Abstract
Objectives
The function of the dental pulp is closely connected to the extracellular matrix (ECM) structure, and ECM has received significant attention due to its biological functions for regulating cells. As such, the interaction between the ECM niche and cells is worth exploring for potential clinical uses.
Materials and methods
In this study, dental pulp stem cell (DPSC)‐derived ECM (DPM) was prepared through cell culture and decellularization to function as the cell niche, and changes in DPSC behaviour and histological analysis of dental pulp tissue regeneration were evaluated following the DPM culture. DPM promoted the replication of DPSCs and exhibited retention of their mineralization. Then, the DPM‐based culture strategy under odontogenic culture medium was further investigated, and the mineralization‐related markers showed that DPSCs were regulated towards odontogenic differentiation. Dental pulp‐like tissue with well‐arranged ECM was harvested after a 2‐month subcutaneous implantation in nude mice with DPM application. Additionally, DPSCs cultured on the plastic culture surface showed the up‐regulation of mineralization makers in vitro, but there was a disorder in matrix formation and mineralization when the cells were cultured in vivo.
Results and Conclusions
DPM‐based cultivation could serve as a cell niche and modulate DPSC behaviour, and this method also provided an alternative to harvest tissue‐specific ECM and provided a strategy for ECM‐cell interaction.
1. INTRODUCTION
Dental pulp is a connective tissue surrounded by dentin, which forms the dentin matrix for life, and its function is closely connected to micro‐environment niche. Dental pulp has a substantial extracellular matrix (ECM) that not only provides a 3‐dimensional structure to maintain the integrity of the tissue1 but also affects the turnover of cells.2 The ECM of dental pulp is rich in fibril, which is mainly composed of collagen type I and III. The mineralization‐related protein and the glycoprotein have a polarized distribution through the odontoblast layer to the core of pulp, and the function of the dental pulp was closely connected to the ECM structure. The ECM provides needed elasticity and consistency for dental pulp,3 which maintains mature dental pulp tissue from abnormal mineralization.4 However, when the dental pulp micro‐environment is invaded by trauma, bacteria or bacterial metabolites, the repair of dental pulp may lead to local or total calcification in the pulp tissue rather than in the original connective tissue. Therefore, the homeostasis of dental pulp tissue is tightly related to the ECM, and it is important to construct a proper ECM micro‐environment when the pulp tissue requires therapy.
Various natural and synthetic material scaffolds were used to simulate the ECM of dental pulp tissue, and in vitro experiments have suggested that these materials facilitated good odontogenesis. Collagen type I, as the main component of natural dental pulp ECM, is the most widely used scaffold material for dental pulp regeneration. The degradation rate of collagen is uncontrollable, and the reconstruction of collagen fibres in regenerated tissue is limited.5 The disordered regenerated collagen fibres in dental pulp tissue may lead to abnormal mineralization. Additionally, the regenerated pulp tissue with platelet rich plasma in the root canal showed characteristics of periodontal tissue.6 Therefore, the micro‐environment of the materials determine the mineralization ability of dental pulp tissue and the long‐term sustainability of the regenerated tissue. Therefore, compared to the improved odontogenesis ability of seeded cells, it is more important to maintain the integrity of a regenerated ECM niche.
The natural dental pulp ECM maintains structural integrity of the tissue and decides the fate of the cells7 as well as plays an essential role in tissue growth, remodelling and maintenance.8 Therefore, a dental pulp tissue‐derived ECM may be a better choice to imitate the natural micro‐environment and help repair and regenerate tissue. The detergent solution treatment is the regular method for obtaining natural tissue ECM. Human dental pulp, however, has an anatomy structure that may be difficult to be standardized. Therefore, a dental pulp stem cell (DPSC)‐derived ECM was harvested from cell culture using ascorbic acid. Unlike decellularized tooth germ tissue, dental pulp matrix (DPM) does not have the structure of blood and lymphatic vessels or the polarized distribution of an odontoblast layer.
In this study, ECM‐based cultured DPSCs were evaluated for dental pulp regeneration. DPM was utilized to imitate the original dental pulp niche, which was compared to a conventional polystyrene culture surface. To the best of our knowledge, the study of tissue‐engineered dental pulp has been investigated using biomaterials, but the native ECM niche interaction has yet to be evaluated. The change in mineralization behaviour of DPSCs in their native niche was performed for the first time, and this cell‐derived ECM model may be useful for clinical applications.
2. MATERIALS AND METHODS
2.1. Animals model
In this study, nude mice were obtained from the Animal Research Centre of Sichuan University. The human dentin and dental pulp tissue were acquired from healthy patients whose premolars were extracted for orthodontic treatment at the West China Hospital of Stomatology, Sichuan University. All the treatments and the study protocol complied with the committee regulations of the Ethics Committee of the Sichuan University. Informed consent was obtained from the participants according to the approved guidelines.
2.2. Cell culture and identification
The premolars were collected from patients (n=10, 12‐16 years of age) with written consent signed by the patients’ parents during orthodontic treatment in the West China Stomatology Hospital. The method to isolate and culture human DPSCs was described previously.9 The dental pulp tissue was cut to approximately 1 mm3 under aseptic conditions and digested with 3 mg/mL collagenase type I (Sigma, St. Louis, MO, USA) as well as 4 mg/mL dispase (Sigma) for 1 hour at 37°C. Single‐cell suspensions were obtained and incubated in α‐MEM (HyClone, Logan, UT, USA) with 10% foetal bovine serum (HyClone) and 100 U/mL of penicillin, streptomycin (HyClone) in a humidified atmosphere at 37°C and 5% CO2. The cell culture medium was changed every 3 days, and the cells from passage 3 were used for the experiments. To characterize the immunophenotype of DPSCs, flow cytometry analysis was used to detect the expression of mesenchymal stem cell‐associated surface markers at passage 3. DPSCs were trypsinized and incubated with CD14 (FITC), CD34 (FITC), CD45 (FITC), CD90 (PE), CD105 (PE) and CD73 (PE). All the antibodies (BD, Franklin Lakes, NJ, USA) were incubated according to the manufacturer's instruction, and flow cytometry was carried out using the Guava easyCyte (Millipore, Bedford, MA, USA). To identify the origin of cultured cells, DPSCs were imaged using immunofluorescence with antibodies against vimentin (Thermo, Waltham, MA, USA) and cytokeratin (Abcam, Cambridge, MA, USA). Subsequent steps were performed according to the manufacturer's recommendations, and all samples were examined under a fluorescence microscope (Nikon, Tokyo, Japan).
2.3. Multipotential differentiation of DPSCs
A total of 1×105 DPSCs were seeded onto each well of a six‐well plate. The control group was cultured in α‐MEM with 10% FBS. At 70% confluence, the cells were changed into osteogenic medium containing 10% FBS, 5 mmol/L l‐glycerophosphate (Sigma), 100 nmol/L dexamethasone (Sigma) and 50 mg/mL ascorbic acid (Sigma) for 21 days.10 After 3 weeks of culture, the cells were fixed in 4% paraformaldehyde for 10 minutes. After being incubated in 0.1% alizarin red solution (Sigma) in Tris‐HCl (pH 8.3) at 37°C for 30 minutes and washed three times in PBS, the cells were photographed under a light microscope (Nikon). A total of 1×105 DPSCs were seeded onto each well of a six‐well plate. At 80% confluence, the cells were cultured in adipogenic medium containing 10% FBS, 2 mmol/L insulin (Sigma), 0.5 mmol/L isobutylmethylxanthine (IBMX; Sigma) and 10 nmol/L dexamethasone (Sigma). After 21 days of culture, the cells were washed three times in PBS after being fixed in 4% paraformaldehyde and then incubated in 0.3% Oil Red O (Sigma) solution for 15 minutes.11 After washing the cells three times in PBS, the cells were routinely observed and photographed under a phase‐contrast inverted microscope (Nikon).
2.4. Preparation of dishes coated with DPM
DPSCs were seeded at 1×105 of each well in a six‐well plate pre‐coated with bovine fibronectin at 2.5 μg/cm2 (Millipore)12 and incubated with 10% FBS α‐MEM. When the cells reached 100% confluence, the culture medium was changed to 20 mmol/L ascorbic acid (Sigma) added 10% FBS α‐MEM. After a 7‐day culture, the cells was exposed by dissolving the cell layer with 0.5% Triton X‐100 and 20 mmol/L NH4OH in PBS at 37°C for 5 minutes then treated with 100 μg/mL DNAse (Sigma) for 1 hour at 37°C, following by three times washes. The DPMs remained, and they were kept in a triple antibiotic (Gibco, Waltham, MA, USA) PBS solution.
2.5. Immunofluorescence staining for DPM
DPMs were harvested on coverslips in a 24‐well plate. The subsequent staining steps were conducted according to the manufacturer's instructions for the antibodies. For the recognition of DPM structures, collagen‐I, collagen‐III, laminin, fibronectin, biglycan (Abcam, Cambridge, MA, USA) and decorin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were stained and examined under a fluorescence microscope (Nikon). DAPI (Sigma) was also used to examine the localization of DNA.
2.6. Scanning electron microscopy for DPM
A scanning electron microscope (SEM) (FEI‐QUANTA 200F, Eindhoven, the Netherlands) was used to visualize the surface morphology of DPM. The DPMs were dehydrated and sputter‐coated with gold for visualization (JEOL JFC‐1200 Fine Coater, Tokyo, Japan).13 Additionally, the DPM fibre diameters were characterized. Briefly, the SEM images of the DPMs were converted to 8‐bit JPG files. Then, these images were captured by nano measure to measure the fibre diameters. Ten sites were collected at every image, and the test was repeated three times.
2.7. DPSCs cultivated on the DPM
The mineralization behaviour change on DPM was also evaluated under 10% FBS α‐MEM and cultivation. The DPSCs were trypsinized and seeded at 2×104 cells/cm2 on both a plastic culture surface (DPSCs group) and DPM (DPSCs+DPM group), and the samples were harvested on days 4 and 7, followed by a Western blot analysis to identify the influence of the matrix‐coated culture.
2.8. Flow cytometric analysis for cell cycle
After culture, the cells were harvested when they reached 60% confluence. The cell pellet was fixed with cold 70% alcohol at 4°C for 48 hour. The cells were stained with 100 mg/mL propidium iodide (Sigma) and a 10 μg/mL RNAse (Takara, Dalian) cocktail at 4°C for 30 minutes. The experiment was repeated at least three times. The flow cytometry was carried out using the Guava easyCyte (Millipore).
2.9. Cell proliferation analysis with CCK‐8
The cell Counting Kit‐8 (CCK‐8) assay (Donjindo, Tabaru, Kumamoto, Japan) was used to evaluate the influence of the DPM on DPSC proliferation. The DPSCs were seeded on a plastic culture surface (DPSCs group) and DPM (DPSCs+DPM group), and both groups were cultured with α‐MEM with 10% FBS from day 1 to day 6. Each sample was added to one well of a 24‐well plate at a density of 3000 cells per well. All procedures were performed according to the manufacturer's recommendations.
2.10. DPSCs cultivated under the dentin matrix liquid extract
Dentin matrix liquid extracts (De) provide the odontogenesis proteins, which contained Collagen‐I, DSP, TGF‐b1, DMP1, biglycan and decorin.10, 14 These factors not only play important roles in differentiation into odontoblasts but also help form dentin tissues as well as induce DPSCs proliferation.15 Therefore, the extract was synthesized according to the previous study14 and used for an odontogenic condition medium. Briefly, DPSCs were cultured under the medium of α‐MEM and 10% FBS at 2×104 cells/cm2 for further odontogenic induction. The cells were grouped as follows: (i) 10% FBS‐MEM on plastic culture surface (DPSCs group), (ii) 10% FBS α‐MEM on a DPM‐coated surface (DPSCs+DPM group), (iii) De and 10% FBS on a plastic culture surface (DPSCs+De group), (iv) De and 10% FBS on a DPM‐coated surface (DPSCs+DPM+De group) for 7 days.
2.11. Real‐time PCR analysis
Real‐time PCR was used to investigate the changes in DPSCs under De. RNA from all the groups was extracted by RNAiso Plus (TaKaRa, Dalian, China). The cDNA was obtained using theμ RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Lithuania). We added 1 μL of a cDNA template, which was diluted 5‐fold with SYBR Green to real‐time PCR analysis expression of the genes: DSPP, DMP1, MEPE, COL‐I, ALP, Sp7 and GAPDH were evaluated. The cycling conditions were as follows: 95°C for 10 minutes, 45 cycles at 95°C (15 seconds each), and 60°C for 1 minutes. All data were analysed using the 2−ΔΔCt method. The assays were repeated three times. The primer sequences for analysed genes were listed in Table 1.
Table 1.
The primer sequences for analysed genes
| Target cDNA | Primer sequence (5′‐3′) | Product size (bp) | NCBI no. |
|---|---|---|---|
| GADPH | F CTTTGGTATCGTGGAAGGACTC | 132 | NM_002046.3 |
| R GTAGAGGCAGGGATGATGTTCT | |||
| ALP | F TAAGGACATCGCCTACCAGCTC | 170 | NM_000478.4 |
| R TCTTCCAGGTGTCAACGAGGT | |||
| DMP1 | F CTCGCACACACTCTCCCACTCAAA | 180 | NM_004407.3 |
| R TGGCTTTCCTCGCTCTGACTCTCT | |||
| DSPP | F GGAGCCACAAACAGAAGCA | 101 | NM_014208.3 |
| R TGGACAACAGCGACATCCT | |||
| Col‐IA1 | F AACATGGAGACTGGTGAGACCT | 145 | NM_000088.3 |
| R CGCCATACTCGAACTGGAATC | |||
| MEPE | F GGATGAAACTGCGAAAGAGG | 149 | NM_001291183.1 |
| R A CCTTCCCTTGGTGAGCATT | |||
| Sp7 | F GAGGTTCACTCGTTCGGATG | 120 | NM_001300837.1 |
| R TGGTGTTTGCTCAGGTGGT |
GADPH (glyceraldehyde‐3‐phosphatedehydrogenase), ALP (alkaline phosphatase), DMP1 (Dentin matrix phosphoprotein 1), DSPP (dentin sialophosphoprotein), Col‐IA1 (collagen‐IA1), MEPE (matrix extracellular phosphoglycoprotein), Sp7 (special protein 7).
2.12. Western blot
All Western blot analyses were conducted according to the manufacturer's instructions. Protein concentrations were measured using the BCA protein assay. Twenty micrograms of protein per lane was loaded on a 10% SDS‐PAGE gel for electrophoresis, and then transferred to 0.2 μm PVDF membranes (Bio‐Rad, Hercules, CA, USA) at 200 mA for 1.5 hour in a blotting apparatus (Bio‐Rad). The membranes were blocked in blocking solution (5% w/v skimmed milk, TBS, 0.1% Tween‐20) at room temperature for 2 hour. For the cell cycle analysis, membranes were incubated with primary antibodies (anti‐Cyclin D1, 1:1000; Santa Cruz Biotechnology; anti‐p21, Abcam, 1:1000; anti‐GAPDH, 1:10 000, Zhengneng, China). For DPSCs cultivated under DPM or De, the membranes were incubated with a primary antibody (DSP, 1:1000, Santa Cruz; Sp7 1:1000, DMP1, Biovision, 1:500; GAPDH, Zhengneng, 1:10 000). Following the treatment with second antibodies, all the immunoreactive proteins were then visualized using an Immobilon Western Chemiluminescent HRP Substrate (Millipore).
2.13. Mineralization‐related marker change under DPM in vivo
To observe the DPM‐modified DPSC in vivo, the DPSCs (DPSCs group) and DPM‐modified DPSCs (DPM group) were mixed with collagen gel and injected into the dermal layer of nude mice. Rat tail collagen‐I (Corning, Corning, NY, USA) was neutralized with 1 mol/L NaOH and 10x MEM (Sigma) at 3 mg/mL on ice. The cells were immersed in the collagen gel at 107 cell/mL on ice. The collagen mixture was ready on ice, and the 200 μL mixture was injected into the dermal layer of nude mice. Each group was repeated three times. The samples were harvested after a 2‐month subcutaneous implantation.
2.14. Evaluation of the DPM‐modified DPSCs in the dentin segments in vivo
The dental pulp tissue regeneration within the dentin segment was also investigated. The human mandibular premolar with a single root canal was used for implantation, which was removed from a healthy patient for prosthodontic treatment. A 7 mm dentin segment was separated from the crown, and the cementum was removed. The root canal was prepared with a 70# file, and a 0.7 mm apical foramen was prepared. Then, all the segments went to radiation sterilization at 25 Gy.16 For the DPSCs group, a total of 1×105 DPSCs were directly seeded inside the dentin segments and incubated under the culture medium for 1 day before the implant operation; For the DPSCs+DPM group, 1×105 DPSCs were seeded on the DPM and culture for 1 day before the operation. The DPSCs‐DPMs was removed from culture surface to dental segments with tweezers 1 day before implantation. The empty dentin segments were also implanted into nude mice as the blank control group. Then, all dentin segments were implanted into the dorsum of nude mice under deep anaesthesia.
2.15. Histological analysis
Eight weeks later, all samples were gained from the mice under deep anaesthesia. The implants were fixed with 4% paraformaldehyde for 5 hours at 4°C. The whole group was demineralized with 10% EDTA (pH 7.6) at 37°C, and embedded in paraffin. All antibodies and staining procedure were used according to the manufacturers’ protocol. DSP and DMP1 were used to identify whether the newly formed tissues followed the process of mineralization. Masson trichrome (MT) was used for collagen fibre staining. For the DPSCs implanted with collagen‐I, paraffin sections were stained with haematoxylin and eosin (H&E) and immunohistochemical stains, including DSP, DMP1 (Santa Cruz) and human mitochondria (Mito) (Millipore). Human mitochondria antibodies reacted with human‐derived cells, and was used to identify whether the seeding cells were in the new regeneration tissue. For the DPSCs implanted with dentin segments, haematoxylin and eosin (H&E), Masson trichrome, and immunohistochemical for human mitochondria (Millipore) were conducted.
2.16. Statistical analysis
All data are expressed as the mean±SD. Statistical significance was analysed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Independent samples were tested using the t test and t′ test, and a value of P<.05 was considered to be statistically significant.
3. RESULTS
3.1. Cell culture
Human DPSCs showed a typical spindle shape morphology at the third in vitro passage. DPSCs were positive for the mesenchymal marker vimentin (Figure 1H) but were negative for CK‐14 (Figure 1G), which is a marker of epithelial cells. Under adipogenic culture conditions for 21 days, lipid droplets formed and were stained with oil red (Figure 1K). When cultured in osteogenic medium for 21 days, DPSCs formed mineralized nodules through alizarin red staining (Figure 1I). Flow cytometric analysis showed that the DPSCs positively expressed the tri‐lineage differentiation potential markers CD90, CD105 and CD7317 (Figure 1A‐C). The lack of receptors for CD14, CD34 and CD45 (Figure 1D‐F) may suggest that DPSCs do not have haematopoietic and angiogenesis lineages.
Figure 1.
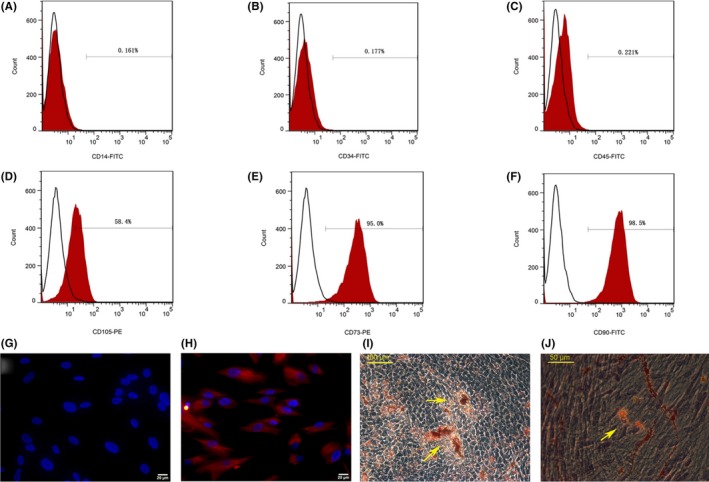
Dental pulp stem cells (DPSCs) culture and identify. FCM analysis (A‐F) showed that DPSCs were positive for CD105 (58.4%), CD73 (95.0%) and CD90 (98.5%), but negative for CD14 (0.161%), CD34 (0.177%) and CD45 (0.221%). The DPSCs were negative for CK‐14 (epithelial cell marker) (G), and positive for vimenten (mesenchymal cell marker) (H). After induction in vitro, DPSCs formed calcium nodules (I) (Yellow arrow) and lipid droplets (J) (Yellow arrows) (n=3)
3.2. Histological analyses and gross anatomy of DPM
The fabricated DPM were harvested with an attachment to the culture surface. Immunofluorescence showed that DPMs had regularly arranged fibres and were positive for collagen‐I (Col‐I), collagen‐III (Col‐III), laminin, decorin, fibronectin and biglycan. Apparently, DAPI staining showed that the nuclei were removed from DPM (Figure 2A). The orientation of fibres followed the trend of former cells. Prepared DPMs were visualized using SEM at 300× and 40 000X (Figure 2B). In this study, the average fibre diameters of the DPMs were 130 nm (ranging from 80 ‐170 nm), while the natural ECMs, which are predominantly composed of intertwined collagen and elastin fibres, ranged from 10 to 300 nm.18
Figure 2.
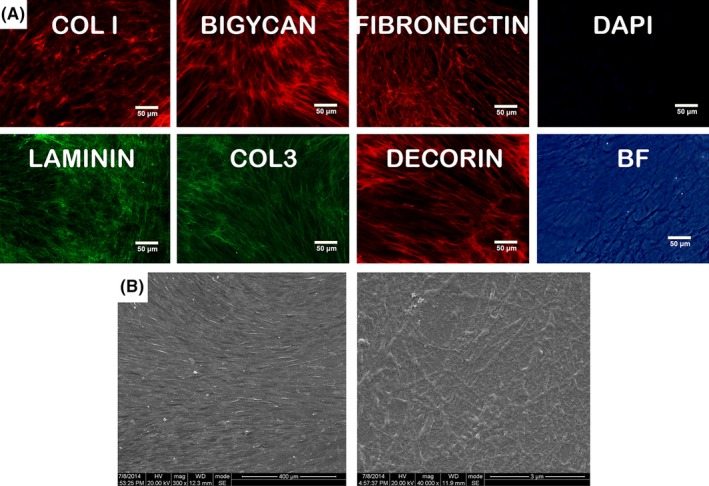
Fabrication of DPM. Immune fluorescence showed the DPM owned net fibrils structure that was positive for Col‐I, Col‐III, laminin, decorin, fibronectin, biglycan, which indicated the decellularization preserved the basic ECM proteins and structure (A). The decellularization was visualized by staining nuclei with the fluorescent dye DAPI (A). SEM image (B) showed the microstructure of DPM under 300× and 40 000× amplification, respectively (n=3)
3.3. Proliferate rate change under DPM and regular culture
As shown in the chart (Figure 3A), all the wells were seeded with the same amount of cells at day 0, and after one day incubation, the OD value did not show an obvious change. During the next 3 days, the DPSCs+DPM group showed a slightly higher value than the DPSCs group. On the fourth day, there was a sharp increase in the proliferation rate, and this effect lasted until the end of culture (day 6), which indicated that DPM had a positive effect on cell proliferation of DPSCs.
Figure 3.
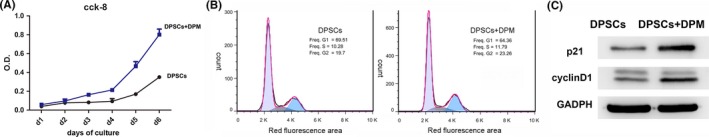
Cell proliferation analysis. DPSCs on the DPM (DPSCs+DPM group) showed a higher value of OD (A) compared with that of DPSCs on the plastic culture surface. The cell cycle analysis with PI staining of DPSCs on a plastic culture surface (DPSCs group) and DPM (DPSCs+DPM group). (B) Cyclin D1 and p21 expression at the protein level by Western blot with GAPDH serving as an internal control (C) (n=3)
3.4. Cell cycle analysis with PI staining flow cytometry
To measure the ratio of G1/S progression, all the cell samples were harvested at 60% confluence. For cells plated on a plastic culture surface, more cells remained in the G1 phase (69.51%) (Figure 3B) compared to cells plated on DPM (64.36%) (Figure 3B). Fewer cells were arrested at the G1 stage in the DPSCs+DPM group, which indicates that DPM was sufficient to initiate cell cycle progression towards expansion without adding growth factors.
3.5. Cell cycle‐related protein analysis
To further illuminate the potential mechanism of DPM‐supported high proliferation, cell cycle‐related proteins were also analysed (Figure 3C). The relative levels of expression of p21, Cdk4/D1 and Cdk6/D1 may determine the proliferative state of the cell.19 Cyclin D1 was higher in the DPSCs+DPM group, which was consistent with the high G1/S transition rate of the DPM group. p21 showed higher expression in the DPSCs+DPM group as well. The higher p21 expression was presumably required to coordinate and form a stable and active cyclin/cdk/kp21 complex that allowed for cells to continue negotiate the S‐phase.20
3.6. DPSCs’ mineral‐related marker expression on DPM in vitro
On Day 4, both the DPSCs on the plastic culture surface and DPM were nearly at 100% confluence, but the ECM on the plastic culture could not be accumulated with each other. At day 7, both groups showed double‐layer growth at the culture surface, and the ECM was being constructed on its own. The mineralization‐related markers DMP1, DSP and Sp7 decreased during DPM cultivation. The DPM culture showed slightly down‐regulated expression on day 4 and a more obvious change on day 7. That change indicated that the origin of the ECM and the micro‐environment for the cells determines the mineralization behaviour. That phenomenon also showed that stemness may be preserved, and fewer DPSCs were involved in the process of occasional mineralization (Figure 4).21
Figure 4.
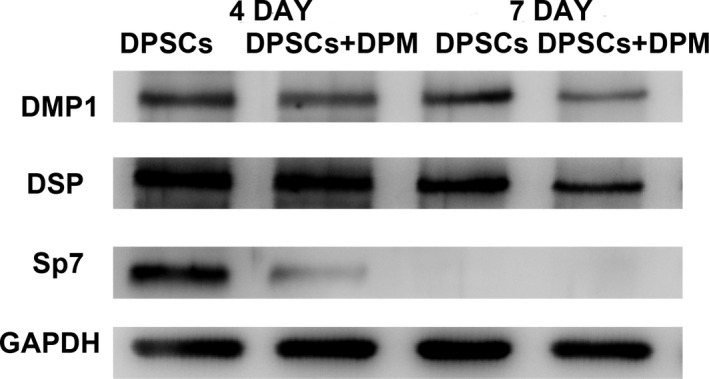
The mineralization marker change during the culture on the DPM on day 4 and day 7 by western blot. The mineralization‐related markers, DMP‐1, DSP and Sp7 were reduced during the DPM cultivation. The DPM culture showed slightly down‐regulated expression on day 4 and a more obvious change on day 7. GAPDH served as an internal control (n=3)
3.7. DPSCs’ mineral‐related marker expression on DPM in vivo
The implanted cells were harvested after a 2‐month implantation. Both groups showed a swirl arrangement with wrapped connective tissue in the collagen implantation model (Figure 5A,B). When the tissues were collected, obvious mineral tissue was not found under the skin. Both groups were positive for human mitochondria (Figure 5D,E), which suggests that the seeded DPSCs had participated. Both groups showed positive DMP1 and DSP expression within the cell cluster. Compared to the DPSCs group (Figure 5J‐G), the DPSCs+DPM group had lower amounts of DMP1 (Figure 5H) and DSP (Figure 5K) since the cells had been implanted and were mainly located around the capsule. Positive sites near the collagen capsule area indicated there was induction of osteogenesis for collagen‐I in vivo.22 Natural dental pulp tissue (C), was used for the positive control for mitochondria (F), DMP1 (I) and DSP (L).
Figure 5.
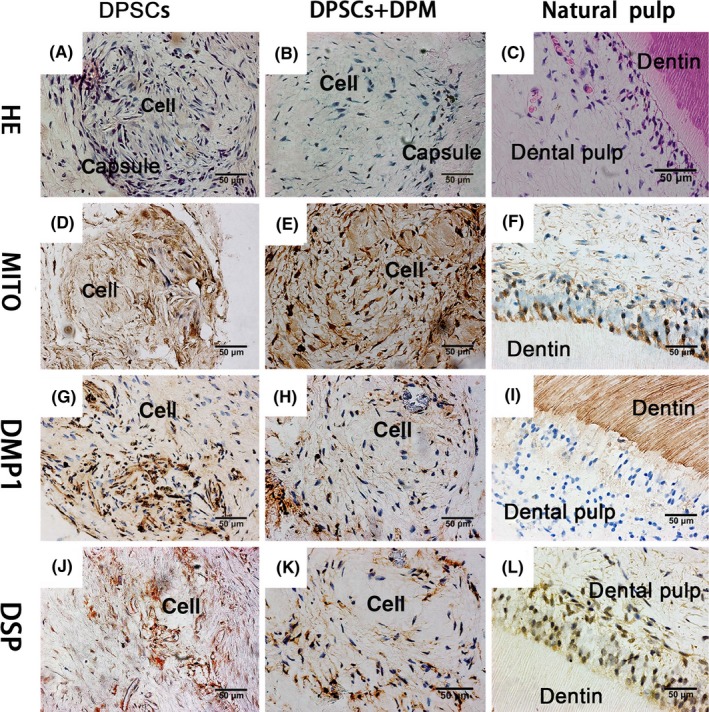
Histological examination of DPM‐treated DPSCs implanted in vivo. The native human dentin pulp structures (C) served as a positive control for the antibodies. The human mitochondria (F), DSP (L), DMP1 (I) was used as the positive control. Through the HE staining, both the DPSCs and DPM‐treated DPSCs formed as a cell cluster with capsule (A, B). Both DPSCs and DPM‐treated DPSCs showed human mitochondria positive (D, E), which indicated that the implanted cells positively participated in the cell cluster. For the DPM‐treated DPSCs, the cell clusters were positive for DSP (K) and DMP‐1(H), but the DPSCs cells possessed stronger DSP (J) and DMP1 (G) expression (n=3)
3.8. DPSC odontogenic behaviour change on the DPM in liquid dentin extracts
Odontogenic‐related markers were compared between the cultures of DPM (DPSCs+DPM+De group) and a regular plastic culture surface (DPSCs+De group) in the liquid dentin matrix extracts (De). Reports on the molecular events of dental injury and reparative dentinogenesis can be simulated by treating DPSC cells with a soluble extract of dentin.8 After a 7‐day induction, differences in genes expression were observed as follows:
Firstly, under the incubation of De, both the DPSCs+DPM+De and DPSCs+De group improved their DSPP (Figure 6B) transcription, which indicates that dentin extract could effectively give rise to odontogenesis markers. Secondly, the early osteogenic marker ALP was down‐regulated when cultured under the induction of De, respectively (Figure 6C), which was also found in a previous study. Interestingly, the mineral‐regulating gene MEPE (Figure 6D) was only down‐regulated in the DPSCs+DPM+De incubation group. Sp7, which is a putative master regulator of bone cell differentiation, showed lower expression in the DPSCs+DPM+De (Figure 6E) group too. Another important osteogenic marker DMP1 (Figure 6A) only improved in the DPSCs+De group.
Figure 6.
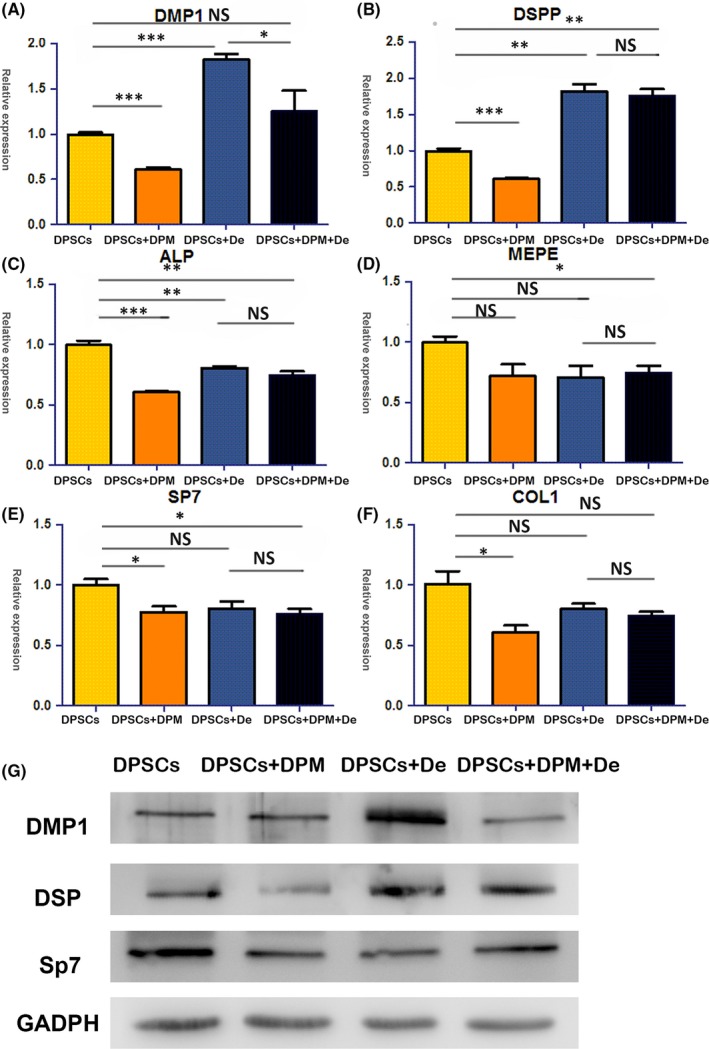
The odontogenic influences of DPM on DPSCs in liquid dentin extract (De). In the liquid dentin extract (DPSCs+De group), DPSCs showed up‐regulated odontogenic markers, including DMP‐1 (A) and DSPP (B) both at the transcript and expression level (G). Meanwhile, early osteogenic markers for ALP were down‐regulated (C) in the DPSCs+De group. During cultivation on the DPM with liquid dentin extract (DPSCs+DPM+De group), ALP(C) and SP7 (E) were down‐regulated. DSPP (B) up‐regulation was accompanied with MEPE (D) down‐regulation, which indicated odontoblast differentiation occurred (*P<.05, **P<.01, ***P<.001, NS=none significance) (n=3)
Immunopositive staining against DSP was detected in both the DPSCs+De and DPSCs+DPM+De groups. The DPSCs+De group presented stronger positive results with DMP1 expression. Under De cultivation, both of the groups showed lower expression of Sp7 than the DPSCs group (Figure 6G).
When De was added to the culture medium, the mineralization showed a different pattern than when DPM was added. A previous investigation demonstrated that down‐regulated MEPE accompanied with up‐regulated DSPP should be considered a marker of DPSC differentiation.23 MEPE regulates the differentiation and mineralization of hard tissue‐related cells, and the expression of MEPE was only observed in immature odontoblasts.24 MEPE becomes down‐regulated when the odontoblasts mature.25, 26 In this study, MEPE was only down‐regulated in the DPSCs+DPM+De group along with a higher expression of DSPP.
3.9. The dental pulp genesis ability on the DPM
The connective tissue‐like tissue was harvested in the DPSCs (Figure 7A,B), DPSCs+DPM (Figure 7C,D) and blank groups (Figure 7G,H). Natural dental pulp (Figure 7E,F) was used as control. In the DPSCs+DPM group, the newly formed tissue had a more ordered fibre arrangement (Figure 7C,K) and possessed a more uniform diameter. The direction of the fibril arrangement had similarity to that of natural dental pulp tissue. A few odontoblast‐like cells (Figure 7D,L, red arrow) were found along the dentin tube side, and collagen fibres are shown in the top of the cells, which indicates there is functional synthesis. In the no‐cell (blank) group, the fibrous tissue grew into the root canal, and a few leucocytes could be found among the tissue (Figure 7H,P, black arrow). For the DPSCs group, the newly formed dental pulp tissue had blood vessels and plenty of erythrocytes among fibrils. However, the typical odontoblast‐like cells were not found in the remolding root canal. The fibres were crossed‐arranged in the full pulp tissue with newly constructed dentin walls. Through Masson staining, strong collagen fibres were observed throughout the full dental pulp tissue along the inner wall of the root canal (Figure 7A,I). Additionally, ectopic mineralization as also found in the regenerated dental pulp (Figure 7B,J, yellow arrow). The immunohistochemistry with human mitochondria yielded positive results in both DPSCs (Figure 7Q) and DPM (Figure 7R) modified DPSCs group as well as the natural human tooth (Figure 7S), but the results were negative in the blank group (Figure 7T), which illustrated that the seed cells succeeded in the regeneration process.
Figure 7.
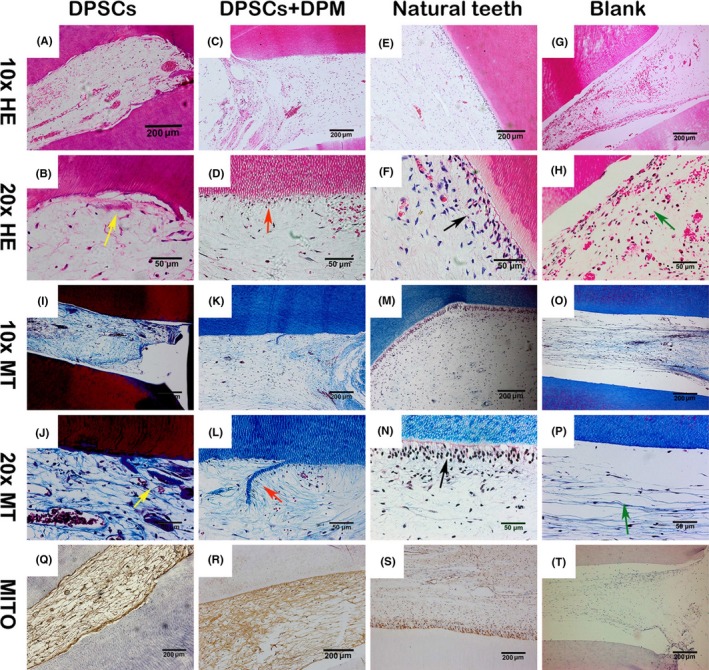
Histological analysis for regenerated dental pulp. There were DPSCs group (A, B), DPSCs+DPM group (C, D), natural dental pulp (E, F), blank group (G, H) with H&E staining. The DPSCs+DPM group (K, L), natural dental pulp (M, N), and blank group (O, P) were also stained with Masson trichrome. Using the staining, the DPSCs+DPM group (red arrow) (L, D) had slighter fibril and a few odontoblast‐like cell layers in the regenerated tissue compared to the DPSCs group, which showed similarity to the natural dental pulp (black arrow) (F, N). Additionally, some collagen‐rich island and strong staining fibrils (yellow arrow) (B, J) could be found in the DPSC‐regenerated dental pulp. In the blank group, the fibrils and some leucocytes were observed in the root canal (green arrow) (H, P). The natural dental pulp tissue served as a positive control for human mitochondria (S). Both the DPSCs (Q) and DPSCs+DPM (R) groups were positive for human mitochondria, and blank group was negative for human mitochondria (T) (n=3)
4. DISCUSSION
The present study described the characterization of DPSCs under a cell‐derived DPM and the contribution of an ECM component to reconstruct dental pulp tissue. DPM preserved the important fibrous portions of ECM, such as collagen‐1, collagen‐3, fibronectin and laminin, and they shared similarity with the component as well as a 3D structure of dental pulp, and these components were involved with regulating the dental pulp matrix and avoiding disordered mineralization.27
The ECM structure contributed to the maintenance of stem cell populations28 through lower expression of differentiation markers, playing an important part in cell proliferation, survival29and stemness preservation.30 In this study, DPM‐based culture made up for the absence of the DPSC niche in vitro. DPSCs possess the capacity for self‐renewal and multi‐lineage differentiation.31Without a native niche, however, DPSCs show great osteogenic differentiation potential in regard to expansion in vitro,32 which may result in failure outcomes in tissue repair.
The regular culture surface is mainly based on polystyrene, whose solid and sound surface characteristics may not be able to imitate the natural milieu well; this could lead to changes in cell behaviour and loss of stemness.9 The proliferation and osteogenic differentiation of DPSCs are sensitive to their extrinsic factors and remolded when the extrinsic factors change.33 This phenomenon highlights the necessity of a proper micro‐environment for dental pulp regeneration.4 During the culture on the DPM, both the enhanced proliferation rate and suppressed mineralization marker expression indicated that DPSCs maintained their stemness.34, 35 The collagens, fibronectin, laminin and biglycan among the DPM activated the integrins of DPSCs. Binding of ECM and cell surface integrin receptors can induce early signalling cascades and gene expression of passage through the G1/S transition.36
Dental pulp is sensitive to the environmental stimuli, and natural dental pulp ECM enables preservation from mineralization. Under physiological conditions, dental pulp is the process of dentin formation throughout an animal's lifetime. Immunostaining and in situ hybridization showed DSP, DPP and DMP1 are mostly expressed in the odontoblast layer and dentin matrix but not in the dental pulp tissue.15 Due to the absence of these proteins, natural dental pulp is unable to mineralize under physiological conditions. When dental pulp is injured or encounters capping material, the local ECM and molecules change. The DPSCs react as reparative dentin, a dentin bridge, and even develop into calcification, leading to closure of the dental pulp rather than repairing the original soft connective tissue. Thus, a proper ECM niche should be established through dental pulp therapy.
The ECM has potential bioactive agents for inducing DPSC reaction, which affects repair and regeneration in dental pulp tissue. Involvement of DPM and DPSCs were further investigated by histological staining in vivo. The results demonstrated that the DPM had an effect on the structural integrity of the regenerated dental pulp ECM. Compared to the DPM‐grafted group, the condensed connective tissue matrix appeared, and thick fibre bundles in the ECM aggregated in the single DPSCs grafted group, which showed there was similarity as tissue age advanced. During cultivation with DPM, the regenerated dental pulp had ordered fibril arrangement in the pre‐dentin area and uniform dental pulp matrix. The results indicated that ECM had an effect on structural integrity of later regenerated dental pulp matrix,37and ECM proteins that resided among the matrix became involved with collagen fibril organization and maintaining the integrity of the dental pulp tissue. Collagens, as the majority of protein found in dental pulp ECM, are also a major component of dentin and other mineralization tissue. For natural dental pulp, large intercellular spaces contain type I collagen (56%),4 and the dentin predominantly contains type I collagen. The breaking balance of ECM collagen fibril bundles potentially brought about the matrix mineralization.38 It was reported that disordered ECM participated in the formation of pulp stones and diffuse calcification.39 Therefore, collagen fibrils should be well organized, and disorganization should be prevented in the dental pulp tissue.40 ECM components regulated structural homeostasis integrity of connective tissue in dental pulp regeneration.41 Therefore, the maintenance of healthy dental pulp matrix rather than the promotion of mineralization of seeded cells is a more crucial question to be addressed in regenerative dental pulp.
Compared to traditional decellularized material, microstructures, such as lymph tubes and small blood vessels, could only be found in decellularized dental pulp tissue.42 The polarized distribution of DPEM has a distinction from the cell‐derived ECM. However, the absence of these microstructures did not affect implantation of the cells at the initiation and later structural reconstruction. This research also highlighted through dental pulp regeneration that the ECM component proteins rather than the polarized decellularized structure are more important.
There are also some limitations to this study. Through in vitro evaluation, the DPM group showed different mineralization markers that changed with or without the De. Most of the mineralization markers belong to the SIBLING family. For example, DMP1 and DSPP are located in the ECM, where they are further cleaved into a functional protein and influence the mineralization behaviour of the cells in their ECM micro‐environment.43, 44 Therefore, both the binding distribution and cleavage of the SIBLING family proteins in the ECM should be explored in future research.
In summary, an ECM‐based substrate provides the niche that supports the balance between the replication and mineralization behaviours of DPSCs. DPM provides an advantageous environment for the DPSCs to preserve themselves before the implantation, and ECM plays an essential part in reconstructing the dental pulp tissue. This ECM‐based system will also be useful for studying the dental pulp scaffold or an ECM‐based capping material, and the DPM can be wrapped around synthesized material to provide a milieu for the cells.
ACKNOWLEDGEMENTS
We thank Dr. Junjie Wu for the instruction of ECM synthesis. This work was supported by Nature Science Foundation of China (31470947), International Cooperation Program of China (2013DFG32770), Program for New Century Excellent Talents by the State Education Commission of China (NCET‐13‐0385), Sichuan University Fund for Distinguished Young Scholars of China.
Zhang X, Li H, Sun J, et al. Cell‐derived micro‐environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif. 2017;50:e12361 10.1111/cpr.12361
Contributor Information
Weihua Guo, Email: guoweihua943019@163.com.
Weidong Tian, Email: drtwd@sina.com.
REFERENCES
- 1. Stern MM, Myers RL, Hammam N, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393‐2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 2003;278:14587‐14590. [DOI] [PubMed] [Google Scholar]
- 3. Lee YH, Kim GE, Cho HJ, et al. Aging of in vitro pulp illustrates change of inflammation and dentinogenesis. J Endod [Internet]. 2013;39:340‐345. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med [Internet]. 2004;15:13‐27. [DOI] [PubMed] [Google Scholar]
- 5. Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337‐4351. [DOI] [PubMed] [Google Scholar]
- 6. Zhu X, Wang Y, Liu Y, Huang GTJ, Zhang C. Immunohistochemical and histochemical analysis of newly formed tissues in root canal space transplanted with dental pulp stem cells plus platelet‐rich plasma. J ndod [Internet]. 2014;40:1573‐1578. [DOI] [PubMed] [Google Scholar]
- 7. Sun Y, Li W, Lu Z, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim. 2005;12: p. 232. [DOI] [PubMed] [Google Scholar]
- 9. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625‐13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li R, Guo W, Yang B, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525‐4538. [DOI] [PubMed] [Google Scholar]
- 11. Jo Y‐Y, Lee H‐J, Kook S‐Y, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767‐773. [DOI] [PubMed] [Google Scholar]
- 12. Vlodavsky I, Folkman J, Sullivan R, et al. Endothelial cell‐derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987;84:2292‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uygun BE, Soto‐Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiao L, Xie L, Yang B, et al. Cryopreserved dentin matrix as a scaffold material for dentin‐pulp tissue regeneration. Biomaterials [Internet]. 2014;35:4929‐4939. [DOI] [PubMed] [Google Scholar]
- 15. Butler WT, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol. 1995;39:169‐179. [PubMed] [Google Scholar]
- 16. De Carlo E, Baiguera S, Conconi MT, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies. Int J Mol Med. 2010;25:195‐202. [PubMed] [Google Scholar]
- 17. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol [Internet]. 2014;32:252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15:467‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan GZ, Ziff EB. Nerve growth factor induces transcription of the p21 WAF1/CIP1 and cyclin D1 genes in PC12 cells by activating the Sp1 transcription factor. J Neurosci. 1997;17:6122‐6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiyama H, Iavarone A, Reeves SA. Regulation of the cdk inhibitor p21 gene during cell cycle progression is under the control of the transcription factor E2F. Oncogene. 1998;16:1513‐1523. [DOI] [PubMed] [Google Scholar]
- 21. Chen X‐D, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow‐derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943‐1956. [DOI] [PubMed] [Google Scholar]
- 22. Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol [Internet]. 2004;2004:24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu M, Sun Y, Liu Y, Yuan M, Zhang Z, Hu W. Modulation of the differentiation of dental pulp stem cells by different concentrations of β‐glycerophosphate. Molecules. 2012;17:1219‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacDougall M, Simmons D, Gu TT, Dong J. MEPE/OF45, a new dentin/bone matrix protein and candidate gene for dentin diseases mapping to chromosome 4q21. Connect Tissue Res. 2002;43:320‐330. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Li W, Shi S, Habelitz S, Gao C, DenBesten P. MEPE is downregulated as dental pulp stem cells differentiate. Arch Oral Biol. 2005;50:923‐928. [DOI] [PubMed] [Google Scholar]
- 26. Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285:E1‐E9. [DOI] [PubMed] [Google Scholar]
- 27. Haruyama N, Sreenath TL, Suzuki S, et al. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biol. 2009;28:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Honoré S, Kovacic H, Pichard V, Briand C, Rognoni JB. α2β1‐Integrin signaling by itself controls G1/S transition in a human adenocarcinoma cell line (Caco‐2): implication of NADPH oxidase‐dependent production of ROS. Exp Cell Res. 2003;285:59‐71. [DOI] [PubMed] [Google Scholar]
- 29. Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue‐specific lineage potential. Tissue Eng Part A. 2011;17:3067‐3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang S, Mu J, Fan Z, et al. Insulin‐like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res [Internet]. 2012;8:346‐356. [DOI] [PubMed] [Google Scholar]
- 31. Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531‐535. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Sha X‐J, Li G‐H, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol [Internet]. 2012;57:1231‐1240. Available at: 10.1016/j.archoralbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 33. Ma D, Ma Z, Zhang X, Wang W. Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J Endod [Internet]. 2009;35:1546‐1553. [DOI] [PubMed] [Google Scholar]
- 34. Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20 19:7370‐7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malik RK, Parsons JT. Integrin‐dependent activation of the p70 ribosomal S6 kinase signaling pathway. J Biol Chem. 1996;271 47:29785‐29791. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin‐dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553‐2560. [DOI] [PubMed] [Google Scholar]
- 37. Yang J, Yamato M, Kohno C, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415‐6422. [DOI] [PubMed] [Google Scholar]
- 38. Khan SZ, Kokubu E, Matsuzaka K, Inoue T. Behaviour of rat‐cultured dental pulp cells in three‐dimensional collagen type‐1 gel in vitro and in vivo. Aust Endod J. 2013;39 3:137‐145. [DOI] [PubMed] [Google Scholar]
- 39. Hillmann G, Geurtsen W. Light‐microscopical investigation of the distribution of extracellular matrix molecules and calcifications in human dental pulps of various ages. Cell Tissue Res. 1997;289 1:145‐154. [DOI] [PubMed] [Google Scholar]
- 40. Wiesmann HP, Meyer U, Plate U, Höhling HJ. Aspects of collagen mineralization in hard tissue formation. Int Rev Cytol. 2004;12:121‐156. [DOI] [PubMed] [Google Scholar]
- 41. Goldberg M, Septier D, Rapoport O, Iozzo RV, Young MF, Ameye LG. Targeted disruption of two small leucine‐rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcif Tissue Int. 2005;77 5:297‐310. [DOI] [PubMed] [Google Scholar]
- 42. Chen G, Chen J, Yang B, et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials [Internet]. 2015;52:56‐70. [DOI] [PubMed] [Google Scholar]
- 43. Maciejewska I, Cowan C, Svoboda K, Butler WT, D'Souza R, Qin C. The NH2‐terminal and COOH‐terminal fragments of dentin matrix protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem. 2009;57 2:155‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki S, Haruyama N, Nishimura F, Kulkarni AB. Dentin sialophosphoprotein and dentin matrix protein‐1: Two highly phosphorylated proteins in mineralized tissues. Arch Oral Biol. 2012;57 9:1165‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]


