Abstract
Objectives
Low concentrations of tumour necrosis factor‐alpha (TNF‐α) have been reported to promote osteogenic differentiation. In this study, a series of in vitro experiments was performed to investigate underlying molecular mechanisms involved.
Materials and methods
MC3T3‐E1 murine preosteoblasts were treated with TNF‐α at doses of 0, 0.1 or 1 ng/mL. The ephrinB2‐EphB4 signalling pathway was activated using ephrinB2‐fc, or inhibited using lentiviruses encoding siRNAs specifically targeting EphB4. Cell proliferation/survival was evaluated using the Cell Counting Kit‐8 (CCK‐8) assay, and expression levels of Runx2, BSP, ephrinB2 and EphB4 were determined using RT‐PCR and Western blotting. ALP activity in these cells was also determined, and mineral nodule formation was evaluated with alizarin red S staining.
Results
Low concentrations of TNF‐α had no influence on cell proliferation/survival. However, expression levels of Runx2, BSP, ephrinB2 and EphB4, as well as ALP activity and mineral nodule formation, were significantly enhanced in MC3T3‐E1 cells treated with low concentrations of TNF‐α. Moreover, activation of the ephrinB2‐EphB4 signalling pathway by ephrinB2‐fc enhanced TNF‐α‐induced osteogenic differentiation, while down‐regulation of EphB4 level reversed the positive effect of TNF‐α.
Conclusions
Low concentrations of TNF‐α promoted osteogenic differentiation via activation of the ephrinB2‐EphB4 signalling pathway.
1. Introduction
Bone defects caused by trauma, surgical removal of tumours, infections and congenital diseases have become serious clinical concerns especially when the damage exceeds the critical size and the bone tissue cannot be completely self‐repaired.1 Therefore, it is of particular clinical significance to thoroughly investigate molecular and cellular mechanisms underlying bone healing process to develop novel and effective therapeutics to accelerate bone regeneration.
Bone healing process is initiated with the release of a mixture of inflammatory substances, followed by granulation tissue formation, matrix formation by osteoblasts and bone remodelling by the interactions between osteoblasts and osteoclasts.2 As the initial step of bone repair, inflammation plays an important role in bone regeneration.3 Tumour necrosis factor‐alpha (TNF‐α), secreted by active monocytes/macrophages and first isolated by Bharat and colleagues in 1984,4 is a pivotal proinflammatory cytokine which actively participates in bone regeneration.5 Although the role of TNF‐α in bone healing and regeneration is controversial,6, 7, 8, 9 it is widely accepted that short‐term exposure to low concentrations of TNF‐α can improve osteogenic differentiation of mesenchymal stem cells (MSCs) and osteoblasts.10, 11 For example, treatment with TNF‐α at the dose of 1 ng/ml for 24 hours significantly promoted osteogenic differentiation of human primary osteoblasts via stimulating a paracrine BMP‐2 loop.10 Our research group also found that low concentrations of TNF‐α enhanced osteogenic differentiation of murine MSCs.11 In addition, in vivo investigations revealed that the level of TNF‐α peaked at 24 hours after bone fracture and this early burst of TNF‐α promoted the activation of bone healing process after bone fracture.12 Moreover, addition of TNF‐α at the dose of 1 ng/ml at the bone fracture site accelerated bone repair.13
Tumour necrosis factor‐alpha has two receptors, and the functional receptor in both osteoblasts and osteoclasts is TNF receptor‐1 (TNFR1).14, 15 Upon binding to TNFR1, TNF‐α triggers a series of signalling cascades which eventually activates NF‐κB signalling pathway and mitogen‐activated protein kinase (MAPK) such as p38, ERK and JNK.11 The activation of numerous signalling pathways by TNF‐α treatment, together with the interactions between these signalling pathways, contributes to the complexity of TNF‐α‐induced effect on osteoblast differentiation.11 Furthermore, our previous findings revealed that blockade of NF‐ĸB signalling via the overexpression of the NF‐ĸB antagonist IĸBα had no impact on the enhanced osteogenic differentiation of ST2 cells induced by low concentration of TNF‐α.11 Therefore, the mechanisms underlying osteogenic differentiation enhanced by low concentrations of TNF‐α remain to be further elucidated.
EphrinB2‐EphB4 signalling pathway is a bidirectional signalling pathway mediating the crosstalk between osteoblasts and osteoclasts and thus plays an important role in maintaining bone homeostasis.16 Briefly, osteoblasts express EphB4, while osteoclasts express ephrinB2. Activation of EphB4 by ephrinB2 in osteoblasts is referred to as forward signalling, which stimulates osteogenic differentiation. However, reverse signalling is the activation of ephrinB2 by EphB4 in osteoclasts, which inhibits the differentiation of osteoclast precursor cells.16 Interaction between EphB4 and ephrinB2 requires direct cell‐cell contact considering both proteins are anchored to the plasma membrane.17 However, the interaction between EphB4 on osteoblasts and ephrinB2 on osteoclasts is limited due to the limited direct surface contact between osteoblasts and osteoclasts based on the fact that the activities of osteoblasts and osteoclasts take place at different time and space during bone remodelling.18 Further investigations revealed that ephrinB2 also exists on the surface of osteoblasts, binds to EphB4 on itself or the neighbouring osteoblasts and promotes bone regeneration by enhancing osteogenic differentiation and mineralization.19, 20 Therefore, ephrinB2 not only exists on the surface of osteoclasts and completes a bidirectional signalling with EphB4 on the surface of osteoblasts, but also expresses on the surface of osteoblasts and act in a paracrine or autocrine manner to promote osteogenic differentiation. Indeed, ephrinB2 expression in osteoblasts is reported to be up‐regulated by PTH and PTH‐related protein (PTHrP), contributing to the anabolic action of PTH and PTHrP.21 However, currently, it is still unknown whether the enhanced osteogenic differentiation induced by low concentrations of TNF‐α is associated with ephrinB2‐EphB4 signalling pathway. In this study, MC3T3‐E1 cells were treated with low concentrations of TNF‐α, and thus, the role of ephrinB2‐EphB4 signalling pathway in the osteogenic differentiation was evaluated in a mild inflammatory microenvironment.
2. Materials and methods
2.1. Cell culture
MC3T3‐E1 cells were maintained in regular culture medium: α‐MEM (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) foetal bovine serum (FBS; Hyclone), 100 U/mL penicillin (Solarbo, Beijing, China) and 100 μg/mL streptomycin (Solarbo) cultured with 5% CO2 at 37°C. For osteogenic differentiation, the cells were cultured in α‐MEM supplemented with 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 10−8 mol/L dexamethasone (Sigma, St. Louis, MO, USA), 50 mg/L ascorbic acid (Sigma) and 10 mmol/L β‐glycerophosphate (Sigma).
2.2. Cell proliferation assay
Cell Counting Kit‐8 (CCK‐8; Dojindo, Kumamoto, Japan) was used to measure cell proliferation. MC3T3‐E1 cells were seeded in 96‐well plates at a density of 5000 cells per well and cultured in regular culture medium. After 24 hours, the medium was switched to α‐MEM supplemented with 0.1% FBS and murine TNF‐α (Peprotech Inc., Rocky Hill, NJ, USA) at concentrations of 0, 0.1 or 1 ng/mL. After 24, 48 and 72 hours, 10 μL of CCK‐8 solution was added to each well and the plates were incubated for 2.5 hours at 37°C. The optical density was measured at 450 nm using the SPECTROstar Nano microplate reader (BMG Labtech Inc., Ortenberg, Germany).
2.3. Activation of ephrinB2‐EphB4 signalling using ephrinB2‐fc
Recombinant Mouse ephrinB2‐fc Chimera (R&D, Minneapolis, MN, USA) was added into the regular culture medium at the concentration of 4 μg/mL to stimulate ephrinB2‐EphB4 signalling in MC3T3‐E1 cells. IgG‐fc (R&D) was used as the negative control.
2.4. Construction of lentiviruses encoding siRNAs specifically targeting murine EphB4 and cell infection
siRNAs specifically targeting murine EphB4 were designed and synthesized by ThermoFisher Scientific (Shanghai, China) and were transferred into the lentiviral expression vector pLenti6.3/V5‐DEST after a series of in vitro recombination assays (ThermoFisher Scientific). The resulted lentiviral vector was named as pLenti6.3‐ephb4siRNA. A lentiviral vector encoding a scrambled shRNA with no homology to any known mouse or human gene, pLenti6.3‐ctrl, was also created to be used as a negative control (ThermoFisher Scientific). pLenti6.3‐ephb4siRNA or pLenti6.3‐ctrl lentiviral vector was transfected into human 293T cells with ViraPower packing mix using Lipofectamine 2000 (ThermoFisher Scientific). Forty‐eight hours after the transfection, the supernatant was collected, centrifuged at 1000 g for 10 minutes to remove cell debris, and filtered with 0.45 μm filters. The lentiviral particles were then concentrated by centrifugation at 50 000 g for 2 hours, resuspended in opti‐MEM, subjected to titration and stored at −80°C.
2.5. RNA isolation and real‐time reverse‐transcriptase polymerase chain reaction assay (RT‐PCR)
MC3T3‐E1 cells were seeded in six‐well plates at a density of 1 × 105 cells per millilitre and then maintained with the aforementioned osteogenic medium supplemented with murine TNF‐α at concentrations of 0, 0.1 and 1 ng/mL, respectively. After being cultured for 24 and 48 hours, total mRNA was extracted using Trizol® reagent (TaKaRa Biotech, Tokyo, Japan) according to the manufacturer's instructions, and used for cDNA synthesis using Reverse Transcriptase (TaKaRa). RT‐PCR was performed using SYBR® Primix Ex Taq™ (TaKaRa) with Roche 480. Each sample was prepared in triplicate, and each experiment was repeated at least three times. GAPDH was used as an internal control. The sequences of the primers for amplification of mouse BSP, Runx2, EphB4, ephrinB2 and GAPDH were as follows: BSP: 5′‐CAGGGAGGCAGTGACTCTTC‐3′ and 5′‐AGTGTGGAAAGTGTGGCGTT‐3′; Runx2: 5′‐CCCAGCCACCTTTACCTACA‐3′ and 5′‐TATGGAGTGCTGCTGGTCTG‐3′; EphB4: 5′‐TGCGGAAAGCAACAAAGTA‐3′ and 5′‐CGGCAGCGTACAGCATAAGT‐3′; ephrinB2: 5′‐TTGCCCCAAAGTGGACTCTAA‐3′ and 5′‐GCAGCGGGGTATTCTCCTTC‐3′; GAPDH: 5′‐AGGTCGGTGTGAACGGATTTG‐3′ and 5′‐TGTAGACCATGTAGTTGAGGTCA‐3′. Relative differences in the PCR product amounts were evaluated by the comparative cycle threshold method, with GAPDH serving as a control.
2.6. Western blot analysis
The proteins were extracted from MC3T3‐E1 cells using RIPA (Solarbo) containing 1% PMSF (Solarbo) for 30 minutes, followed by centrifugation at 4°C, 12 000 g for 20 minutes to collect supernatant. The concentrations of protein samples were measured using the bicinchoninic acid protein assay kit (Solarbo), and the protein samples were then mixed with 5× SDS‐PAGE loading buffer (Beyotime, Shanghai, China) at 100°C for 5 minutes. The samples were then run on 10% SDS‐PAGE gel (Beyotime) and electrotransferred to polyvinylidene fluoride (PVDF) membranes (Invitrogen, Cartlsbad, CA, USA) for 1 hour at 100 V. After the PVDF membrane was blocked in 5% defatted milk for 1 hour at room temperature, it was probed with rabbit anti‐BSP antibody (catalogue no. Sc‐292394; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti‐Runx2 antibody (catalogue no. 12556S; CST, Danvers, MA, USA), rabbit anti‐EphB4 antibody (catalogue no. 20883‐1‐AP; Proteintech, Wuhan, China) and rabbit anti‐ephrinB2 antibody (catalogue no. Ab131536; Abcam, Cambridge, MA, USA) overnight at 4°C, and then incubated with HRP‐labelled goat anti‐rabbit IgG (catalogue no. 7074P2; CST). Blots were visualized using the Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) and GAPDH was used as an internal control.
2.7. ALP activity
MC3T3‐E1 cells were washed with 1× PBS and extracted using 1% Triton X‐100 for 30 minutes after incubated for 7 and 14 days. The cells were then sonicated and cell lysates were centrifuged at 12 000 g for 5 minutes at 4°C. ALP activity was assayed according to the instructions of the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, 30 μL of supernatant, 50 μL of buffer solution and 50 μL of matrix liquid were added to a 96‐well plate. The plate was incubated in dark place for 15 minutes at 37°C. Colouration solution (150 μL) was then added to each well, and the absorbance of each sample was measured with a spectrophotometer at 520 nm wavelength. The supernatant was replaced with standard phenol solution and deionized water in the standard wells and negative control wells. ALP activity was calculated according to the concentration of the phenol in a standard well and adjusted according to the protein concentration of each sample.
2.8. Alizarin red S staining
MC3T3‐E1 cells were seeded in a 12‐well plate at the density of 5 × 104 cells per millilitre. After 4 weeks, cells were washed with 1× PBS twice, fixed with 4% paraformaldehyde for 30 minutes, washed with deionized water twice and stained with 2% Alizarin Red S (pH 4.2) (Sigma) for 15 minutes at room temperature. Stained cells were photographed. For quantifying the relative amount of calcium, 300 μL of 10% (w/v) cetylpyridinium chloride (Sigma) was added to the stained wells and the absorbance of extracted dye was assayed at 562 nm wavelength with a spectrophotometer.
2.9. Statistical analysis
All data were expressed as mean ± SEM from at least three replicates for each experiment. One‐way ANOVA was used to test significance between the experimental groups and the control group using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). A P value <.05 was considered statistically significant.
3. Results
3.1. Low concentrations of TNF‐α showed no effects on proliferation/survival of MC3T3‐E1 cells
As indicated by CCK‐8 assay, no statistically significant difference in cell proliferation/survival was detected in MC3T3‐E1 cells treated with TNF‐α at concentrations of 0.1 or 1 ng/mL when compared with the control cells (Figure 1), indicating that low concentrations of TNF‐α had no effect on the proliferation/survival of MC3T3‐E1 cells.
Figure 1.
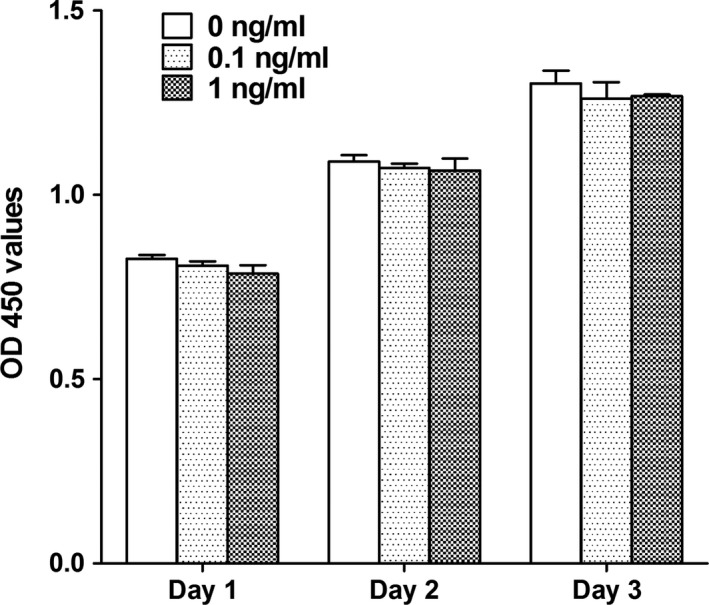
The effect of TNF‐α on the proliferation/survival of MC3T3‐E1 cells. No statistically significant difference in cell proliferation/survival was detected in MC3T3‐E1 cells treated with TNF‐α at concentrations of 0.1 or 1 ng/mL when compared with the control cells
3.2. Low concentrations of TNF‐α promoted osteogenic differentiation of MC3T3‐E1 cells
After treated with TNF‐α at concentrations of 0.1 or 1 ng/mL for 24 or 48 hours, the mRNA and protein levels of Runx2 and BSP in MC3T3‐E1 cells were significantly up‐regulated when compared with the control cells (Figure 2A‐D). We then treated MC3T3‐E1 cells with TNF‐α at concentrations of 0.1 or 1 ng/mL for 7 or 14 days and determined ALP activity in these cells. Our results indicated that after treated with low concentrations of TNF‐α for 7 and 14 days, ALP activity in MC3T3‐E1 cells was significantly increased when compared with the control cells (Figure 2E). Furthermore, mineral nodule formation was significantly enhanced in MC3T3‐E1 cells treated with low concentrations of TNF‐α when compared with the control cells (Figure 2F). These results clearly indicated that the treatment of TNF‐α at low concentrations enhanced osteogenic differentiation and in vitro mineralization.
Figure 2.
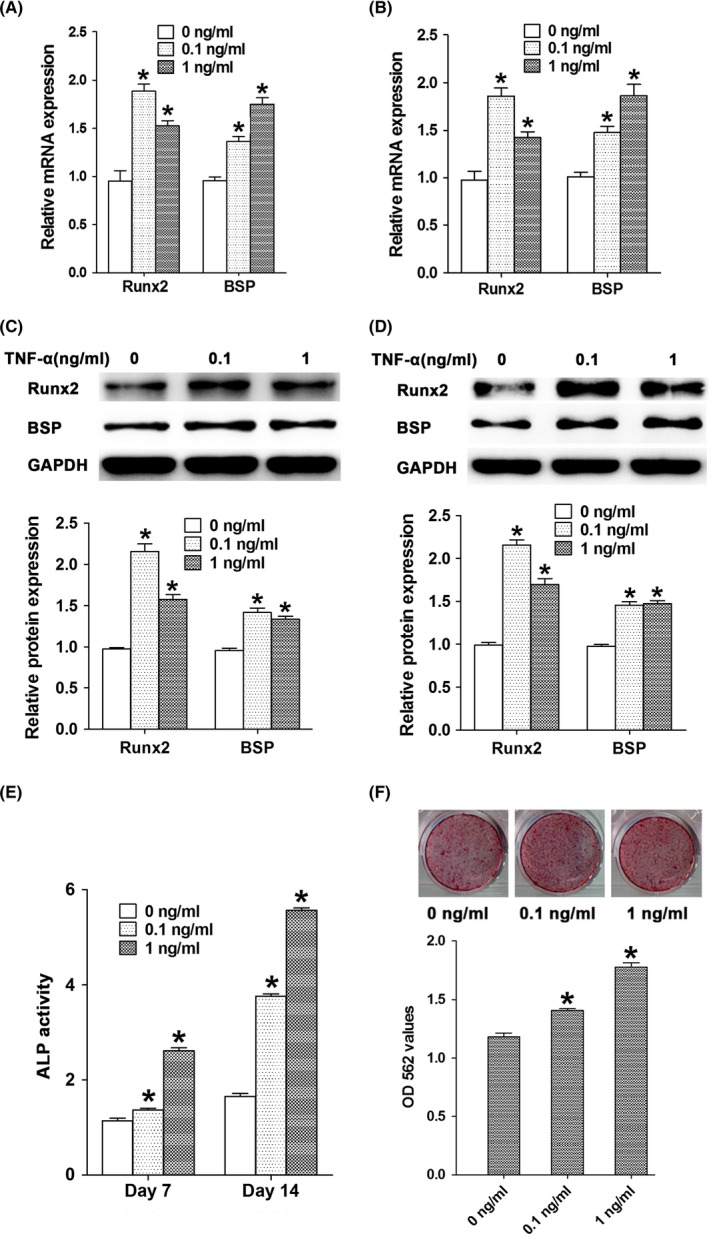
The effect of TNF‐α at low concentrations on osteogenic differentiation of MC3T3‐E1 cells. The mRNA levels of Runx2 and BSP in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 24 h (A) and 48 h (B). Protein levels of Runx2 and BSP in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 24 h (C) and 48 h (D). (E) The ALP activity in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 7 and 14 days. (F) The mineral nodule formation and the calcium ion concentrations in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 4 weeks. *P<.05 vs the control group
3.3. Low concentrations of TNF‐α activated ephrinB2‐EphB4 signalling by up‐regulating expression levels of ephrinB2 and EphB4 in MC3T3‐E1 cells
MC3T3‐E1 cells were treated with TNF‐α at concentrations of 0.1 or 1 ng/mL for 24 or 48 hours, and RT‐PCR and Western blot assays were performed to monitor expression levels of ephrinB2 and EphB4. We found that both mRNA and protein levels of ephrinB2 and EphB4 were higher in MC3T3‐E1 cells treated with low concentrations of TNF‐α than those in the control group (Figure 3).
Figure 3.
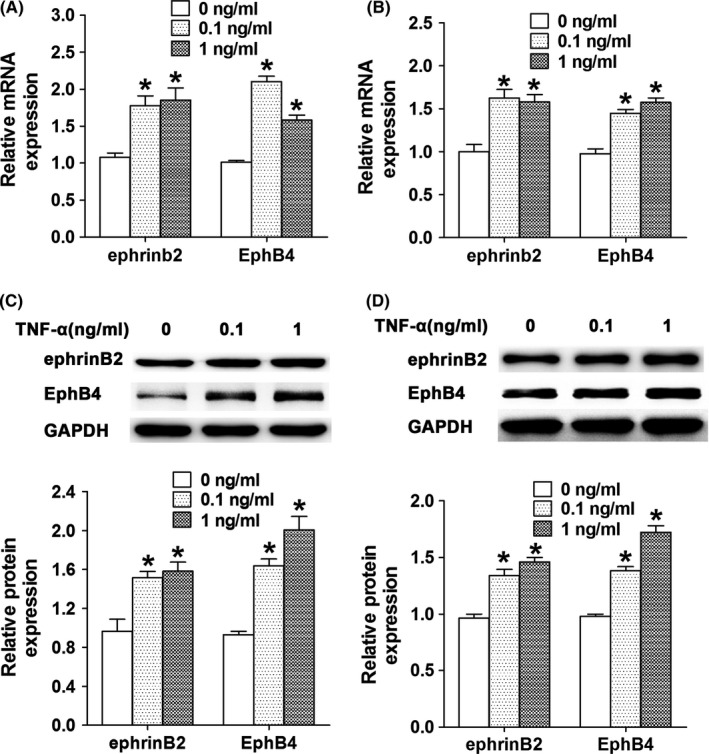
The effect of TNF‐α at low concentrations on the expression of ephrinB2 and EphB4 in MC3T3‐E1 cells. The mRNA levels of ephrinB2 and EphB4 in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 24 h (A) and 48 h (B). Protein levels of ephrinB2 and EphB4 in MC3T3‐E1 cells after treated with TNF‐α at concentrations of 0.1 and 1 ng/mL for 24 h (C) and 48 h (D). *P<.05 vs the control group
3.4. The positive effects of TNF‐α on osteogenic differentiation were mediated by the activation of ephrinB2‐EphB4 signalling pathway
To investigate the role of ephrinB2‐EphB4 signalling pathway in osteogenic differentiation of MC3T3‐E1 cells treated with TNF‐α at low concentrations, ephrinB2‐fc was added into the medium to stimulate the ephrinB2‐EphB4 signalling. The results showed that protein levels of EphB4, Runx2 and BSP were significantly higher in MC3T3 cells treated with TNF‐α (1 ng/mL) or ephrinB2‐fc (4 μg/mL) than those in the control cells. In MC3T3‐E1 cells treated with both TNF‐α (1 ng/mL) and ephrinB2‐fc (4 μg/mL), the protein levels of EphB4, Runx2 and BSP were further increased (Figure 4A,B). ALP activity and mineral nodule formation in MC3T3‐E1 cells treated with TNF‐α (1 ng/mL) or ephrinB2‐fc (4 μg/mL) were also promoted when compared with the control cells. Moreover, these two indicators in MC3T3‐E1 cells treated with both TNF‐α (1 ng/mL) and ephrinB2‐fc (4 μg/mL) were further enhanced when compared with the control cells (Figure 4C,D).
Figure 4.
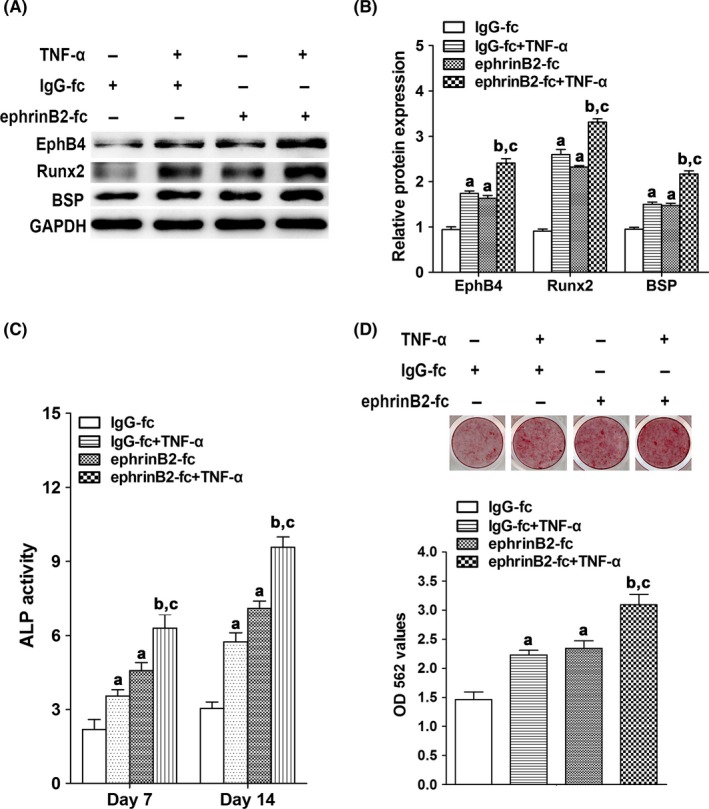
The effect of 1 ng/mL of TNF‐α and 4 μg/mL ephrinB2‐fc on osteogenic indicators in MC3T3‐E1 cells. A, Protein levels of EphB4, Runx2 and BSP in MC3T3‐E1 cells after treated with TNF‐α and/or ephrinB2‐fc for 48 h. B, Quantitative analysis of protein levels of Runx2 and BSP in MC3T3‐E1 cells after treated with TNF‐α and/or ephrinB2‐fc for 48 h. C, ALP activity in MC3T3‐E1 cells after treated with TNF‐α and/or ephrinB2‐fc for 7 and 14 d. D, The mineral nodule formation and the calcium ion concentrations in MC3T3‐E1 cells after treated with TNF‐α and/or ephrinB2‐fc for 4 wk. a P<.05 vs the IgG‐fc group; b P<.05 vs the IgG‐fc+TNF‐α group; c P<.05 vs the ephrinB2‐fc group
We then inhibited ephrinB2‐EphB4 signalling in MC3T3‐E1 cells by knocking‐down the expression levels of EphB4 using pLenti6.3‐ephb4siRNA encoding siRNAs specifically targeting EphB4 (Figure 5). MC3T3‐E1 cells infected with EphB4 knocked‐down lentiviruses were named as MC3T3‐E1‐ephb4siRNA cells. MC3T3‐E1 cells infected with the negative control lentiviruses (pLenti6.3‐ctrl) served as controls (named as MC3T3‐E1‐ctrl cells). Expression levels of Runx2 and BSP, ALP activity and in vitro mineral nodule formation were also determined in MC3T3‐E1‐ephb4siRNA cells and MC3T3‐E1‐ctrl cells treated with or without TNF‐α (1 ng/mL). We found that the protein levels of Runx2 and BSP, ALP activity and in vitro mineral nodule formation were all significantly down‐regulated in MC3T3‐E1‐ephb4siRNA cells when compared with MC3T3‐E1‐ctrl cells (Figure 6). Moreover, the positive effects of TNF‐α (1 ng/mL) on osteogenic differentiation were reversed in MC3T3‐E1‐ephb4siRNA cells when compared with MC3T3‐E1‐ctrl cells (Figure 6). Taken together, our results indicated that the enhanced osteogenic differentiation induced by low concentrations of TNF‐α is mediated by the activation of ephrinB2/EphB4 signalling pathway.
Figure 5.
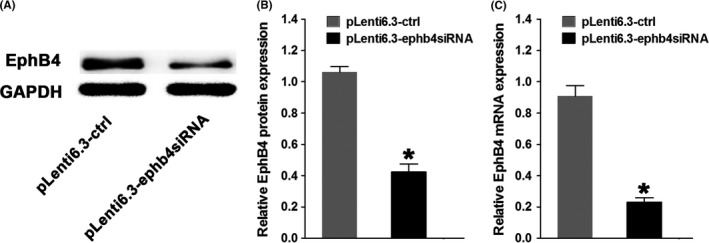
The levels of EphB4 in MC3T3‐E1‐ephb4siRNA cells. A, Protein levels of EphB4 in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells. B, Quantification of EphB4 protein levels in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells. C, The mRNA level of EphB4 in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells. *P<.05 vs the pLenti6.3‐ctrl group
Figure 6.
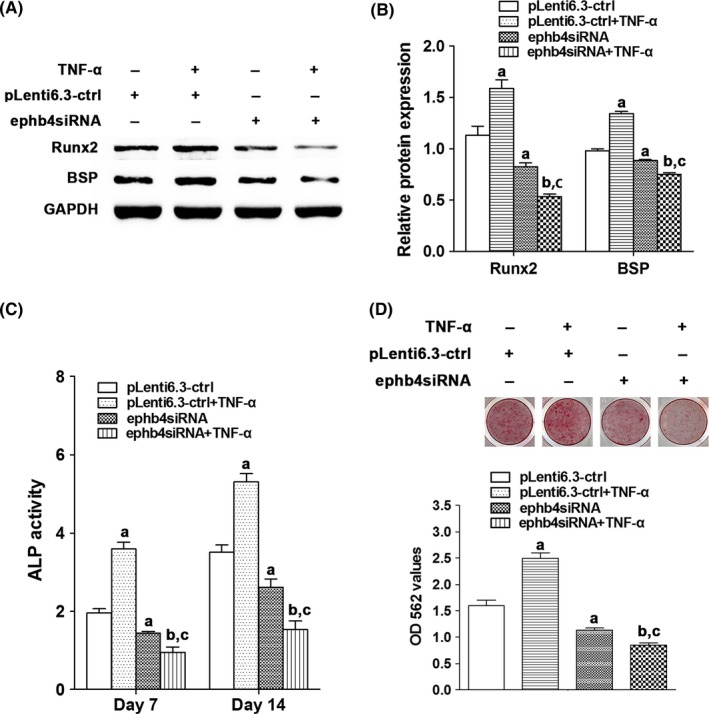
The effect of TNF‐α on osteogenic differentiation of MC3T3‐E1‐ephb4siRNA cells. A, Protein levels of Runx2 and BSP in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells treated with or without TNF‐α for 48 h. B, Quantification of Runx2 and BSP protein levels in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells treated with or without TNF‐α for 48 h. C, ALP activity in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells treated with or without TNF‐α for 7 and 14 d. D, The mineral nodule formation and the calcium ion concentrations in MC3T3‐E1‐ctrl cells or MC3T3‐E1‐ephb4siRNA cells treated with or without TNF‐α for 4 wk. a P<.05 vs the pLenti6.3‐ctrl group; b P<.05 vs the pLenti6.3‐ctrl+TNF‐α group; c P<.05 vs the pLenti6.3‐ephb4siRNA group (ephb4siRNA=pLenti6.3‐ephb4siRNA)
4. Discussion
During the process of osteogenic differentiation, two distinguishable events occur: proliferation and osteogenic differentiation of bone forming cells, which eventually leads to the extracellular matrix maturation and mineralization.22, 23 In this study, we first investigated the effect of TNF‐α on proliferation/survival of MC3T3‐E1 cells. In accordance with our previous study,11 no significant changes in proliferation/survival were detected in MC3T3‐E1 cells treated with low concentrations of TNF‐α for up to 3 days, suggesting that mildly elevated TNF‐α level at the early stage of bone healing process has no adverse effect on osteoblast proliferation.
We then investigated the effect of TNF‐α on osteogenic differentiation. Our results showed that TNF‐α treatment in MC3T3‐E1 cells at low concentrations (0.1 and 1 ng/mL) up‐regulated expression levels of Runx2 and BSP for up to 48 hours and enhanced ALP activity for up to 2 weeks. Moreover, mineral nodule formation was increased and calcium ion concentration was elevated in MC3T3‐E1 cells after the cells were treated with TNF‐α at low concentrations for 4 weeks. Consistent with previous findings,8, 11, 13, 24 our results further indicated that low concentrations of TNF‐α promoted osteogenic differentiation and mineralization in MC3T3‐E1 cells. Furthermore, most studies revealed that high concentrations of TNF‐α inhibit osteogenic differentiation.7, 25, 26 Interestingly like the concentration, the exposure duration of TNF‐α is also a critical element in determining the effects of TNF‐α on bone regeneration. Lu et al.10 demonstrated that 24 hours of TNF‐α (1 ng/mL) treatment significantly up‐regulated BSP and osteocalcin gene expression levels in human primary osteoblasts at day 7, but these genes were significantly down‐regulated after continuous TNF‐α treatment for 7 days. Our previous study also revealed that short‐term exposure of ST2 murine MSCs to TNF‐α (0.1 ng/mL) for 24 and 48 hours significantly enhanced expressions of Runx2, Osx, OC and ALP, while long‐term treatment of these cells with TNF‐α (0.1 ng/mL) for 28 days inhibited mineral nodule formation.11 In contrast, in this study, we found that both short‐ and long‐term exposure of MC3T3‐E1 cells to low concentrations of TNF‐α promoted osteogenic differentiation and mineralization in these cells. Potential reasons for these inconsistent results may include different cell types, different cell origins, differentiation stages of bone forming cells and the experimental conditions used by different researchers.
As we have mentioned previously, numerous signalling pathways were activated after TNF‐α treatment, and our previous findings showed that the positive effects of TNF‐α treatment at low concentrations on osteogenic differentiation is not mediated by NF‐κB signalling pathway.11 As a bidirectional signalling pathway playing an important role in the crosstalk between osteoblasts and osteoclasts, ephrinB2‐EphB4 signalling pathway can also be activated upon surface contact between adjacent osteoblasts in an autocrine or paracrine manner and promote osteogenic differentiation.16, 19 To investigate whether ephrinB2‐EphB4 signalling pathway plays a role in the positive effects of TNF‐α on osteogenic differentiation, we first monitored expression levels of ephrinB2 and EphB4 in MC3T3‐E1 cells treated with low concentrations of TNF‐α. We found that both mRNA and protein levels of ephrinB2 and EphB4 were elevated in MC3T3‐E1 cells treated with TNF‐α at low concentrations. In addition, enhanced ephrinB2‐EphB4 signalling by recombinant mouse ephrinB2‐fc chimera further enhanced the positive effects on osteogenic differentiation induced by TNF‐α treatment. However, inhibition of ephrinB2‐EphB4 signalling by lentiviruses encoding siRNAs specifically targeting EphB4 significantly reversed the positive effects of TNF‐α on osteogenic differentiation. All of these findings strongly indicated that ephrinB2‐EphB4 signalling is activated in osteoblasts by TNF‐α treatment, and the enhanced osteogenic differentiation induced by low concentrations of TNF‐α is mediated, at least partly, by ephrinB2‐EphB4 signalling pathway.
EphrinB2‐EphB4 signalling can activate various downstream signalling pathways and play roles in a broad spectrum of biological processes.16, 17, 27, 28 In human MSCs, ephrinB2‐EphB4 signalling activated JNK, which in turn suppressed activated T cells.27 EphrinB2‐EphB4 signalling can also activate Erk‐dependent VEGF pathway in hepatic stellate cells to promote chemotaxis of sinusoidal endothelial cells.28 During the crosstalk between osteoblasts and osteoclasts mediated by ephrinB2‐EphB4 signalling, Erk pathway is also identified as a potential downstream effector of the forward signalling in osteoblasts stimulated with ephrinB2.16 Previous reports indicated that besides NF‐κB signalling pathway, TNF‐α can also activate mitogen‐activated protein kinase (MAPK) such as p38, Erk and JNK,29 among which Erk and JNK have been widely validated to be critical signalling in promoting osteogenic differentiation30, 31, 32 while NF‐κB signalling pathway mainly mediated the inhibitory effect of TNF‐α on osteogenic differentiation.33 Taken together, our results suggested that enhanced osteogenic differentiation in response to low concentrations of TNF‐α treatment in MC3T3‐E1 cells is mediated by ephrinB2‐EphB4 signalling pathway, with JNK and Erk acting as the potential downstream effecters. When the ephrinB2‐EphB4 signalling is blocked, the activation of the downstream Erk and JNK signalling is reversed, and the inhibitory effect of NF‐κB signalling pathway on osteogenic differentiation may be activated.
In summary, an early, mild inflammatory microenvironment is important for the healing of bone defects, which is evidenced by that obliterating TNF‐α signalling exerts detrimental influence on bone healing in vivo,34 and local addition of TNF‐α at bone defect sites would promote bone formation.13 Moreover, short‐term of TNF‐α preconditioning could mimic the short boost of inflammation normally occurring after bone injury, which suggested that controlled TNF‐α treatment might serve as a feasible approach to promote bone regeneration.35 In this study, we found that ephrinB2‐EphB4 signalling within osteoblasts mediated the enhancement of osteogenic differentiation induced by lower concentrations of TNF‐α. Our findings cast a significant light on the molecular mechanisms underlying osteogenic differentiation enhanced by lower concentrations of TNF‐α, which may be a novel drug target to promote bone healing processes. However, the detailed mechanisms underlying the interplay between TNF‐α and ephrinB2‐EphB4 signalling pathway still need further investigations.
Financial disclosure and Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 81271141) to PY and the Construction Engineering Special Fund of “Taishan Scholars” to XX. The authors declare that there is no conflict of interests.
Contributor Information
Chengzhe Yang, Email: yangchengzhe19@163.com.
Pishan Yang, Email: yangps@sdu.edu.cn.
References
- 1. Dodde RN, Yavuzer R, Bier UC, Alkadri A, Jackson IT. Spontaneous bone healing in the rabbit. J Craniofac Surg. 2000;11:346–349. [DOI] [PubMed] [Google Scholar]
- 2. Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;46(suppl 355):S7–S21. [DOI] [PubMed] [Google Scholar]
- 3. Thomsen P, Gretzer C. Macrophage interactions with modified material surfaces. Curr Opin Solid State Mater Sci. 2001;5:163–176. [Google Scholar]
- 4. Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259:686–691. [PubMed] [Google Scholar]
- 5. Brunetti G, Colucci S, Pignataro P, et al. T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J Periodontol. 2005;76:1675–1680. [DOI] [PubMed] [Google Scholar]
- 6. Li W, Yu B, Li M, et al. NEMO‐binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem Bioph Res Co. 2010;391:1228–1233. [DOI] [PubMed] [Google Scholar]
- 7. Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthr Cartil. 2009;17:735–742. [DOI] [PubMed] [Google Scholar]
- 8. Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNF alpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF‐kappa B signaling pathway. Bone. 2009;45:367–376. [DOI] [PubMed] [Google Scholar]
- 9. Feng X, Feng G, Xing J, et al. TNF‐alpha triggers osteogenic differentiation of human dental pulp stem cells via the NF‐kappaB signalling pathway. Cell Biol Int. 2013;37:1267–1275. [DOI] [PubMed] [Google Scholar]
- 10. Lu Z, Wang G, Dunstan CR, Zreiqat H. Short‐term exposure to tumor necrosis factor‐alpha enables human osteoblasts to direct adipose tissue‐derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21:2420–2429. [DOI] [PubMed] [Google Scholar]
- 11. Huang H, Zhao N, Xu X, et al. Dose‐specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karnes JM, Daffner SD, Watkins CM. Multiple roles of tumor necrosis factor‐alpha in fracture healing. Bone. 2015;78:87–93. [DOI] [PubMed] [Google Scholar]
- 13. Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF‐alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle‐derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han D, Ybanez MD, Ahmadi S, Yeh K, Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxid Redox Signal. 2009;11:2245–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nanes MS. Tumor necrosis factor‐alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. [DOI] [PubMed] [Google Scholar]
- 16. Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2‐EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. [DOI] [PubMed] [Google Scholar]
- 17. Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. [DOI] [PubMed] [Google Scholar]
- 18. Tonna S, Takyar FM, Vrahnas C, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB J. 2014;28:4482–4496. [DOI] [PubMed] [Google Scholar]
- 19. Martin TJ, Allan EH, Ho PW, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. [DOI] [PubMed] [Google Scholar]
- 20. Takyar FM, Tonna S, Ho PW, et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28:912–925. [DOI] [PubMed] [Google Scholar]
- 21. Allan EH, Hausler KD, Wei T, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. [DOI] [PubMed] [Google Scholar]
- 22. Karner E, Backesjo CM, Cedervall J, Sugars RV, Ahrlund‐Richter L, Wendel M. Dynamics of gene expression during bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Biochim Biophys Acta. 2009;1790:110–118. [DOI] [PubMed] [Google Scholar]
- 23. Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. [DOI] [PubMed] [Google Scholar]
- 24. Briolay A, Lencel P, Bessueille L, Caverzasio J, Buchet R, Magne D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF‐alpha in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430:1072–1077. [DOI] [PubMed] [Google Scholar]
- 25. Mukai T, Otsuka F, Otani H, et al. TNF‐alpha inhibits BMP‐induced osteoblast differentiation through activating SAPK/JNK signaling. Biochem Biophys Res Commun. 2007;356:1004–1010. [DOI] [PubMed] [Google Scholar]
- 26. Lu X, Beck GJ, Gilbert LC, et al. Identification of the homeobox protein Prx1 (MHox, Prrx‐1) as a regulator of osterix expression and mediator of tumor necrosis factor alpha action in osteoblast differentiation. J Bone Miner Res. 2011;26:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen TM, Arthur A, Hayball JD, Gronthos S. EphB and Ephrin‐B interactions mediate human mesenchymal stem cell suppression of activated T‐cells. Stem Cells Dev. 2013;22:2751–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das A, Shergill U, Thakur L, et al. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk‐dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol. 2010;298:G908–G915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Micheau O, Tschopp J. Induction of TNF receptor I‐mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. [DOI] [PubMed] [Google Scholar]
- 30. Xia L, Yin Z, Mao L, et al. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci Rep. 2016;6:22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li CS, Zheng Z, Su XX, et al. Activation of the extracellular signal‐regulated kinase signaling is critical for human umbilical cord mesenchymal stem cell osteogenic differentiation. Biomed Res Int. 2016;2016:3764372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y, Zhou J, Li Y, et al. Rap1A regulates osteoblastic differentiation via the ERK and p38 mediated signaling. PLoS ONE. 2015;10:e0143777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor‐kappaB. Nat Med. 2009;15:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF‐alpha signaling: the role of TNF‐alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. [DOI] [PubMed] [Google Scholar]
- 35. Lu Z, Wang G, Dunstan CR, et al. Activation and promotion of adipose stem cells by tumour necrosis factor‐alpha preconditioning for bone regeneration. J Cell Physiol. 2013;228:1737–1744. [DOI] [PubMed] [Google Scholar]


