Abstract
Objectives
We previously reported that conditioned medium from cultures of bone marrow‐derived mesenchymal stem cells have strong potential to accelerate bone regeneration. We now examine in vitro and in vivo a defined cytokine cocktail that mimics the effects of conditioned medium on bone regeneration.
Materials and methods
A cocktail of recombinant human insulin‐like growth factor‐1, vascular endothelial growth factor‐A and transforming growth factor‐β1 was prepared at concentrations similar to those in conditioned medium. Conversely, these cytokines were depleted from conditioned medium, and the effects of the cocktail, the conditioned medium and the cytokine‐depleted conditioned medium on bone regeneration were evaluated in vitro and in vivo.
Results
The cytokine cocktail and conditioned medium enhanced cell migration, tube formation, and expression of osteogenic and angiogenic genes. Depletion of cytokines significantly decreased the effects of conditioned medium in vitro. Similarly, the cytokine cocktail and conditioned medium, but not cytokine‐depleted medium, increased bone regeneration in damaged rat calvarial bone. Immunohistochemistry indicated that the cytokine cocktail and conditioned medium strongly enhanced recruitment of endogenous stem cells and endothelial cells.
Conclusions
The data indicate that the cytokine cocktail and conditioned medium enhance the migration of stem cells and endothelial cells to damaged bone, and elicit osteogenesis and angiogenesis.
1. Introduction
Cytokines and stem cells, especially bone marrow‐derived mesenchymal stem cells, have been extensively investigated in the clinic as tools to regenerate bone.1, 2 However, several issues with stem cells remain to be addressed, including tumorigenesis,3 poor survival of implanted cells4, 5 and transmission of infectious disease. However, as implanted mesenchymal stem cells may contribute to tissue regeneration not only via pluripotency but also via paracrine effects,6, 7 conditioned media from stem cell cultures may help address these issues. For example, conditioned media from cultures of amniotic fluid‐derived mesenchymal stem cells and adipose‐derived stem cells significantly enhanced wound healing.8 Similarly, conditioned media from endothelial progenitor cells induced neovascularization in a rat model of hindlimb ischaemia.9 We note that the secretomes of various mesenchymal stem cells have been described.10, 11, 12
We previously reported that serum‐free conditioned media from human bone marrow‐derived mesenchymal stem cells (hMSCs) contain numerous cytokines, including insulin growth factor‐1 (IGF‐1), vascular endothelial growth factor‐A (VEGF) and transforming growth factor‐β1 (TGF‐β1). These cytokines regulate migration, angiogenesis and osteogenesis in host mesenchymal stem cells, and thus may accelerate regeneration of bone and periodontal tissue.13, 14, 15, 16, 17 Indeed, the conditioned medium enhances cell migration in vitro, stem cell expression of angiogenic and osteogenic genes, including angiopoietin 1, vegf, collagen I, osteocalcin and Runt‐related transcription factor 2 (Runx2), as well as tube formation in human umbilical cord vein endothelial cells (HUVECs). In vivo, the conditioned medium elicits regeneration of bone and periodontal tissue, not only in animal models but also in preliminary human study.18 We note, however, that high doses of cytokines are typically required to achieve clinical effects.19 Unfortunately, such doses may also trigger severe inflammation, as was reported when recombinant human bone morphogenetic protein‐2 was administered after head and neck surgery.20, 21
It is possible that only a small number of specific factors in the conditioned medium regenerate bone. Indeed, we hypothesized that IGF‐1, VEGF and TGF‐β1 are the relevant active factors, because they were included in CM from hMSCs relatively at higher concentrations than the other factors according to our previous reports and they were well known to affect cellular migration, angiogenesis and osteogenesis. However, other well‐documented factors relevant in osteogenesis like bone morphogenic protein, fibroblast growth factors‐2, platelet‐derived growth factors‐BB and stromal cell‐derived factor‐1 were not found in CM from hMSCs.14 To test this hypothesis, we investigated the activity of conditioned media depleted of these cytokines, as well as that of a defined cocktail of recombinant cytokines.
2. Materials and methods
2.1. Animal experiments
All animal experiments were in accordance with the Guidelines for Animal Experimentation of Nagoya University School of Medicine (approval no. 27351).
2.2. Preparation of cells
Human bone marrow‐derived mesenchymal stem cells were purchased from Lonza, Inc. (Walkersville, MD, USA) and cultured at 37°C and 5% CO2/95% air, in MSC basal medium (Lonza, Inc.) containing MSCGM SingleQuots (Lonza, Inc.). Cells were then subcultured at approximately 1 × 104 cells/cm2, and used in experiments after 3‐5 passages.
Rat mesenchymal stem cells (rMSCs) were isolated from five male Wistar/ST rats, 8 weeks old and weighing 180‐210 g (Japan SLC, Shizuoka, Japan), as described previously.22 Briefly, rats were euthanized and the femora were dissected. Basal medium containing MSCGM SingleQuots was then injected aseptically into the bone marrow using an 18‐gauge syringe to flush bone marrow cells. Cells collected from three flushes were seeded into a cell culture dish (Greiner Bio‐One International GmbH, Kremsmünster, Austria) containing MSC basal medium supplemented with MSCGM SingleQuots and 10% foetal bovine serum (FBS), and cultured at 37°C and 5% CO2/95% air. Floating cells were removed after 3 days, and the medium was refreshed. When nearly confluent, adherent, spindle‐shaped cells were harvested with 0.05% trypsin/ethylenediaminetetraacetic acid, resuspended in fresh medium, and transferred to new dishes at 1 × 104 cells/cm2. rMSCs obtained after 2‐4 passages were used in subsequent experiments.
2.3. Preparation of conditioned medium
Human bone marrow‐derived mesenchymal stem cells, 70‐80% confluent, were rinsed with phosphate‐buffered saline (PBS), and fed serum‐free Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) containing 100 U/mL penicillin G, 100 mg/mL streptomycin and 0.25 mg/mL amphotericin B (Thermo Fisher Scientific). The medium was collected after 48 hours, centrifuged for 5 minutes at 407 g and then for another 3 minutes at 1630 g to remove cells, filtered at 0.22 μm (Millex™ GP Filter Unit; Merck Millipore Ltd, Darmstadt, Germany), and stored at 4°C within 2 weeks or at −80°C within 1 month until use.
2.4. Cytokine depletion
Cytokines were depleted from conditioned medium using rabbit anti‐human polyclonal antibodies against IGF‐1 (LS‐C36891; LifeSpan BioSciences, Inc., Seattle, WA, USA), VEGF (ab39250; Abcam, Cambridge, UK) and TGF‐β1 (ab125287; Abcam). Briefly, Protein G Mag Sepharose (GE Healthcare Ltd, Little Chalfont, UK) was pre‐coated with 28 ng/mL anti‐IGF‐1, and gently mixed with conditioned medium overnight at 4°C, using an MTR‐103 rotator (AS ONE Co., Osaka, Japan). Beads were then removed by centrifugation for 5 minutes at 1630 g, and the supernatant was depleted in a similar manner using 10 ng/mL anti‐VEGF and 7 ng/mL anti‐TGF‐β1. Enzyme‐linked immunosorbent assay was used to confirm depletion.
2.5. Cytokine cocktail
Recombinant human IGF‐1 (Somazon™; Astellas Pharma Inc., Tokyo, Japan), VEGF‐A (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and TGF‐β1 (R&D Systems, Inc., Minneapolis, MN, USA) were prepared at 1400, 500 and 350 pg/mL to match the respective concentration in conditioned medium.13
2.6. Cell migration
Rat mesenchymal stem cells (5 × 105 cells/cm2) passaged 2‐3 times in DMEM with 0.25% FBS were seeded into the upper chambers of transwell dishes with 8 μm pores (BD BioCoat Control Inserts; Becton Dickinson and Co., Franklin Lakes, NJ, USA). Lower chambers were filled with conditioned medium, depleted conditioned medium or cytokine cocktail, using DMEM with or without 30% FBS as positive and negative control. After 48 hours, the upper surfaces of transwell membranes were rinsed with PBS, cleared of cells with a cotton swab, stained with haematoxylin, and mounted on microscope slides. Migrating cells were counted in three randomly selected fields using a light microscope (CL40; Olympus, Tokyo, Japan) at 200× magnification. Assays were repeated five times and data are represented as mean ± SD.
2.7. Endothelial tube formation
Endothelial tube formation was assessed using an in vitro angiogenesis kit (Cell Biolabs, Inc. San Diego, CA, USA), following the manufacturer's instruction. Briefly, 50 μL endothelial cell medium gel solution (CBA‐200; Cell Biolabs) was placed in a 96‐well plate, solidified at 37°C for 1 hour, seeded with HUVECs (3 × 104 cells/well), and cultured for 10 hours at 37°C and 5% CO2 in endothelial cell medium (ScienCell Research Laboratories, Carlsbad, CA, USA) supplemented with or without conditioned medium, depleted conditioned medium, cytokine cocktail or 500 ng/mL VEGF. Endothelial tube formation was evaluated on microphotographs acquired with a light microscope (CL40; Olympus) at 40× magnifications. Straight cellular extensions connecting two cell masses or branch points were considered to be tubes, and total tube length and number of branching points were quantified (n=5).
2.8. Real‐time reverse transcriptase‐polymerase chain reaction
rMSCs were cultured for 48 hours with conditioned medium, depleted conditioned medium, DMEM containing cytokine cocktail or DMEM containing 10% FBS, and total RNA was extracted using RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. Real‐time reverse transcriptase‐polymerase chain reaction (real‐time RT‐PCR) was performed as previously reported, using specific primers and probes for alkaline phosphatase, Runx2, osteocalcin, VEGF‐A, angiopoietin 1 and GAPDH (Table 1). Reactions were prepared using Taqman Fast Virus 1‐Step Master Mix (Applied Biosystems, Thermo Fisher Scientific), and monitored in real‐time using a 7000 Sequence Detector (Perkin‐Elmer, Forster City, CA, USA). Targets were amplified over one cycle at 50°C for 2 minutes, one cycle at 60°C for 30 minutes, one cycle at 95°C for 5 minutes, 50 cycles at 95°C for 20 seconds (denaturation) and 60°C for 1 minute (annealing and extension). The relative amount of target mRNAs in one sample was obtained according to the corresponding standard curves, which were constructed using 625, 125, 25, 5 and 1 ng total RNA. Relative expression was normalized to GAPDH. Assays were repeated five times and data are represented as mean ± SD.
Table 1.
Real‐time RT‐PCR primers and probes
| Gene | Sequence | Product length | Tm | GenBank accession no. | |
|---|---|---|---|---|---|
| ALP | F | GACAGTCATTGAATACAAAAC | 132 | 40.6 | M_19070 |
| R | ACGGAATTCTTGGTTAGTA | 41.6 | |||
| Probe | TAAGCCATCTCGCCTGCCAT | 50.2 | |||
| OCN | F | GACTCTGAGTCTGACAAA | 86 | 40.6 | NM_013414 |
| R | AGTCCATTGTTGAGGTAG | 40.6 | |||
| Probe | CGGAGTCTATTCACCACCTTACTGC | 54.2 | |||
| Runx2 | F | CCTCTTATCTGAGCCAGA | 123 | 42.9 | NM_053470 |
| R | GCAGTGTCATCATCTGAA | 40.6 | |||
| Probe | CATCCATCCATTCCACCACGC | 51.2 | |||
| VEGF | F | ATCCCGGTTTAAATCCTG | 94 | 40.6 | NM_031836 |
| R | GGAACATTTACACGTCTG | 40.6 | |||
| Probe | CACTGTGAGCCTTGTTCAGAGC | 51.6 | |||
| Ang‐1 | F | GAAGGAGGAGAAAGAAAAC | 81 | 41.6 | NM_053546 |
| R | TCTGCTAAGTTGCTTCTC | 40.6 | |||
| Probe | TGGTTACTCGTCAGACATTCATCATCC | 53.1 | |||
| GAPDH | F | GTTCCAGTATGACTCTACC | 129 | 43.8 | NM_017008 |
| R | TCACCCCATTTGATGTTA | 38.3 | |||
| Probe | TTCAACGGCACAGTCAAGGC | 48.7 |
ALP, alkaline phosphatase; OCN, osteocalcin; Runx2, Runt‐related transcription factor 2; VEGF, vascular endothelial growth factor; Ang‐1, angiopoietin 1; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; Tm, melting temperature (°C).
2.9. Rat model of calvarial bone defect
Wistar/ST rats, 8 weeks old, were anesthetized by intraperitoneal injection of 20 mg/kg pentobarbital (Somnopenthl™; Kyoritsu Seiyaku, Tokyo, Japan). After shaving, a straight incision was made below the ear, and the periosteum was raised to expose the surface of the calvarial bone. Two circular defects (full thickness, diameter 5 mm) were created in the calvarial bone using a high‐speed trephine bur, with constant saline irrigation. Subsequently, an absorbable atelocollagen sponge (TERUDERMIS; Olympus Terumo Biomaterials, Tokyo, Japan) pre‐soaked in 30 μL conditioned medium, depleted conditioned medium, cytokine cocktail or PBS were implanted (n=8 per group). Rats were sacrificed at 1 or 2 weeks after implantation.
2.10. Radiography and histology
Damaged bones were dissected after 2 weeks, fixed in 10% formalin, and analysed by micro‐computed tomography (micro‐CT) on a laboratory X‐ray CT device (micro‐CT; RIGAKU, Tokyo, Japan). 3D images were reconstructed using software supplied with the instrument, from which the ratio of the area (mm2) of newly formed bone to the area of the surgically created defect (mm2) was computed. After radiography, specimens were decalcified with K‐CX solution (Falma Co., Tokyo, Japan), dehydrated with graded ethanol, cleared with xylene, embedded in paraffin, sectioned sagittally at 4 μm, stained with haematoxylin and eosin, and imaged under a light microscope (CL40; Olympus).
2.11. Immunohistochemistry
Damaged bones were dissected 1 week after surgery, and fresh‐frozen sections were prepared according to Kawamoto's method,23 using a Multi‐Purpose Cryosection Preparation Kit. Briefly, frozen blocks were coated with cryofilm type 2C, and sectioned with a tungsten carbide knife at −25°C in a cryostat chamber (CM3050S; Leica Microsystems GmbH, Wetzlar, Germany). Sections were then fixed in 100% ethanol for 10 minutes at room temperature, washed with PBS, blocked with 5% bovine serum albumin in PBS for 30 minutes, and probed overnight at 4°C with primary antibodies in blocking buffer. A mouse monoclonal antibody against CD31 (BD Pharmingen™, Tokyo, Japan) was used to mark rat endothelial cells, while a polyclonal rabbit antibody against CD105 (Santa Cruz Biotechnology Inc., Dallas, TX, USA) was used to mark rat stem cells. Sections were then stained for 30 minutes with goat anti‐mouse IgG conjugated to Alexa Fluor 633 (Invitrogen™, Carlsbad, CA, USA) and donkey anti‐rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen™) respectively. After washing with PBS, sections were stained with 4′‐6‐diamidino‐2‐phenylindole, washed with PBS, and mounted between a glass slide and an adhesive film. The section was sealed with the mounting resin SCMM R2 (Leica), and the resin was hardened by ultraviolet irradiation for 1 min on a UV Quick Cryosection Mounter (ATTO Corp., Tokyo, Japan). Finally, specimens were imaged using a fluorescence microscope (BZ9000; Keyence Co., Osaka, Japan).
2.12. Statistical analysis
Data were analysed in spss program for Windows version 23.0 (SPSS, Chicago, IL, USA). Treatments were compared using Tukey's honestly significant difference test. Data are reported as mean ± standard deviation, with P<.05 considered to indicate statistical significance.
3. Results
3.1. Effect of cytokine cocktail on rMSC migration
Conditioned medium (63.0 ± 9.99) and the cytokine cocktail (61.4 ± 7.16) significantly increased the number of migrating rMSCs in comparison to serum‐free DMEM (7.44 ± 3.75) and depleted conditioned medium (44.1 ± 9.01). There was no statistical difference between conditioned medium and cytokine cocktail. Migration also increased in cells exposed to DMEM supplemented with 30% FBS (82.4 ± 5.77), as expected (Figure 1).
Figure 1.
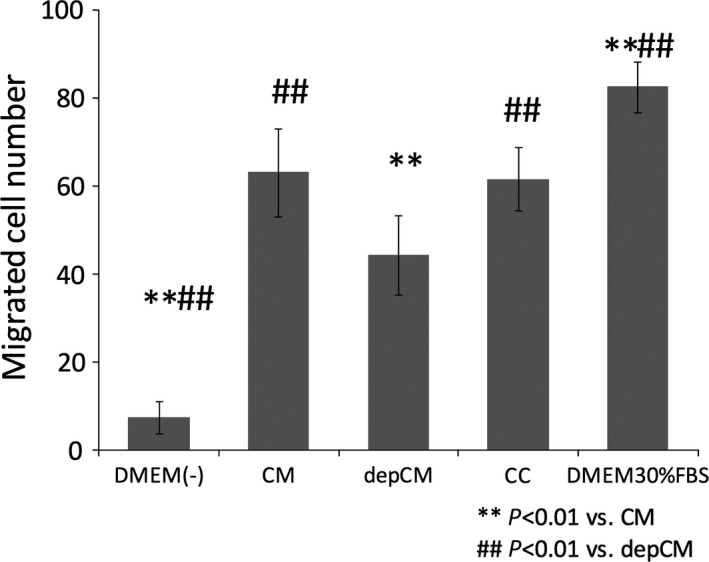
Conditioned medium (CM) and a cytokine cocktail (CC) promote cell migration. Conditioned medium and a cocktail of insulin growth factor‐1 (IGF‐1), vascular endothelial growth factor‐A (VEGF) and transforming growth factor‐β1 (TGF‐β1) significantly increased rat mesenchymal stem cell migration in comparison to conditioned medium from which IGF‐1, VEGF and TGF‐β1 had been depleted (depCM) (data are mean ± SD, n=5. P<.01)
3.2. Effect of cytokine cocktail on endothelial tube formation
Vascular networks were formed in the presence of conditioned medium, cytokine cocktail and VEGF (Figure 2A). Tube length was 7248.20 ± 1825.34 μm, 12 586.38 ± 1841.74 μm, 8901.78 ± 892.5 μm, 13 764.12 ± 1993.28 μm and 18 115.33 ± 1870.62 μm in cells cultured with endothelial cell medium, conditioned medium, depleted conditioned medium, cytokine cocktail and VEGF respectively (Figure 2B), with 25.75 ± 6.60, 59.50 ± 16.78, 29.00 ± 4.55, 57.50 ± 10.47 and 91.75 ± 16.52 branching points (Figure 2C). Tube formation was comparable between cells exposed to the cytokine cocktail and to conditioned medium, although cytokine depletion from the latter significantly suppressed tube formation.
Figure 2.
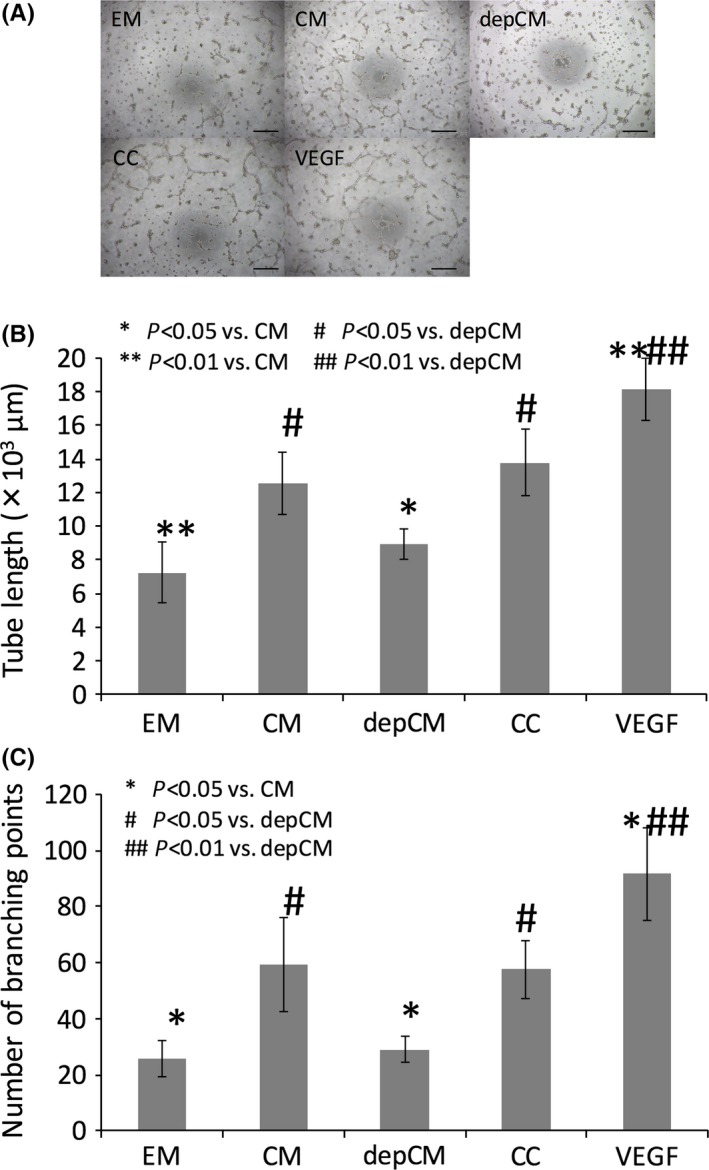
Conditioned medium (CM) and a cytokine cocktail (CC) promote tube formation. (A) Tube formation in human umbilical cord vein endothelial cells cultured in endothelial cell medium (EM), conditioned medium (CM), cytokine‐depleted conditioned medium (depCM), cytokine cocktail (CC) or 500 ng/mL VEGF. Scale bar, 500 μm. Developing new blood vessels were observed under a microscope after 10 h, and cells were photographed under phase contrast at 40× magnification. The CC and CM increased both tube length (B) and branching points (C), although depletion of cytokines from conditioned medium (depCM) blocked this effect (data are mean ± SD, n=5. P<.05)
3.3. Cytokine cocktail regulates expression of osteogenic and angiogenic marker genes
Expression of alkaline phosphatase, osteocalcin, Runx2, VEGF and angiopoietin‐1 was significantly and comparably upregulated in cells exposed to conditioned medium and cytokine cocktail, in comparison to cells grown in basal DMEM (Figure 3). Depletion of cytokines from conditioned medium also significantly suppressed expression of alkaline phosphatase, osteocalcin and angiopoietin‐1, but not Runx2 and VEGF.
Figure 3.
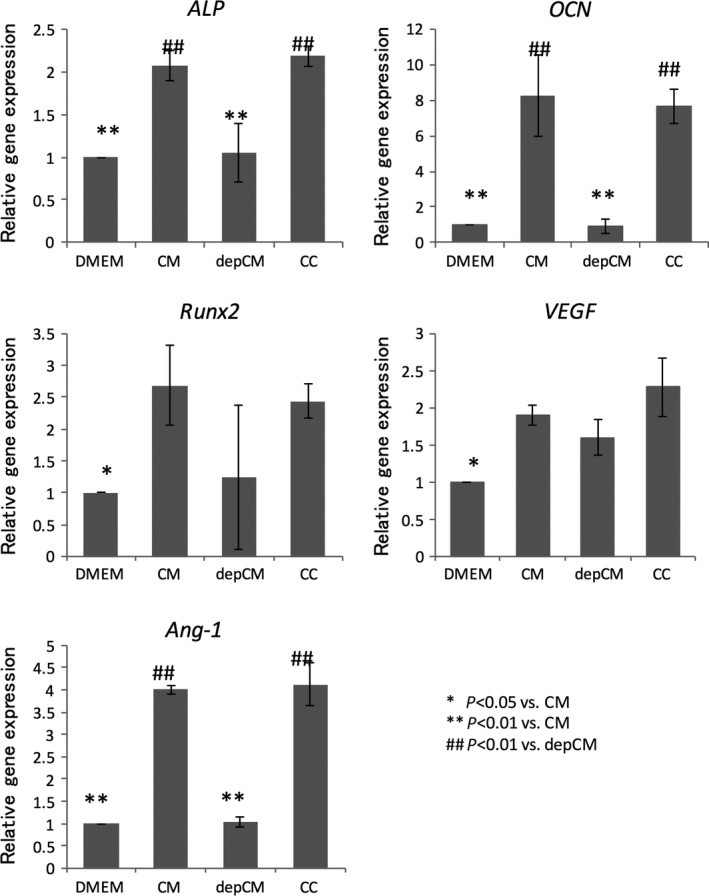
Conditioned medium (CM) and a cytokine cocktail (CC) enhance expression of osteogenic and angiogenic markers. mRNA levels of alkaline phosphatase (ALP), osteocalcin (OCN), Runx2, VEGF‐A and angiopoietin 1 (Ang‐1) in rMSCs cultured in the presence of CM, cytokine‐depleted conditioned medium (depCM), and CC, or in DMEM with 10% foetal bovine serum, as measured by real‐time RT‐PCR. Data are mean ± SD, n=5. Like CM, the CC increased expression of alkaline phosphatase, osteocalcin and angiopoietin 1, although depletion of cytokines from conditioned medium blocked the increase (P<.01)
3.4. Effect of cytokine cocktail on regeneration in damaged rat calvarial bone
Absorbable TERUDERMIS scaffolds soaked in cytokine cocktail or other treatments were implanted into surgically damaged rat calvarial bones. The area of newly regenerated bone was then quantified by micro‐CT as a percentage of the total graft area 2 weeks after implantation (Figure 4A). We found that pre‐treatment of scaffolds with conditioned medium and cytokine cocktail significantly increased the area of newly regenerated bone to 74.94 ± 19.11% and 73.15 ± 11.95%, relative to damaged bone without scaffolds (Defect) (15.27 ± 8.21%) and to implants pre‐soaked in PBS (31.61 ± 5.23%). In contrast, the area of newly regenerated bone in scaffolds pre‐soaked with depleted conditioned medium (35.65 ± 7.62%) was comparable to the latter two (Figure 4B). Histology also showed that implants treated with cytokine cocktail and conditioned medium were well covered with newly regenerated bone, while damaged bone without scaffolds (Defect) was covered mostly with connective tissue, as were damaged bone implanted with scaffolds soaked in PBS or depleted conditioned medium (Figure 4C).
Figure 4.
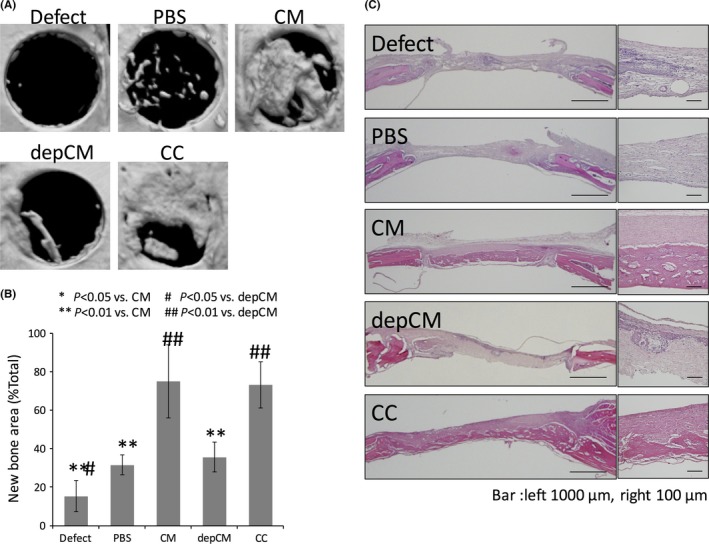
Conditioned medium (CM) and a cytokine cocktail (CC) elicit bone regeneration in damaged rat calvarial bone. A, Micro‐CT indicated that newly regenerated bone had almost covered the defect 2 wk after implantation with scaffolds pre‐soaked in cytokine cocktail (CC) and conditioned medium (CM), and to a lesser extent, after implantation of scaffolds pre‐treated with cytokine‐depleted conditioned medium (depCM). B, Per cent bone regenerated as measured by micro‐CT at 2 wk, indicating that the CC and CM increase bone regeneration. Data are mean ± SD, n=8. C, Histological sections of the damaged calvarial bone were stained with haematoxylin and eosin to evaluate bone regeneration 2 wk without treatment, or 2 wk after implantation of scaffolds pre‐soaked with PBS, CM, cytokine‐depleted conditioned medium (depCM) and CC. In untreated bone or in bone implanted with scaffolds pre‐soaked with cytokine‐depleted medium and PBS, the defect was filled with connective tissue and was infiltrated with inflammatory cells, especially in untreated bone (Defect). In bone treated with implants pre‐soaked in CC and CM, newly regenerated bone had begun to cover the defect and ossify without infiltration by inflammatory cells
3.5. CD31 and CD105 expression in damaged rat calvarial bone
Scaffolds pre‐soaked in cytokine cocktail and conditioned medium and implanted into surgically damaged calvarial bone were colonized with numerous CD31‐ or CD105‐expressing cells. Fewer such cells were observed in scaffolds pre‐soaked in cytokine‐depleted conditioned medium (Figure 5).
Figure 5.
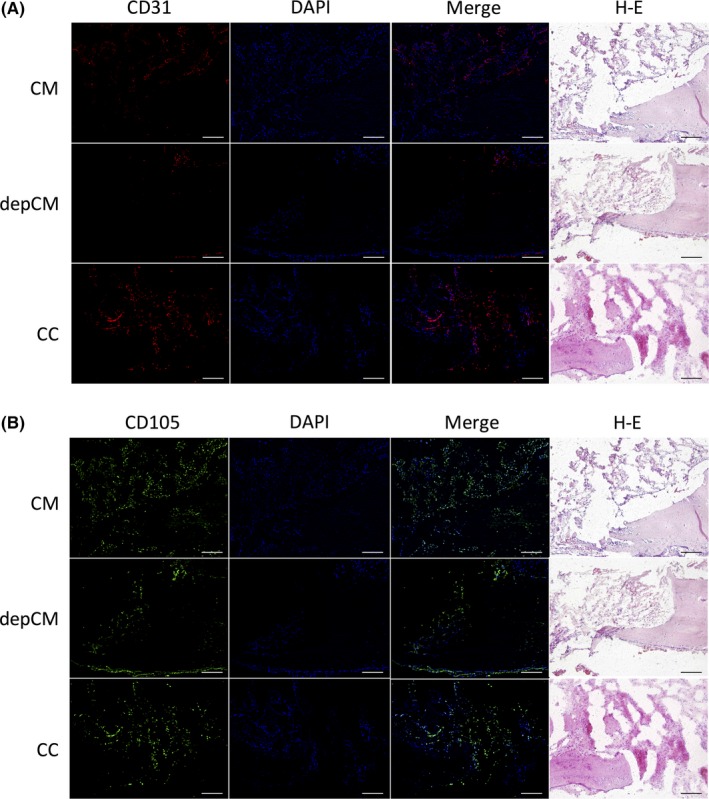
Conditioned medium (CM) and a cytokine cocktail (CC) enhance migration of endogenous endothelial and mesenchymal stem cells. One week after implantation into damaged calvarial bone, tissues were immunostained for (A) CD31 (red), a marker of endothelial cells, and (B) CD105 (green), a marker of stem cells. Cell nuclei were labelled with DAPI (blue). Scale bar, 200 μm. In bone implanted with scaffolds pre‐soaked in CC and CM, CD31‐ and CD105‐expressing cells had accumulated at the edges of the damage. Accumulation of these cells was less pronounced in bone implanted with scaffolds pre‐soaked in cytokine‐depleted conditioned medium (depCM)
4. Discussion
The therapeutic value of mesenchymal stem cells in tissue engineering and regenerative medicine is attributable in part to paracrine pathways6, 7 triggered by several secreted factors secreted into culture media.24 Indeed, we have reported that conditioned media from hMSCs contains cytokines such as IGF‐1, VEGF and TGF‐β1, which may synergistically affect migration, angiogenesis and osteogenesis in host mesenchymal stem cells. Thus, conditioned media have great potential to regenerate bone and periodontal tissue.13, 14, 15, 16, 17, 18 The effects of these three cytokines are thought to be very complex, although IGF‐1 is believed to regulate migration of osteoblasts25 and mesenchymal stem cells,26 and sustained systemic or local infusion of IGF‐1 was shown to enhance bone formation.27 On the other hand, VEGF is a master regulator of angiogenesis, and enhances survival and differentiation in endothelial cells that promote osteogenesis,28 while TGF‐β1 stimulates migration of osteoprogenitor cells and regulates cellular proliferation, differentiation and production of extracellular matrix.29
However, conditioned media contain numerous other soluble factors that may positively and/or negatively regulate bone regeneration, so it is essential to identify the specific factors that regenerate bone. In this study, we hypothesized that IGF‐1, VEGF and TGF‐β1 are the relevant factors. Thus, we evaluated the activity and pharmacologic effects of a defined mix of these cytokines, as well as those of conditioned medium from which these cytokines had been depleted. The concentration of these cytokines in conditioned medium was carefully measured by ELISA to be 1386 ± 465, 468.5 ± 109 and 339.8 ± 14.4 pg/mL, respectively, and the cytokine cocktail was prepared with 1400 pg/mL IGF‐1, 500 pg/mL VEGF‐A and 350 pg/mL TGF‐β1 to match these concentrations.
Some recombinant cytokines, including bone morphogenetic protein‐2 and platelet‐derived growth factor‐BB, are already on the market, and are widely used in the clinic to effectively regenerate bone or periodontal tissue.19, 30 However, high doses are typically required to achieve sufficient bone volume.31 For example, INFUSE® Bone Graft (Medtronic, Minneapolis, MN, USA) contains 1.5 mg/mL recombinant human bone morphogenetic factor‐2 in a bovine collagen carrier, and is about a 1000‐fold more concentrated than conditioned medium. Similarly, the periodontal implant GEM21S® (Osteohealth, Shirley, NY, USA) contains a significantly concentrated amount (0.3 mg/mL) of recombinant human platelet‐derived growth factor‐BB in a porous beta‐tricalcium phosphate matrix. Unfortunately, such high doses may also evoke adverse events such as severe oedema, as was reported for bone morphogenetic protein‐2.20, 21 Accordingly, various cytokines have been tested preclinically over the last 20 years and more, but with little progress to the clinic. Notably, we found by histology that infiltration of inflammatory cells was less pronounced in scaffolds treated with the cytokine cocktail (Figure 4C), presumably because the mix contains a well‐defined set of cytokines at a lower dose.
On the other hand, a combination of appropriate cytokines may synergize to exert multiple effects,32 and thereby elicit bone regeneration at a lower dose of each. For example, IGF‐1 was reported to enhance VEGF expression via hypoxia‐inducible factor‐2a.33 Similarly, TGF‐β promotes VEGF expression in human cerebral microvascular endothelial cells,34 and increases the amount of available IGF by downregulating expression of IGF‐binding protein.35 In line with these, we observed that a mix of these three cytokines promoted bone regeneration in vitro and in vivo more strongly than a single cytokine or a mix of two (data not shown). Hence, we hypothesized that a combination of these three cytokines was ideal.
Importantly, our data indicate that like conditioned medium, a defined cytokine cocktail enhanced migration (Figure 1) and expression of osteogenic and angiogenic marker genes in rMSCs in vitro (Figure 3), as well tube formation in HUVECs (Figure 2A,B).
According to the cytokine antibody array analysis that we performed before,36 as CC includes numerous factors except these three cytokines that will affect the cellular behaviour, depCM has some residual activities in vitro and in vivo (Figures 1, 2, 3, 4). However, these data showed the significant decrease compared with the data from both CM and CC, and strongly suggested that the depletion of these three cytokines was successful. CC also elicited bone regeneration, as indicated by early migration of stem cells and endothelial cells (Figure 4A‐C, and Figure 5). These synergistic effects are due to the combination of cytokines each, and appear to be unachievable with a single cytokine. Therefore, CC may overcome the current issues with cytokine therapy, and offer more significant clinical value. As clinical studies of CM did not evoke adverse events,18 CC will promise to be a well‐defined alternative with similar properties.
We have been reported the advantages of CM for bone and periodontal tissue regeneration.13, 14, 15, 16, 17, 18 Bone and periodontal tissue regeneration by CM was achieved with angiogenesis and endogenous stem cells proliferation and differentiation. In other words, these findings indicated the possibilities that CM will be an alternative method to exogenous stem cell transplantation for tissue regeneration. Our previous study indicated that the potential CM to promote bone regeneration was at least equal to that of mesenchymal stem cell transplantation in rat calvarial bone defect model.14 CM will also eliminate several problems of the current stem cell therapy, such as safety, expense and regulatory problems associated cell handling.
On the other hand, as described above, CM includes numerous factors according to the results from cytokine antibody array assay.36 It will be very important to define the active ingredients of CM for the future clinical use. In this study, we used three cytokines, which were included at relatively high concentrations in CM and were previously proven to contribute bone and periodontal tissue regeneration. We performed this study as a part of preclinical study of CC. For the pharmaceutical manufacturing, these three well‐known cytokines may be suitable combination from the point of safety and availability as well as therapeutic potential.
CC will provide a unique and exciting alternative to existing procedures for bone regeneration.
Conflict of interest
The authors declare that this work was supported in part by a research fund from Showa Yakuhin Kako Co., Ltd (Tokyo, Japan).
Acknowledgements
The authors thank Drs Kenichi Ogata and Takeshi Tsuruta from the Department of Oral and Maxillofacial Surgery of Nagoya University Graduate School of Medicine, and Hiroyuki Kogami, Mikio Taniguchi, and Mutsumi Shibuya from Showa Yakuhin Kako Co., Ltd, for kind help and contributions to the study. This work was supported in part by JSPS KAKENHI Grant Number JP15K11213.
Katagiri W, Sakaguchi K, Kawai T, Wakayama Y, Osugi M, Hibi H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow‐derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017;50:e12333 10.1111/cpr.12333
References
- 1. Yamada Y, Nakamura S, Ito K, et al. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells. 2013;31:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voss P, Sauerbier S, Wiedmann‐Al‐Ahmad M, et al. Bone regeneration in sinus lifts: comparing tissue‐engineered bone and iliac bone. Br J Oral Maxillofac Surg. 2010;48:121–126. [DOI] [PubMed] [Google Scholar]
- 3. Wislet‐Gendebien S, Poulet C, Neirinckx V, et al. In vivo tumorigenesis was observed after injection of in vitro expanded neural crest stem cells isolated from adult bone marrow. PLoS ONE. 2012;7:e46425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ide C, Nakai Y, Nakano N, et al. Bone marrow stromal cell transplantation for treatment of sub‐acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. [DOI] [PubMed] [Google Scholar]
- 5. Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture‐expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow‐derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. [DOI] [PubMed] [Google Scholar]
- 8. Yoon BS, Moon JH, Jun EK, et al. Secretory profiles and wound healing effects of human amniotic fluid‐derived mesenchymal stem cells. Stem Cells Dev. 2010;19:887–902. [DOI] [PubMed] [Google Scholar]
- 9. Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell‐free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4:e5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barcelos LS, Duplaa C, Kränkel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai L, Johnstone BH, Cook TG, et al. IFATS collection: human adipose tissue‐derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perin EC, Silva GV. Autologous cell‐based therapy for ischemic heart disease: clinical evidence, proposed mechanisms of action, and current limitations. Catheter Cardiovasc Interv. 2009;73:281–288. [DOI] [PubMed] [Google Scholar]
- 13. Katagiri W, Osugi M, Kawai T, Ueda M. Novel cell‐free regeneration of bone using stem cell‐derived factors. Int J Oral Maxillofac Implants. 2013;28:1009–1016. [DOI] [PubMed] [Google Scholar]
- 14. Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inukai T, Katagiri W, Yoshimi R, et al. Novel application of stem cell‐derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430:763–768. [DOI] [PubMed] [Google Scholar]
- 16. Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M. Secretomes from bone marrow‐derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369–381. [DOI] [PubMed] [Google Scholar]
- 17. Katagiri W, Osugi M, Kinoshita K, Hibi H. Conditioned medium from mesenchymal stem cells enhances early bone regeneration after maxillary sinus floor elevation in rabbits. Implant Dent. 2015;24:657–663. [DOI] [PubMed] [Google Scholar]
- 18. Katagiri W, Osugi M, Kawai T, Hibi H. First‐in‐human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nevins M, Giannobile WV, McGuire MK, et al. Platelet‐derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. [DOI] [PubMed] [Google Scholar]
- 20. Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh‐BMP‐2 in anterior cervical discectomy and fusion: a case study. Spine J. 2006;7:235–239. [DOI] [PubMed] [Google Scholar]
- 21. Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein‐2. Eur Spine J. 2007;16:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats‐similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawamoto T. Use of a new adhesive film for the preparation of multi‐purpose fresh‐frozen sections from hard tissues, whole‐animals, insects and plants. Arch Histol Cytol. 2003;66:123–143. [DOI] [PubMed] [Google Scholar]
- 24. Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. [DOI] [PubMed] [Google Scholar]
- 25. Cornish J, Grey A, Callon KE, et al. Shared pathways of osteoblast mitogenesis induced by amylin, adrenomedullin, and IGF‐1. Biochem Biophys Res Commun. 2004;318:240–246. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin‐like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. [DOI] [PubMed] [Google Scholar]
- 27. Fowlkes JL, Thrailkill KM, Liu L, et al. Effects of systemic and local administration of recombinant human IGF‐I (rhIGF‐I) on de novo bone formation in an aged mouse model. J Bone Miner Res. 2006;21:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2007;19:665–667. [DOI] [PubMed] [Google Scholar]
- 29. Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95–103. [DOI] [PubMed] [Google Scholar]
- 30. Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein‐2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67:1947–1960. [DOI] [PubMed] [Google Scholar]
- 31. Cowan CM, Aghaloo T, Chou YF, et al. MicroCT evaluation of three‐dimensional mineralization in response to BMP‐2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007;13:501–512. [DOI] [PubMed] [Google Scholar]
- 32. Ozaki Y, Nishimura M, Sekiya K, et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119–129. [DOI] [PubMed] [Google Scholar]
- 33. Akeno N, Robins J, Zhang M, Czyzyk‐Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF‐I in osteoblast‐like cells is mediated by the PI3K signaling pathway through the hypoxia‐inducible factor‐2alpha. Endocrinology. 2002;143:420–425. [DOI] [PubMed] [Google Scholar]
- 34. Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, Weller M. Modulation of cerebral endothelial cell function by TGF‐ß in glioblastoma: VEGF‐dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget. 2015;6:22480–22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ortiz CO, Chen BK, Bale LK, Overgaard MT, Oxvig C, Conover CA. Transforming growth factor‐beta regulation of the insulin‐like growth factor binding protein‐4 protease system in cultured human osteoblasts. J Bone Miner Res. 2003;18:1066–1072. [DOI] [PubMed] [Google Scholar]
- 36. Ogata K, Katagiri W, Hibi H. Secretomes from mesenchymal stem cells participate in the regulation of osteoclastogenesis in vitro. Clin Oral Investig. 2016. in press. [DOI] [PubMed] [Google Scholar]


