite the relative high response for cytotoxicity agents in tumour treatment, chemotherapy is nowadays being questioned by a growing incidence of drug resistance and tumour relapse. Clinically, a large number of responders of ovarian cancer relapse within a short period after completing first‐line chemotherapy and require further systemic therapy.26 For decades, researchers have studied the fundamental mechanism of tumour repopulation from different perspectives such as inflammation, angiogenesis.27 Until recently, “Phoenix Rising” pathway was thrown out28 and clearly defined the possible cell death‐mediated tumour repopulation in melanoma via a caspase 3‐mediated mechanism.29 Consistent with this concept, our study defined that the caspase 3‐mediated growth of living tumour cell stimulated by dying cells also happened in ovarian cancer cells treated with chemotherapy drugs. Patients with recurrent ovarian cancer are rarely curable and often have only short‐term progression‐free survival.6 Therefore, preventing recurrence and improving quality of life have become key objectives in the treatment and management of patients with ovarian cancer. In our study, we first investigated the efficacy of Berberine, a natural product with lower cytotoxicity in normal human cells, against the repopulation of ovarian cancer cells after chemotherapy.19, 30 We found that Berberine showed a significant ability against the VP16‐induced repopulation of human ovarian cancer cells SKOV3. This confirmed the beneficial role of Berberine against recurrent ovarian cancer, indicating the combination of chemotherapeutics and Berberine might be a possible way to effectively prevent recurrence.
In our study, we meticulously studied the related mechanism underlying the repopulation of ovarian cancer cells exposed to chemotherapy drug VP16. Our result indicated that the chemotherapy drug VP16 treatment led to the activation of caspase 3, induction of iPLA2 and COX‐2, as well as the increase in PGE2 level in ovarian cancer. Considering the contribution of caspase 3 and PGE2 in “Phoenix Rising” pathway of cancer recurrence upon radiotherapy and other cytotoxic treatments,9, 10 targeting “Phoenix Rising” pathway may offer better perspective strategy against cancer relapse. Indeed, caspase 3 inhibition of genetic manipulation was indicated successful to attenuate the repopulation of tumour cells in experimental mice model. The combination of chemotherapy and pan caspase inhibitor may be beneficial in cancer treatment, especially the promising result from pan caspase inhibitor in clinical trial for hepatitis C, non‐alcoholic steatohepatitis and liver reperfusion injury.31, 32 However, the activity of caspase 3 often is required for chemotherapy‐, radiotherapy‐ and many targeted therapy‐induced apoptosis and may directly associate the patient's response or survival, to say nothing of the issue with female fertility or development of eye and ear due to lack of caspase 3.33 Therefore, targeting another key molecular of “Phoenix Rising” pathway would be more reasonable and safer. Being a potent promoter of cell proliferation, motility, invasion and angiogenesis,22 the effect of PGE2 promoting the growth of tumour cells has been reported in variety of cancers, such as colon cancer, lung cancer and ovarian cancer.34, 35 COX‐2 is an important enzyme in the AA metabolic pathway which converts AA into endoperoxide intermediates that are ultimately converted to prostanoids, including PGE2. As a natural product and a COX‐2‐specific inhibitor, our result showed that Berberine not only maintained the activity of caspase 3, but also retarded the VP16‐triggered abnormal PGs production catalysed by COX‐2, which is confirmed by the effectiveness of Berberine on inhibiting the expression of COX‐2.36 Given the importance of AA metallic pathway in recurrence of ovarian cancer upon chemotherapy, our result also warranted combined cytotoxic with other therapy targeting AA‐COX‐2‐PGE2 pathway in the future.
As an accepted key regulator of cellular signalling pathways that control cell proliferation, FAK has been connected with proteins of AA metabolic pathway by many studies. For example, there have been studies showing that exogenous PGE2 can increase the FAK phosphorylation at Tyr397 in the PGE2‐concentration‐dependent manner in HCC cells. At the same time, selective COX‐2 inhibitor celecoxib was found to decrease the expression of FAK protein. These results implied that FAK phosphorylation may be regulated by the level of PGE2, 37 although the phosphorylation of FAK is traditionally thought to be mediated by integrins.23 In our study, enhanced phosphorylation of FAK was observed in both VP16 and exogenous PGE2 treatment condition. More importantly, Berberine was found to abrogate the increasing of phosphorylation of FAK following the inhibition of AA‐COX‐2‐PGE2 pathway. Further study will be needed to explore whether inhibitory effect of Berberine on the phosphorylation of FAK in ovarian cancer cells due to its direct inhibition on integrin β1, since down regulated the levels of integrin β1 in colorectal carcinoma (CRC) cells was shown with efficacy of Berberine before.23
In conclusion, Berberine contributed to the inhibition of repopulation of a small number of surviving ovarian cancer cells after the chemotherapy, which was achieved mainly by suppressing the two rate‐limiting enzymes iPLA2 and COX‐2 in the dying cells, subsequently reduced the production of PGE2 in tumour microenvironment. In the meanwhile, further inhibition of PGE2‐FAK in ovarian cancer cells with recurrence potential also played a critical role on tumour relapse. Our work provided a promising strategy on the recurrence prevention of ovarian cancer with the combination of Berberine and caspase 3‐activated chemotherapy.
DISCLOSURE STATEMENT
The authors have no conflict of interest.
ACKNOWLEDGEMENTS
This work is sponsored by Norman Bethune Program of Jilin University (2015224) and the undergraduate innovative programme of Jilin University (2015741091) Most of experiments were carried out at Nanomedicine Engineering Laboratory of Jilin Province.
Zhao Y, Cui L, Pan Y, et al. Berberine inhibits the chemotherapy‐induced repopulation by suppressing the arachidonic acid metabolic pathway and phosphorylation of FAK in ovarian cancer. Cell Prolif. 2017;50:e12393 10.1111/cpr.12393
Contributor Information
Kan He, Email: hek@jlu.edu.cn.
Li Chen, Email: chenl@jlu.edu.cn.
REFERENCES
- 1. Hansen JM, Coleman RL, Sood AK. Targeting the tumour microenvironment in ovarian cancer. Eur J Cancer. 2016;56:131‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozga M, Aghajanian C, Myers‐Virtue S, et al. A systematic review of ovarian cancer and fear of recurrence. Palliat Support Care. 2015;13:1771‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton‐Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220:940‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466‐478. [DOI] [PubMed] [Google Scholar]
- 7. Yeung TL, Leung CS, Li F, Wong SS, Mok SC. Targeting stromal‐cancer cell crosstalk networks in ovarian cancer treatment. Biomolecules. 2016;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015;31:84‐88. [DOI] [PubMed] [Google Scholar]
- 9. Zimmerman MA, Huang Q, Li F, Liu X, Li CY. Cell death‐stimulated cell proliferation: a tissue regeneration mechanism usurped by tumors during radiotherapy. Semin Radiat Oncol. 2013;23:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Q, Li F, Liu X, et al. Caspase 3–mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang P, Cartwright CA, Li J, et al. Arachidonic acid metabolism in human prostate cancer. Int J Oncol. 2012;41:1495‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2‐induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai X, Zhang W, Liu N, et al. Focal adhesion kinase: important to prostaglandin E2‐mediated adhesion, migration and invasion in hepatocellular carcinoma cells. Oncol Rep. 2009;21:129‐136. [PubMed] [Google Scholar]
- 14. Yoon H, Dehart JP, Murphy JM, Lim ST. Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights. J Histochem Cytochem. 2015;63:114‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stokes JB, Adair SJ, Slack‐Davis JK, et al. Inhibition of focal adhesion kinase by PF‐562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. 2011;10:2135‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yarla NS, Bishayee A, Sethi G, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40–41:48‐81. [DOI] [PubMed] [Google Scholar]
- 17. Kuo C‐L, Chi C‐W, Liu T‐Y. The anti‐inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127‐137. [DOI] [PubMed] [Google Scholar]
- 18. Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS, Lim JS. Berberine‐induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX‐2 protein expression. Biochem Pharmacol. 2012;83:385‐394. [DOI] [PubMed] [Google Scholar]
- 19. Hu Y, Wang S, Wu X, et al. Chinese herbal medicine‐derived compounds for cancer therapy: a focus on hepatocellular carcinoma. J Ethnopharmacol. 2013;149:601‐612. [DOI] [PubMed] [Google Scholar]
- 20. Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti‐inflammatory drugs. Adv Pharmacol. 1997;39:1‐20. [DOI] [PubMed] [Google Scholar]
- 21. Sinha M, Gautam L, Shukla PK, Kaur P, Sharma S, Singh TP. Current perspectives in NSAID‐induced gastropathy. Mediators Inflamm. 2013;2013:258209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Li O, Kan M, et al. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol Med Rep. 2015;12:4572‐4577. [DOI] [PubMed] [Google Scholar]
- 23. Park JJ, Seo SM, Kim EJ, et al. Berberine inhibits human colon cancer cell migration via AMP‐activated protein kinase‐mediated downregulation of integrin beta1 signaling. Biochem Biophys Res Commun. 2012;426:461‐467. [DOI] [PubMed] [Google Scholar]
- 24. Ho YT, Yang JS, Li TC, et al. Berberine suppresses in vitro migration and invasion of human SCC‐4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF‐kappaB, u‐PA and MMP‐2 and ‐9. Cancer Lett. 2009;279:155‐162. [DOI] [PubMed] [Google Scholar]
- 25. Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016:343‐346. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Ge T, Cui X, et al. Prediction of advanced ovarian cancer recurrence by plasma metabolic profiling. Mol BioSyst. 2015;11:516‐521. [DOI] [PubMed] [Google Scholar]
- 27. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li F, Huang Q, Chen J, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donato AL, Huang Q, Liu X, Li F, Zimmerman MA, Li CY. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J Invest Dermatol. 2014;134:1686‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh T, Vaid M, Katiyar N, Sharma S, Katiyar SK. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase‐2, prostaglandin E(2) and prostaglandin E(2) receptors. Carcinogenesis. 2011;32:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Baskin‐Bey ES, Washburn K, Feng S, et al. Clinical trial of the pan‐caspase inhibitor, IDN‐6556, in human liver preservation injury. Am J Transplant. 2007;7:218‐225. [DOI] [PubMed] [Google Scholar]
- 32. Keoni CL, Brown TL. Inhibition of apoptosis and efficacy of pan caspase inhibitor, Q‐VD‐OPh, in models of human disease. J Cell Death. 2015;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armstrong PA, Wood SJ, Shimizu N, Kuster K, Perachio A, Makishima T. Preserved otolith organ function in caspase‐3‐deficient mice with impaired horizontal semicircular canal function. Exp Brain Res. 2015;233:1825‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs‐axin‐beta‐catenin signaling axis. Science. 2005;310:1504‐1510. [DOI] [PubMed] [Google Scholar]
- 35. Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen‐activated protein kinase/Erk pathway signaling and cell proliferation in non‐small cell lung cancer cells in an epidermal growth factor receptor‐independent manner. Can Res. 2005;65:6275‐6281. [DOI] [PubMed] [Google Scholar]
- 36. Kuo C‐L, Chi C‐W, Liu T‐Y. Modulation of apoptosis by berberine through inhibition of cyclooxygenase‐2 and Mcl‐1 expression in oral cancer cells. In Vivo. 2005;19: 247‐252. [PubMed] [Google Scholar]
- 37. Kajimoto M, Ichiyama T, Ueno Y, Shiraishi M, Hasegawa M, Furukawa S. Enhancement of activated beta1‐integrin expression by prostaglandin E2 via EP receptors in isolated human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. Inflamm Res. 2009;58:224‐228. [DOI] [PubMed] [Google Scholar]


