Abstract
Objectives
SAV1 is a human homologue of Salvador that contains two protein‐protein interaction modules known as WW domains and acts as a scaffolding protein. SAV1 participates in the development of diverse types of cancer. We aimed to investigate the role of SAV1 in human colorectal cancer.
Materials and methods
Human colorectal cancer samples were used to study the expression of SAV1 and YAP. Loss‐of‐function and gain‐of‐function strategies were used to study the effects of SAV1 on colorectal cancer cell growth. Rapamycin was used to treat cells and mice to investigate the effect of mTOR signalling.
Results
SAV1 represses the development of colorectal cancer by inhibiting the Akt‐mTOR signalling in a YAP‐dependent manner. The mRNA and protein levels of SAV1 are down‐regulated in human colorectal cancer tissues compared with adjacent non‐cancer tissues. SAV1 knockdown promotes the growth of colorectal cancer cells in vitro and in vivo, whereas SAV1 overexpression leads to opposing results. SAV1 represses the activation of the Akt‐mTOR signalling, and rapamycin treatment blunts the effects of SAV1 on in vitro and in vivo growth of colorectal cancer cells. Finally, we show that SAV1 promotes the phosphorylation and inactivation of YAP, which contributes to the effect of SAV1 on Akt‐mTOR signalling pathway.
Conclusions
SAV1 is a repressor during the development of human colorectal cancer by inhibiting the YAP‐Akt‐mTOR signalling pathway.
1. INTRODUCTION
Colorectal cancer is a significant cause of mortality globally. More than 1.2 million patients are diagnosed with colorectal cancer every year, and more than 600 000 die from this disease.1 The disparities are pronounced worldwide, with vastly different colorectal cancer mortality rates and trends among nations.2 In countries with programmatic screening, it has seen a declining incidence and mortality.3 Molecular profiling of colorectal cancer has become increasingly important for identification of prognostic and predictive biomarkers, as well as for insights into the biology that drives the tumour.3, 4 DNA, proteins, messenger RNA and micro‐RNA have all been evaluated in the development of colorectal cancer.3 However, the mechanisms underlying the development of colorectal cancer are still not fully understood. Novel therapeutic targets remain to identify.
The Salvador family WW domain‐containing 1 (SAV1, also known as WW45) is a WW domain‐containing protein. This protein contains two WW domains and a coiled‐coil region. SAV1 belongs to the core kinase components of Hippo signalling pathway.5 The transcriptional co‐activators YAP1 and TAZ are the downstream effectors of the SAV1 and regulate target gene expression. SAV1 promotes the phosphorylation of YAP and inactivates it by cooperating with the other core kinase components, including MOB, MST1/2 and LATS1/2.6, 7 SAV1 contributes to establishing a polarized cell structure in addition to regulating proliferation.8 In murine renal tubules, deficiency of Sav1 causes structural and cellular abnormalities of the epithelial cells, including significant enlargement of their nuclei through inactivation of the Hippo pathway.9 In addition, SAV1‐MST2‐NEK2A and Eg5 have distinct, but complementary functions, in centrosome disjunction.10
Accumulating evidence show that SAV1 participates in the development of diverse types of cancer. In mucinous tubular and spindle cell carcinoma, SAV1 is involved in a mutually exclusive fashion.11 In patients with pancreatic ductal adenocarcinoma (PDAC), the SAV1 expression is reduced and SAV1 is a potential prognostic marker and target for PDAC therapy.12 In human hepatocellular carcinoma, inactivation of SAV1 is significantly associated with poor prognosis.13 In addition, down‐regulation of SAV1 plays a role in the pathogenesis of high‐grade clear cell renal cell carcinoma.14 However, the roles of SAV1 in other types of cancer remain unknown.
Here we aimed to study the roles of SAV1 in human colorectal cancer and the underlying mechanism. We show that SAV1 expression is down‐regulated in human colorectal cancer. SAV1 inhibits the growth of colorectal cancer cells in vitro and in vivo. Mechanism study reveals that SAV1 inhibits YAP to repress the activation of Akt‐mTOR signalling, which contributes to the anti‐cancer function of SAV1.
2. MATERIALS AND METHODS
2.1. Patient tissues
Twelve colorectal cancer patients with full information were involved in this study from April 2010 to May 2011 at the First Affiliated Hospital of Zhengzhou University. The colorectal cancer tissues and matched adjacent non‐cancer colorectal tissues were obtained from these patients and stored at −80°C until usage. A written form of informed consent was obtained from all patients. The study was approved by the Clinical Research Ethics Committee of Zhengzhou University. The information of patients is shown in Table 1.
Table 1.
Clinicopathologic characteristics of patients with colorectal cancer
| Characteristics | No. patients (%) |
|---|---|
| Age | |
| >60 | 15 (75) |
| ≤60 | 5 (25) |
| Gender | |
| Male | 13 (65) |
| Female | 7 (35) |
| Tumour size (mm) | |
| >100 | 8 (40) |
| ≤100 | 12 (60) |
| pTNM stage | |
| I | 2 (10) |
| II | 5 (25) |
| III | 8 (40) |
| IV | 5 (25) |
| Tumour differentiation | |
| Well | 6 (30) |
| Moderate | 7 (35) |
| Poor | 7 (35) |
| Lymphovascular invasion | |
| Negative | 9 (45) |
| Positive | 11 (55) |
| Tumour shape | |
| Massive | 6 (30) |
| Ulcerative | 10 (50) |
| Infiltrating | 4 (20) |
| Recurrence | |
| Yes | 14 (70) |
| No | 6 (30) |
2.2. Cell lines and culture
The colorectal cancer cell lines DLD‐1, HCT1116, SW480, Caco‐2 and HT‐29 were purchased from ATCC. HEK293FT cells were purchased from Thermo. The colorectal cancer cell lines and HEK293FT cells were cultured in advanced DMEM medium (Thermo, #12491‐015) supplied with 10% foetal bovine serum (Thermo, #12484‐010) and penicillin‐streptomycin (Thermo, #15140163).
2.3. Perpetration of lentivirus and transduction
The ViraPower™ II Lentiviral Gateway® Expression System was purchased from Thermo (#K36720). The shRNA sequence targeting SAV1 is 5′‐GGAGATGTAGTTTCAAGAAAC‐3′; and the shRNA sequence targeting YAP is 5′‐GCAGGTTGGGAGATGGCAAAG‐3′. The oligos were synthesized by Invitrogen (Carlsbad, CA, USA). To overexpress SAV1, the human SAV1 (NM_021818.3) was cloned and inserted into the expressing vector. For lentivirus packaging, HEK293FT cells were co‐transfected with the lentiviral particles. For transduction, DLD‐1 and HT‐29 cells were incubated with virus‐containing supernatant with 8 mg/mL polybrene (Sigma, St. Louis, MO, USA, #107689). Forty‐eight hours later, the infected cells were then selected for additional 72 hours with puromycin.
2.4. Western blot
Fresh tissues from patients, tumour tissues from nude mice and cultured cells were subjected to protein extraction with RIPA (Beyotime, #P0013B) supplied with proteinase inhibitor cocktail (Promega, #G6521). Western blot was performed as described previously.15 The following primary antibodies were used: anti‐SAV1 antibody (Cell Signaling Technology, #3507); anti‐GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti‐p‐Akt antibody (Cell Signaling Technology, #13038); anti‐Akt antibody (Cell Signaling Technology, #4691); anti‐p‐mTOR antibody (Cell Signaling Technology, #5536); anti‐mTOR antibody (Cell Signaling Technology, #2983); anti‐p‐S6K1 antibody (Abcam, #ab126818); anti‐S6K1 antibody (Thermo, #PA1‐31167); anti‐p‐YAP antibody (Abcam, # 13008); anti‐YAP antibody (Abcam, #07‐2114); anti‐GSK3β (Cell Signaling Technology, #12456); anti‐p‐GSK3β (Cell Signaling Technology, #9336); anti‐β‐catenin (Cell Signaling Technology, #8480); and anti‐p‐β‐catenin (Cell Signaling Technology, #2009).
2.5. Quantitative real‐time PCR
Total RNA was extracted from fresh tissues and cells by using the TRIzol reagent (Thermo, #10296010). About 1 μg of total RNA was subjected to cDNA synthesis with the PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa, #6110A). The quantitative real‐time PCR (qRT‐PCR) was carried out by using SYBR® Premix Ex Taq™ (TaKaRa, #RR420A). The primers used for qRT‐PCR are listed below:
SAV1 forward: 5′‐ATGCTGTCCCGAAAGAAAACC‐3′
SAV1 reverse: 5′‐AGGCATAAGATTCCGAAGCAGA‐3′
GAPDH forward: 5′‐TGTGGGCATCAATGGATTTGG‐3′
GAPDH reverse: 5′‐ ACACCATGTATTCCGGGTCAAT‐3′
2.6. Measurement of cell number
The number of colorectal cancer cells was monitored by Cell Counting Kit‐8 kit (Sigma, #96992) to evaluate the growth rate of cells in vitro.
2.7. Colony formation assay
Transduced DLD‐1 and HT‐29 cells were subjected to colony formation assay as described previously.15
2.8. In vivo tumour xenograft experiment
To analyse the in vivo growth of colorectal cancer cells, xenograft experiment was carried out. Transduced DLD‐1 cells in 100 μL of a 1:1 mixture of culture medium and growth factor‐reduced Matrigel were implanted subcutaneously into the forelegs of 6‐week‐old male BALB/c athymic nu/nu mice (Vital River). When the tumours reached approximately 7‐10 mm in diameter, they were prepared to form a brie and then injected subcutaneously into nude mice. Tumour growth (size) was monitored using callipers every 3 days. At the end of the experiment, the tumour weight was analysed. For rapamycin treatment, 5 mg/kg/d rapamycin was given through intraperitoneal administration route. The study was approved by the Animal Research Ethics Committee of Zhengzhou University.
2.9. Statistical analysis
All the values are expressed and mean±SEM of at least three independent experiments if no other statement is provided. Normality tests were assessed via the Shapiro‐Wilk test. The difference between two groups was analysed by paired or unpaired Student's t‐test when the data matched the standard. One‐way ANOVA analysis followed by Bonferroni post hoc test was applied for analysing the difference among groups more than three when the data matched the standard. All the statistical analysis was carried out by using the spss 20. version.
3. RESULTS
3.1. SAV1 is down‐regulated in human colorectal cancer tissues
The roles of the SAV1 protein in human colorectal cancer remain unknown. In this study, we aimed to investigate the potential functions of the SAV1 protein in human colorectal cancer. To this end, we initially analysed the protein level of SAV1 in human colorectal cancer tissues and adjacent non‐cancer tissues by western blot. The paired tissues from 12 patients with colorectal cancer were analysed. The results showed that the protein level of SAV1 was significantly down‐regulated in human colorectal cancer tissues compared with adjacent non‐cancer tissues (Figure 1A,B). To further study whether the down‐regulation of SAV1 protein is a result of the decline in its mRNA level. We performed quantitative real‐time PCR to analyse the mRNA level of SAV1 in human colorectal cancer tissues and adjacent non‐cancer tissues. The results indicated that the mRNA level of SAV1 was remarkably decreased in colorectal cancer tissues compared with matched non‐cancer tissues (Figure 1C). Taken together, our findings demonstrated that the mRNA and protein level of SAV1 were decreased in human colorectal cancer, implicating that SAV1 is involved in human colorectal cancer.
Figure 1.

The expression of SAV1 is down‐regulated in human colorectal cancer. A, Western blot results showing the protein level of SAV1 in human colon cancer (C) and matched adjacent non‐cancer (NC) tissues. B, Quantitative results in (A). The data were analysed by paired Student's t‐test. **P<.01. n=12 in each group. C, Quantitative real‐time PCR results showing the mRNA level of SAV1 in human colon cancer (C) and matched adjacent NC tissues. The data were analysed by paired Student's t‐test. ***P<.001. n=12 in each group
3.2. SAV1 inhibits the growth of colorectal cancer in vitro
As the potential involvement of SAV1 in human colorectal cancer, we further aimed to analyse the effects of the SAV1 protein on the growth of colorectal cancer cells. We first determined the expression of SAV1 and its target YAP in five colon cancer cell lines, DLD‐1, HL‐29, HCT1116, SW480 and Caco‐2 cells. The results showed that the expression levels of SAV1 and p‐YAP/YAP were comparable among these cell lines (Supplementary Figure S1). Therefore, we chose DLD‐1 and HT‐29 cells for further study. The expression of SAV1 was knocked down by lentivirus‐mediated short hairpin RNA in two colorectal cancer cell lines, DLD‐1 and HT‐29 (Figure 2A). We performed cell proliferation assay with CCK‐8 kit and found that SAV1 knockdown increased the cell number since day 2 in DLD‐1 and HT‐29 cells (Figure 2B), indicating that SAV1 knockdown promoted the growth of colorectal cancer cells. We also obtained colorectal cancer cells with stable SAV1 knockdown and performed colony formation assay with these cells. The results demonstrated that SAV1 knockdown increased colony number of DLD‐1 and HT‐29 cells (Figure 2C), indicating that SAV1 knockdown promoted the colony formation capacity of colorectal cancer cells.
Figure 2.
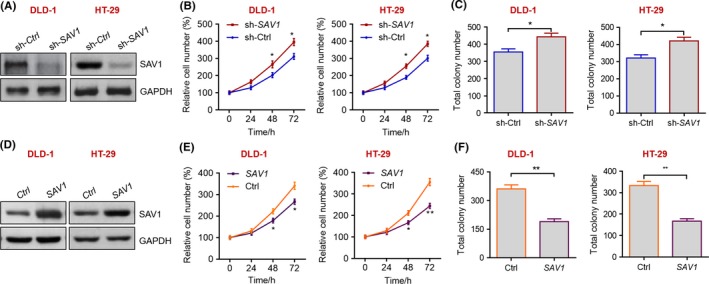
SAV1 represses the growth of colorectal cancer cells in vitro. A, Western blot results showing the knockdown of SAV1 in human DLD‐1 and HT‐29 cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing sh‐SAV1 or control sh‐Ctrl for 24 h, and then protein was extracted and subjected to Western blot analysis with the anti‐SAV1 antibody. B, Knockdown of SAV1 promotes the growth of colon cancer cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing sh‐SAV1 or control sh‐Ctrl, and cell number were monitored with CCK‐8 kit at the indicated time points. The data were analysed by one‐way ANOVA. *P<.05 vs sh‐Ctrl. C, Knockdown of SAV1 promotes colony formation of colon cancer cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing sh‐SAV1 or control sh‐Ctrl and subjected to colony formation assay, and the cells were cultured for 14 d. Then, the total number in each well of the six‐well plate was evaluated. The data were analysed by unpaired Student's t‐test. *P<.05 vs sh‐Ctrl. D, Western blot results showing the overexpression of SAV1 in human DLD‐1 and HT‐29 cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing SAV1 or control vector (Ctrl) for 24 h, and then protein was extracted and subjected to Western blot analysis with the anti‐SAV1 antibody. E, Overexpression of SAV1 represses the growth of colon cancer cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing SAV1 or control vector (Ctrl), and cell number was monitored with CCK‐8 kit at the indicated time points. The data were analysed by one‐way ANOVA. *P<.05, **P<.01 vs Ctrl. F, Overexpression of SAV1 reduces colony number of colon cancer cells. DLD‐1 and HT‐29 cells were infected with lentivirus expressing SAV1 or control vector (Ctrl) and were subjected to colony formation assay, and the cells were cultured for 14 d. Then, the total number in each well of the six‐well plate was evaluated. The data were analysed by unpaired Student's t‐test. **P<.01 vs Ctrl
To further evaluate whether overexpression of SAV1 protein could repress the development of colorectal cancer, we overexpressed SAV1 protein in DLD‐1 and HT‐29 cell with lentivirus (Figure 2D). We found that SAV1 overexpression in colorectal cancer cells decreased their growth rate and repressed the capacity of colony formation (Figure 2E,F).
Taken together, these in vitro findings demonstrated that SAV1 could inhibit the growth and colony formation of colorectal cancer cells.
3.3. SAV1 inhibits the growth of colon cancer in vivo
We were still very interested whether SAV1 could regulate cancer cell growth in vivo. Therefore, we subjected the DLD‐1 cells with a stable SAV1 knockdown to in vivo tumour growth assay by performing xenograft experiment in nude mice. We monitored the tumour size for 4 weeks and found that SAV1 knockdown significantly increased the tumour size from the day 21 compared with the control group (Figure 3A). In addition, the tumour size was larger in the SAV1 knockdown group compared with the control group at the end of the experiment (Figure 3B). We also calculated the weight of tumours at the end of experiments, and the results showed that the tumour weight of SAV1 knockdown group was much larger than that of the control group (Figure 3C). Therefore, SAV1 knockdown promoted the growth of colorectal cancer cells in vivo.
Figure 3.
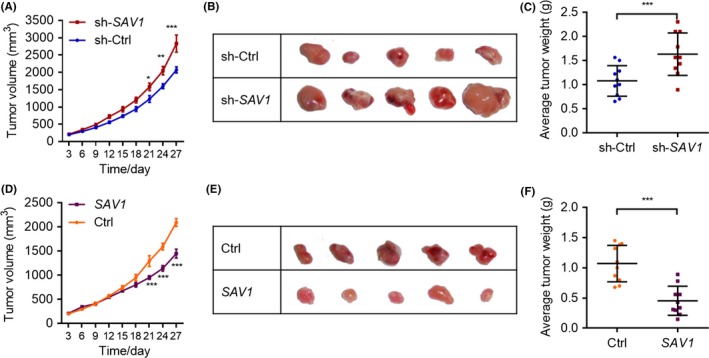
SAV1 inhibits the growth of colorectal cancer cells in vivo. A, The curve of the tumour growth in vivo. DLD‐1 cells with/without stable SAV1 knockdown were subjected to tumour growth assay in vivo. The volume of tumours was monitored every 3 days. The data were analysed by one‐way ANOVA. *P<.05, **P<.01, ***P<.001 vs sh‐Ctrl at the same time point. n=10 in each group. B, Representative image showing tumour size. DLD‐1 cells with/without stable SAV1 knockdown were subjected to tumour growth assay in vivo. The tumours were harvested at the end of the experiment, and the image was captured. C, Tumour weight of colon cancer. DLD‐1 cells with/without stable SAV1 knockdown were subjected to tumour growth assay in vivo. The tumours were harvested at the end of the experiment, and tumour weight was evaluated. The data were analysed by unpaired Student's t‐test. ***P<.001. n=10 in each group. D, The curve of the tumour growth in vivo. DLD‐1 cells with/without stable SAV1 overexpression were subjected to tumour growth assay in vivo. The volume of tumours was monitored every 3 days. The data were analysed by one‐way ANOVA. ***P<.001 vs Ctrl at the same time point. n=10 in each group. E, Representative image showing tumour size. DLD‐1 cells with/without stable SAV1 overexpression were subjected to tumour growth assay in vivo. The tumours were harvested at the end of the experiment, and the image was captured. F, Tumour weight of colon cancer. DLD‐1 cells with/without stable SAV1 overexpression were subjected to tumour growth assay in vivo. The tumours were harvested at the end of the experiment, and tumour weight was evaluated. The data were analysed by unpaired Student's t‐test. ***P<.001. n=10 in each group
We also investigated whether overexpression of SAV1 could inhibit the development of colorectal cancer in vivo. As thus, DLD‐1 cells with or without SAV1 stable overexpression were subjected to in vivo tumour growth assay. We found that SAV1 overexpression repressed the in vivo growth of DLD‐1 cells from the day 21 (Figure 3D). In addition, the tumour size in SAV1 overexpression group was smaller than that of the control group at the end of the experiments (Figure 3E). In addition, the average tumour weight was also decreased by overexpressing SAV1 (Figure 3F). Similarly, SAV1 knockdown repressed the in vivo growth rate and final tumour weight of HT‐29 cells (Supplementary Figure S2A,B). Therefore, SAV1 overexpression repressed the growth of colorectal cancer cells in vivo.
Taken together, our in vitro and in vivo evidence showed that SAV1 inhabited the development of colorectal cancer.
3.4. SAV1 represses Akt‐mTOR pathway in colorectal cancer
The next question we wanted to answer is how SAV1 inhibits the development of colorectal cancer. The Akt‐mTOR signalling pathway is a core mechanism underlying colorectal cancer development.16, 17 Therefore, we analysed the potential effects of SAV1 on Akt‐mTOR signalling. Indeed, we observed that the phosphorylation levels of Akt, mTOR and S6K1 were up‐regulated in colorectal cancer tissues compared with non‐cancer colon tissues (Figure 4A,B). We also analysed the activation of the Akt‐mTOR signalling pathway in tumours obtained from nude mice. Consistently, when SAV1 was knocked down, the phosphorylation levels of Akt, mTOR and S6K1 were remarkably increased in tumour tissues compared with the control group (Figure 4C,D). By contrast, SAV1 overexpression significantly reduced the phosphorylation level of Akt, mTOR and S6K1 (Figure 4E,F). Therefore, SAV1 could repress the activation of the Akt‐mTOR signalling pathway. The dysregulation of Wnt/β‐catenin signalling plays a vital role in the pathogenesis of colorectal cancer.18 Therefore, we also determined whether SAV1 regulates the phosphorylation levels of glycogen synthase kinase 3 beta (GSK3β) and β‐catenin, two compartments of the Wnt/β‐catenin pathway. The results showed that SAV1 knockdown did not alter the phosphorylation levels of GSK3β and β‐catenin (Supplementary Figure S3), indicating that SAV1 was unable to change the Wnt/β‐catenin signalling.
Figure 4.
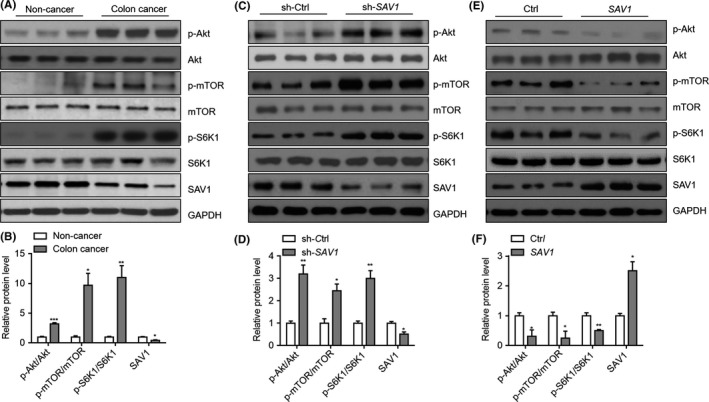
SAV1 inactivates Akt‐mTOR signalling pathway in colorectal cancer cells. A, Western blot results showing the phosphorylation of Akt, mTOR, and S6K1 in colorectal cancer tissues compared with non‐cancer tissues. B, Quantitative results of relative protein levels in (A). *P<.05, **P<.01, ***P<.001 vs non‐cancer tissues. The data were analysed by unpaired Student's t‐test. n=3 in each group. C, Western blot results showing the phosphorylation of Akt, mTOR, and S6K1 in tumour tissues with the SAV1 knockdown. DLD‐1 cells with/without SAV1 knockdown were growth in vivo in nude mice, and the tumour tissues were isolated from nude mice at the end of the experiment. The protein was harvested and subjected to Western blot analysis. D, Quantitative results of relative protein levels in (C). *P<.05, **P<.01 vs non‐cancer tissues. The data were analysed by unpaired Student's t‐test. n=3 in each group. E, Western blot results showing the Akt‐mTOR signalling pathway is inhibited in colon cancer cells with SAV1 overexpression. DLD‐1 cells with/without SAV1 overexpression were growth in vivo in nude mice, and the tumour tissues were isolated from nude mice at the end of the experiment. The protein was harvested and subjected to Western blot analysis. F, Quantitative results of relative protein levels in (C). *P<.05, **P<.01, ***P<.001 vs non‐cancer tissues. The data were analysed by unpaired Student's t‐test. n=3 in each group
To further confirm whether activation of the mTOR signalling accounts for SAV1‐mediated effects on colorectal cancer, we inhibited mTOR with rapamycin. Rapamycin treatment repressed the phosphorylation of S6K1 and blocked the activation of S6K1 by SAV1 knockdown in DLD‐1 cells (Figure 5A). Inhibition of mTOR with rapamycin reduced the growth rate of DLD‐1 cells, and SAV1 knockdown was unable to promote the growth of DLD‐1 cells in the presence of rapamycin (Figure 5B). In addition, we observed that rapamycin treatment reduced the numbers of DLD‐1 colonies and blocked the effects of SAV1 on colony formation of DLD‐1 cells (Figure 5C). Therefore, the mTOR signalling is important for SAV1‐mediated effects on colorectal cancer cells in vitro. To further validate this finding in vivo, the tumour‐bearing nude mice were treated with rapamycin at the dose of 5 mg/kg/day. The results showed that rapamycin treatment reduced the tumour weight (DLD‐1 cells) in vivo, and knockdown of SAV1 was unable to promote in vivo growth of DLD‐1 cells when mTOR was inhibited by rapamycin (Figure 5D).
Figure 5.
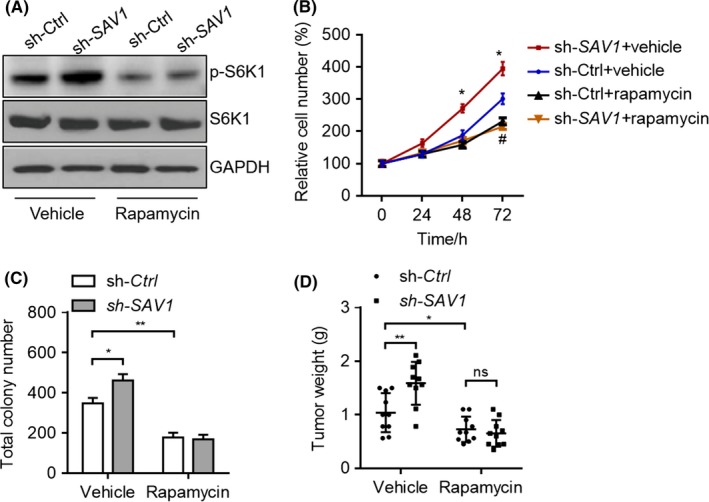
Rapamycin inhibits the effect of SAV1 in vitro and in vivo. A, Rapamycin represses the activation of S6K1 by SAV1 knockdown. DLD‐1 cells were infected with lentivirus expressing sh‐SAV1 or sh‐Ctrl for 24 h and then treated with rapamycin (500 nmol/L) for additional 24 h. B, Rapamycin inhibits the promotion of DLD‐1 cell growth by the SAV1 knockdown. DLD‐1 cells with/without SAV1 stable knockdown were treated with/without rapamycin (500 nmol/L), and the cell number was monitored by CCK‐8 kit at the indicated time points. *P<.05 vs sh‐Ctrl+Vehicle at the same time point. #indicates P<.05 of sh‐Ctrl+Vehicle vs sh‐Ctrl+Rapamycin. C, Rapamycin reduces the colony number increased by the SAV1 knockdown. DLD‐1 cells with/without SAV1 stable knockdown were cultured for 14 d in the presence of rapamycin (500 nmol/L) or not, and the cell number was monitored at the indicated time points. The data were analysed by one‐way ANOVA. *P<.05 and **P<.01 vs sh‐Ctrl+Vehicle at the same time point. D, Rapamycin blocks the effect of SAV1 on colon cancer growth in vivo. DLD‐1 cells with/without SAV1 stable knockdown were subjected to in vivo tumour growth assay in the presence of rapamycin or not. The tumour weight was calculated at the end of the experiment, and the data were analysed by one‐way ANOVA. *P<.05 and **P<.01, ns: not significant. n=10 in each group
Taken together, our findings demonstrated that SAV1 repressed Akt‐mTOR signalling pathway to inhibit the development of colorectal cancer.
3.5. The inhibition of Akt‐mTOR by SAV1 is mediated by YAP
Finally, we investigated how SAV1 represses the activation of Akt‐mTOR signalling. The YAP is a classic downstream of SAV1.19 SAV1 could promote the phosphorylation of YAP and repress its activation.5 Indeed, we observed that the phosphorylation level of YAP was decreased by SAV1 knockdown and increased by SAV1 overexpression in DLD‐1 cells (Figure 6A). YAP is an activator of Akt‐mTOR signalling by promoting the expression of miR‐29 and inhibition of PTEN.19 We knocked down the expression of YAP and investigated its contribution to SAV1‐mediated inhibition of Akt‐mTOR signalling. Knockdown of YAP reduced the phosphorylation of Akt, mTOR and S6K1 in DLD‐1 cells (Figure 6B). Interestingly, SAV1 knockdown was unable to activate the Akt‐mTOR signalling when YAP was deficient (Figure 6B). Therefore, SAV1 knockdown activates AKT‐mTOR signalling in a YAP‐dependent manner. Finally, we analysed the phosphorylation level of YAP in colorectal cancer tissues and matched adjacent non‐cancer tissues. The results showed that the phosphorylation level of YAP was reduced in colorectal cancer tissues compared with non‐cancer tissues (Figure 6C,D), indicating that YAP was activated in human colorectal cancer tissues.
Figure 6.
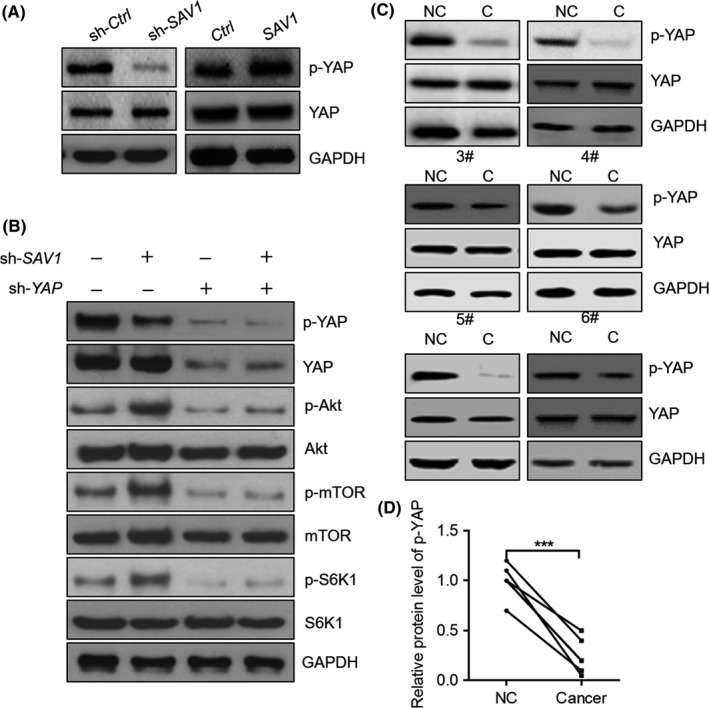
YAP contributes to the activation of Akt‐mTOR by SAV1 knockdown. A, Western blot results showing SAV1 regulates the phosphorylation of YAP. DLD‐1 cells were infected with lentivirus expressing the indicated vectors for 24 h, and then the protein was harvested that subjected to western blot. B, Western blot results showing YAP is involved in the activation of the Akt‐mTOR signalling pathway by the SAV1 knockdown. DLD‐1 cells were infected with lentivirus expressing the indicated vectors for 24 h, and then the protein was harvested that subjected to western blot. C, Western blot results showing YAP phosphorylation level is reduced in colon cancer tissues. D, Quantitative results of phosphorylated YAP (p‐YAP) level in (C). The data were analysed by paired Student's t‐test. ***P<0.001. n=6 in each group
4. DISCUSSION
In the present work, we provide evidence that SAV1 is a tumour suppressor in human colorectal cancer partially by inactivating YAP‐Akt‐mTOR signalling pathway. SAV1 mRNA and protein levels are down‐regulated in human colorectal cancer tissues compared with matched adjacent non‐cancer tissues. SAV1 knockdown promotes the growth of colorectal cancer cells in vitro and in vivo. By contrast, overexpression of SAV1 in colorectal cancer cells could repress the development of cancer in vitro and in vivo. Mechanistically, we show that SAV1 represses the activation of Akt‐mTOR signalling in a YAP‐dependent manner, and rapamycin treatment blocks the effects on SAV1 in colorectal cancer.
The functions of SAV1 in several types of cancer have been reported. In breast cancer, SAV1 expression was down‐regulated in breast cancer samples compared to normal breast tissues.20 In human PDAC, lower expression of SAV1 is involved in the progression of PDAC, suggesting that SAV1 may be a potential prognostic marker and target for PDAC therapy.12 Additionally, SAV1 is frequently down‐regulated in high‐grade clear cell renal cell carcinoma.14 In this study, we also found that the mRNA and protein levels of SAV1 were significantly down‐regulated in human colorectal cancer tissues compared with adjacent non‐cancer tissues. These findings implicate that down‐regulation of SAV1 is involved in diverse cancers, and may be a core mechanism for these cancers. However, the diagnostic and prognostic values of SAV1 in human colorectal cancer remain to elucidate.
By using loss‐of‐function and gain‐of‐function strategies, we demonstrated that SAV1 repressed the growth of colorectal cancer both in vitro and in vivo. The Hippo pathway could cooperate with the mTOR signalling in different cells and tissues to regulate cell proliferation and organ size.19 Activation of mTOR promotes the development of colorectal cancer.21 Therefore, we tested the effects of SAV1 on the Akt‐mTOR signalling. Initially, we found that the phosphorylation of Akt, mTOR and the S6K1 was up‐regulated in colorectal cancer tissues, where the expression of SAV1 was reduced. Additionally, the activation of Akt‐mTOR signalling was also activated in tumours isolated from nude mice of the shSAV1 group compared with the control group. In contrast, SAV1 overexpression reduced the phosphorylation of Akt, mTOR and S6K1. These findings reveal that SAV1 could inhibit the Akt‐mTOR signalling pathway. To evaluate the importance of this axis for SAV1 function in colorectal cancer cells, we inhibited the activation of mTOR with rapamycin. The results showed that rapamycin treatment blocked the effects of SAV1 on the growth of colorectal cancer cell in vitro and in vivo. Therefore, the activation of Akt‐mTOR signalling is an important mechanism underlying the hyperproliferative phenotype in colorectal cells with SAV1 low expression. In other types of cancer, SAV1 regulates survival of cancer cells. For instance, in breast cancer, activation of SAV1 promotes cell death.20, 22 The overexpression of SAV1 attenuated the HAX1 (hematopoietic cell‐specific protein 1 [HS1]‐associated protein X‐1) protective effects from hydrogen peroxide (H2O2)‐induced cell death in MCF‐7 cells, while knockdown of SAV1 significantly enhanced the anti‐apoptotic function of HAX1.20 We did not determine the effects of SAV1 on cell death of colorectal cancer. Further works may be needed to address this question. Interestingly, a recent work reported that Sav1 knockout alone was unable to induce spontaneous tumours following colitis induction in mice,23 indicating that other factors are important for the functions of SAV1 in colorectal cancer. Indeed, Kim et al.23 showed that increased prostaglandin E2 (PGE2) and dysregulation of Hippo pathway cooperates to induce colon cancer development. Therefore, it is also interesting to investigate the potential cooperation between SAV1 and PGE2 in colorectal cancer.
Overexpression of YAP/TAZ is an independent predictor of prognosis in colorectal cancer.7 In addition, we found that SAV1 repressed the activation of Akt‐mTOR in a YAP‐dependent manner, and phosphorylation of YAP was down‐regulated in human colorectal cancer tissues. SAV1‐mediated repression of YAP was also observed in human clear cell renal cell carcinoma.14 Low expression of SAV1 in renal cell carcinoma led to the frequently nuclear localization of YAP, and high proliferation rate of cancer cells.14 In addition, ablation of SAV1 induced YAP localization to the nucleus in oval cells, accounting for their increased proliferative capacity, but not in hepatocytes. Liver tumours that developed in mice heterozygous for SAV1 deletion or with liver‐specific SAV1 ablation developed hepatocellular carcinoma and cholangiocarcinoma that are originating from oval cells.24, 25 Therefore, the repression of YAP by SAV1 accounts for the tumour repressor roles of SAV1 in diverse cancers. YAP alone could regulate the activation of the Akt‐mTOR pathway. We showed that knockdown of YAP repressed the activation of Akt‐mTOR signalling in colorectal cancer cells, indicating that the YAP‐Akt‐mTOR axis is critically important for colorectal cancer. Interestingly, YAP could also serve as a downstream target of the Akt signalling. Inhibition of YAP through an Akt‐dependent process by 3, 3′‐diindolylmethane represses the growth of human colon cancer cells.26 Therefore, there may be a YAP‐Akt‐YAP feedback loop existing in the development of human colorectal cancer. In conclusion, we identify SAV1 as a tumour suppressor in human colorectal cancer. SAV1 represses the growth of colorectal cancer cells by inactivating Akt‐mTOR signalling in a YAP‐dependent manner. Therefore, SAV1 may serve as a potential target for treatment of human colorectal cancer.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by China Postdoctoral Science Foundation (No.2015M572121).
Jiang J, Chang W, Fu Y, et al. SAV1 represses the development of human colorectal cancer by regulating the Akt‐mTOR pathway in a YAP‐dependent manner. Cell Prolif. 2017;50:e12351 10.1111/cpr.12351
Contributor Information
Xiefu Zhang, Email: zhangxiefu@medmail.com.cn.
Shuijun Zhang, Email: zhangshuijun@hotmail.com, Email: shuijun_zhang@sohu.com.
REFERENCES
- 1. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490‐1502. [DOI] [PubMed] [Google Scholar]
- 2. Potter MB. Strategies and resources to address colorectal cancer screening rates and disparities in the United States and globally. Annu Rev Public Health. 2013;34:413‐429. [DOI] [PubMed] [Google Scholar]
- 3. Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83‐95. [DOI] [PubMed] [Google Scholar]
- 4. Dickinson BT, Kisiel J, Ahlquist DA, Grady WM. Molecular markers for colorectal cancer screening. Gut. 2015;64:1485‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246‐257. [DOI] [PubMed] [Google Scholar]
- 7. Moroishi T, Hansen CG, Guan K‐L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim M, Kim M, Lee MS, Kim CH, Lim DS. The MST1/2‐SAV1 complex of the Hippo pathway promotes ciliogenesis. Nat Commun. 2014;5:5370. [DOI] [PubMed] [Google Scholar]
- 9. Kai T, Tsukamoto Y, Hijiya N, et al. Kidney‐specific knockout of Sav1 in the mouse promotes hyperproliferation of renal tubular epithelium through suppression of the Hippo pathway. J Pathol. 2016;239:97‐108. [DOI] [PubMed] [Google Scholar]
- 10. Mardin BR, Lange C, Baxter JE, et al. Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat Cell Biol. 2010;12:1166‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehra R, Vats P, Cieslik M, et al. Biallelic alteration and dysregulation of the hippo pathway in mucinous tubular and spindle cell carcinoma of the kidney. Cancer Discov. 2016;6:1258‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Wang Y, Li P‐P, et al. Expression profile and prognostic value of SAV1 in patients with pancreatic ductal adenocarcinoma. Tumor Biol. 2016;37:16207‐16213. [DOI] [PubMed] [Google Scholar]
- 13. Sohn BH, Shim JJ, Kim SB, et al. Inactivation of hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22:1256‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuura K, Nakada C, Mashio M, et al. Downregulation of SAV1 plays a role in pathogenesis of high‐grade clear cell renal cell carcinoma. BMC Cancer. 2011;11:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kugel S, Sebastián C, Fitamant J, et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell 2016;165:1401‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horng CT, Wu YJ, Chen PN, Chu SC, Tsai CM, Hsieh YS. Koelreuteria Formosana Extract Induces Growth Inhibition and Cell Death in Human Colon Carcinoma Cells via G2/M Arrest and LC3‐II Activation‐Dependent Autophagy. Nutr Cancer. 2017;69:44‐55. [DOI] [PubMed] [Google Scholar]
- 17. Foley TM, Payne SN, Pasch CA, et al. Dual PI3K/mTOR inhibition in colorectal cancers with APC and PIK3CA mutations. Mol Cancer Res 2017; 10.1158/1541-7786.MCR-16-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531‐7537. [DOI] [PubMed] [Google Scholar]
- 19. Csibi A, Blenis J. Hippo‐YAP and mTOR pathways collaborate to regulate organ size. Nat Cell Biol. 2012;14:1244‐1245. [DOI] [PubMed] [Google Scholar]
- 20. Luo X, Li Z, Li X, et al. hSav1 interacts with HAX1 and attenuates its anti‐apoptotic effects in MCF‐7 breast cancer cells. Int J Mol Med. 2011;28:349‐355. [DOI] [PubMed] [Google Scholar]
- 21. Crunkhorn S. Cancer: mTOR inhibition curbs colorectal cancer. Nat Rev Drug Discov. 2015;14:14‐15. [DOI] [PubMed] [Google Scholar]
- 22. Park BH, Lee YH. Phosphorylation of SAV1 by mammalian ste20‐like kinase promotes cell death. BMB Rep. 2011;44:584‐589. [DOI] [PubMed] [Google Scholar]
- 23. Kim H‐B, Kim M, Park Y‐S, et al. Prostaglandin E2 activates YAP and a positive‐signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology. 2017;152:616‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee K‐P, Lee J‐H, Kim T‐S, et al. The Hippo‐Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:8248‐8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li XJ, Leem SH, Park MH, Kim SM. Regulation of YAP through an Akt‐dependent process by 3, 3'‐diindolylmethane in human colon cancer cells. Int J Oncol. 2013;43:1992‐1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


