Abstract
Despite numerous remarkable achievements in the field of anti‐cancer therapy, tumour relapse and metastasis still remain major obstacles in improvement of overall cancer survival, which may be at least partially owing to epithelial‐mesenchymal transition (EMT). Multiple signalling pathways have been identified in EMT; however, it appears that the role of the Hedgehog and WNT/β‐catenin pathways are more prominent than others. These are well‐known preserved intracellular regulatory pathways of different cellular functions including proliferation, survival, adhesion and differentiation. Over the last few decades, several naturally occurring compounds have been identified to significantly obstruct several intermediates in Hedgehog and WNT/β‐catenin signalling, eventually resulting in suppression of signal transduction. This article highlights the current state of knowledge associated with Hedgehog and WNT/β‐catenin, their involvement in metastasis through EMT processes and introduction of the most potent naturally occurring agents with capability of suppressing them, eventually overcoming tumour relapse, invasion and metastasis.
1. Introduction
Currently, only one in five patients suffering from metastatic cancers survive more than 5 years1 and by the time of diagnose confirmation, primary tumour would have already been infiltrated to distant organs and survived as disseminated seeds, resulting in presumptive relapsing even after complete remission. Aberrant control of epithelial proliferation and angiogenesis has been proposed as the most important factor in initiation and expansion of primary carcinomas,2 nevertheless, not enough for a complete metastatic tumour. Process of metastasis consists of several interrelated steps, each of which can be assumed as a unique target for anti‐metastasis therapy. The first step includes breaking free from primary tumour. For this purpose, cancer cells reduce their adhesion to neighbour cells and prepare a clear pathway for migration to the vascular‐rich stroma region.3 By the time presented in vasculature‐rich stroma region, they easily intravasate into blood stream via discontinuous regions of tumour vasculature. Being present in blood stream, they can easily migrate to secondary organs. Cancer cells induce retraction of endothelial cells by secretion of several mediators including pro‐inflammatory cytokines, VEGF and induction of endothelial cell death by reactive oxygen species or matrix metalloproteinases (MMPs).4 Blood flow and secondary colonized organ cancer cells are two important factors determining primary tumour cells' distributing position. For instance, narrow capillary beds presented in lung and liver are places in which cancer cells can easily become entrapped. In another form, circulatory cancer cells can express receptors with high affinity to platelets surface ligands, which protect them from immune cells invasion and metastasis supporting site.5, 6, 7 Presented in secondary site, cancer cells can easily extravasate through retracted or dead endothelial cells,8 initiate pro‐inflammatory cytokines and proteinases secretion, stimulating their neighbour cells to secret growth factors, co‐opting the local environment for migrated primary cancer cells proliferation.4, 9, 10
Epithelial‐to‐mesenchymal transition [EMT], a fundamental step in metastasis, is defined as a group of changes in epithelial cells, preparing them to adopt a pluripotent mesenchymal phenotype with characteristics including enhanced migration capability, invasiveness, resistance to apoptosis and secretion of extra cellular matrix components.11 Enhancement in metastasis due to EMT mostly takes part because of increased invasiveness and migratory abilities of mesenchymal cells.12 During EMT, downregulation of E‐cadherin results in less cell adhesiveness and achieving of the mesenchymal markers such as N‐cadherin, Fibronectin, Vimentin and transcription factors result in more cells motility.11 Zinc finger transcription factors SNAIL, SLUG, ZEB1 and TWIST are responsible for repressing genes involved in epithelial phenotype.13 EMT induction has been mostly proposed to take part by various signalling pathways. WNT, Hedgehog and Notch are three developmental pathways, whose genes alteration results in facilitation of EMT.13
Recent discovery of naturally occurring compounds with potent anti‐cancer activity presented in consuming food‐based dietary resulted in an outbreak in designing chemotherapy regimen utilizing these compounds together with conventional chemotherapy agents. It has been proposed that approximately one third of cancers can be prevented by controlling diet and regular physical activities.14 These unique effects partly have been linked to their suppressing effect on Wnt and Hedgehog signalling pathways that modulate the EMT and CSCs proliferation and consequently invasion and metastasis. Some of these well‐known nutritional compounds include curcumin, green tea polyphenols, resveratrol, soy isoflavones and lycopene, all belonging to group of bioactive compounds categorized as “phytochemicals”.
Overall, phytochemicals are referred to group of compounds, donating flavour, smell and texture to the plants. More importantly, these agents protect plants from specific stress conditions including bacteria, viral and fungal invasion and oxidative stress. More than 250,000 plant species exist on earth; nevertheless, only 1–10% of this rate are edible and source of medical benefits in humans. Based on their structure, these compounds can be classified as alkaloids, isoprene derivatives and phenol compounds. Unlike proteins, fats and vitamins, these agents are not vital for life maintenance. However, their consumption has shown to be together with body protection against reactive oxygen and nitrogen species “preludes” and reduction of occurrence of several life‐threatening events including cancer, cardiovascular disorders and neurodegenerative disorders. Consequently, routine consumption of these nutraceuticals, products derived from dietary consuming food with extra health advantages, including fruits, coffee and cruciferous vegetables can provide lots of these agents for body. Other specific beneficial actions of these agents include protection against carcinogenesis, inflammatory conditions, oxidative stress and situations such as hypercholesterolemia and hypertension.12 Foods containing these nutraceuticals or more widely, any food either formulated or presented naturally, are referred as functional foods. According to US institute of medicines, foods and nutrition can supply and/or significantly improve health benefits. According to statistical reports, retail value of functional foods are now more than 50 billion Dollars in USA and the rate is increasing more and more each year.13
As mentioned earlier, utilizing current nutraceuticals, phytochemicals or more widely naturally occurring compounds can result in synergistic effects through blocking different ENT‐involved signalling pathways.14 For instance, concurrent administration of curcumin with Tamoxifen, Daunorubicin, Cisplatin and Vincristine was shown to re‐sensitize resistant cancer cells to these conventional compounds.15 In other studies by li et al. and Youngjoo et al.,16, 17 it was demonstrated that oligogalactans of apple increase the growth inhibitory effect of COX‐2 inhibitors in a colitis‐associated colorectal cancer. Addition of other phytochemicals such as quercetins together with curcumin resulted in even more beneficial effects compared to curcumin therapy alone.18 Consequently, it has been proposed that foods with multiple phytochemicals result in more significant effects and can be consumed as the first‐line defence against cancer. From other side, specific phytochemicals such as curcumin can result in DNA protection from breach during radiation therapy and provide extended protection window.19, 20 Studies have reported that phytochemicals as standalone agents and without concurrent administration with chemotherapies can again demonstrate anti‐cancer effects. For instance, apple seed extract showed a G0/G1 phase arrest in breast cancer and prostate cancer. Additionally, levels of maspin, an important tumour suppressing protein, was significantly increased by apple seed extract and resulted in suppression of cell invasion, metastasis and angiogenesis sufficiently.21 The purpose of this mini‐review was to first describe different pathways involved in EMT and metastasis and then describe different effects of phytochemicals with potent inhibitory activities on pathways involved in EMT including Wnt/β‐catenin and Hedgehog pathways.
2. EMT, tumours microenvironment and signalling pathways
Dynamic cellular transition is an essential phenomenon for cell maintenance with environmental signals and neighbouring cells interactions (Fig. 1). The most important reactions involved in dynamic cellular transition include EMT and mesenchymal‐to‐epithelial transition. Molecular pathways discovered up to now responsible for initiation or acceleration of EMT to completion include transcription factors activation, specific genes overexpression, reorganization of specific cytoskeletal proteins, secretion of ECM‐degrading enzymes and many more alterations. The word “transition” instead of “transformation” is used to emphasize that this process is reversible, known as mesenchymal‐epithelial transition (MET); however, current knowledge about this process is still very little.22
Figure 1.
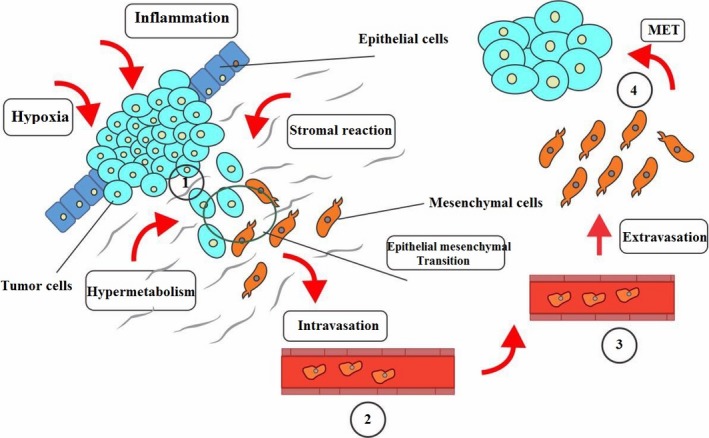
EMT in microenvironment and metastasis. (1) In carcinoma, there exist a primary epithelial phenotype containing a small group of mesenchymal‐like cancer stem cells. If not stimulated, they will undergo EMT, through genetic, autocrine and paracrine factors. These cells more easily escape from tumour mass by two ways, seeding nearby vasculature or stroma taking part by EMT to achieve mesenchymal features. Through migration, they can surpass the surgical region and consequently avoid surgical removal and radiotherapy and can form local recurrence in any place where re‐epithelialization take place during MET creating new malignancies. (2) The mesenchymal form is the best form to intravasate. (3) and a mixture of phenotypes is observed in blood stream. (4) The next step is extravasation. They may stay dormant; however, in any condition, they may be finally re‐epithelialized by MET and begin a macro‐metastasis with bone destruction abilities
EMT can be categorized in three unique biological settings. Although the functional consequences are completely different, the basic biological alterations seem to be approximately the same. First group consists of EMTs associated with implantation, embryo formation and organ development. This class of EMTs neither induces fibrosis nor an invasive phenotype. Second group consists of EMTs which are associated with wound healing, tissue regeneration and organ fibrosis. This group of EMT start with initiation of inflammation and cease by attenuation of inflammation and is part of a repair‐associated event. The third, takes part in neoplastic cells which has previously undergone genetic or epigenetic changes. These changes mostly affect oncogenes and tumour suppressor genes.23
Induction of both EMT‐ and MET‐like events is initiated with loss of cells' polarity, partially or completely subversion of cell‐cell junctions, renewal and remodelling of cytoskeletal elements, achieving the ability of motility and suppressing apoptosis process. Several in vitro and in vivo processes have linked activation of various pathways with induction of EMT or EMT‐like phenotypes; however, five pathways appear to be more important and approximately trigger majority of mentioned events.24 These include tyrosine kinase inhibitors including epidermal growth factors, hepatocyte growth factors, platelet‐derived growth factors, fibroblast growth factors, insulin‐like growth factors and platelet‐derived growth factors, NF‐κB, Wnt/β‐catenin, TGF‐β and integrin pathways.
During EMT, downregulation of junctional components seems to be the most important event, accompanying EMT process in several systems.24, 25 However, it is not fully understood whether this downregulation in transcription is a cause or an event of EMT‐like proceedings. Downregulation of E‐cadherin expression, one of these junctional components, has been strongly linked with cell‐cell dissociation and invasion in pancreas, mammary gland and prostate mouse cancer models.26, 27 Specific transcription factors consisting of Snail (Snail1), Twist, Slug (Snail2), E47 and SIP1/Zeb have been specifically related to downregulation of E‐cadherin expression.28 Mentioned factors seems to be elaborated in majority of physiological EMT situations; however, their overexpression in epithelial cells has been strongly correlated with induction of EMT.24, 25, 28 In addition to downregulation of E‐cadherin expression, members of Snail families have shown to be entailed in cell motility, proliferation, differentiation and apoptosis both in vivo and in vitro.29, 30, 31
Except above‐mentioned signalling pathway controlling EMT process, other distinct pathways have also been discovered recently which are mostly depended on tumour cells microenvironment. These pathways have shown to be mostly activated during tumour growth and progression. Tumour cells growth is in debt of an increase in metabolites and oxygen demand which is supplied by tumour‐enhanced vasculature. Cells presented in a hypoxic and not well‐nourished environment activate specific pathways associated with hypermetabolism, glycolysis, resistance to acidosis‐induced toxicity and neo‐angiogenesis.32 More careful examination resulted in discovery of hypoxia‐related gene expression locally in solid tumour resulting in contribution to tumour heterogeneity.33 This process results in expression of HIF‐1 which in turn activates Snail and Twist expression, relating hypoxia with EMT induction.34, 35 The other precise characteristic of tumour microenvironment is stromal reactions through which epithelial‐mesenchymal interactions initiate or regulate diverse group of pathways including integrins, growth factors and cytokines, which are crucial for tumour expansion and metastasis.33 Finally, inflammatory cells presented in tumour environment are other important group of cells taking part in secretion of activating factors and NF‐κB has been shown to directly regulate Slug, Snail and Twist transcription factors.
3. MET, the reversal of EMT
During cancer development, activation of EMT usually results in adoption of a phenotype which is more suitable for escaping from tumour's primary site and developing metastasis in secondary site.36 Presented at metastatic site, mesenchymal phenotype is not necessary anymore. In this level, MET takes part and results in differentiation of metastatic cells and push further metastasis progression. Although essential for development in secondary metastatic site, MET in primary tumour site can play an important role, as a reversal pathway of EMT, in inhibition of migratory metastatic cells formation. Consequently, activation of this pathway in primary site can inhibit further progression of tumour and metastasis. One of the most important signalling molecules and contributors of saving cell‐cell adhesion is E‐cadherin. Downregulation of E‐cadherin expression has shown to result in evasion of cells to the neighbour sites. The second necessary component is β‐catenin, whose complexation with E‐cadherin molecules is the most important factor adhering neighbour cells together.37 Studies on invasive breast cancer has shown significant decrease in expression of E‐cadherin and phosphorylated active form of β‐catenin.38, 39 The most important β‐catenin downstream effector genes, c‐Myc and CyclinD1, have shown to be significantly increased in TNBC subtype, resulting in progression of cancer.40 Based on these findings, study on phytochemicals for reversing EMT and induction of MET is a challenging and attractive criteria. In a study performed by Strinivasan et al., it was shown that triple‐negative breast cancer cells treated with quercetin demonstrated higher levels of E‐cadherin, decreased β‐catenin's nuclear localization and decreased expression of c‐Myc and cyclin D1 compared to untreated cells.41
Dorai et al. demonstrated that curcumin either in prostate cancer cell line or in bone marrow can increase bone morphogenetic protein 7 (BMP‐7) levels and through this, alter the ratio between BMP‐7 and TGF‐β and consequently tip the MET process in prostate tumour cells. Studies on mammary tumours induced by 7, 12‐dimethylbenz(a)anthracene treatment by Belguise et al. demonstrated that treatment with EGCG can result in downregulation of c‐Rel, CK‐2 and downstream targets such as hydrocarbon receptors and SLUG decrease, reversing the EMT phase to MET.42
Several pathways have been identified to be involved in induction of EMT; however, the most studied ones include WNT and Hedgehog pathways. These pathways are mostly involved in developmental pathways and alteration in them has been shown to be common in various cancers and facilitate EMT.43 WNT signalling pathway consists of two pathways, canonical and non‐canonical pathways.44 Non‐canonical pathway is supposed to be independent from β‐catenin but the canonical pathway is β‐catenin dependent.
4. Complexity of EMT signalling pathways in tumour microenvironment
As mentioned earlier, as EMT consists of different steps, existence of different pathways taking part in modulating this process is not plausible, some of which include TGF‐β, NF‐κB, Hedgehog, Wnt/β‐catenin and Notch pathways (Fig. 2).45 TGF‐β is one of the key pathways in promotion of tumour progression and metastasis 46 which affect EMT by Smad‐dependent/‐independent transcriptional pathways. In Smad‐dependent pathway, TGF‐β results in phosphorylation of Smad 2 and Smad 3, which in next step forms complexes with Smad 4 and translocate to the nucleus where they interact with specific binding domains including Snail, Slug, ZEB and others.47 In non‐Smad pathway, TGF‐β directly activates Ras/Erk, phosphatidyl inositol‐3 (PI3) kinase, C‐Jun N‐terminal kinase (JNK) and Cdc42 GTPase, playing important role in induction of EMT.48
Figure 2.
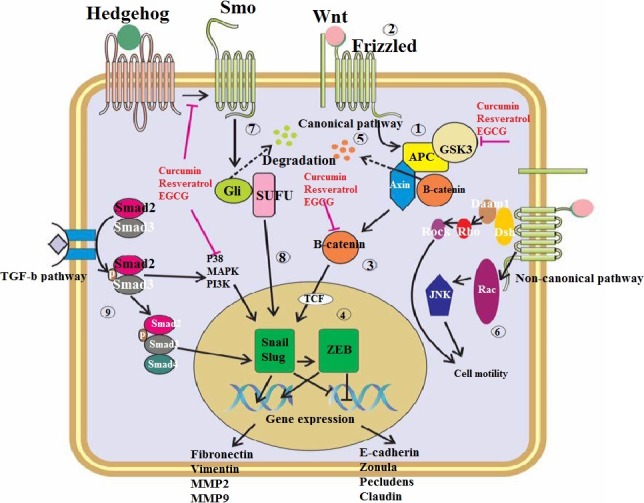
Signalling pathways involved in EMT and the naturally occurring compounds mechanism in suppressing them. (1) In normal condition, Wnt/β‐catenin pathway is kept inactive as the β‐catenin is in complex with APC/Axin complex. (2) by the time Wnt or other specific ligands bind with membrane receptors including frizzled and LRP, or by situations in which APC/Axin become unstable, cascade become activated and result in increasing of free form of β‐catenin and is not anymore phosphorylated and can easily accumulate in cytoplasm. (4) Eventually, this free β‐catenin associate with TCF in nuclease and promotes several Wnt target genes expression. (5) If cell remains in inactive state, APC helps Axin to phosphorylate β‐catenin and results in subsequent degradation of β‐catenin through ubiquitination. (6) Wnt/β‐catenin non‐canonical signalling pathway is transduced independent from β‐catenin activation. After ligation, Dsh by means of Doam 1 activates Rho kinase (ROCK). Dsh also activates Rac, resulting in subsequent activation of JNK. The integrity of these pathways results in cytoskeletal changes and induction of cell polarization and mutilations during “Gastrulation”. (7) In the absence of Hedgehog receptors ligand, Gli is complexed with several proteins including SUFU and create a large complex. In this condition, Ptch proteins result in Gli degradation and formation of repressor form of Gli (Rep‐Gli2/3). (8) By the time Hedgehog ligands interact with Ptch, Smo gets activated and Gli 2 and 3 suppressing events become inhibited. Consequently, Gli translocation in its long form results in inhibition of targeted genes. (9) Overall, after ligation of ligand to TGF‐β receptor, signalling takes part by oligomerization of serine/threonine receptor kinases and subsequent phosphorylation of Smad2 and Smad3. This results in their partnering with Smad4 and translocation to nucleus and activation of related genes transcription
Presence of inflammatory cytokines in tumour microenvironment including TNF‐α, IL‐6 and LPS are important factors in induction of NF‐κB pathway which in turn activates expression of EMT inducers including Snail and ZEB.49 NF‐κB suppresses E‐cadherin and elevates expression of Vimentin, a specific mesenchymal gene. Also NF‐κB by activating Snail, a central transcription factor during epithelial phenotype loss, results in repression of E‐cadherin.50 ZEB1 and ZEB2, both of which suppress E‐cadherin expression, are also activated by NF‐κB pathway. NF‐κB activation also results in overexpression of TGF‐β.
Since Wnt/β‐catenin and Hedgehog pathways are the pathways that regulate important adhesion molecules such as β‐catenin and E‐cadherin, these pathways can be promising targets for regulating EMT in carcinoma cell lines. It has been shown that translocation of β‐catenin to the nucleus together with E‐cadherin loss result in alteration of epithelial phenotype, and β‐catenin is also an important factor in maintaining stemness characteristics of CSCs in skin cancer.51 Also studies have shown that Wnt blockade results in promotion of epithelial differentiation.52 Recent studies have proposed that Notch pathway can also target Snail‐2 and through this, induce EMT. Consequently, inhibition of Notch pathway by pharmacological inhibitors of C‐secretases can result in repression of CD133 stem‐like cells in embryonal brain tumours.53
5. Wnt/β‐catenin signalling pathway and EMT
In the absence of Wnt ligands, a complex multiprotein encompassing kinases consisting of glycogen synthase kinase‐3β (GSK3β) and casein kinase 1 (CK1) and scaffolding proteins consisting of adenomatous polyposis coli, Axin 1 and Axin 2 controlling β‐catenin levels are in bond with β‐catenin. Mentioned complexes phosphorylate β‐catenin at N‐terminal serine and threonine residues and propose it for further ubiquitination and proteolytic degradation. However, in the presence of Wnt ligands, frizzled and low‐density lipoprotein receptor‐related proteins co‐activate and inhibit mentioned complexes, resulting in stabilization of β‐catenin. Accumulation of β‐catenin in cytoplasm results in translocation of β‐catenin to the nucleus, where it activates expression of Wnt/β‐catenin target genes. Abnormalities in Wnt pathway result in elevation of β‐catenin as well as mutations in genes involved in Wnt pathway detected in various cancer histologies.54 It has been shown that cancer cell invasion and metastasis can be directly mediated by activation of Wnt/β‐catenin signalling pathway.55
Canonical Wnt pathway has shown to facilitate EMT through several mechanisms.56 Wnt/β‐catenin has the potency to upregulate several mesenchymal markers such as SLUG, ZEB1 and TWIST which in next step suppress E‐cadherin and elevate levels of several matrix metalloproteinases (MMP) including MMP‐3, MMP‐7 and MMP‐9, remodelling extracellular matrix, enhancing invasion and inactivation of E‐cadherin by cleaving its ectodomain. At the end, it is important to mention that Wnt signalling pathway can lead to upregulation of SNAIL through inhibiting its phosphorylation by GSK3β and eventually inhibition of its degradation. Excessive data have demonstrated that WNT signalling can end in activation of EMT and in turn increase cancer metastasis.
6. Hedgehog signalling pathway and EMT
Hedgehog signalling pathway is one of the essential pathways in cell growth and patterning of different tissues through embryonic progress 57 and it has been shown that disruption of this pathway in embryonic era is together with several anomalies in central nervous system and axial skeleton. Activation pathway has not been completely understood yet; however, it has been demonstrated that this pathway sequestration usually takes place in cytoplasm with a highly synchronised network of Hedgehog proteins including Sonic Hh, Indian Hh and Desert Hh, 12‐transmembrane receptors including ptched1 and ptched2, 7‐transmembrane smoothened (Smo) and Gli family transcription factors including Gli1, Gli2 and Gli3.58 In the absence of Hedgehog ligands, Gli2 and Gli3 interact with several specific proteins including serine threonine kinase fused, kinesin‐like Costal 2 and suppressor of fused (SUFU) and construct a large complex. In addition, in the absence of Hedgehog, ptch proteins indorse degradation of Gli family transcription and generation of repressor form of Glis (Rep‐Gli2/3). Rep‐Gli2/3 translocations to nucleus results in inhibition of Gli1, Ptch and several tumour proliferation‐ and invasion‐related genes including cyclin D, Snail, Bcl2 and Myc.59 As Hedgehog ligands interact with Ptch, Smo becomes activated, and all Gli2 and Gli3 suppressing events become inhibited.60 Translocation of Gli transcription family in their full length into nucleus results in initiation of target genes expression.61
Several data resulted from studying different human tumours including breast cancer, glioblastoma, multiple myeloma and adenocarcinoma have demonstrated that Hedgehog signalling pathway activation can result in cancer stem cell (CSC) regulation.62, 63 Hedgehog signalling can act through multiple pathways within same cancer and facilitate the interactions between CSCs, tumour differentiated cells and tumour microenvironment.64 Important conclusion of this debate is that Hedgehog signalling pathway can take part as a key factor in tissues which are continuous self‐renewal by stem cells, persisting into post‐natal and adult life.
7. The crosstalk between Hedgehog and Wnt/β‐catenin signalling pathway
Mentioning the fact that both Wnt/β‐catenin and Hedgehog pathways take part in physiological and pathological development of stem cells, it seems logical to encounter crosstalk between these two overlapping pathways. Several efforts have been focused on the question that how Wnt/β‐catenin and Hedgehog signals converge into one response? Furthermore, several studies have represented that there exist fundamental similarities between Wnt/β‐catenin and Hedgehog signalling pathways.65 First similarity is that both pathways are activated by G‐protein‐coupled receptors including frizzled and smoothened receptors.66 Second similarity comes from the fact that both pathways activation results in inhibition of phosphorylation‐dependent proteolysis of the effector proteins including Cubitus interruptus and β‐catenin which convert suppression to activation of transcription.67 It has also been demonstrated that Wnt/β‐catenin pathway is downstream of Hedgehog signalling pathway in bone development.68 Furthermore, Gli1 activation results in transcription activation of ligands taking part in Wnt/β‐catenin pathway, proposing that Wnt/β‐catenin pathway is downstream of Hedgehog signalling pathway in specific conditions.69 Also it is important to mention that mediators presented in Wnt pathway regulate agents presented in Hedgehog signalling pathway too. For instance, GSK‐3β is one of these regulatory molecules, which are presented in both signalling pathways.52 Finally, it has been shown that response to oncogenic Hedgehog signalling is also reliant on canonical Wnt/β3‐catenin signalling pathway.70 Mentioned findings suggest that Wnt/β‐catenin and Hedgehog pathways are interrelated pathways in tumour cells, which can induce aggressiveness and metastasis. Consequently, common inhibitors of these pathways can inhibit cancer progression more vigorously lessening resistance and complications development resulted from chemotherapy agents.
8. Emerging of Naturally Occurring Agents for suppressing Wnt/β‐catenin and Hedgehog signalling pathways
Among naturally occurring compounds, phytochemicals, especially polyphenols, are one of the vast and most studied classes consisting of flavonoids and non‐flavonoids subgroups. These agents overcome the need for introduction of foreign compounds with several complications to individuals and from other side can result in prevention of several disorders occurrence in healthy individuals as they are generally non‐toxic, more accessible and cost‐effective compared to synthetic agents. Recent studies have also resulted in discovery of several potent agents including curcumin, resveratrol, epigallocatechin‐3‐gallate (EGCG), lycopene, retinoids, deguelin and sulforaphane as potent inhibitors of Wnt/β‐catenin and Hedgehog pathways. Analysis of these plant ingredients resulted in discovery of several unique suppressing agents of Wnt/β‐catenin and Hedgehog signalling pathways that are introduced in this section.
8.1. Flavonoids
The word “Flavonoid” comes from a Latin word meaning “yellow”. The basis for this nomenclature deals with the fact that leafs, flowers and fruits colours come from the pigments which are full of abundant amounts of flavonoids. The backbone of flavonoids consist of a C6‐C3‐C6 formula. These molecules are mostly presented in conjugated form taking part as an important agent, with several physiochemical and biochemical role in plant cells or organelles; the most differences between variety of flavonoids consists of heteroaromatic ring C pattern or the overall hydroxylation pattern.71 These agents can be presented in hydroxylated, C‐glycosylated form of direct carbon atom of skeleton or O‐glycosylated of hydroxy group. In addition, in some cases, the alkyl group of flavonoids skeleton can be covalently attached to the remaining moieties and result in formation of additional rings. Classifying flavonoids according to the position of aromatic rings attached to Benzopyrano moiety, four subgroups of flavonoids are formed including flavonols, isoflavonoids, neuflavonoids and chalcones.72 Further modification of these four groups have resulted in formation of more than 6000 different compounds belonging to this category.73
8.1.1. Apigenin and Fisetin
Apigenin was the first flavonoid reported with regulating effect on Wnt pathway through inhibiting function of casein kinase II (CK2). Studies on breast cancer has shown that 40 μmol/L from apigenin is enough for decreasing levels of β‐catenin and Dsh protein through accelerating their degradation.74 Recently, fisetin has shown to increase endogenous Wnt inhibitors such as Axin and β‐TrCp in addition to Wnt and Wnt co‐receptors downregulation.75
8.1.2. Green tea
Epidemiological investigations have demonstrated that consumption of tea is together with significant decrease in prevalence of cancer, related to the presence of polyphenolic compounds in tea.76 Catechins presented in green tea are one of the most important currently characterized flavonoids in recent years. About 30–40% dry weight of a cup of brewed green tea consists of catechins including Epigallo catechin 3‐gallate (EGCG) and Epigallocatechin (EGC). EGCG has shown to be the most abundant catechin presented in green tea. EGCG has shown to significantly suppress Wnt‐β‐catenin pathway; however, the pathway has not been completely understood yet. Studies on breast cancer has shown that EGCG can result in blockade of Wnt/β‐catenin through induction of a specific transcriptional repressor known as HBP‐1 without affecting β‐catenin levels.77
In a study performed by Singh et al., it was demonstrated that EGCG can increase β‐catenin of serine 33/37 residue through activation of GK1/GSK‐3β. This results in increased degradation of β‐catenin and subsequent decrease in nuclear accumulation of β‐catenin phosphorylation in HT29 colon cancer.78 It has been shown that two APC‐dependent pathways are involved in intracellular β‐catenin protein stability. First one is the (APC/Axin/CK1/GSK‐3β)‐dependent pathway and the other is (APC/Siah‐1)‐dependent pathway. Studies have shown that EGCG‐mediated β‐catenin degradation is independent from the later pathway.77 Oh et al. reported that mutated β‐catenin in CK‐1 phosphorylation site (Ser45) or GSK‐3β phosphorylation site (Ser37) were not downregulated in response to treatment with EGCG. Consequently, it can be concluded that phospho‐regulation in these unique moieties is essential for EGCG‐induced β‐catenin degradation. Furthermore, inhibiting GSK‐3β activity or its depletion before EGCG treatment did not affect intracellular β‐catenin protein complex stability. Converging two findings, they concluded that EGCG‐mediated degradation of β‐catenin is independent of GSK‐3β. Further studies demonstrated that EGCG can induce degradation of β‐catenin through β‐TrCp‐dependent proteasome pathway and subsequent proliferation inhibition. Among catechins, EGCG seems to be more potent in inhibiting oxidative stress and carcinogenesis. Studies have shown that EGCG can potently inhibit expression of β‐catenin and β‐catenin/TCF‐4 receptor activity in a concentration‐dependent manner, confirming EGCG to be a potent Wnt inhibitor.79 Decreasing cytosolic β‐catenin levels is also another result of treating lung cancer H460 and A549 cell lines with EGCG.80 Treating HT29 cells in colon cancer has shown to result in inhibition of GSK3‐α and GSK3‐β activities.81 Furthermore, it has been shown that EGCG by inhibiting TCF/LEF‐associated luciferase expression can inhibit the canonical Wnt signalling.82 Studies conducted by microarray gene expression profiling analysis demonstrated two pathways involved in cell proliferation including Wnt and Id both of which can be inhibited by EGCG. In breast cancer, EGCG inhibited Wnt signalling by HBP‐1 transcription repressor, a potent Wnt signalling pathway suppressor.83, 84 This has shown to be mediated by increasing mRNA stability of HBP1.85 Considering Hedgehog signalling pathway, EGCG significantly suppress mRNA expression of Gli1 and downregulate Gli receptor activity in prostate cancer. Recent studies have shown that by Indian Hedgehog pathway suppression and downregulation of Gli1 and Ptch expression, EGCG significantly inhibit progression of chondrosarcoma.86
8.1.3. Genistein
Genistein is an important isoflavone, which is found in abundance in number of plants including soybeans and tofu and Broccoli isoflavones consists of “kaempferol rhamnetin”. Genistein with chemical formula of [4′, 5, 7‐trihydroxy Isoflavonoid] is presented in food both in free and/or esterified form.87 From long ago it was discovered that usage of soy products is together with lower risk of cancer incidence, mostly due to the presence of abundant amounts of genistein structurally almost similar to 17‐β‐estradiol. This compound was discovered in plant Genista tinctoria L. and it is soluble in several polar solvents. As mentioned earlier, the most abundant source of genistein are foods with soy base.88 From the aspect of bioavailability, genistein has a desired bioavailability; however, considering toxicokinetics, no specific safety profile has yet been published for genistein. Studies have demonstrated that isoflavones can extensively suppress β‐catenin/Tcf‐driven transcription with dose‐dependent manner in AGS gastric cancer cells.89 Sarker et al. have also demonstrated that isoflavones, especially genistein, can also upregulate expression of GSK‐3β, enhance β‐catenin attachment to GSK‐3β and elevate β‐catenin phosphorylation levels and through this suppress prostate cancer growth.90, 91 Genistein can also reduce Wnt‐1‐induced proliferation and their targets including VIZ, c‐Myc and cyclin D1.91, 92, 93
8.2. Non‐flavonoids
8.2.1. Resveratrol
For many years, resveratrol was known as an anti‐inflammatory agent under the name of Polygonum cuspidatum.94 The process of resveratrol formation is unique for plant systems as the formation of hydroxy‐cinnamoic acid from phenylalanine is only possible in plants. After this step, either chalcone synthase or pinosylvin synthase by consumption of adequate number of hydroxy‐cinnamoic acid groups result in formation of resveratrol. The main role of resveratrol in plant is protection from fungal attack. Under condition of Bothrytis cinerae attack, this bioactive compound is considered as expressed in grape skin and is consequently presented in red wine. This agent belongs to the phytoalexin family and is a secondary metabolite in plants, playing role of a defensive agent in certain stress conditions including pathogens attack, induction of wounds, ultra‐violet irradiation and several studies have demonstrated numerous beneficial effects of this agent as anti‐cancer, anti‐inflammatory, anti‐oxidant and anti‐asthmatic effects.95, 96, 97, 98 This agent is also a potent suppressor of tumour metastasis,99 platelets aggregation inhibitor, mucine regulator and secretion of inflammatory mediators including IL‐6 and IL‐8.100, 101, 102 Studies have shown that treating SGC‐7901 cells with resveratrol resulted in suppression of metastasis and invasion. Further studies demonstrated that GLi‐1 expression was significantly restricted by this phytochemical. Additionally, Snail and N‐Cadherin were decreased after treatment with resveratrol too. Snail takes part as a key component in induction of EMT through binding with promoter E‐Box and repressing E‐cadherin transcription. Consequently, increase in E‐cadherin expression takes part when Snail is inhibited.103 It has been shown that low concentrations of this agent can significantly decrease β‐catenin levels in nucleus and downregulate expression of Igs and pygoI, two β‐catenin localization regulators in colon‐derived cells.104 From other side, by inhibiting Gli1 mRNA expression and Gli1 receptor downregulation, resveratrol can effectively suppress Hedgehog pathway and consequently inhibit prostate cancer cell growth.105
8.2.2. Quercetin
It has been hypothesized that this agent demonstrates its anti‐colon cancer effects through downregulation of β‐catenin/TCF pathway. Quercetin can result in downregulation of Cyclin D1 and Survivin mRNA expression plus protein expression through inhibition of β‐catenin/TFC signalling.106 In another study by Wei et al., it was reported that quercetin in a dose‐dependent manner could result in decrease of both mRNA and Gli‐1 protein expression consequently inhibiting the Hedgehog signalling pathway.107 Reported by Srivastava et al. for the first time, administration of sulforaphane (SFN), an effective anti‐cancer compound presented in cruciferous vegetables, together with quercetin resulted in synergistically inhibitory effects on self‐renewal capacity of pancreatic CSC. Administration of SFN alone has shown to be together with dysregulation of pancreatic CSCs renewal process through several mechanisms, including inhibition of Bcl2 and xIAP expression and induction of apoptosis, phosphorylation of FKFR and activation of Caspase‐3. In addition, expression of several proteins involved in Wnt/β‐catenin pathway and subsequent EMT process including β‐catenin, vimentin, ZEB1 and Twist‐1 was significantly inhibited by SFN. Consequently, they reported that SFN by inhibiting β‐catenin pathway result in synergistic effects in inhibition of pancreatic CSCs. This synergistically effects can be interpreted by two facts. First, quercetin has reported to be a significant chemosensitizing agent and an effective therapeutic molecule in resistant cancer cells and second, quercetin itself has direct inhibitory effects on Wnt/β‐catenin pathway involved proteins including Cyclin D1 and Survivin.108
8.2.3. Curcumin
Extracted from the rhizome of Curcuma longa L., curcumin is an extensive hydrophobic agent extracted from turmeric. Structurally, this polyphenol is a bis‐α, β‐unsaturated β‐diketone, known as diferuloylmethane demonstrating a keto/enol tatumerism. The efficacy of curcumin in several cancers has been well studied and reported in literature.109 Curcumin administration has shown to result in significant reduction of Ptched and Smo expression in hepatic stellate cells (HSCs). From other side, curcumin can cause elevation in levels of H/hip protein expression in cultured HSCs. Furthermore, it has been shown that nuclear translocation, DNA‐specific sequence binding and targeted genes transcription from activation of Gli was significantly restricted in cells being treated with curcumin.110 Curcumin can also modulate Bcl2 protein family and caspase cascade activation in dependence to Hh signalling pathway blockade. Curcumin can also result in G0/G1 cell cycle arrest and induce Bcl2‐mediated apoptosis again by pharmacological blockade of Hedgehog pathway in HSC cell lines.111 In a study with the purpose of determining whether osteosarcoma progression can be disrupted by inhibition of Wnt/β‐catenin pathway through curcumin consumption or not, it was demonstrated that curcumin could suppress intrinsic and activated β‐catenin/TCF transcriptional activities and its nuclear concentration was drastically decreased. Another mechanism was proposed to be inhibition of osteosarcoma invasiveness by downregulation of Wnt/β‐catenin and matrix metalloproteinases (MMP)‐9, cyclin D1, c‐Myc and survivin.112 In another study, it was shown that Axin 2 and FRA1 (FOS‐like antigen 1), two components of Wnt/β‐catenin pathway, were downregulated after treatment with curcumin.113
As another example, in medulloblastoma, an aggressive primary brain tumour, sonic Hedgehog pathway takes part in pathology of the aggressive disease and curcumin can significantly suppress Shh‐Gli1 through Shh protein downregulation and the most important downstream targets including c‐Myc and Cyclin D1 suggesting that curcumin can significantly enhance the upper non‐toxic doses of cis‐platin and γ‐Ray.114 Mentioning the fact Bcl2 is one of the downstream genes of Hedgehog pathway approve above‐mentioned results again. Studies on prostate cancer demonstrated that curcumin can suppress expression of Gli‐1 mRNA and in turn downregulate Gli receptor activity through inhibition of prostate cancer cell growth.115
8.3. Miscellaneous
Lycopene, the deep‐red compound presented in tomato and its related products, is another potent agent suppressing Wnt and Hedgehog pathways. Experimental studies have demonstrated that in addition to inflammatory signalling suppression and prevention of oxidative DNA damage, lycopene can regulate expression of IGF/AKT, AR and Wnt/β‐catenin pathways.116 In addition, lycopene by attenuation of IGF‐1 effects on phosphorylation of AKT and GSK‐3β can reduce IGF‐1‐stimulated prostate cancer growth.115 Vitamin D (Vit D) is another potent inhibitor of Wnt/β‐catenin and Hedgehog signalling pathways. Vitamin D has the potency to inhibit several signalling pathways including Notch, NF‐kB and IGF‐1 signalling pathway, putting aside Wnt and Hedgehog signalling pathways.117 Furthermore, Vit D receptor can also inhibit β‐catenin and its receptor activity suppression enhanced by wild‐type APC is another proof of Wnt signalling pathway inhibition by Vit D. Macrophage‐secreted soluble factors can initiate Wnt canonical pathway by crosstalk between STAT1 and IL‐1β.118 However, this pathway can be completely inhibited in macrophages and suppress Wnt signalling pathway in colon carcinoma cells. Vitamin D can also be a potent suppressor in human cancers possessing high activity of Hedgehog signalling pathway.119, 120
9. Barriers to the routine usage of phytochemicals in cancer therapy
Unfortunately, in spite of several newly beneficial effects achieved by administration of phytochemicals either through monotherapy or combinatorial therapy, a number of problems still remains unsolved which must be overcome before progressing any further for routine cancer therapy. Very low solubility, poor penetration and distribution in tumours site, and rapid uptake by scavenging cells are some of the most important restrictions associated with phytochemicals. Additionally, poor pharmacokinetic profile of phytochemicals including fast metabolism and low half‐life in body result in additional restriction in use of these agents. Consequently, the need for derivatization of these phytochemicals to improve their pharmacokinetic profile seems to be essential. The ideal derivate is the one possessing fair solubility, bioavailability, tumour penetration and distribution with equivalent or more effectiveness compared to the primary prototype. Emergence of nanotechnology has brought several promising results in improving pharmacokinetic of low‐solubility and low‐bioavailable drugs, and in the case of phytochemicals, can significantly overcome restrictions of pharmacokinetic. However, further studies are need for confirmation of this theory.121
10. Conclusion and future challenges
As discussed here, Wnt/β‐catenin and Hedgehog are two indispensable signalling pathways playing prominent role in tumour relapse and metastasis which may be in part due to the fact that both of these pathways are directly involved in EMT. Furthermore, downstream molecules and several resultant products of these pathways have been shown to be directly linked with EMT, tumour invasion and metastasis. Consequently, establishing new promising drugs with specific targeting abilities of their upstream and downstream signalling seems to be an attractive challenge for overcoming metastasis and prevention of cancer progression. Naturally occurring compounds due to their low‐toxic profile, cost‐effectiveness and easy accessibility appear to be a valuable source for searching new leading compounds. The future challenges associated with tumour invasion and metastasis therapy with naturally occurring agents would be mostly related to in vitro and in vitro mechanistic studies to unravel new gates for discovery of new lead compounds with more potency and less toxicity.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 2. Feitelson MA, Arzumanyan A, Kulathinal RJ, et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol 2015;35:S25–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obenauf AC, Massagué J. Surviving at a distance: organ‐specific metastasis. Trends cancer. 2015;1:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Y, Ma L. The emerging molecular machinery and therapeutic targets of metastasis. Trends Pharmacol Sci. 2015;36:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128:24–31. [DOI] [PubMed] [Google Scholar]
- 8. García‐Román J, Zentella‐Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013;335:259–269. [DOI] [PubMed] [Google Scholar]
- 9. Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol. 2010;32:125–127. [PubMed] [Google Scholar]
- 10. Pein M, Oskarsson T. Microenvironment in metastasis: roadblocks and supportive niches. Am J Physiol Cell Physiol. 2015;309:C627–C638. [DOI] [PubMed] [Google Scholar]
- 11. Aceto N, Toner M, Maheswaran S, Haber DA. En route to metastasis: circulating tumor cell clusters and epithelial‐to‐mesenchymal transition. Trends Cancer. 2015;1:44–52. [DOI] [PubMed] [Google Scholar]
- 12. Oh J, Hlatky L, Jeong YS, Kim D. Therapeutic Effectiveness of Anticancer Phytochemicals on Cancer Stem Cells. Toxins (Basel). 2016;8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Boer A, Urlings MJ, Bast A. Active ingredients leading in health claims on functional foods. J Funct Foods. 2016;20:587–593. [Google Scholar]
- 14. Maru GB, Hudlikar RR, Kumar G, Gandhi K, Mahimkar MB. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: from experimental models to clinical trials. World J Biol Chem. 2016;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mock CD, Jordan BC, Selvam C. Recent advances of curcumin and its analogues in breast cancer prevention and treatment. RSC Advances. 2015;5:75575–75588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon Y. Curcumin as a cancer chemotherapy sensitizing agent. J Korean Soc Appl Biol Chem. 2014;57:273–280. [Google Scholar]
- 17. Li Y, Niu Y, Sun Y, et al. An Apple Oligogalactan Potentiates the growth inhibitory effect of celecoxib on colorectal cancer. Nutr Cancer. 2014;66:29–37. [DOI] [PubMed] [Google Scholar]
- 18. Basnet P, Skalko‐Basnet N. Curcumin: an anti‐inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moiseeva EP, Almeida GM, Jones GD, Manson MM. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007;6:3071–3079. [DOI] [PubMed] [Google Scholar]
- 20. Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. [DOI] [PubMed] [Google Scholar]
- 21. Reagan‐Shaw S, Eggert D, Mukhtar H, Ahmad N. Antiproliferative effects of apple peel extract against cancer cells. Nutr Cancer. 2010;62:517–524. [DOI] [PubMed] [Google Scholar]
- 22. Diepenbruck M, Christofori G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. [DOI] [PubMed] [Google Scholar]
- 23. Kalluri R, Neilson EG. Epithelial‐mesenchymal transition and its implications for fibrosis. J Clin Investig. 2003;112:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Toit A. Cell signalling: when signals cross. Nat Rev Mol Cell Biol. 2015;16:204. [DOI] [PubMed] [Google Scholar]
- 25. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. [DOI] [PubMed] [Google Scholar]
- 26. Tang Y, Herr G, Johnson W, Resnik E, Aho J. Induction and analysis of epithelial to mesenchymal transition. J Vis Exp. 2013;27:e50478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derksen PW, Liu X, Saridin F, et al. Somatic inactivation of E‐cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez‐Tilló E, Liu Y, de Barrios O, et al. EMT‐activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naber HP, Drabsch Y, Snaar‐Jagalska BE, ten Dijke P, van Laar T. Snail and Slug, key regulators of TGF‐β‐induced EMT, are sufficient for the induction of single‐cell invasion. Biochem Biophys Res Commun. 2013;435:58–63. [DOI] [PubMed] [Google Scholar]
- 30. McGrail DJ, Mezencev R, Kieu QM, McDonald JF, Dawson MR. SNAIL‐induced epithelial‐to‐mesenchymal transition produces concerted biophysical changes from altered cytoskeletal gene expression. FASEB J. 2015;29:1280–1289. [DOI] [PubMed] [Google Scholar]
- 31. Unternaehrer JJ, Zhao R, Kim K, et al. The epithelial‐mesenchymal transition factor SNAIL paradoxically enhances reprogramming. Stem Cell Rep. 2014;3:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arias JI, Aller MA, Arias J. Cancer cell: using inflammation to invade the host. Mol Cancer. 2007;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Liu J. Tumor stroma as targets for cancer therapy. Pharmacol Ther. 2013;137:200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Renaud S, Guenot D, Falcoz PE, Massard G, Beau‐Faller M. Role of hypoxia in epithelial‐to‐mesenchymal transition (EMT) in non‐small cell lung cancer (NSCLC). Eur Respir J. 2014;44(Suppl 58):P814. [Google Scholar]
- 35. Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor‐1 (HIF‐1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. [DOI] [PubMed] [Google Scholar]
- 36. Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. [DOI] [PubMed] [Google Scholar]
- 37. Daugherty RL, Gottardi CJ. Phospho‐regulation of β‐catenin adhesion and signaling functions. Physiology. 2007;22:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kashiwagi S, Yashiro M, Takashima T, et al. Significance of E‐cadherin expression in triple‐negative breast cancer. Br J Cancer. 2010;103:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakopoulou L, Mylona E, Papadaki I, et al. Study of phospho‐β‐catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod Pathol. 2006;19:556–563. [DOI] [PubMed] [Google Scholar]
- 40. Li Z, Jiao X, Wang C, et al. Cyclin D1 induction of cellular migration requires p27KIP1. Cancer Res. 2006;66:9986–9994. [DOI] [PubMed] [Google Scholar]
- 41. Srinivasan A, Thangavel C, Liu Y, et al. Quercetin regulates β‐catenin signaling and reduces the migration of triple negative breast cancer. Mol Carcinog. 2016;55:743–756. [DOI] [PubMed] [Google Scholar]
- 42. Dorai T, Diouri J, O'Shea O, Doty SB. Curcumin inhibits prostate cancer bone metastasis by up‐regulating bone morphogenic protein‐7 in vivo. J Cancer Ther. 2014;5:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shan S, Lv Q, Zhao Y, et al. Wnt/β‐catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int J Clin Exp Pathol. 2015;8:12357. [PMC free article] [PubMed] [Google Scholar]
- 44. MacDonald BT, Tamai K, He X. Wnt/β‐catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duan P, Bonewald LF. The role of the wnt/β‐catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strzyz P. Cancer biology: TGF [beta] and EMT as double agents. Nat Rev Mol Cell Biol. 2016;17:202–203. [DOI] [PubMed] [Google Scholar]
- 47. Macias MJ, Martin‐Malpartida P, Massagué J. Structural determinants of Smad function in TGF‐β signaling. Trends Biochem Sci. 2015;40:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hasan M, Neumann B, Haupeltshofer S, et al. Activation of TGF‐β‐induced non‐Smad signaling pathways during Th17 differentiation. Immunol Cell Biol. 2015;93:662–672. [DOI] [PubMed] [Google Scholar]
- 49. Panahi Y, Darvishi B, Ghanei M, Jowzi N, Beiraghdar F, Varnamkhasti BS. Molecular mechanisms of curcumins suppressing effects on tumorigenesis, angiogenesis and metastasis, focusing on NF‐κB pathway. Cytokine Growth Factor Rev. 2016;28:21–29. [DOI] [PubMed] [Google Scholar]
- 50. Zhang K, Zhaos J, Liu X, et al. Activation of NF‐B upregulates Snail and consequent repression of E‐cadherin in cholangiocarcinoma cell invasion. Hepatogastroenterology. 2010;58:1–7. [PubMed] [Google Scholar]
- 51. Ingvarsson S, Chen H, Kristjánsdóttir S, Jónasson JG, Magnússon JB, Egilsson V. Alterations of E‐cadherin and β‐catenin in gastric cancer. BMC Cancer. 2001;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kazi MM, Trivedi TI, Kobawala TP, Ghosh NR. The Potential of Wnt Signaling Pathway in Cancer: A Focus on Breast Cancer. Cancer Transl Med. 2016;2:55. [Google Scholar]
- 55. Spranger S, Gajewski TF. A new paradigm for tumor immune escape: β‐catenin‐driven immune exclusion. J Immunother Cancer. 2015;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marcucci F, Stassi G, De Maria R. Epithelial‐mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discovery. 2016;15:311–325. [DOI] [PubMed] [Google Scholar]
- 57. Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget. 2015;6:13899–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. di Magliano MP, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. [DOI] [PubMed] [Google Scholar]
- 60. Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yao E, Chuang PT. Hedgehog signaling: from basic research to clinical applications. J Formos Med Assoc. 2015;114:569–576. [DOI] [PubMed] [Google Scholar]
- 62. Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin Cancer Res. 2015;21:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Merchant AA, Matsui W. Targeting Hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma J, Cheng J, Gong Y, Tian L, Huang Q. Downregulation of Wnt signaling by sonic hedgehog activation promotes repopulation of human tumor cell lines. Dis Model Mech. 2015;8:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sokol SY. Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Semin Cell Dev Biol. 2015;42:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. [DOI] [PubMed] [Google Scholar]
- 68. Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg. 2008;90(Supplement 1):19–24. [DOI] [PubMed] [Google Scholar]
- 69. Cohen MM. The hedgehog signaling network. Am J Med Genet A. 2003;123:5–28. [DOI] [PubMed] [Google Scholar]
- 70. Yang SH, Andl T, Grachtchouk V, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β‐catenin signaling. Nat Genet. 2008;40:1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG. Flavonoids: potential Wnt/beta‐catenin signaling modulators in cancer. Life Sci. 2011;89:545–554. [DOI] [PubMed] [Google Scholar]
- 72. Vezza T, Rodríguez‐Nogales A, Algieri F, Utrilla MP, Rodriguez‐Cabezas ME, Galvez J. Flavonoids in inflammatory bowel disease: a review. Nutrients. 2016;8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31:206–238. [DOI] [PubMed] [Google Scholar]
- 74. Liu X, Li L, Lv L, et al. Apigenin inhibits the proliferation and invasion of osteosarcoma cells by suppressing the Wnt/β‐catenin signaling pathway. Oncol Rep 2015;34:1035–1041. [DOI] [PubMed] [Google Scholar]
- 75. Lall RK, Adhami VM, Mukhtar H. Dietary flavonoid fisetin for cancerprevention and treatment. Mol Nutr Food Res. 2016;60:1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lai C‐S, Pan M‐H. Mechanism for possible chemopreventive effects of natural dietary compounds on smoking‐induced tumorigenesis. J Exp Clin Med. 2011;3:262–271. [Google Scholar]
- 77. Oh S, Gwak J, Park S, et al. Green tea polyphenol EGCG suppresses Wnt/β‐catenin signaling by promoting GSK‐3β‐and PP2A‐independent β‐catenin phosphorylation/degradation. BioFactors. 2014;40:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gao Z, Xu Z, Hung MS, et al. Promoter demethylation of WIF‐1 by epigallocatechin‐3‐gallate in lung cancer cells. Anticancer Res. 2009;29:2025–2030. [PubMed] [Google Scholar]
- 79. Dashwood WM, Orner GA, Dashwood RH. Inhibition of β‐catenin/Tcf activity by white tea, green tea, and epigallocatechin‐3‐gallate (EGCG): minor contribution of H 2 O 2 at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. [DOI] [PubMed] [Google Scholar]
- 80. Gao Z, Xu Z, Hung MS, et al. Promoter demethylation of WIF‐1 by epigallocatechin‐3‐gallate in lung cancer cells. Anticancer Res. 2009;29:2025–2030. [PubMed] [Google Scholar]
- 81. Pahlke G, Ngiewih Y, Kern M, et al. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54:7075–7082. [DOI] [PubMed] [Google Scholar]
- 82. Mount JG, Muzylak M, Allen S, et al. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235:1390–1399. [DOI] [PubMed] [Google Scholar]
- 83. Bose M, Hao X, Ju J, et al. Inhibition of tumorigenesis in Apc Min/+ mice by a combination of (–)‐epigallocatechin‐3‐gallate and fish oil. J Agric Food Chem. 2007;55:7695–7700. [DOI] [PubMed] [Google Scholar]
- 84. Liu L, Lai CQ, Nie L, et al. The modulation of endothelial cell gene expression by green tea polyphenol‐EGCG. Mol Nutr Food Res. 2008;52:1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim J, Zhang X, Rieger‐Christ KM, et al. Suppression of Wnt signaling by the green tea compound (–)‐epigallocatechin 3‐gallate (EGCG) in invasive breast cancer cells requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–10875. [DOI] [PubMed] [Google Scholar]
- 86. Tang GQ, Yan TQ, Guo W, et al. (−)‐Epigallocatechin‐3‐gallate induces apoptosis and suppresses proliferation by inhibiting the human Indian Hedgehog pathway in human chondrosarcoma cells. J Cancer Res Clin Oncol. 2010;136:1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Inbaraj BS, Chen BH. Isoflavones in foods and ingestion in the diet In: Victor R. Preedy, ed. Isoflavones: Chemistry, Analysis, Function and Effects. RCS publication, UK: 2012:28. [Google Scholar]
- 88. Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, Release 3.1. Beltsville: US Department of Agriculture; 2011. [Google Scholar]
- 89. Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park CH, Hahm ER, Lee JH, et al. Inhibition of β‐catenin‐mediated transactivation by flavanone in AGS gastric cancer cells. Biochem Biophys Res Commun. 2005;331:1222–1228. [DOI] [PubMed] [Google Scholar]
- 91. Li Y, Wang Z, Kong D, et al. Regulation of Akt/FOXO3a/GSK‐3β/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283:27707–27716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sarkar FH, Li Y, Wang Z, et al. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E‐cadherin expression and attenuates β‐catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–339. [DOI] [PubMed] [Google Scholar]
- 94. Su Y, Simmen FA, Xiao R, et al. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16. [DOI] [PubMed] [Google Scholar]
- 95. Qadri SM, Föller M, Lang F. Inhibition of suicidal erythrocyte death by resveratrol. Life Sci. 2009;85:33–38. [DOI] [PubMed] [Google Scholar]
- 96. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discovery. 2006;5:493–506. [DOI] [PubMed] [Google Scholar]
- 97. Bisht K, Wagner K‐H, Bulmer AC. Curcumin, resveratrol and flavonoids as anti‐inflammatory, cyto‐and DNA‐protective dietary compounds. Toxicology. 2010;278:88–100. [DOI] [PubMed] [Google Scholar]
- 98. Michels G, et al. Resveratrol induces apoptotic cell death in rat H4IIE hepatoma cells but necrosis in C6 glioma cells. Toxicology. 2006;225:173–182. [DOI] [PubMed] [Google Scholar]
- 99. Busquets S, Ametller E, Fuster G, et al. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007;245:144–148. [DOI] [PubMed] [Google Scholar]
- 100. Olas B, Wachowicz B, Tomczak A, et al. Comparative anti‐platelet and antioxidant properties of polyphenol‐rich extracts from: berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets. 2008;19:70–77. [DOI] [PubMed] [Google Scholar]
- 101. Knobloch J, Hag H, Jungck D, et al. Resveratrol impairs the release of steroid‐resistant cytokines from bacterial endotoxin‐exposed alveolar macrophages in chronic obstructive pulmonary disease. Basic Clin Pharmacol Toxicol. 2011;109:138–143. [DOI] [PubMed] [Google Scholar]
- 102. Lee SY, Lee HJ, Sikder MA, et al. Resveratrol inhibits mucin gene expression, production and secretion from airway epithelial cells. Phytother Res. 2012;26:1082–1087. [DOI] [PubMed] [Google Scholar]
- 103. Thiery JP, Acloque H, Huang RY, et al. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139:871–890. [DOI] [PubMed] [Google Scholar]
- 104. Hope C, Planutis K, Planutiene M, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon‐derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52(Suppl. 1):S52–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Slusarz A, Shenouda NS, Sakla MS, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. [DOI] [PubMed] [Google Scholar]
- 106. Shan B‐E, Wang M‐X, Li R‐Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β‐catenin signaling pathway. Cancer Invest. 2009;27:604–612. [DOI] [PubMed] [Google Scholar]
- 107. Li W, Zhao Y, Tao B, et al. Effects of quercetin on hedgehog signaling in chronic myeloid leukemia KBM7 cells. Chin J Integr Med. 2014;20:776–781. [DOI] [PubMed] [Google Scholar]
- 108. Srivastava RK, Tang SN, Zhu W, et al. Sulforaphane synergizes with quercetin to inhibit self‐renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an “old‐age” disease with an “age‐old” solution. Cancer Lett. 2008;267:133–164. [DOI] [PubMed] [Google Scholar]
- 110. Xu J, Fu Y, Chen A. Activation of peroxisome proliferator‐activated receptor‐γ contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–G30. [DOI] [PubMed] [Google Scholar]
- 111. Lian N, Jiang Y, Zhang F, et al. Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells. Lab Invest. 2015;95:790–803. [DOI] [PubMed] [Google Scholar]
- 112. Leow PC, Tian Q, Ong ZY, et al. Antitumor activity of natural compounds, curcumin and PKF118‐310, as Wnt/β‐catenin antagonists against human osteosarcoma cells. Invest New Drugs. 2010;28:766–782. [DOI] [PubMed] [Google Scholar]
- 113. Shin HW, Park SY, Lee KB, et al. Down‐regulation of Wnt signaling during apoptosis of human hepatic stellate cells. Hepatogastroenterology. 2008;56:208–212. [PubMed] [Google Scholar]
- 114. Elamin MH, Shinwari Z, Hendrayani SF, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49:302–314. [DOI] [PubMed] [Google Scholar]
- 115. Banerjee M, Tripathi LM, Srivastava VM, et al. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003;25:213–224. [DOI] [PubMed] [Google Scholar]
- 116. Wertz K. Lycopene effects contributing to prostate health. Nutr Cancer. 2009;61:775–783. [DOI] [PubMed] [Google Scholar]
- 117. Kovalenko PL, Zhang Z, Cui M, et al. 1, 25 dihydroxyvitamin D‐mediated orchestration of anticancer, transcript‐level effects in the immortalized, non‐transformed prostate epithelial cell line, RWPE1. BMC Genom. 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kaler P, Augenlicht L, Klampfer L. Macrophage‐derived IL‐1β stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bijlsma MF, Peppelenbosch MP, Arnold Spek C. (Pro‐) vitamin D as treatment option for hedgehog‐related malignancies. Med Hypotheses. 2008;70:202–203. [DOI] [PubMed] [Google Scholar]
- 120. Tang JY, So P‐L, Epstein EH. Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xie J, Yang Z, Zhou C, et al. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol Adv. 2016;34:343–353. [DOI] [PubMed] [Google Scholar]


