Abstract
Objectives
The aim of the study was to investigate the role of the JAK/STAT3 signalling pathway in angiogenesis.
Materials and methods
The model established in vitro, involved a 3D collagen gel being implanted with endothelial cells (ECs) from red fluorescent protein‐labelled mice, and adipose‐derived stromal cells (ASCs) from green fluorescent protein‐labelled mice. Phenomena of angiogenesis, after treatment by the inhibitor and the activator of JAK/STAT3 pathway respectively, were observed using confocal laser scanning microscopy. Transwell co‐culture of ECs and ASCs was used to elucidate mechanisms.
Results
Stattic, inhibitor of JAK/STAT3 pathway, attenuated angiogenesis in the model. In contrast, angiogenesis was promoted after treatment of Olanzapine, an activator. We found that protein levels of VEGFA and cyclin D1 were regulated by the JAK/STAT3 pathway, and flow cytometry further confirmed variations in cell cycle parameters of ECs and ASCs. Genes VEGFA/B,VEGFR2, MMP‐2,MMP‐9,IGF‐1 and b‐FGF were down‐regulated by Stattic in ECs, while Olanzapine significantly up‐regulated mRNA levels of these genes. As for ASCs, genes VEGFA,MMP‐2,MMP‐9,IGF‐1 and b‐FGF were modulated by the JAK/STAT3 pathway.
Conclusions
Angiogenesis in the 3D collagen gel was regulated by the JAK/STAT3 pathway which involved changes in vessel length, vessel diameter and sprout number. The underlying mechanism was that the JAK/STAT3 signalling pathway regulated angiogenesis by modulation of numbers of angiogenesis‐related growth factors and by direct regulation of cell cycle.
Abbreviations
- ASCs
adipose‐derived stromal cells
- b‐FGF
basic fibroblast growth factor
- BSA
bovine serum albumin
- CLSM
confocal laser scanning microscope
- DMSO
dimethyl sulfoxide
- ECs
endothelial cells
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- HIF‐1α
hypoxia inducible factor‐1α
- IGF‐1
insulin‐like growth factor‐1
- JAK
Janus kinase
- MMP‐2/9
matrix metalloproteinase‐2/9
- PI
propodium iodide
- PVDF
polyvinylidene difluoride
- PVD
peripheral vascular disease
- R/GFP
red/green fluorescent protein
- STAT
signal transducer and activator of transcription
- VE‐ca
vascular endothelial cadherin
- VEGFA/B
vascular endothelial growth factor‐A/B
- VEGFR2
vascular endothelial growth factor receptor 2
- β‐actin
beta‐actin
1. Introduction
Angiogenesis is a key step for tissue regeneration and complete organ engineering. The establishment of a functional vascularization represents one of the major challenges to be overcome for the broad implementation of tissue engineering applications into clinical practice. This neovascularization occurs through a series of steps, including stimulation of endothelial cells (ECs) by autocrine and paracrine growth factors, proteolytic degradation of the basement membrane and surrounding extracellular matrix, ECs proliferation and migration,1 and structural reorganization into a three‐dimensionally tubular structure.2
Recent studies focused on angiogenesis has been elucidated that adipose‐derived stromal cells (ASCs) have a great potential in vascular regeneration. ASCs can promote endothelial tubulogenesis and eventually promote neovascularization or angiogenesis by secreting angiogenic cytokines and growth factors in a paracrine manner.3 Even in transplantations in vivo and early clinical trials, ASCs are thought to have a promising angiogenic effect. Combination of ASCs and ECs can provide possible methods for cell‐based revascularization therapies to treat various diseases, such as peripheral vascular disease (PVD) and ischaemic disease.4, 5 In our previous study, we proved that angiogenesis was promoted when ECs were co‐cultured with ASCs at 1:1 ratio in 3D collagen gel, and amounts of several angiogenic growth factors were also increased.6
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is essential for many developmental processes including cellular proliferation, innate immune responses, stem cell differentiation and maintenance.7, 8 The STAT proteins were identified as latent cytoplasmic transcription factors in response to all cytokine‐driven signalling. Seven mammalian STAT proteins such as STAT1, 2, 3, 4, 5a, 5b and 6 act as multifunctional mediators to regulate various cellular processes.9, 10 Growing evidence suggests the robust angiogenic effects of JAK/STAT cytokine signals.11 Especially, among all these seven STAT proteins, STAT3 plays a critical role in angiogenesis.12, 13 Several studies have demonstrated the role of STAT3 as a mediator and biomarker in endothelial activation that regulates many aspects of angiogenesis.14 The principal functional remodelling events, namely EC proliferation, migration as well as the selective degradation of the basement membrane and extracellular matrix, require STAT3 activation.15 Thus, further investigations to clarify the function of STAT3 pathway in angiogenesis are eagerly awaited.
In this regard, a 3D collagen gel model was established to investigate whether JAK/STAT3 signalling pathway can influence vascular network formation. Furthermore, by using transwell co‐culture, we further elucidated the underlying mechanism of how ASC‐mediated angiogenesis was regulated. Results presented in this article can provide relevant data to better understand the role of JAK/STAT3 pathway in vascular regeneration.
2. Materials and methods
2.1. Cell culture
Animal materials used for this study were obtained according to governing ethical principles and the protocol was reviewed and approved by the Institutional Review Board (IRB).
To obtain purified ECs at high density, brain microvascular tissue was collected from neonatal mice. Tissue samples were cut into small pieces and treated with 0.5% type II collagenase for 1 hour. The EC‐containing suspension was collected and mixed 1:1 (v/v) with fresh high‐glucose Dulbecco's modified Eagle's media (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% FBS and 1% penicillin‐streptomycin solution. Then the mixed suspension was centrifuged at 179 g for 8 minutes. After removing the supernatant, remaining tissue fragments were mixed with 20% bovine serum albumin (BSA) and centrifuged at 1000 g for 20 minutes. Once more the supernatant was removed and fresh 10% FBS DMEM was added to centrifuge tubes to re‐suspend the ECs. Then the EC‐containing suspension was then added into plates or T25 flasks at 37°C in a humidified atmosphere of 5% CO2. Passage 0 was used in the study.
ASCs were obtained from subcutaneous adipose tissue of 4‐week female mice. Briefly, the subcutaneous adipose tissue from 4‐week female mice was aseptically collected and cut into small pieces and treated with 0.75% type I collagenase for 30 min. This ASC‐containing suspension was then collected and mixed 1:1 (v/v) with fresh α‐glucose Dulbecco's modified Eagle's media (α‐MEM; Hyclone) containing 10% foetal bovine serum (FBS) and 1% penicillin‐streptomycin solution (Hyclone). The mixed suspension was centrifuged at 180 g for 5 minutes. After removing the upper supernatant containing adipose debris, 10% FBS α‐MEM was added to re‐suspend the ASCs. Then the suspension was added into plates or T25 flasks at 37°C in a humidified atmosphere of 5% CO2. Purified ASCs should be subcultured to passage 2 for further experiments.16
To obtain DsRed‐Express‐positive ECs (here we called it RFP) and green fluorescent protein (GFP)‐positive ASCs, brain microvascular tissue and subcutaneous adipose tissue were collected from DsRed‐Express transgenic mice (The Genetic Centre of Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences and Centre of Comparative Medicine, Peking Union Medical College, Beijing, China) and enhanced GFP transgenic mice (The Centre of Genetically Engineered Mice, West China Hospital, Sichuan University, Chengdu, China) respectively. Procedures were the same as described above.
2.2. Co‐culture system
After 24 hours of equilibration, the culture media were replaced with 2% FBS culture media for a 12 hour starvation. Then cell samples were divided into three groups: Stattic group, Olanzapine group and control group.
Stattic (Abcam, Cambridge, UK), inhibitor of STAT3 pathway, was dissolved in dimethyl sulfoxide (DMSO, 100 mm), and added to cell culture media by 0.1 volume % at a final concentration of 10 μm. Olanzapine (Abcam), the activator, was dissolved in DMSO, and added to cell culture media by 0.1 volume % at a final concentration of 300 nm. The same final concentration (100 μm) of DMSO (AMRESCO, Solon, OH, USA) was used as vehicle control.
GFP‐ASCs from fat tissues and RFP‐ECs from brain microvascular tissues were co‐cultured in a 3D collagen gel model. Briefly, the ECs and ASCs were mixed at a 1:1 ratio and suspended in DMEM and rat tail tendon collagen type I (Shengyou Biotechnology, Shanghai, China). The cell suspension was then transferred into 96‐well plates to form gel samples at 37°C and cultured for 7 days. The morphologies of vascular‐like structures were captured using a Leica DMIRE2 confocal laser scanning microscope (CLSM, TCS SP2; Leica Microsystems, Wetzlar, Germany, parameter: 20×, Leica Microsystems original image: 1024×1024, 100 μm) equipped with a 60× oil immersion objective lens. Image analysis software Imaris 7.0.0 (Bitplane, Zurich, Switzerland) was used for three‐dimensional reconstruction.
To detect the underlying mechanism which can elucidate the morphology of angiogenesis in 3D collagen gel model, here we used transwell co‐culture. ECs were seeded on six‐well plates at 2 × 105 cells per well and ASCs were also seeded at 2 × 105 cells per well in transwell chambers. Samples for further experiments were all collected after 4 days co‐culture.
2.3. Western blot
Protein samples were mixed with Bio‐Rad Laemmli Sample Buffer and then boiled at 100°C for 5 minutes. Proteins were separated by electrophoresis in 8% acrylamide gels containing SDS and transferred to polyvinylidene difluoride (PVDF) membranes at 200 mA for 1 hour. Membranes were blocked with 5% non‐fat dry milk for 2 hours prior to incubation with 1:500~1000 antibodies including STAT3/p‐STAT3 (phospho Y705), vascular endothelial growth factor‐A (VEGFA), cyclin D1 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Abcam) for 3 hours at RT. Then the membranes were washed with TBST and incubated with 1:3000 anti‐IgG‐HRP (alex series; Abcam) for 1 hour at RT. The blots were developed using Western Blotting Luminol Reagent Kit (sc‐2048). Signals were visualized using Kodak X‐AR and optical density (OD) method with Quantity One 4.6.3 software (Bio‐Rad, Hercules, CA, USA) was used for quantization.
2.4. Cell cycle assay
Cell samples of ECs and ASCs were harvested by Trypsin‐EDTA Solution (GIBCO, Carlsbad, CA, USA) and suspended in ice‐cold 70% ethanol which served as fixation solution. RNase (keyGen, Nanjing, China) and propodium iodide (PI) were then added and incubated at 37°C for half an hour. The cell cycle was analysed by Flow Cytometry FACS Calibur (BD Biosciences, Franklin Lakes, NJ, USA). The percentage of cell populations at G0/G1, S and G2/M phases were examined by Modfit LT 4.0 trial cell cycle analysis software.
2.5. Quantitative real‐time PCR
Total RNA samples were extracted from ASCs and ECs with RNeasy Plus Mini Kit (Qiagen, Shanghai, China) with a genomic DNA eliminator. Dissolved in RNase‐free water, extracted RNA samples were quantified by measuring the absorbance at 260 nm with a spectrophotometer. Then, RNA samples were treated with DNase I (Mbi, Glen Burnie, MD, USA), and cDNA was prepared from each sample, using 0.5 μg total RNA and cDNA synthesis kit (Mbi) at 20 μL final volume. The selected sets of primers are listed in Table 1. All primers were determined through BLAST.
Table 1.
Primer sequences of β‐actin and target genes
| Target gene (mouse) | Primer pairs |
|---|---|
| β‐actin (266 bp) | Forward: GTCCCTCACCCTCCCAAAAG |
| Reverse: GCTGCCTCAACACCTCAACCC | |
| VEGFA (106 bp) | Forward: CTGCTGTGGACTTGTGTTGG |
| Reverse: AAAGGACTTCGGCCTCTCTC | |
| VEGFB (128 bp) | Forward: GCAACACCAAGTCCGAATG |
| Reverse: CTGGCTTCACAGCACTCTCC | |
| VEGFR2 (133 bp) | Forward: TTTGGCAAATACAACCCTTCAGA |
| Reverse: GCAGAAGATACTGTCACCACC | |
| MMP‐2 (171 bp) | Forward: CAAGTTCCCCGGCGATGTC |
| Reverse: TTCTGGTCAAGGTCACCTGTC | |
| MMP‐9 (145 bp) | Forward: CTGGACAGCCAGACACTAAAG |
| Reverse: CTCGCGGCAAGTCTTCAGAG | |
| VE‐ca (102 bp) | Forward: CATCGCAGAGTCCCTCAGTT |
| Reverse: TCAGCCAGCATCTTGAACCT | |
| IGF‐1 (171 bp) | Forward: CTGCTTGCTCACCTTCACC |
| Reverse: TCATCCACAATGCCTGTCTG | |
| b‐FGF (138 bp) | Forward: AGGAAGATGGACGGCTGCT |
| Reverse: GCCCAGTTCGTTTCAGTGC | |
| HIF‐1a (129 bp) | Forward: TGAACATCAAGTCAGCAACG |
| Reverse: CACAAATCAGCACCAAGCAC |
Quantitative real‐time PCR (qPCR) amplification of target message RNA was performed using the SYBR Green I PCR master mix (Takara, Tokyo, Japan). Reactions were carried out on an ABI 7300 (Applied Biosystems, Shanghai, China) under the following procedures: denaturation for 3 minutes at 94°C, followed by 40 cycles, consisting 5 seconds at 94°C and 34 seconds at 60°C. For each reaction, a melting curve was generated to test the primer dimer formation and false priming. Relative mRNA levels were calculated after normalizing to beta‐actin (β‐actin) and the results were expressed as relative fold changes.
2.6. Statistical analysis
All experiments were performed in triplicate and reproduced at least three separate times. Statistical analysis of data was accomplished with SPSS 16.0 using one‐way ANOVA. The Student‐Newman‐Keuls (SNK‐q) test was used to evaluate significance between each two groups. Data were considered significantly different if the two‐tailed P value was <.05.
3. Results
3.1. Angiogenesis by co‐culturing ECs and ASCs in 3D collagen model
First, ECs and ASCs were successfully isolated from normal and fluorescent protein‐labelled mice respectively. Factor VIII immunofluorescence confirmed positive EC marker in isolated ECs. ASCs were identified to be positive for stromal cell markers, i.e. CD34, CD146, CD44 and Sca‐1. In in vitro 3D gels, co‐culture of RFP‐ECs and GFP‐ASCs formed vessel‐like structures after 7 days co‐culture. Compared with control group, the formations of extended branches and vessel‐like structures were suppressed after the treatment of Stattic (Figure 1A). Moreover, the average length and diameter were shorter (Figure 1B,C), and the sprout number was fewer (Figure 1D). On the other hand, in Olanzapine group, the numbers of extended branches and vessel‐like structures, the average length of vessel‐like structures and sprout number were all significantly increased (Figure 1B,D). The results of CLSM showed that JAK/STAT3 pathway can prominently regulate angiogenesis in the 3D gel model.
Figure 1.
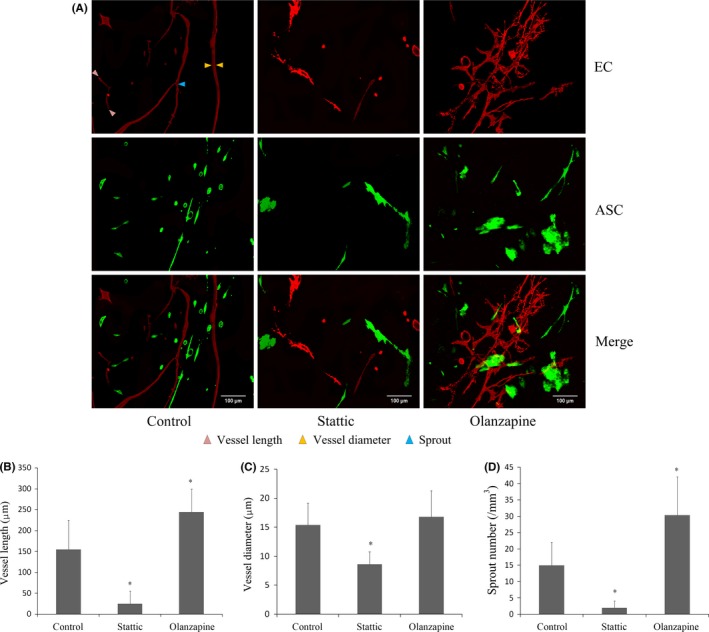
Angiogenesis was modulated by JAK/STAT3 regulators in 3D co‐culture model. A, Formation of vessel‐like structures in 3D gel after 7‐d co‐culture between ECs and ASCs. 3D construction morphologies showed that the treatment with Stattic significantly reduced the formation of vessel‐like structures. Olanzapine promoted angiogenesis in 3D collagen gel. The experiments shown are representative of three different experiments (n=3). B–D, Analyses of vessel‐like structure length (B), lumen diameter (C) and sprout formation (D), using Image‐Pro Plus Software 6.0. The data were expressed as the mean of three different experiments (n=3). *Significant difference with respect to normal control (P<.05)
3.2. Effects of Stattic and Olanzapine on protein levels of STAT3 and p‐STAT3
To confirm the inhibition and activation of JAK/STAT3 pathway, STAT3 and p‐STAT3 (Y705) were examined by Western blot analysis after transwell co‐culture of ECs and ASCs (Figure 2A).
Figure 2.
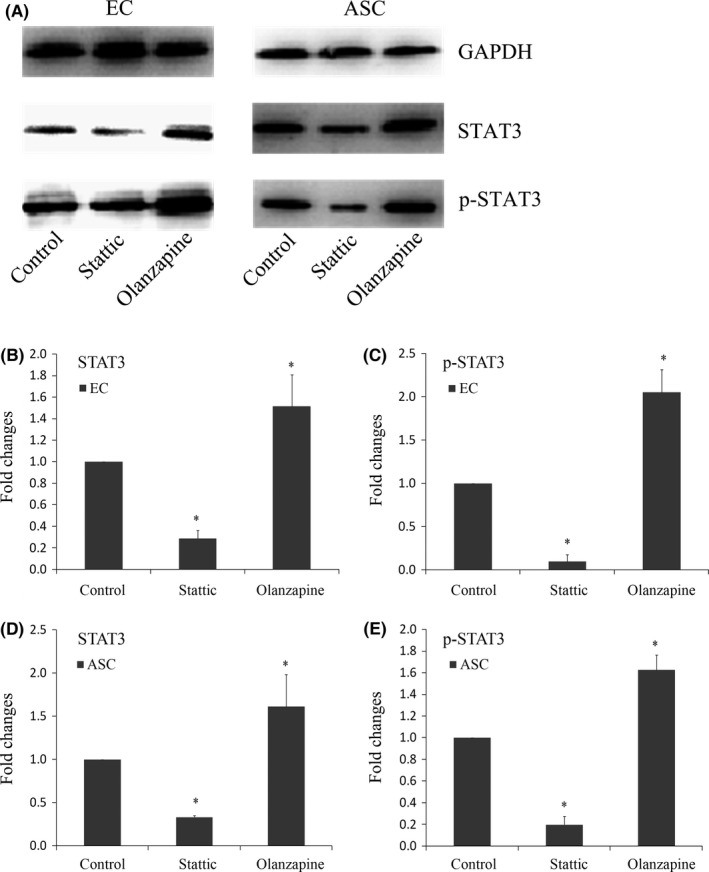
STAT3 and phosphorylated STAT3 were regulated by Stattic and Olanzapine in both ECs and ASCs. A, Western blot showed that in both ECs and ASCs, protein levels of STAT3 and p‐STAT3 were decreased by Stattic and increased by Olanzapine. B, C, Quantification of STAT3 (B) and p‐STAT3 (C) in co‐cultured ECs was done by OD method. Data shown were representative of three independent experiments (n=3). *Significant difference with respect to normal control (P<.05). D, E, Quantification of STAT3 (D) and p‐STAT3 (E) in ASCs was done by OD method. Data shown were representative of three independent experiments (n=3). *Significant difference with respect to normal control (P<.05)
We observed that the treatment of Stattic significantly decreased total STAT3 protein level in ECs (Figure 2B). And p‐STAT3 level was also decreased in ECs (Figure 2C). In contrast, the presence of Olanzapine significantly increased total STAT3 level and STAT3 phosphorylation in ECs (Figure 2B,C). The quantities were up to 1.513‐ and 2.049‐fold higher respectively. Similar patterns were seen when we examined the protein samples of ASCs, as shown in Figure 2, both STAT3 and p‐STAT3 were inhibited by Stattic. Olanzapine induced significant activations of both STAT3 and p‐STAT3.
3.3. Expressions of downstream protein targets of p‐STAT3
To elucidate the mechanism of how Stattic and Olanzapine modulate angiogenesis, we focused on the expressions of downstream proteins of JAK/STAT3 pathway such as cyclin D1 and VEGFA (Figure 3A).
Figure 3.
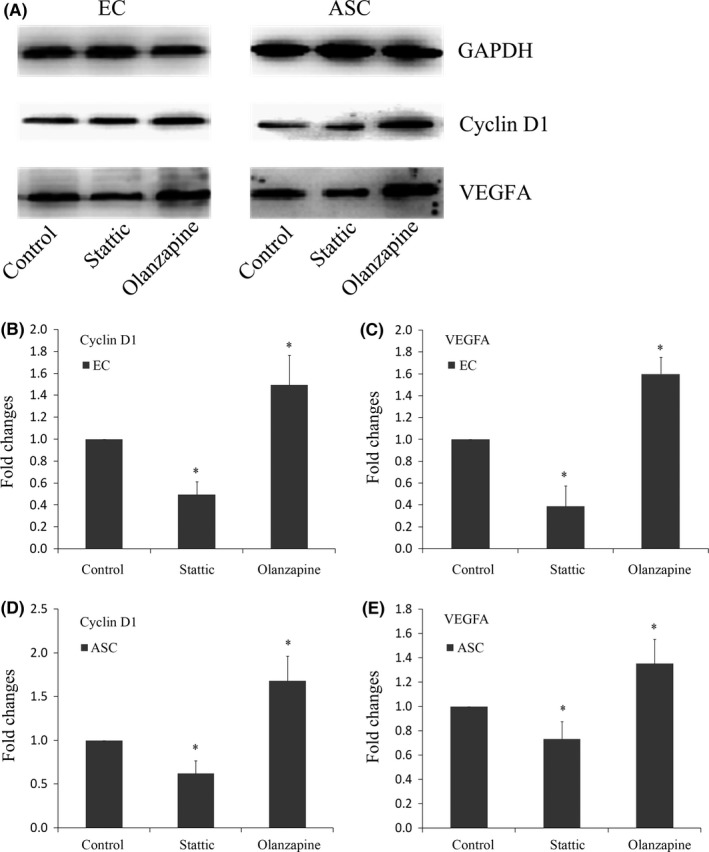
Variations of downstream proteins of JAK/STAT3 pathway. A, Western blot showed that Stattic significantly inhibited the protein expressions of cyclin D1 and VEGFA in both ECs and ASCs. The up‐regulations of cyclin D1 and VEGFA were induced by Olanzapine in both ECs and ASCs. B, C, Quantification of cyclin D1 (B) and VEGFA (C) in co‐cultured ECs was done by OD method. Data shown were representative of three independent experiments (n=3). *Significant difference with respect to normal control (P<.05). D, E, Quantification of cyclin D1 (D) and VEGFA (E) in ASCs was done by OD method. Data shown were representative of three independent experiments (n=3). *Significant difference with respect to normal control (P<.05)
We found that the treatment with Stattic decreased the expression of cyclin D1 in ECs, and the expression of VEGFA was also decreased by Stattic (Figure 3B,C). In contrast, Olanzapine induced a strong up‐regulation of cyclin D1 in ECs, and the expression of VEGFA was also significantly up‐regulated (up to 1.495‐fold and 1.600‐fold respectively; Figure 3B,C).
Similarly, expressions of both cyclin D1 and VEGFA in ASCs were decreased in Stattic group (Figure 3D,E). On the other hand, cyclin D1 and VEGFA in ASCs were up‐regulated after the treatment of Olanzapine (up to 1.680‐ and 1.355‐fold, respectively; Figure 3D,E). At this point, we proved that the treatment of Stattic and Olanzapine was functional.
3.4. Cell cycle analysed by flow cytometry
Based on the results of variations of cyclin D1, we performed flow cytometric analysis to assess the cell population at various stages of cell cycle.
In ECs, cell cycle of Stattic group showed a higher proportion of cells in G0/G1 phase (72.8%) compared with control group (66.1%) (Figure 4A), and a statistically significant decline in G2/M phase was also noticeable (Figure 4B). It can be concluded that Stattic had a negative effect on cell proliferation. Meanwhile, the exposure of Olanzapine caused a significant decline in G0/G1 phase (Figure 4B), indicating that cell cycle progression can be promoted by Olanzapine.
Figure 4.
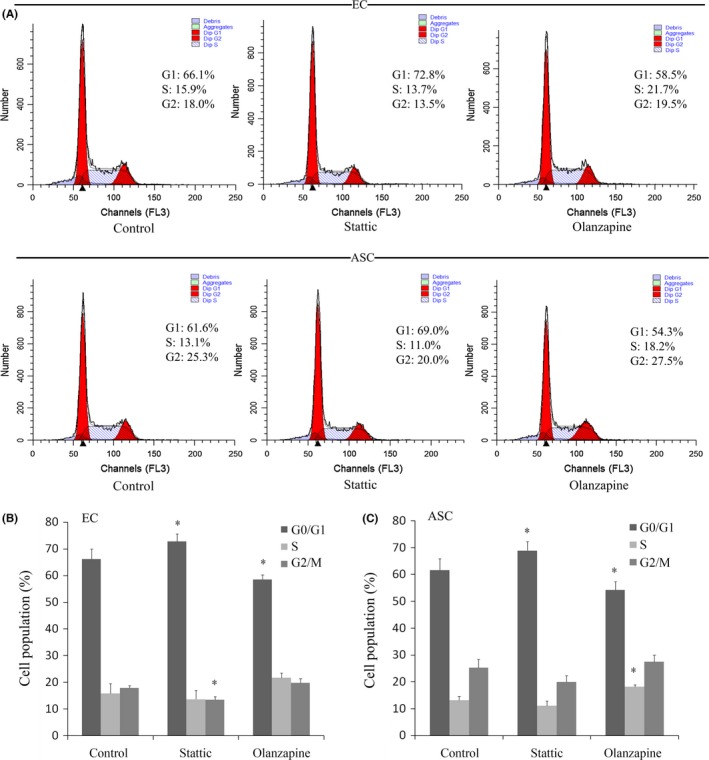
Effects of Stattic and Olanzapine on cell cycle distribution. The cell cycle was detected by flow cytometry. The percentage of cell populations at different phases was examined by Modfit LT 4.0. A, The peaks with different DNA content represent cells in G1 and G2 phases. Data shown were representative of three independent experiments (n=3). B, C, The proportion of G0/G1 period frequency in both ECs and ASCs treated with Stattic was significantly increased (*P<.05). The proportion of G0/G1 period frequency in both ECs and ASCs was significantly decreased in Olanzapine group (*P<.05)
As predicted, Stattic significantly inhibited the cell cycle progression of ASCs. The percentage of G0/G1 phase (69.0%) was higher than that of the control group (61.6%) (Figure 4A). In contrast, the treatment with Olanzapine caused a decrease in G0/G1 phase (54.3%) in comparison with control group (61.6%). An accumulation of cells in S phase was also observed (Figure 4C). Therefore, the fact that JAK/STAT3 pathway can coordinate cell cycle progressions of both ECs and ASCs was further confirmed by flow cytometry.
3.5. Relevant growth factors were modulated in both ECs and ASCs by JAK/STAT3 pathway
To better reveal downstream events of STAT3 signalling that are involved in the regulation of angiogenesis, we examined several angiogenesis‐related genes. Transwell chambers were used to achieve gene detections in ECs and ASCs.
Compared with control group, we found that in co‐cultured ECs, variations of VEGFA and insulin‐like growth factor‐1 (IGF‐1) were prominent. Levels of VEGFB, vascular endothelial growth factor receptor 2 (VEGFR2), matrix metalloproteinase‐2/9 (MMP‐2/9) and basic fibroblast growth factor (b‐FGF) also showed significant variations. There were no significant differences on mRNA levels of vascular endothelial cadherin (VE‐ca) and hypoxia inducible factor‐1α (HIF1‐α) (Figure 5, Table 2).
Figure 5.
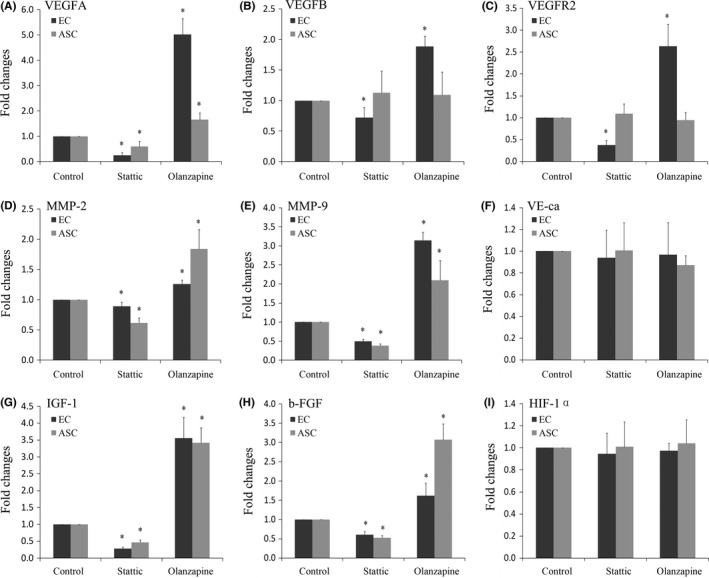
Gene changes of angiogenesis‐related growth factors by quantitative real‐time PCR. Effects of Stattic and Olanzapine on mRNA level of VEGFA (A), VEGFB (B), VEGFR2 (C), MMP‐2 (D), MMP‐9 (E), VE‐ca (F), IGF‐1 (G), b‐FGF (H) and HIF‐1α (I) in ECs and ASCs. Data shown were representative of three independent experiments (n=3). *Significant difference with respect to normal control (P<.05)
Table 2.
Fold changes of gene expressions of angiogenesis‐related growth factors
| Gene profile of growth factors | EC | ASC | ||
|---|---|---|---|---|
| Stattic | Olanzapine | Stattic | Olanzapine | |
| VEGFA | 0.250 ↓a | 5.018 ↑a | 0.605 ↓a | 1.658 ↑a |
| VEGFB | 0.723 ↓a | 1.889 ↑a | 1.131 — | 1.093 — |
| VEFGR2 | 0.374 ↓a | 2.626 ↑a | 1.092 — | 0.940 — |
| MMP‐2 | 0.890 ↓a | 1.259 ↑a | 0.615 ↓a | 1.841 ↑a |
| MMP‐9 | 0.488 ↓a | 3.150 ↑a | 0.379 ↓a | 2.095 ↑a |
| VE‐ca | 0.940 — | 0.967 — | 1.007 — | 0.871 — |
| IGF‐1 | 0.279 ↓a | 3.558 ↑a | 0.463 ↓a | 3.423 ↑a |
| b‐FGF | 0.604 ↓a | 1.613 ↑a | 0.520 ↓a | 3.069 ↑a |
| HIF‐1α | 0.944 — | 0.974 — | 1.008 — | 1.041 — |
Significant difference with respect to control group (P<.05).
In the case of ASCs, we found that JAK/STAT3 pathway can regulate the expressions of VEGFA, MMP‐2, MMP‐9, IGF‐1 and b‐FGF, especially IGF‐1. Meanwhile, other soluble growth factors related to angiogenesis, including VEGFB, VEGFR2, VE‐ca and HIF‐1α, showed no significant variations (Figure 5, Table 2).
4. Discussion
Adipose‐derived stromal cells are thought as one of the most promising stem cell populations identified thus far. It has been demonstrated that ASCs possessed several advantages over bone marrow‐derived stromal cells (BMSCs), including a less invasive harvesting procedure, an increased number of stem cell progenitors yielded from an equivalent amount of tissue harvested, increased proliferation and differentiation capacities, and better angiogenic/osteogenic properties. Furthermore, the use of ASCs as research tools and cell therapeutics has been shown to be both safe and efficacious.17, 18 In recent years, intensive efforts have been made to use ASCs for the improvement of angiogenesis. A number of articles have described that cultured ASCs at relatively early passages secreted soluble factors such as VEGF, IGF‐1, b‐FGF, granulocyte‐macrophage colony‐stimulating factor and interleukins.19, 20 These released factors are believed to play an essential role in early stages of vascular system formation. In our study, the formation of blood vessel networks was completed by remodelling through interactions between ECs and surrounding ASCs. There were two kinds of interactions between ECs and ASCs in 3D collagen gel: cell‐cell contact interaction and non‐contact interaction. In cell‐cell contact interaction, ASCs can express smooth muscle proteins and provide microvascular beds for ECs. This interaction can markedly expedite the formation of vascular network. On the other hand, ASCs can still induce angiogenesis through paracrine effects in non‐contact interaction. Many soluble factors secreted by ASCs could modulate ECs through paracrine effects.
JAK/STAT3 pathway can be activated by various growth factors or cytokines, mediated by receptor‐associated JAK kinases, as well as by non‐receptor Tyr kinases. STAT3 is phosphorylated on the Tyr residue at position 705, followed by dimerization via reciprocal phosphoTyr‐SH2 interactions, translocation into the nucleus, and binding to the STAT‐binding motifs in the promoter regions of the target genes such as cyclin D1.21 Olanzapine is an atypical antipsychotic, approved for the treatment of schizophrenia and bipolar disorder.22 Olanzapine has also been studied as the activator of STAT3 signalling. Olanzapine treatment in cell culture increases phospho‐STAT binding to the putative promoter region of RGS7, increases RGS7 mRNA levels and membrane‐associated RGS7 protein levels and causes JAK/STAT‐dependent desensitization.23 Stattic is a well‐known STAT3 inhibitor. It is a non‐peptide, small‐molecule inhibitor reported to inhibit STAT3 dimerization by selectively interacting with the STAT3 SH2 domain.24
The results of Western blot showed that the presence of Stattic and Olanzapine both had significant effects on STAT3 and p‐STAT3. The inhibition of STAT3 can lead to endothelial dysfunction while on the other hand reducing vasa vasorum neovascularization and, thereby, plaque progression.25 Conversely, Olanzapine contributed to the activation of STAT3, which emerged as a factor that promoted angiogenesis by regulating the release of factors that impacted endothelial regeneration.26 As expected from the changes of p‐STAT3 abundance and DNA‐binding activity, the exposure of Stattic and Olanzapine led to the variations of STAT3 target protein products. Cyclin D1, for example, a cell cycle regulator, was regulated in both ECs and ASCs. In agreement with the findings of Western blot, the analysis of flow cytometry demonstrated that cell cycles of ECs and ASCs were both regulated in this co‐culture model, indicating that JAK/STAT3 pathway can influence angiogenesis by affecting the progression of cell proliferation.
The crosstalk between ECs and ASCs in vascular wall is thus critical for the vascular maturation. This entire process is tightly controlled by various growth factors. VEGF has been widely reported as a key angiogenic factor with the strongest and most significant biological activity in enhancing blood formation.27, 28 VEGFA exhibits two major biological activities: one is the capacity to stimulate vascular EC proliferation,29, 30, 31 and the other is the ability to increase vascular permeability.32 In our study, significant changes of VEGFA were detected at both gene and protein level. We believe that the modulation of VEGFA by JAK/STAT3 pathway plays a key role in this co‐culture model. VEGFR2 is a crucial VEGF‐kinase receptor which plays an important role in vascular development. Activation of VEGFR2 results in the promotion of cell migration and vascular permeability.33 In our study, gene expression of VEGFR2 was regulated by Stattic and Olanzapine in ECs. Furthermore, differences of gene expressions of MMP‐2, MMP‐9 and b‐FGF were also detected. By degrading surrounding extracellular matrix, MMP‐2 and MMP‐9 assist the movement of vascular ECs into surrounding site for formation of micro‐vessels.34, 35 b‐FGF has been attributed as a key player in angiogenesis by promoting EC proliferation,36, 37 altering intercellular adhesion and communication by affecting cadherins, gap junctions and by modulating integrin expression.38 Apart from all these growth factors, IGF‐1, a potent angiogenic inducer that can enhance VEGF‐dependent signalling events,39, 40 showed obvious variation in both ECs and ASCs. The phenomenon that IGF‐1 can be regulated by STAT3 pathway in angiogenesis has never been reported before.
To sum up, we obtained mechanistic insights into the function of JAK/STAT3 in angiogenesis by showing that (a) JAK/STAT3 pathway directly modulates cell cycle of both ECs and ASCs; and (b) JAK/STAT3 pathway regulates several critical angiogenesis‐related growth factors such as VEGFA, MMP‐2, MMP‐9, IGF‐1 and b‐FGF.
There were some limitations in our study. First, angiogenesis in 3D gels involves cell‐cell contact crosstalk. Here, to isolate gene expressions between ECs and ASCs, non‐contact transwell co‐culture was used. This could be inaccurate to some extent due to the gaps between contact and non‐contact co‐cultures. Secondly, the growth factor profile we screened was based on a common gene bank; other unscreened growth factors may also play vital roles in angiogenesis. Thirdly, gene and protein expressions by murine cells have many differences from those of human cells; results achieved from murine cells might not be applicable to human cells. Fourthly, exact mechanism of how growth factors like IGF‐1 and b‐FGF were regulated by JAK/STAT3 pathway need to be further explored.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (81471803, 81470721), Sichuan Science and Technology Innovation Team (2014TD0001).
References
- 1. Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert SR, Camara J, Camara R, et al. Contaminated open fracture and crush injury: a murine model. Bone Res. 2015;3:14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Traktuev DO, Prater DN, Merfeld‐Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420. [DOI] [PubMed] [Google Scholar]
- 4. Melero‐Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P. Engineering robust and functional vascular networks in vivo with human adult and cord blood derived progenitor cells. Circ Res. 2008;103:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gimble JM, Katz AJ, Bunnell BA. Adipose‐derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cun X, Xie J, Lin S, Fu N, Deng S, Xie Q. Gene profile of soluble growth factors involved in angiogenesis, in an adipose‐derived stromal cell/endothelial cell co‐culture, 3D gel model. Cell Prolif. 2015;48:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. [DOI] [PubMed] [Google Scholar]
- 8. Amoyel M, Anderson AM, Bach EA. JAK/STAT pathway dysregulation in tumors: a Drosophila perspective. Semin Cell Dev Biol. 2014;28:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu TY, Pang JH, Wu KP, Lin LP, Tseng WC, Tsai WC. Platelet‐rich plasma increases proliferation of tendon cells by modulating Stat3and p27 to up‐regulate expression of cyclins and cyclin‐dependent kinases. Cell Prolif. 2015;48:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohr A, Chatain N, Domoszlai T, Rinis N, Sommerauer M. Dynamics and non‐canonical aspects of JAK/STAT signalling. Eur J Cell Biol. 2012;91:524–532. [DOI] [PubMed] [Google Scholar]
- 11. Grote K, Luchtefeld M, Schieffer B. Janus under stress—role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol. 2005;43:357–363. [DOI] [PubMed] [Google Scholar]
- 12. Chen Z, Han Z. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200. [DOI] [PubMed] [Google Scholar]
- 13. Bartoli M, Platt D, Lemtalsi T. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–1564. [DOI] [PubMed] [Google Scholar]
- 14. Chen S, Murphy DA, Lassoued W. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol Ther. 2008;7:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yue Y, Yang X, Wei X, et al. Osteogenic differentiation of adipose‐derived stem cells prompted by low‐intensity pulsed ultrasound. Cell Prolif. 2013;46:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tobita M, Orbay H, Mizuno H. Adipose‐derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–170. [PubMed] [Google Scholar]
- 18. Zhou C, Cai X, Fu Y, et al. Tetraploid complementation proves pluripotency of induced pluripotent stemcells derived from adipose tissue. Cell Prolif. 2015;48:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jia C, Koh AJ, Roca H, McCauley LK. Juxtacrine interaction of macrophages and bone marrow stromal cells induce interleukin‐6 signals and promote cell migration. Bone Res. 2015;3:15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. [DOI] [PubMed] [Google Scholar]
- 21. Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a smallmolecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. [DOI] [PubMed] [Google Scholar]
- 22. Littrell Kimberly H, Petty Richard G, Wolf Nicole M. Olanzapine: a 5‐year perspective. Expert Rev Neurother. 2006;6:811–821. [DOI] [PubMed] [Google Scholar]
- 23. Muma NA, Singh RK, Vercillo MS, D'Souza DN. Olanzapine increases RGS7 protein expression via stimulation of the janus tyrosine kinase‐signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322:133–140. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JS. A new small‐molecule Stat3 inhibitor. Chem Biol. 2006;13:1123–1124. [DOI] [PubMed] [Google Scholar]
- 25. Dutzmann J, Daniel J‐M. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc Res. 2015;106:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilfiker‐Kleiner D, Limbourg A, Drexler H. STAT3‐mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15:152–157. [DOI] [PubMed] [Google Scholar]
- 27. Bauer SM, Bauer RJ, Liu ZJ. Vascular endothelial growth factor‐C promotes vasculogenesis, angiogenesis, and collagen constriction in three‐dimensional collagen gels. Vasc Surg. 2005;41:699–707. [DOI] [PubMed] [Google Scholar]
- 28. Sakurai MK, Lee S, Arsenault DA. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292:L742–L747. [DOI] [PubMed] [Google Scholar]
- 29. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. [DOI] [PubMed] [Google Scholar]
- 30. Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005;109:227–241. [DOI] [PubMed] [Google Scholar]
- 32. Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. [DOI] [PubMed] [Google Scholar]
- 33. Bhanushali U, Rajendran S. 5‐Benzylidene‐2,4‐thiazolidenedione derivatives: design, synthesis and evaluation as inhibitors of angiogenesis targeting VEGR‐2. Bioorg Chem. 2016;67:139–147. [DOI] [PubMed] [Google Scholar]
- 34. Page‐McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. [DOI] [PubMed] [Google Scholar]
- 36. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. [DOI] [PubMed] [Google Scholar]
- 37. Lang I, Hoffmann C, Olip H, et al. Differential mitogenic responses of human macrovascular and microvascular endothelial cells to cytokines underline their phenotypic heterogeneity. Cell Prolif. 2001;34:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med (Berl). 2008;86:785–789. [DOI] [PubMed] [Google Scholar]
- 39. Tan X, Fan S, Wen Wu, Zhang Y. MicroRNA‐26a inhibits osteosarcoma cell proliferation by targeting IGF‐1. Bone Res. 2015;3:15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin‐like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–1020. [DOI] [PubMed] [Google Scholar]


