Abstract
Background and objectives
MicroRNAs are small non‐coding RNAs involved in pathogenesis and progression of human malignancies. MicroRNA‐491‐5p (miR‐491‐5p) is down‐regulated in many human cancers where it would serve as a tumour suppressor. However, the role played by miR‐491‐5p in pathogenesis of human osteosarcoma has remained largely unknown. This study has been conducted to examine effects of miR‐491‐5p on migration and proliferation of cells of the SAOS‐2 and MG63 osteosarcoma lines, and mechanisms of those effects.
Materials and methods
Levels of miR‐491‐5p expression in osteosarcoma tissues and in human osteosarcoma cell lines were studied using qualitative real‐time polymerase chain reaction (qRT‐PCR) methods. Cell viability was detected using the CCK‐8 and EdU assays, while the transwell assay was used to evaluate migration and invasion. Apoptosis was analysed uing flow cytometry and the Hoechst 33342 nuclear staining method. A dual‐luciferase reporter system was used to confirm the target gene of miR‐491‐5p. The electrophoretic mobility shift assay (EMSA) with DIG‐labelled double‐stranded FOXP4 oligonucleotides was used to confirm whether or not miR‐491‐5p suppressed FOXP4 activation.
Results
Cells of osteosarcoma tissues and cell lines had low levels of miR‐491‐5p expression, but high levels of forkhead‐box P4 (FOXP4) expression. Transfection of SAOS‐2 and MG63 cells with miR‐491‐5p mimics inhibited expression of FOXP4 protein, which suppressed cell growth and migration, but induced apoptosis. Dual‐luciferase reporter assays confirmed FOXP4 as the target gene for miR‐491‐5p. Overexpression of miR‐491‐5p suppressed FOXP4 activity in SAOS‐2 and MG63 cells. Knockdown of FOXP4 in SAOS‐2 and MG63 cells using an RNAi strategy resulted in reduced levels of cell proliferation and migration, but increased levels of apoptosis.
Conclusion
Our in vitro studies showed that up‐regulation of miR‐491‐5p suppressed proliferation of the human osteosarcoma cells and induced apoptosis by targeting FOXP4. These findings suggest that miR‐491‐5p could be further studied as a potential clinical diagnostic or predictive biomarker for human osteosarcoma.
1. Introduction
Osteosarcoma, although rare, is the most common primary bone tumour in children and young adults1 and is characterized by osteoid production and osteoblastic differentiation.2 In recent decades, the incidence of osteosarcoma has increased nearly 1.4% each year,3 and while surgical excision is the primary treatment, many osteosarcoma patients have a poor clinical outcome.4, 5 Although the 5‐year survival rate for patients with non‐metastatic osteosarcoma has increased >50% with the use of neoadjuvant chemotherapy, patients with recurrent or metastatic osteosarcoma continue to have a poor prognosis.6, 7 Furthermore, only a small number of established diagnostic and prognostic osteosarcoma biomarkers are available. Therefore, there is an urgent need to identify new biomarkers that can be used to improve the treatment and prognosis of osteosarcoma patients, and especially high‐risk patients.
MicroRNAs (miRNAs) are endogenous small non‐coding RNAs, which are 22‐24 nucleotides in length and play crucial roles in post‐transcriptional regulation.8 MiRNAs bind to the 3′‐untranslated region (3′‐UTR) of their target mRNA molecules and thereby suppress their translation or stimulate their degradation.9, 10 Down‐regulation of a miRNA usually results in overexpression of its target gene. Previous studies have shown that 98 miRNAs are located at different genomic regions involved in human tumorigenesis, suggesting that miRNAs are involved in the development and pathogenesis of malignancies, and could possibly serve as diagnostic or prognostic tumour biomarkers.11 Furthermore, miRNAs play critical roles in a variety of other cellular processes, including tumour growth,12 cell apoptosis13 and tumour cell metastasis,14 and can act as either oncogenes or anti‐oncogenes.15 Additionally, the expression profiles of different miRNAs in plasma, serum and tumour samples have been used to accurately classify human cancers, suggesting that miRNAs could possibly serve as biomarkers for diagnosing human cancers and developing a prognosis for cancer patients.16, 17
When miRNA‐491‐5p was first identified in 2005,18 its expression was shown to be down‐regulated in certain malignant tumours.19, 20, 21, 22 Other studies have reported that miR‐491‐5p overexpression is associated with inhibited cell growth and promotion of apoptosis in colorectal,19 ovarian22 and breast carcinomas.21 A recent study that employed microRNA microarray methodologies found that miRNA‐491‐5p expression was significantly decreased in human osteosarcoma tissue.23 However, the actual mechanism by which microRNA‐491‐5p affects the behaviour of osteosarcoma cells remains obscure.
We performed this study to define the role of miR‐491‐5p in human osteosarcoma tissue and investigate the effects of miR‐491‐5p overexpression on human osteosarcoma cell proliferation, metastasis and apoptosis. As literature reports have identified forkhead‐box P4 (FOXP4) as a potential target gene for miR‐491‐5p, we studied how miR‐491‐5p affects FOXP4 gene expression.
2. Methods
2.1. Tissue collection
Between 2014 and 2015, 43 samples of osteosarcoma and associated pericarcinomatous tissue were obtained from individual patients who had undergone surgery. The patients included in this study had not received any previous chemotherapy, radiotherapy, immunotherapy or systemic treatment for their disease. The clinical stage of each osteosarcoma patient was classified based on criteria developed by the Union for International Cancer Control (UICC).24 Each sample of carcinoma and pericarcinomatous tissue was examined by a trained pathologist (Table 2).
Table 2.
Correlation of miR‐491 expression with clinicopathological feature of osteosarcoma
| Parameters | No. of cases | miR‐491 expression | P | |||
|---|---|---|---|---|---|---|
| High | Percentage | Low | Percentage | |||
| Age | ||||||
| <20 years | 19 | 9 | 47.37 | 10 | 52.63 | 0.6112 |
| ≥20 years | 24 | 15 | 62.50 | 9 | 37.50 | |
| Sex | ||||||
| Male | 30 | 18 | 60.00 | 12 | 40.00 | 0.52 |
| Female | 13 | 6 | 46.15 | 7 | 53.85 | |
| Tumour size | ||||||
| >8 cm | 26 | 9 | 34.62 | 17 | 65.38 | <0.01 |
| ≤8 cm | 17 | 15 | 88.24 | 2 | 11.76 | |
| Anatomical location | ||||||
| Tibia/femur | 33 | 17 | 51.52 | 16 | 48.48 | 0.3025 |
| Elsewhere | 10 | 7 | 70.00 | 3 | 30.00 | |
| Clinical stage | ||||||
| I/II | 27 | 20 | 74.07 | 7 | 25.93 | <0.01 |
| III/IV | 16 | 4 | 25.00 | 12 | 75.00 | |
| Distant metastasis | ||||||
| Absent | 25 | 9 | 36.00 | 16 | 64.00 | 0.0191 |
| Present | 18 | 13 | 72.22 | 5 | 27.78 | |
All patients included in the study provided a signed informed consent document, allowing for the use of their clinical specimens in this research. The study protocol was approved by the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, China.
2.2. Cell lines and culture conditions
Human osteoblast cell line hFOB (OB3) was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in Ham's F12 Medium Dulbecco's modified Eagle's medium (ATCC) containing 2.5 mm l‐glutamine (Sigma, St. Louis, MO, USA) and 20% FBS (Hyclone, Logan, UT, USA). Human osteosarcoma cell lines SAOS‐2, MG63 and U‐2OS were obtained from ATCC and cultured according to the vendor's instructions. All culture medium contained penicillin (100 IU/mL) and streptomycin (100 IU/mL). All cells were cultured and maintained in a humidified incubator set at 37°C and containing an atmosphere of 5% CO2.
2.3. Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis
Total RNA was extracted from tumour tissues and cultured cells using Trizol reagent (Invitrogen Inc, Carlsbad, CA, USA) according to the manufacturer's instructions. The levels of miRNA‐491‐5p expression were determined using a SYBR PrimeScript miRNA RT PCR Kit (TAKARA, Dalian, China), and the results were normalized to those obtained for U6 snRNA. The cDNA used for qRT‐PCR analysis was synthesized from 2 μg of total RNA with the aid of M‐MLV reverse transcriptase (Promega, Fitchburg, WI, USA). qRT‐PCR was performed using 2× SYBR green PCR Master Mix (TOYOBO, Osaka, Japan) and an ABI PRISM 7500 Sequence Detection system. The relative levels of FOXP4 mRNA were normalized to those for GAPDH. The sequence‐specific PCR primers used for qRT‐PCR are shown in Table 1. All calculated values were normalized using the 2−ΔΔCt method.
Table 1.
Primer sequences used for miRNA and mRNA expression analysis
| Name | Primer sequence (5′‐3′) |
|---|---|
| miR‐491‐5p‐RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCTCATG |
| U6‐RT | CGCTTCACGAATTTGCGTGTCAT |
| U6‐F | CTCGCTTCGGCAGCACA |
| U6‐R | AACGCTTCACGAATTTGCGT |
| miR‐491‐5p‐F | ACACTCCAGCTGGGAGTGGGGAACCCTTC |
| Universal‐R | TGGTGTCGTGGAGTCG |
| FOXP4‐F | ATCGGCAGCTGACGCTAAATGAGA |
| FOXP4‐R | AAACACTTGTGCAGGCTGAGGTTG |
| GAPDH‐F | ACACCCACTCCTCCACCTTT |
| GAPDH‐R | TTACTCCTTGGAGGCCATGT |
F, forward primer; R, reverse primer; RT, reverse transcription primer.
2.4. Protein extraction and Western blot
Total proteins from tissues and cells were extracted on ice for 30 minutes using SDS lysis buffer (Beyotime, Nantong, China), and the total amounts of extracted soluble protein were determined using a BCA Protein Assay kit (Pierce, Rockford, IL, USA). Equal amounts of total extracted protein were boiled in loading buffer for 5 minutes and then separated by electrophoresis on a 12% SDS‐polyacrylamide gel at 120V for 2 hours. Following separation, the proteins were transferred onto 0.22 μm polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were washed three times for 20 minutes each time with TBST and then incubated with FOXP4 antibody (1:1000) (ab17726; Abcam, Cambridge, MA, USA) or GAPDH antibody (1:1500) (ab8245; Abcam) at room temperature for 1 hour. Next, the membranes were washed three additional times with TBST and then incubated with a secondary antibody (goat anti‐rabbit IgG‐HRP; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1/3000 dilution for 1 hour at room temperature. Following incubation, the membranes were washed three more times, and the immunostained proteins were detected using Beyo ECL Plus reagent (Beyotime) and a gel imaging system. The staining intensities of individual protein bands were quantified using Bio‐Rad Quantity One software (Bio‐Rad, Hercules, CA, USA).
2.5. Plasmid construction
The full‐length FOXP4 3′‐untranslated region (3′‐UTR) was cloned into a psi‐CHECK2 vector (Promega) and designated as FOXP4‐3′‐UTR‐WT. A QuikChange Site‐Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA) was used to construct a mutant 3′‐UTR of FOXP4. The predicted target sites (UCCCCACC) were mutated to AGGGCCU. All plasmids were confirmed by DNA sequencing.
2.6. Cell transfection
The miR‐491‐5p mimics and scrambled sequences (NCs) were obtained from Guangzhou Ribobio, China. Small‐interfering RNA (siRNA) molecules targeting the FOXP4 gene (5′‐UGUAGAACUCAUGAUUCUGGGTT‐3′) and negative control gene (5′‐AGGUAGUGUAAUCGCCUUGTT‐3′) were obtained from GenePharma (Shanghai, China). Osteosarcoma cells (1 × 105) were seeded into the wells of a 24‐well culture plate and incubated for 24 hours; after which, the cells were transfected with either mimics (50 mm) or FOXP4‐siRNA (100 mm). The transfections were performed by using Lipofectamine 2000 (Invitrogen) in medium without FBS.
2.7. Luciferase assay
For the luciferase reporter assay, aliquots of cells (100 μL) were cultured in 24‐well plates and then co‐transfected with 100 ng of FOXP4‐3′‐UTR‐WT or ‐MUT psi‐CHECK2 vectors plus 50 nM miR‐491‐5p mimics or scrambled sequences using Lipofectamine 2000 reagent. After 48 hours, the cells were harvested and lysed. Luciferase activity was detected using the Dual‐Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. Firefly luciferase activity was used as an internal reference standard.
2.8. Cell viability assay
The proliferative capabilities of cells which received different treatments were analysed using a Cell Counting Kit‐8 (CCK‐8) (Dojindo, Japan) according to the manufacturer's instructions. Briefly, exponentially growing cells (1 × 104) that had been transfected with either miR‐491‐5p or FOXP4‐siRNA were seeded into individual wells of a 96‐well culture plate and incubated for 24, 48 or 72 hours, respectively. Three replicates were used for each time point. After incubation, CCK‐8 solution (100 μL) was added to each well, and the cells were incubated at 37°C for an additional 60 minutes. Next, the OD value at 450 nm for each well was recorded by a Microplate Reader (Rayto Life and Analytical Science C. Ltd, Shenzhen, China).
2.9. 5‐ethynyl‐2‐deoxyuridine (EdU) assay
SAOS‐2 and MG63 cells were seeded into 96‐well plates and transfected with miR‐491‐5p mimics or FOXP4‐siRNA. At 48 h after transfection, 5‐ethynyl‐2′‐deoxyuridine (EdU) (100 μm) (Cell Light EdU DNA imaging Kit; Guangzhou RiboBio, China) was added to each well, and the cells were cultured for an additional 2 hours; after which, they were stained as previously described.25 EdU‐positive cells were identified in parallel by staining with Apollo® 643 azide and flow cytometric analysis.
2.10. Cell apoptosis assay
Apoptosis was assessed as previously described.22 Briefly, aliquots of cells (1 × 107) that had received different types of treatment were pelleted by centrifugation and washed with PBS. The cells were then stained with Annexin V‐FITC plus 1 mg/mL PI and analysed with a FACS Calibur flow cytometer (Becton‐Dickinson, Franklin Lakes, NJ, USA). Annexin V‐FITC(+)/PI(−) and Annexin V‐FITC(+)/PI(+) staining identified cells that were in the early and later stages of apoptosis, respectively.
The Hoechst 33342 nuclear staining method was also used to detect apoptotic cells. Briefly, cells were seeded into six‐well plates and cultured with Hoechst 33342 blue fluorescent nuclear dye (Sigma) for 30 minutes; after which, their nuclear morphology was examined with a fluorescence microscope equipped to detect Hoechst 33342 staining at 365 nm.
2.11. Cell migration and invasion assays
Cell migration and invasion assays were performed as previously described.26
2.12. Electrophonic mobility shift assay (EMSA)
EMSAs were performed using a second‐generation DIG Gel Shit Kit (Roche Diagnostics, Mannheim, Germany). Briefly, double‐stranded oligonucleotide probes containing the FOXP4 binding sites were obtained from Sangon Biotech (Shanghai, China). The nuclear proteins in SAOS‐2 and MG63 cells were extracted using a CelLytic™ NuCLEAR™ Extraction Kit (Sigma) according to the manufacturer's instructions. The EMSA assays were performed as previously described.27 The oligonucleotide was electro‐blotted onto a positively charged nylon membrane (Roche Diagnostics, Indianapolis, IN, USA) and immunodetected using anti‐digoxigenin‐AP (Roche Diagnostics).
2.13. Statistical analysis
The data were analysed using PASW Statistics for Windows, Version 18.0. (SPSS Inc., Chicago, IL, USA). All experiments were performed at least three times, and each experimental sample was tested three times before the results were included in a final analysis. All values for experimental results are expressed as the mean ± SD. The statistical significance of differences between independent groups was determined by one‐way analysis of variance (ANOVA) or either a paired or unpaired Student's t‐test. The Mann‐Whitney U test was used to compare differences related to miR‐491 in samples of osteosarcoma tissue vs. pericarcinomatous tissue. P‐values <.05 were considered statistically significant.
3. Results
3.1. Osteosarcoma tissue and cell lines had reduced levels of miR‐491‐5p expression
The levels of miR‐491‐5p expression as determined by qRT‐PCR were lower in samples of tumour tissue when compared with their levels in matched samples of normal tissue (n=43) (Figure 1A) and were down‐regulated in osteosarcoma cells (Figure 1B). These results suggested that miR‐491‐5p might play a role in human osteosarcoma and formed the basis for our further investigations using SAOS‐2 and MG‐63 cells.
Figure 1.
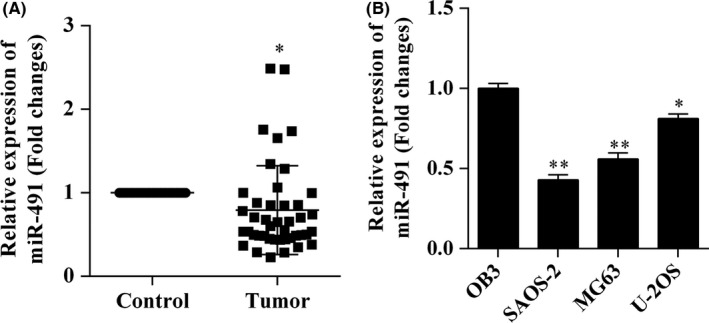
Expression of miR‐491‐5p was decreased in osteosarcoma tissue and cell lines. The levels of miR‐491‐5p expression in human osteosarcoma tissue and cell lines were analysed using the quantitative reverse transcription polymerase chain reaction (qRT‐PCR). A, Expression of miR‐491‐5p in human osteosarcoma tissues and matched adjacent normal tissues. B, Expression of miR‐491‐5p in human osteosarcoma cell lines; *P<.05, **P<.01
3.2. miR‐491‐5p expression and clinicopathological features in osteosarcoma patients
Clinical pathology findings in the 43 osteosarcoma patients included in our study are shown in Table 2. The patients were classified into two groups based on the median relative ratio of miR‐491‐5p expression (0.602). The results showed that patients with a low level of miR‐491‐5p expression had larger tumours (P<.01), were at an advanced clinical stage (P<.01), and had distant metastases (P=.0191). However, a patient's miR‐491‐5p expression level was not associated with other parameters, such as age (P=.6112), sex (P=.52), or the tumour's anatomical location (P=.3025) (Table 2).
3.3. Overexpression of miR‐491‐5p inhibited osteosarcoma cell proliferation
SAOS‐2 and MG‐63 osteocarcinoma cells transfected with miR‐491‐5p mimics and then cultured in vitro showed overexpression of miR‐491‐5p (Figure 2A). SAOS‐2 and MG‐63 cells transfected with miR‐491‐5p displayed suppressed proliferation when compared with NC‐transfected cells (Figure 2B). Additionally, EdU results showed that SAOS‐2 and MG‐63 cells which overexpressed miR‐491‐5p displayed reduced levels of proliferation (Figure 2C).
Figure 2.
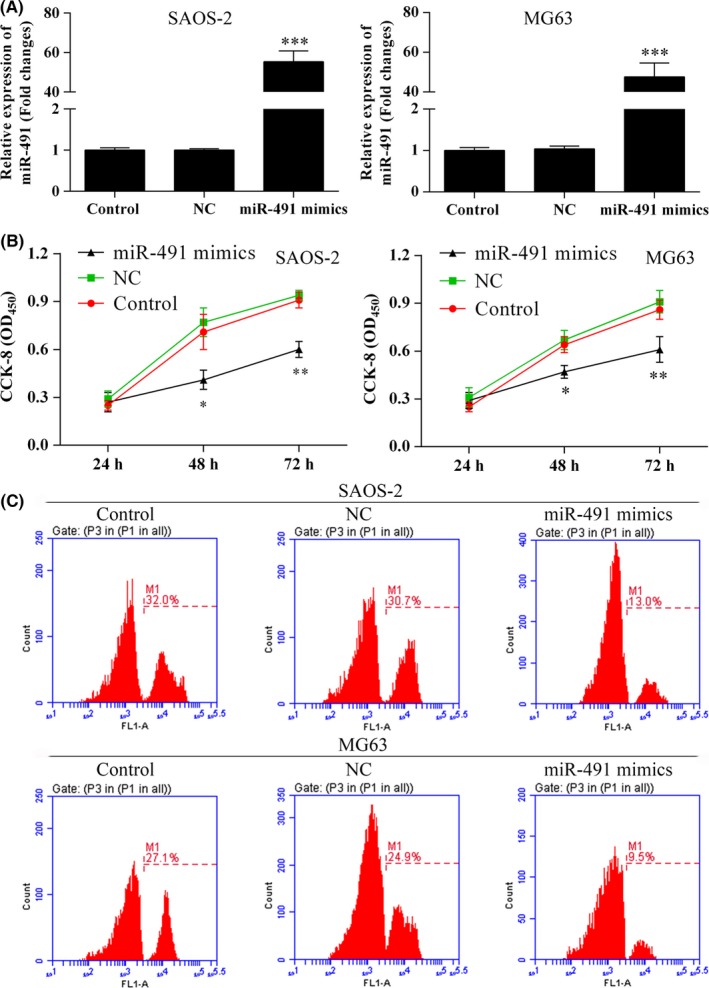
Osteosarcoma cells which overexpressed miR‐491‐5p showed reduced rates of proliferation. A, miR‐491‐5p was overexpressed in SAOS‐2 and MG63 cells; ***P<.001. B, The Cell Counting Kit‐8 (CCK‐8) assay was used to determine the proliferation rates of SAOS‐2 and MG63 cells transfected with miR‐491‐5p mimics; *P<.05, **P<.01. C, Cell proliferation rates as determined using the EdU flow cytometry assay
3.4. Overexpression of miR‐491‐5p suppressed osteosarcoma cell migration and invasion
The relationship between miR‐491‐5p expression levels and the migration/invasion capabilities of osteosarcoma cells was examined in vitro using the transwell assay. The results showed that the migration and invasion capabilities of SAOS‐2 and MG‐63 cells were inhibited following transfection with miR‐491‐5p mimics (Figure 3).
Figure 3.
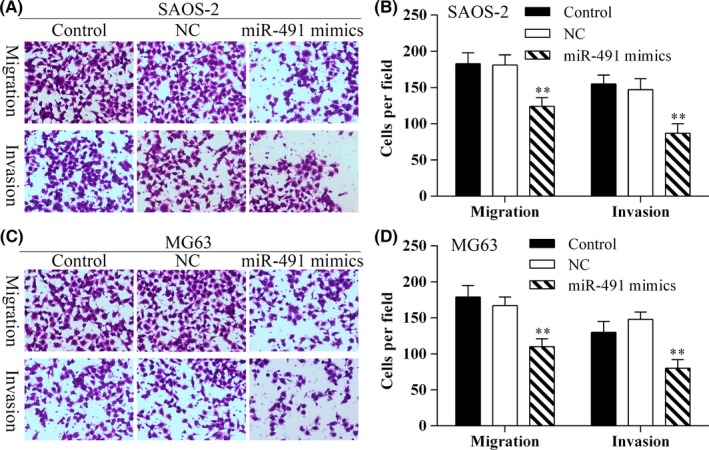
Osteosarcoma cells which overexpressed miR‐491‐5p showed reduced migration. A, The transwell assay was used to detect SAOS‐2 cell migration. Photomicrographs of migrating SAOS‐2 cells (×40 magnification). B, The average migration of SAOS‐2 cells (shown as the number of cells per field) in the different experimental groups; **P<.01. C, The transwell assay was used to detect MG63 cell migration. Photomicrographs showing the migration ability of MG63 cells (×40 magnification). D, The average migration of MG63 cells (shown as the number of cells per field) in the different experimental groups; **P<.01. Data represent results obtained from three independent experiments
3.5. Overexpression of miR‐491‐5p induced apoptosis in osteosarcoma cells
The effect of miR‐491‐5p on cell apoptosis was first examined using the Annexin V‐FITC/PI staining method. The apoptosis assays showed that transfection with miR‐491‐5p mimics induced increased levels of cellular apoptosis (Figure 4A‐D). The Hoechst 33342 nuclear staining method was also used to detect the effect of miR‐491‐5p on apoptosis in SAOS‐2 and MG63 cells. The results showed that overexpression of miR‐491‐5p induced apoptosis in SAOS‐2 and MG63 cells (Figure 4E,F).
Figure 4.
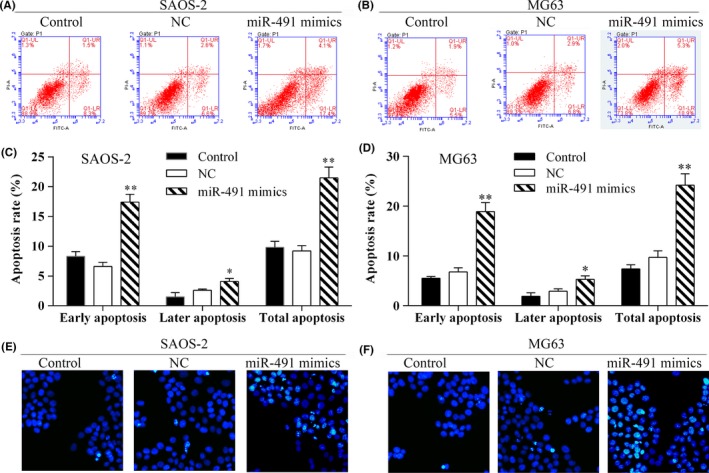
Overexpression of miR‐491‐5p induced apoptosis in osteosarcoma cells. Annexin V‐FITC/PI staining was performed to detect cell apoptosis via flow cytometry. A, B, Flow cytometric analyses of SAOS‐2 and MG63 cells for apoptosis after miR‐491‐5p transfection. C, D, The percentages of early, late and total apoptotic cells in each experimental group; *P<.05, **P<.01. Data are shown as the mean value ± SD. LR (lower right): early apoptotic cells, UR (upper right): late apoptotic cells, LL (lower left): viable cells, UL (upper left): necrotic cells. E, F, Hoechst staining to detect apoptosis in SAOS‐2 and MG63 cells transfected with miR‐491‐5p mimics
3.6. Forkhead‐box P4 (FOXP4) is a target of miR‐491‐5p
The levels of FOXP4 protein expression in human osteosarcoma tissue and osteosarcoma cell lines were detected in Western blot studies which showed that FOXP4 expression was increased in both the tissues samples (Figure 5A) and cell lines (Figure 5B). Furthermore, the levels of FOXP4 mRNA expression in SAOS‐2 and MG63 cells transfected with miR‐491‐5p mimics were detected using qRT‐PCR (Figure 5C,D). The results showed that FOXP4 mRNA expression in SAOS‐2 and MG63 cells was significantly decreased after the cells had been transfected with miR‐491‐5p mimics (Figures 4D and 5C). Moreover, Western blot results showed that overexpression of miR‐491‐5p suppressed FOXP4 protein expression (Figure 5E). When taken together, these results indicate that miR‐491‐5p suppresses FOXP4 expression at mRNA and protein levels.
Figure 5.
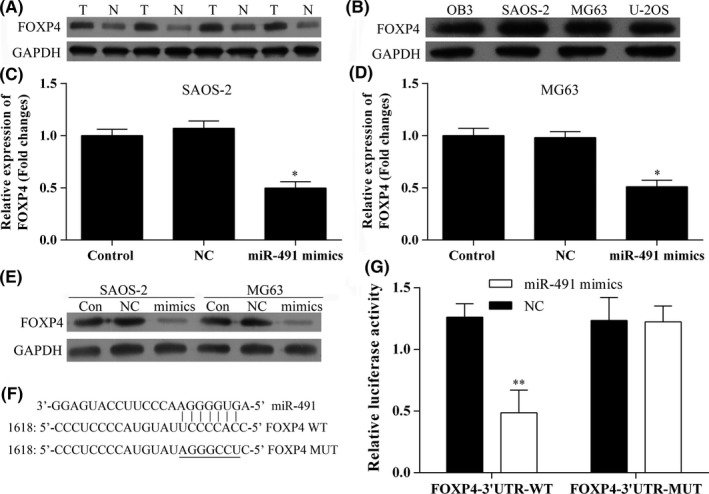
FOXP4 is a target gene for miR‐491‐5p. A, Western blot analysis of FOXP4 protein expression in samples of human osteosarcoma tissue and normal tissue; T, tumour; N, normal. B, Western blot analysis of FOXP4 protein expression in human osteosarcoma cell lines. C, D, The levels of FOXP4 mRNA expression in SAOS‐2 and MG63 cells transfected with miR‐491‐5p mimics; *P<.05, **P<.01. E, The levels of FOXP4 protein expression in SAOS‐2 and MG63 cells transfected with miR‐491‐5p mimics. F, G, Luciferase activity in the FOXP4T‐3′‐UTR‐WT group was significantly decreased after transfection with miR‐491‐5p mimics; **P<.01
To determine whether FOXP4 a target gene for miR‐491‐5p, we used the miRNA target predication databases (TargetScan: www.targetscan.org and miRDB: www.mirdb.org) to perform a computational analysis which showed that miR‐491‐5p has one predicted target site in the human FOXP4 3′‐UTR (Figure 5F). Next, a dual‐luciferase reporter system assay was performed to confirm that FOXP4 is a direct target of miR‐491‐5p. The assay results showed that miR‐491 mimics suppressed the reporter's luciferase activity and that the luciferase expression displayed by the mutant 3′‐UTR of FOXP4 was no longer regulated by miR‐491‐5p (Figure 5G). These results confirmed that the selected site in the 3′‐UTR of FOXP4 was a miR‐491‐5p regulation site and that FOXP4 is a target gene for miR‐491‐5p.
3.7. miR‐491‐5p suppressed FOXP4 activity in SAOS‐2 and MG63 cells
FOXP4 is a transcription factor involved in cell‐specific and tissue‐specific gene regulation. Therefore, we examined whether miR‐491‐5p would inhibit FOXP4 activation. To answer this question, we performed EMSAs with DIG‐labelled double‐stranded FOXP4 oligonucleotides to confirm whether miR‐491‐5p could inhibit FOXP4 activity in SAOS‐2 and MG63 cells. Our results showed that overexpression of miR‐491‐5p suppressed constitutive FOXP4 binding activity in both cell lines (Figure 6).
Figure 6.
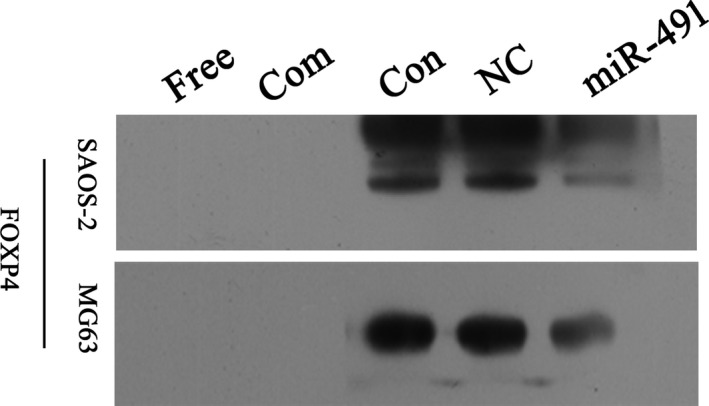
miR‐491‐5p suppressed FOXP4 activity in SAOS‐2 and MG63 cells. SAOS‐2 and MG63 cells were transfected with miR‐491‐5p and then assessed for FOXP4 DNA binding activity with the EMSA as performed using a FOXP4‐specific oligonucleotide probe. Presentation: Free, without probe; Com, competitor; Con, control; NC, negative control; miR‐491, miR‐491‐5p mimics
3.8. Knockdown of FOXP4 suppressed osteosarcoma cell proliferation
We next examined the effects of FOXP4 knockdown resulting from RNA interference following transfection with siRNA (Figure 7A,B). The results showed that the levels of FOXP4 mRNA and protein expression were significantly decreased in SAOS‐2 and MG63 cells transfected with FOXP4‐siRNA (Figure 7A,B,E). Furthermore, the results of both CCK‐8 and EdU assays showed that knockdown of FOXP4 inhibited the proliferation of SASO‐2 and MG63 cells (Figure 7C,E).
Figure 7.
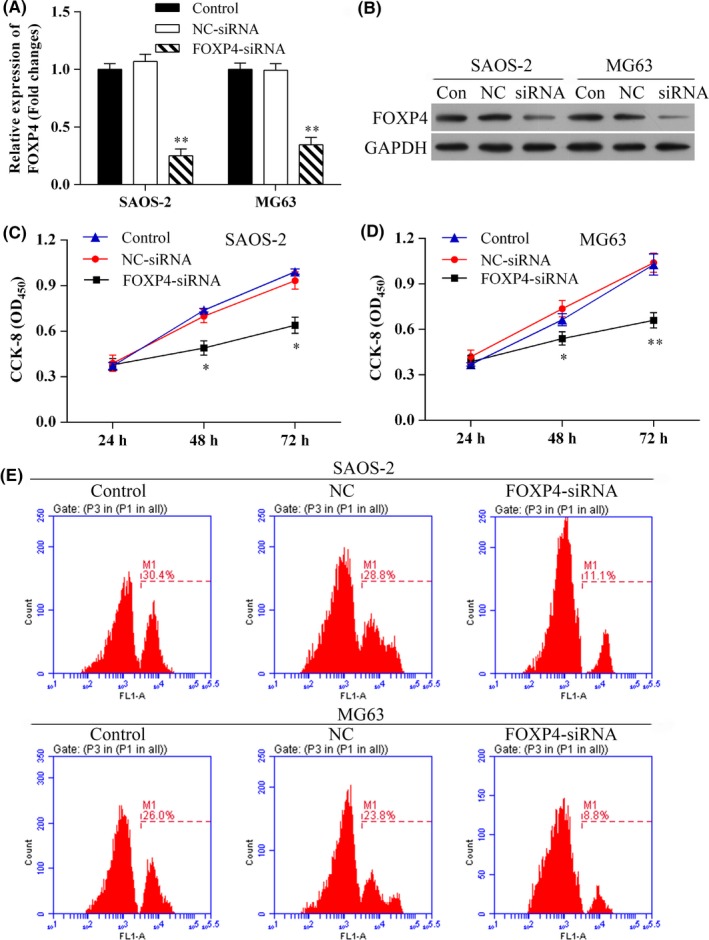
Knockdown of FOXP4 inhibited osteosarcoma cell proliferation in vitro. A, The levels of FOXP4 mRNA expression in SAOS‐2 and MG63 cells transfected with FOXP4‐siRNA were detected by qRT‐PCR; **P<.01. B, Western blot analysis of FOXP4 protein expression after transfection with FOXP4‐siNRA. C, D, Osteosarcoma cells showed reduced proliferation after knockdown of FOXP4 expression; *P<.05, ** P<.01. E, Cell proliferation was measured using the EdU flow cytometry assay
3.9. Knockdown of FOXP4 inhibited osteosarcoma cell migration and invasion
Transwell assays showed that SAOS‐2 and MG‐63 cells transfected with FOXP4‐siRNA had reduced migration and invasion capabilities (Figure 8).
Figure 8.
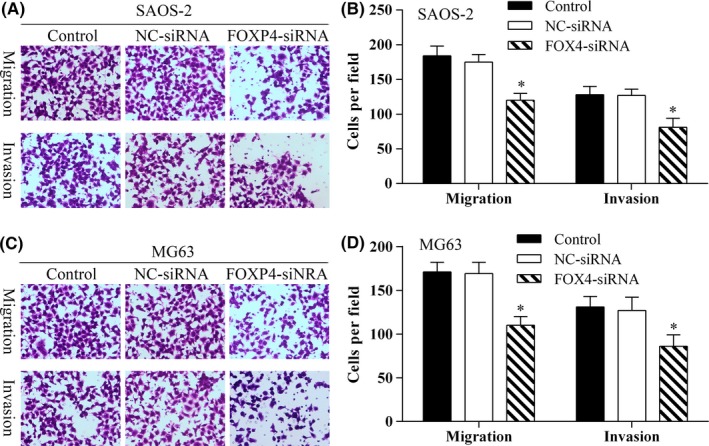
Knockdown of FOXP4 inhibited osteosarcoma cell migration. A, The transwell assay was used to detect SAOS‐2 cell migration. Photomicrographs showing the migration capabilities of SAOS‐2 cells (×40 magnification). B, The average migration of SAOS‐2 cells as determined by the number of cells per field in the different experimental groups; *P<.05. C, The transwell assay was used to detect MG63 cell migration. Photomicrographs showing the migration capabilities of MG63 cells (×40 magnification). D, The average migration of MG63 cells as determined by the number of cells per field in the different experimental groups; *P<.05. Data represent results from three independent experiments
3.10. Knockdown of FOXP4 promoted osteosarcoma cell apoptosis
The Annexin V‐FITC/PI staining method used for performing apoptosis assays showed that knockdown of FOXP4 promoted apoptosis in SAOS‐2 and MG63 cells (Figure 9A‐D), and similar results were found when using the Hoechst 33342 nuclear staining method (Figure 9E,F).
Figure 9.
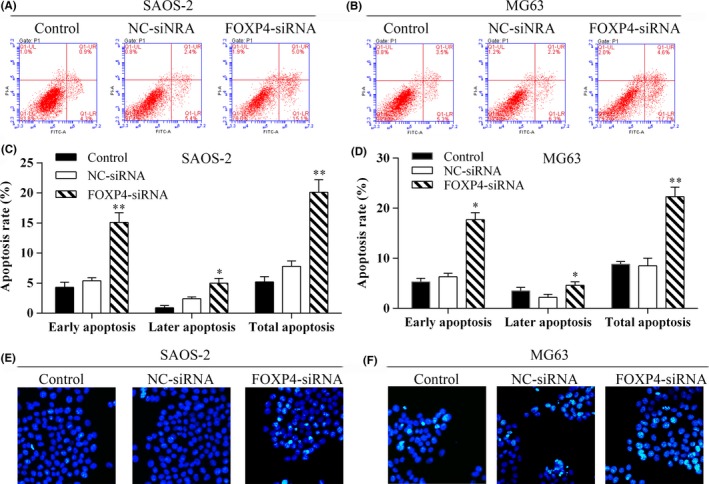
Knockdown of FOXP4 promoted osteosarcoma cell apoptosis. A, B, Flow cytometric analyses of apoptosis levels among SAOS‐2 and MG63 cells transfected with FOXP4‐siNRA. C, D, The percentages of early, late and total apoptotic cells in each experimental group; *P<.05, **P<.01. Data represent the mean value ± SD. LR (lower right): early apoptotic cells, UR (upper right): late apoptotic cells, LL (lower left): viable cells, UL (upper left): necrotic cells. E, F, Hoechst staining to detect apoptosis in SAOS‐2 and MG63 cells transfected with FOXP4‐siRNA
4. Discussion
Several recently published studies have supported a role for microRNAs (miRNAs) in regulating fundamental normal and reactive cellular processes.28, 29 Moreover, an increasing number of studies support a role for miRNA in the origination and progression of human cancers.12, 13, 14 Dysregulation of miRNA activity may result in progressive and uncontrolled tumour growth.12, 13 The ability to control both cell growth and cell survival may play important future roles in preventing and treating human various malignancies, including osteosarcoma.
Our current study showed that up‐regulation of miR‐491‐5p suppressed the proliferation of human osteosarcoma cells and increased the levels of apoptosis in those cells in vitro by targeting FOXP4. Additionally, we found that miR‐491‐5p expression was decreased in osteosarcoma tissue specimens and cell lines. Overexpression of miR‐491‐5p suppressed osteosarcoma cell proliferation and migration and also induced cellular apoptosis. Transfection of miR‐491‐5p mimics reduced FOXP4 protein expression, suggesting FOXP4 as a target gene for miR‐491‐5p. Knockdown of FOXP4 inhibited osteosarcoma cell proliferation and migration and promoted cellular apoptosis.
Abnormal levels of miRNA expression have been reported in a variety of human cancers. The gene that encodes miR‐491‐5p is located in the 9p21.3 region30 and functions as a tumour‐suppressor gene. Reduced levels of miR‐491‐5p expression have been reported in samples of human ovarian carcinoma tissue, and overexpression of miR‐491‐5p has been shown to suppress ovarian cell proliferation and promote cell apoptosis by targeting BCL‐XL.22 Previous studies have reported that miR‐491‐5p exerts similar effects on apoptosis and proliferation in breast cancer,21 colorectal cancer19 and pancreatic carcinoma20 cells. Furthermore, miR‐491‐5p was shown to inhibit invasive capabilities of human oral squamous cancer31 and breast cancer32 cells. Moreover, as an anti‐tumour gene, miRNA‐491‐5p has been reported to target the matrix metalloproteinase 9 (MMP9) in osteosarcoma33 and hepatocellular carcinoma,34 which involved in osteosarcoma and hepatocellular carcinoma metastasis.33, 34 In addition, miR‐491‐5p attenuated the angiogenic ability in hepatocellular carcinoma cells by inhibiting TGF‐β/SMAD3/NF‐κB signal pathway through targeting the SMAD3‐3′‐UTR.35 What's more, miR‐491 targets G‐protein‐coupled receptor kinase‐interacting protein 1 (GIT‐1), which blocked the activation of NF‐κB to attenuate cancer stem cell‐like properties of hepatocellular carcinoma.36 Besides, miR‐491 can also regulate its target genes, such as IGF2BP1,37 CTGF,38 TPX39 and Notch,40 functioning as tumour suppressor. Our study showed miR‐491‐5p suppressed the proliferation of human osteosarcoma cells and increased the cell apoptosis in vitro by targeting FOXP4. Our work will be a strong addition to the anti‐tumour literature as well as the miR‐491 literature.
The Forkhead‐box (FOX) family comprises a group of transcription factors involved in cell‐specific and tissue‐specific gene regulation.41 Forkhead‐box P4 (FOXP4) is a member of the FOXP subfamily of winged‐helix transcription factors and encodes a 685 amino acid protein with similarities to FOXP1 and FOXP2.42 FOXP4 is primarily expressed in gut, neural and pulmonary tissues during embryonic development.43 Wiehagen et al.44 demonstrated that FOXP4 is required for normal T‐cell antigenic memory to pathogens, and also for T‐cell development. Long et al.45 studied the association between FOXP4 and prostate cancer in Eastern Asian populations. Up‐regulated levels of FOXP4 expression have been identified in human hepatocellular carcinoma (HCC) tissue, and knockdown of FOXP4 has been shown to inhibit HepG2 and Hep‐3B cell proliferation and colony formation in vitro by causing cell cycle arrest.46 Increased levels of FOXP4 expression have been found in human non‐small‐cell lung cancer tissue and cell lines. Furthermore, knockdown of FOXP4 was shown to markedly reduce the proliferation and invasion capabilities of A549 and H1703 cells and induce cell cycle arrest. These findings further confirm that FOXP4 is target gene for miR‐138.47
In this study, we found significantly increased levels of FOXP4 gene and protein expression in osteosarcoma tissue and cell lines. Knockdown of FOXP4 by RNA interference suppressed the proliferation and migration of osteosarcoma cells and was associated with increased levels of apoptosis in those cells. When taken together, these findings support the hypothesis that down‐regulation of miR‐491‐5p promotes human osteosarcoma cell proliferation via a FOXP4‐mediated signalling pathway.
5. Conclusion
In conclusion, these in vitro studies showed that up‐regulation of miR‐491‐5p suppressed human osteosarcoma cell proliferation and increased the levels of apoptosis in human osteosarcoma cells by targeting FOXP4. Our findings justify further studying miR‐491‐5p as a potential clinical diagnostic or predictive biomarker for human osteosarcoma.
Competing interests
The authors declare that they have no competing interests.
Acknowledgement
We thank Guangzhou Vipotion Biotechnology Co., Ltd. for assistance in constructing vectors used in the luciferase reporter assay.
Funding informationFinancial support was kindly provided by the Department of Anatomy, Guangzhou Medical University.
References
- 1. Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis. 2007;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walkley CR, Qudsi R, Sankaran VG, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Gene Dev. 2008;22:1662–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caudill JS, Arndt CA. Diagnosis and management of bone malignancy in adolescence. Adolesc Med State Art Rev. 2007;18:62–78, ix. [PubMed] [Google Scholar]
- 4. Huang J, Liu K, Yu Y, et al. Targeting HMGB1‐mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy. 2012;8:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ando K, Heymann M‐F, Stresing V, Mori K, Rédini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers. 2013;5:591–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma In: Jaffe N, Bruland OS, Bielack S, eds. Pediatric and Adolescent Osteosarcoma, Vol 152 Newyork, NY: Springer; 2010:3–13. [Google Scholar]
- 7. Tan ML, Choong PF, Dass CR. Osteosarcoma: conventional treatment vs. gene therapy. Cancer Biol Ther. 2009;8:106–117. [DOI] [PubMed] [Google Scholar]
- 8. Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 10. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. [DOI] [PubMed] [Google Scholar]
- 11. Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu H, Li W, Chen C, Pei Y, Long X. MiR‐335 acts as a potential tumor suppressor miRNA via downregulating ROCK1 expression in hepatocellular carcinoma. Tumor biol. 2015;36:6313–6319. [DOI] [PubMed] [Google Scholar]
- 13. Tu Y, Liu L, Zhao D, et al. Overexpression of miRNA‐497 inhibits tumor angiogenesis by targeting VEGFR2. Sci Rep. 2015;5:13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin J‐Y, Kim Y‐I, Cho S‐J, et al. MicroRNA 135a suppresses lymph node metastasis through down‐regulation of ROCK1 in early gastric cancer. PLoS ONE. 2014;9:e85205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark edition). 2012;17:2508–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong J, Liu Y, Liao W, Liu R, Shi P, Wang L. miRNA‐223 is a potential diagnostic and prognostic marker for osteosarcoma. J Bone Oncol. 2016;5:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. [DOI] [PubMed] [Google Scholar]
- 19. Nakano H, Miyazawa T, Kinoshita K, Yamada Y, Yoshida T. Functional screening identifies a microRNA, miR‐491 that induces apoptosis by targeting Bcl‐X(L) in colorectal cancer cells. Int J Cancer. 2010;127:1072–1080. [DOI] [PubMed] [Google Scholar]
- 20. Guo R, Wang Y, Shi WY, Liu B, Hou SQ, Liu L. MicroRNA miR‐491‐5p targeting both TP53 and Bcl‐XL induces cell apoptosis in SW1990 pancreatic cancer cells through mitochondria mediated pathway. Molecules (Basel, Switzerland) 2012;17:14733–14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leivonen S‐K, Sahlberg KK, Mäkelä R, et al. High‐throughput screens identify microRNAs essential for HER2 positive breast cancer cell growth. Mol Oncol. 2014;8:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denoyelle C, Lambert B, Meryet‐Figuiere M, et al. miR‐491‐5p‐induced apoptosis in ovarian carcinoma depends on the direct inhibition of both BCL‐XL and EGFR leading to BIM activation. Cell Death Dis. 2014;5:e1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Namlos HM, Meza‐Zepeda LA, Baroy T, et al. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS ONE. 2012;7:e48086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Wu S, Shi X. Expression of matrix metalloproteinase regulator, RECK, and its clinical significance in osteosarcoma. J Orthop Res. 2010;28:1621–1625. [DOI] [PubMed] [Google Scholar]
- 25. Feng S, Cong S, Zhang X, et al. MicroRNA‐192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011;39:6669–6678.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruan WD, Wang P, Feng S, Xue Y, Zhang B. MicroRNA‐497 inhibits cell proliferation, migration, and invasion by targeting AMOT in human osteosarcoma cells. Onco Targets Ther. 2016;9:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto H, Kariya R, Shimamoto M, et al. Antitumor effect of berberine against primary effusion lymphoma via inhibition of NF‐kappaB pathway. Cancer Sci. 2012;103:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer SU, Thirion C, Polesskaya A, et al. TNF‐α and IGF1 modify the microRNA signature in skeletal muscle cell differentiation. Cell Commun Adhes. 2015;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS ONE. 2013;8:e75885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li X, Liu Y, Granberg KJ, et al. Two mature products of MIR‐491 coordinate to suppress key cancer hallmarks in glioblastoma. Oncogene. 2015;34:1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang WC, Chan SH, Jang TH, et al. miRNA‐491‐5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res. 2014;74:751–764. [DOI] [PubMed] [Google Scholar]
- 32. Rutnam ZJ, Yang BB. The non‐coding 3' UTR of CD44 induces metastasis by regulating extracellular matrix functions. J Cell Sci. 2012;125:2075–2085. [DOI] [PubMed] [Google Scholar]
- 33. Tian X, Zhang X. A single nucleotide polymorphism (rs1056629) in 3'‐UTR of MMP‐9 is responsible for a decreased risk of metastatic osteosarcoma by compromising its interaction with microRNA‐491‐5p. Cell Physiol Biochem. 2016;38:1415–1424. [DOI] [PubMed] [Google Scholar]
- 34. Zhou Y, Li Y, Ye J, et al. MicroRNA‐491 is involved in metastasis of hepatocellular carcinoma by inhibitions of matrix metalloproteinase and epithelial to mesenchymal transition. Liver Int. 2013;33:1271–1280. [DOI] [PubMed] [Google Scholar]
- 35. Jiang F, Wang X, Liu Q, et al. Inhibition of TGF‐beta/SMAD3/NF‐kappaB signaling by microRNA‐491 is involved in arsenic trioxide‐induced anti‐angiogenesis in hepatocellular carcinoma cells. Toxicol Lett. 2014;231:55–61. [DOI] [PubMed] [Google Scholar]
- 36. Yang X, Ye J, Yan H, et al. MiR‐491 attenuates cancer stem cells‐like properties of hepatocellular carcinoma by inhibition of GIT‐1/NF‐kappaB‐mediated EMT. Tumour Biol. 2016;37:201–209. [DOI] [PubMed] [Google Scholar]
- 37. Gong F, Ren P, Zhang Y, Jiang J, Zhang H. MicroRNAs‐491‐5p suppresses cell proliferation and invasion by inhibiting IGF2BP1 in non‐small cell lung cancer. Am J Transl Res. 2016;8:485–495. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Sun D, Han S, Liu C, et al. Microrna‐199a‐5p functions as a tumor suppressor via suppressing connective tissue growth factor (CTGF) in follicular thyroid carcinoma. Med Sci Monit. 2016;11:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J, Liu Z, Dou C, et al. miR‐491 inhibits the proliferation, invasion and migration of hepatocellular carcinoma cell via down‐regulating TPX2 expression. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:512–517. [PubMed] [Google Scholar]
- 40. Zhang Q, Li Q, Xu T, Jiang H, Xu LG. miR‐491‐5p suppresses cell growth and invasion by targeting Notch3 in nasopharyngeal carcinoma. Oncol Rep. 2016;35:3541–3547. [DOI] [PubMed] [Google Scholar]
- 41. Chokas AL, Trivedi CM, Lu MM, et al. Foxp1/2/4‐NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin‐6. J Biol Chem. 2010;285:13304–13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teufel A, Wong EA, Mukhopadhyay M, Malik N, Westphal H. FoxP4, a novel forkhead transcription factor. Biochim Biophys Acta. 2003;1627:147–152. [DOI] [PubMed] [Google Scholar]
- 43. Lu MM, Li S, Yang H, Morrisey EE. Foxp4: a novel member of the Foxp subfamily of winged‐helix genes co‐expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Gene Expr Patterns. 2002;2:223–228. [DOI] [PubMed] [Google Scholar]
- 44. Wiehagen KR, Corbo‐Rodgers E, Li S, et al. Foxp4 is dispensable for T cell development, but required for robust recall responses. PLoS ONE. 2012;7:e42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Long QZ, Du YF, Ding XY, et al. Replication and fine mapping for association of the C2orf43, FOXP4, GPRC6A and RFX6 genes with prostate cancer in the Chinese population. PLoS ONE. 2012;7:e37866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang G, Sun Y, He Y, Ji C, Hu B, Sun Y. MicroRNA‐338‐3p inhibits cell proliferation in hepatocellular carcinoma by target forkhead box P4 (FOXP4). Int J Exp Pathol. 2015;8:337–344. [PMC free article] [PubMed] [Google Scholar]
- 47. Yang T, Li H, Thakur A, et al. FOXP4 modulates tumor growth and independently associates with miR‐138 in non‐small cell lung cancer cells. Tumour Biol. 2015;36:8185–8191. [DOI] [PubMed] [Google Scholar]


