Abstract
Objective
The mechanism of Schisandrin B on the proliferation and migration of airway smooth muscle cells (ASMCs) in asthmatic rats was explored.
Methods
SD rats were divided into three groups: control (group 1), model (group 2) and model + Schisandrin B (group 3). miR‐150 and lncRNA BCYRN1 levels were measured by qRT‐PCR. The combination of BCYRN1 and miR‐150 was detected by RNA pull down. ASMCs’ viability/proliferation/migration were examined by WST‐1 assay and 24‐well Transwell system.
Results
Schisandrin B up‐regulated miR‐150 expression and down‐regulated BCYRN1 expression in sensitized rats. Schisandrin B reversed the expression of miR‐150 and BCYRN1 in MV‐treated ASMCs. In addition, Schisandrin B inhibited the viability, proliferation and migration of MV‐induced ASMCs. We also found miR‐150 inhibited BCYRN1 expression which was proved by experiments using ASMCs transfected with miR‐150 inhibitor.
Conclusion
Schisandrin B increased miR‐150 expression and decreased BCYRN1, and BCYRN1 expression was inhibited by miR‐150, which indicated that Schisandrin B could regulate BCYRN1 through miR‐150.
1. INTRODUCTION
Asthma is a chronic airway disorder, which is characterized by airflow obstruction, inflammation and airway hyperreactivity.1 It affects 5% of adults and 10% of children with continues rising prevalence.2 There are many factors that can cause asthma, including exercise, infection, allergens, occupational exposures and smoking,3 and the symptoms mainly include shortness of breath, wheezing, coughing and tightness of the chest.4 Airway smooth muscle (ASM) has hyperplastic and hypertrophic characteristics, and it may be an important contributor to airflow obstruction, which leads to increased thickening and narrowing of airways.5 ASM cells (ASMCs) is also associated with the inflammatory response in the airways through secreting proteases, cytokines and growth factors that can induce ASM phenotypic modulation.5
In addition to these symptoms, ASM in asthma is infiltrated by mast cells (MCs).6 MCs can release a lot of pleiotropic autacoid mediators, proteases and cytokines that interact with smooth muscle cells and induce proliferation and contraction that can limit airway narrowing reversibility,7 so it plays a significant role in the pathophysiology of asthma.8 Researchers also thought the infiltration of ASM by MCs is one of the essential determinants of asthmatic phenotype.9, 10, 11
Schisandrin B is one of the most commonly used lignans in Chinese folk medicine, which is extracted from Schisandra chinensis Baill fruit.12 It can be used for anti‐hepatitis, anti‐asthma, anti‐ageing, antioxidant and treating myocardial disorders.13 In 2015, Lv et al proved that Schisandrin B inhibited the proliferation of human lung adenocarcinoma A549 cells.15 In 2016, Zhang et al14 reported Schisandrin B could inhibit the proliferation of ASMCs. Later, Jiang et al16 found that Schisandrin B inhibited the proliferation and invasion of glioma cells.
Researchers found that multiple pathways of asthma were regulated by abnormal miRNAs, such as miRNA‐155, miRNA‐26a, miR‐133a, miR‐148a and miR‐126.17, 18 Zhou et al19 found that a number of abnormal miRNAs were found in CD4+T cells in asthmatic mice, and miR‐150 was one of the down‐regulated expression miRNA. However, the role of miR‐150 in asthma is not fully understood. Few studies demonstrated the importance of both long non‐coding RNAs (lncRNAs) and miRNAs in ASM function.20 In 2013, Perry et al21 conducted a transcriptomic approach to measure the expression of miRNAs, lncRNAs and mRNAs in primary ASM cells. In 2016, Zhang et al22 proved that lncRNAs BCYRN1 promoted the proliferation and migration of ASMCs in asthma through up‐regulating TRP1 expression.
Here we demonstrate that Schisandrin B inhibits the proliferation and migration of ASMCs in asthmatic rats via mast cells, and further explain the reason for the changes of BCYRN1 expression in ASMCs of asthmatic rats.
2. MATERIALS AND METHODS
2.1. Animals and groups
All the experiments were performed at the department of Respiratory Medicine, People's Hospital Affiliated to Zhengzhou University. All experimental procedures were complied with NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Ethics Committee of People's Hospital Affiliated to Zhengzhou University. Sixty female SD rats were purchased from Shanghai Institute of Biochemistry and Cell Biology of Chinese Academy of Science, and randomly divided into control group (n = 20), model group (n = 20) and model + Schisandrin B group (n = 20). Rats were given Schisandrin B dissolved in dimethyl sulfoxide (DMSO, Sigma‐Aldrich, St. Louis, MO) by intragastric administration. The ovalbumin (OVA) model was established as previously reported.14 Rats were treated with OVA (10 mg, Thermo Scientific, Waltham, MA) subcutaneously, and aluminium hydroxide (200 mg) that dissolved in NaCl solution (0.9%, 1 mL) and inactivated Bordetella pertussis (1 mL) at the first and eighth day. In control group, rats were treated with an equal volume of saline. In group treated with Schisandrin B, rats were treated with 80 mg/kg/day of Schisandrin B (AMQUAR, Shanghai) by intragastric administration. In the next 6 weeks, rats were excited by ultrasonic atomizing inhalation containing 2% OVA for 30 minute, and this procedure repeated 3 times/week. Finally, the rats were sacrificed within 18‐24 hour in each group.
2.2. Cell culture and stimulation
Mast cells and ASMCs were isolated from SD rats in accordance with the protocol approved by the Ethics Committee of People's Hospital Affiliated to Zhengzhou University, and cultured as previously described.23, 24 At confluence, ASMC cultures exhibited a typical “hill‐and‐valley” appearance. ASMC suspensions were seeded at a density of 1‐5 × 105 cells/mL, and ASMCs were harvested for study when >95% of the cells displayed the characteristics of smooth muscle cells. MCs were harvested for the study when the concentration was >95%. Cells at passages 6‐10 were used for all experiments.
Airway smooth muscle cells were pre‐treated with dexamethasone (10−7 mol/L) for 1 hour, then stimulated with 2.5% foetal bovine serum (FBS, Gibco) for 24 hour. MCs were stimulated by IgE (2.5 μg/mL) and goat anti‐human IgE (1 μg/mL) (Calbiochem, CA) as previously described.25
2.3. Measurements of cell viability, proliferation and migration
The viability and proliferation of ASMCs were detected by WST‐1 assay and Roche real‐time cell analyser (RTCA) DP assay. ASMCs were seeded into 96‐well plates with DMEM containing 10% FBS. ASMCs were arrested by removing serum for 24 hour, stimulated with PDGF‐BB (25 ng/mL, Gibco) for 24 hour, added WST‐1 directly to the culture wells and incubated for 120 minutes at 37°C. Plates were read by a scanning multi‐well spectrophotometer with a wavelength of 450 nm and a reference wavelength of 630 nm. Percentage of viability was obtained by comparing OD values to that of ASMCs in a control group. The ASMCs proliferation variation was obtained using the Roche RTCA DP assay by calculating the changes of ASMCs to baseline. Each experiment was repeated at least three times.
The migration of ASMCs was performed using a 24‐well Transwell system (Costar, Badhoevedorp, The Netherlands). It allowed cells to migrate through 8 μm pore sized polycarbonate membrane. ASMCs suspensions (5×104 cells) were added to the upper chamber of the Transwells. The lower chamber was filled with 600 μL DMEM containing 1% FBS with IgE/anti‐IgE, or IgE/anti‐IgE+Schisandrin B. After 48 hours, the upper compartment was removed and the culture medium in the lower compartment was further investigated. ASMCs were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet for 10 minutes. Analysis was performed on three wells for each condition and each experiment was repeated at least three times.
2.4. RNA pull‐down assays
Biotin‐labelled BCYRN1 was transcribed with the biotin RNA Labeling mix (Roche) and T7 or SP6 RNA polymerase, and purified with RNeasy Mini Kit (QIAGEN). Then protein (1 mg) from ASMCs extracts was mixed with biotinylated RNA biotin‐labelled RNAs (50 pmol), and incubated with streptavidin agarose beads (Invitrogen). The standard Western blotting technique was used for measuring proteins binding to the streptavidin‐coupled dynabeads.
2.5. Western blot
Protein concentrations were determined by BCA Protein Assay kit (Pierce Biotechnology). Western blot was performed according to standard procedures. The Ago 2 antibody against BCYRN1 was purchased from Abcam (Cambridge, United Kingdom) and used as the first primary antibody. The corresponding horseradish peroxidase‐conjugated secondary antibody (Invitrogen) was incubated for 1 hour. Protein‐antibody complexes were detected by the enhanced chemiluminescence system (ECL, Roche Molecular Biochemicals). β‐actin (Invitrogen) was served as a control protein to quantify the expression of related proteins.
2.6. Quantitative real‐time PCR
RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed using the SuperScript First Strand Synthesis System for RT‐PCR (Invitrogen). An ABI Prism 5700 Sequence Detection System (Applied Biosystems) was used for reverse‐transcription of total RNA and the subsequent real‐time PCR. Total fluid volume (25 μL) and TaqMan RT‐PCR Master Mix Reagents (Applied Biosystems) were used for amplification. β‐actin gene was used as an internal control to normalize the difference in the amount of total RNA in each sample. miR‐150 and BCYRN1 expressions were measured by two‐step cycle protocol. It consisted of a denaturing phase at 95°C for 15 seconds, 30 seconds at 60°C for primer annealing, and an extension temperature of 72°C for 30 seconds for 40 cycles. Results were analysed using ABI Prism 5700 SDS software (Applied Biosystems). The comparative method 2‐ΔΔCt was used to calculate the relative expressions of miR‐150 and BCYRN1 gene.26
2.7. Transfection with miR‐150 inhibitor
Cells were transfected with miR‐150 inhibitor or the negative control at 70%‐80% confluence using Lipofectamine 2000 (Sigma) according to the manufacturer's instructions. Stable clones were acquired at 2 weeks after antibiotic selection. The miR‐150 inhibitor and negative control were designed and synthesized by Invitrogen.
3. RESULTS
3.1. Schisandrin B reverses the expressions of miR‐150 and BCYRN1 levels in MCs and ASMCs
To confirm the molecular mechanism of Schisandrin B on the inhibition of asthma, miR‐150 and BCYRN1 expressions in MCs and ASMCs were measured in control, model and model + Schisandrin B groups. Compared with control group, miR‐150 expressions in model, and model + Schisandrin B groups were down‐regulated in both MCs and ASMCs; moreover, Schisandrin B reversed the expression of miR‐150 (Figure 1A). There were significant differences in three different groups in both MCs and ASMCs (P < .01). BCYRN1 expression was up‐regulated in ASMCs, and the expression in model group was lower than model + Schisandrin B group, which indicated that Schisandrin B reversed the expression of BCYRN1 (Figure 1B). There were significant differences in three different groups in ASMCs (P < .01). These findings suggested that Schisandrin B up‐regulated miR‐150 expression and down‐regulated BCYRN1 expression in asthma model rats.
Figure 1.
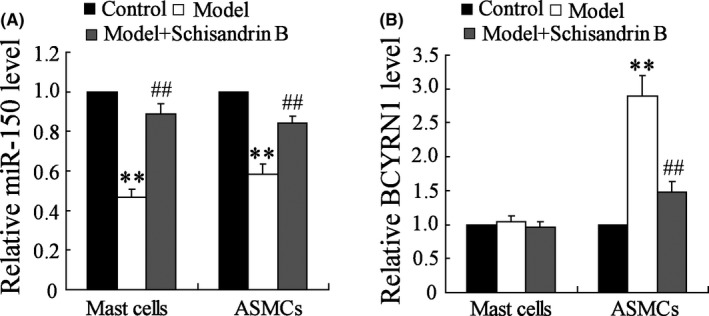
Expressions of miR‐150 and BCYRN1 levels in MCs and ASMCs in different subject groups: healthy control (N = 20), model (N = 20) and model + Schisandrin B (N = 20). Expressions of miR‐150 and BCYRN1 levels are shown in A and B. The dose of Schisandrin B was 80 mg/kg/d, intragastric administration. A, Compared with control group, expressions of miR‐150 levels in model and model + Schisandrin B groups were down‐regulated in both MCs and ASMCs, and the expression of miR‐150 levels in model + Schisandrin B group was higher than model group. Schisandrin B reversed this effect. B, Expressions of BCYRN1 levels were up‐regulated in ASMCs, and the expression in model + Schisandrin B group was lower than model group. Schisandrin B reversed this effect. ** P < .01 compared to control group; ## P < .01 compared to model group
3.2. Schisandrin B reverses the expression of miR‐150 in bronchial MCs
The expression of miR‐150 in MCs was down‐regulated after treated with IgE/anti‐IgE. After treated with Schisandrin B, the expression of miR‐150 was up‐regulated than MCs without treatment (Figure 2). In addition, miR‐150 level was up‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. Hence, Schisandrin B could increase miR‐150 expression in MCs.
Figure 2.
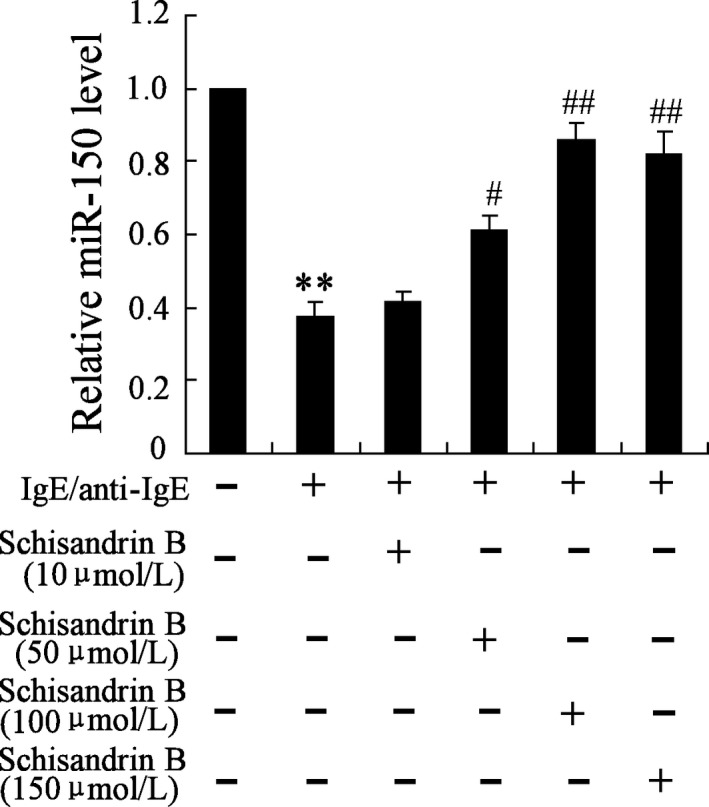
Schisandrin B reverses the expressions of miR‐150 in bronchial MCs. Bronchial MCs were stimulated by IgE/anti‐IgE (IgE 2.5 μg/mL, goat anti‐human IgE 1 μg/mL), with or without Schisandrin B (100 μmol/L, dissolved in dimethyl sulfoxide, DMSO). IgE/anti‐IgE down‐regulated the expression of miR‐150, and Schisandrin B reversed the effect. miR‐150 level was up‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. ** P < .01 compared to control group; ## P < .01 compared to IgE/anti‐IgE (+)+Schisandrin B (−) group
3.3. Schisandrin B reverses the expressions of miR‐150 and BCYRN1 level in MV‐induced ASMCs
According to Figure 3A, the expression of miR‐150 in ASMCs was down‐regulated after induced by MV, and Schisandrin B reversed this effect. And miR‐150 level was up‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. The expression of BCYRN1 in ASMCs was up‐regulated, and Schisandrin B reversed this effect (Figure 3B). And BCYRN1 level was down‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. Hence, MVs, which were isolated from IgE/anti‐IgE‐treated MCs, could down‐regulate the expression of miR‐150 and up‐regulated the expression of BCYRN1, while Schisandrin B reversed these effects, which further proved that Schisandrin B up‐regualted miR‐150 expression and down‐regulated BCYRN1 expression in ASMCs.
Figure 3.
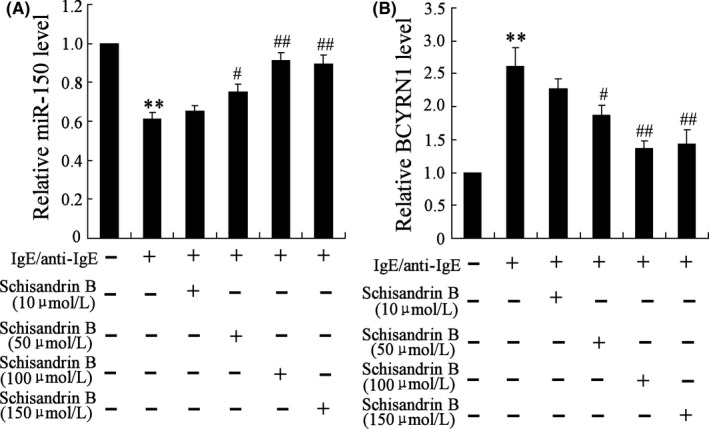
Schisandrin B reverses the expressions of miR‐150 and BCYRN1 in MV‐induced ASMCs. A, The expressions of miR‐150 were down‐regulated in ASMCs stimulated by MV isolated from IgE/anti‐IgE‐treated MCs, and Schisandrin B reversed this effect. miR‐150 level was up‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. B, The expressions of BCYRN1 were up‐regulated in ASMCs, and Schisandrin B reversed this effect. BCYRN1 level was down‐regulated with the increase of Schisandrin B concentration before the concentration reached 100 μmol/L. ** P < .01 compared to control group; ## P < .01 compared to IgE/anti‐IgE (+)+Schisandrin B (−) group
3.4. Schisandrin B inhibits the viability, proliferation and migration of MV‐induced ASMCs
Next, we examined the effect of MVs on the viability, proliferation and migration of ASMCs. Viability, proliferation and migration of ASMCs were increased, and Schisandrin B reversed this effect (Figure 4A‐D). There were significant differences in ASMCs treated with or without Schisandrin B (P < .01). These data indicated that Schisandrin B played critical roles in inhibiting viability, proliferation and migration of ASMCs.
Figure 4.
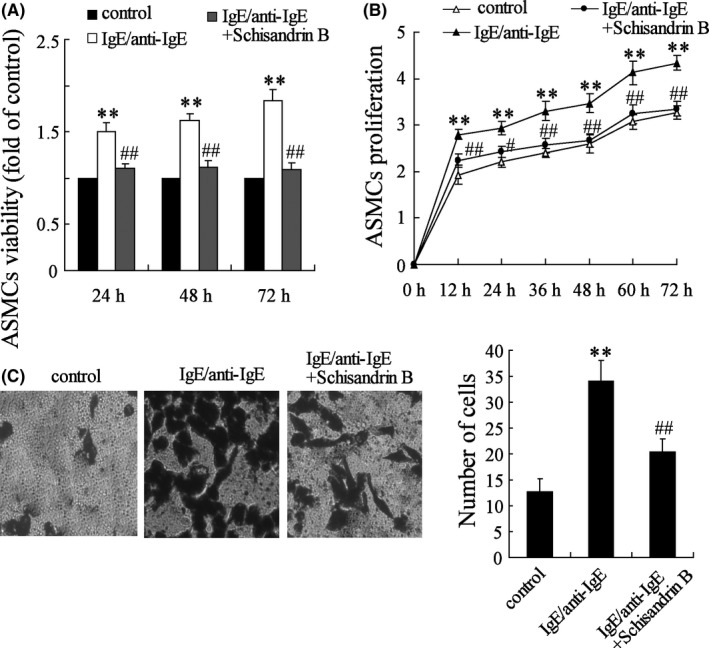
Schisandrin B inhibits the viability, proliferation and migration of MV‐induced ASMCs. MCs were stimulated by IgE/anti‐IgE, and MV was isolated from MCs to treat ASMCs. The dose of Schisandrin B was 100 μmol/L. A, Viability of ASMCs was increased, and Schisandrin B reversed this effect. B, Proliferation of ASMCs was increased, and Schisandrin B reversed this effect. C, The number of ASMCs was increased, and Schisandrin B reversed this effect. ** P < .01 compared to control group; ## P < .01 compared to IgE/anti‐IgE groups
3.5. Effect of miR‐150 on BCYRN1 expression on MV‐induced ASMCs
According to bioinformatics software, 11 direct combination sites were found between miR‐150 and BCYRN1 (Figure 5A). We performed an RNA pull‐down assay to identify the binding between Ago 2 and BCYRN1. The result showed that protein Ago 2 was specifically associated with BCYRN1 (Figure 5B). miR‐150 inhibitor increased the expression of BCYRN1 level (Figure 5C), and there were significant difference between vectors transfected with or without miR‐150 inhibitor (P < .01), which indicated that miR‐150 negatively regulated BCYRN1 expression.
Figure 5.
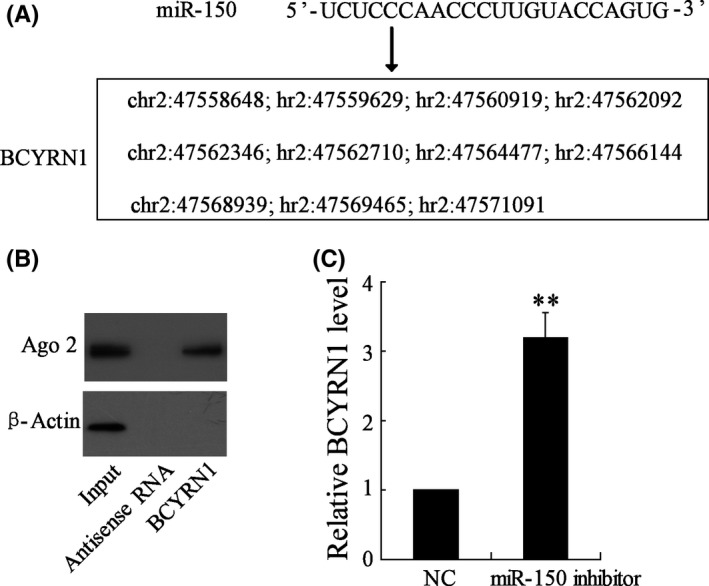
Effect of miR‐150 on BCYRN1 expression on MV‐induced ASMCs. A, Eleven direct combination sites were found between miR‐150 and BCYRN1. MEG3‐modified biotin was incubated with cell lysis buffer. B, The effect of anti‐sense RNA and BCYRN1 on the protein level of Ago 2 in ASMCs. C, miR‐150 inhibitor increased the expression of BCYRN1, ** P < .01 compared to NC group
3.6. Effect of Schisandrin B and miR‐150 inhibitor on MV‐induced ASMCs
Schisandrin B up‐regulated the expression of miR‐150 on MV‐induced ASMCs, and miR‐150 inhibitor reversed this effect (Figure 6A), while Schisandrin B down‐regulated the expression of BCYRN1 on MV‐induced ASMCs, and miR‐150 inhibitor reversed this effect (Figure 6B), indicating BCYRN1 expression was inhibited by miR‐150, and Schisandrin B could regulate BCYRN1 through miR‐150. In addition, Schisandrin B inhibited ASMCs viability, proliferation and migration, while miR‐150 inhibitor reversed this effect (Figure 6C‐E), suggesting Schisandrin B could regulate the viability, proliferation and migration of ASMCs via miR‐150.
Figure 6.
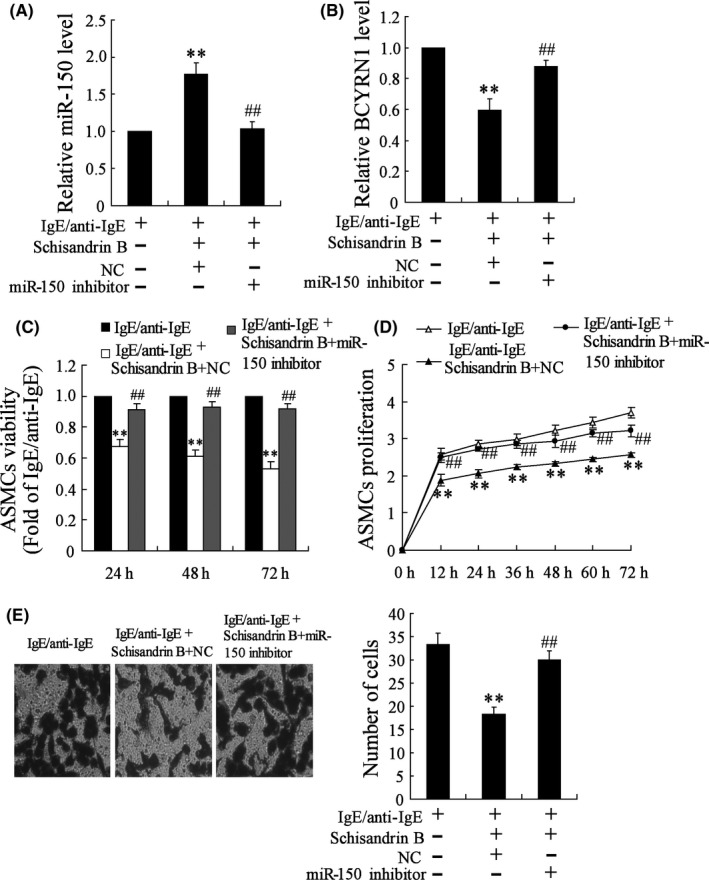
Effect of Schisandrin B and miR‐150 inhibitor on MV‐induced ASMCs. Bronchial MC was treated with IgE/anti‐IgE, with or without Schisandrin B, and MV were separated to stimulate ASMCs. ASMCs were transfected with or without miR‐150 inhibitor or empty vector. The dose of Schisandrin B was 100 μmol/L. A, Schisandrin B up‐regulated the expression of miR‐150, and miR‐150 inhibitor reversed this effect. B, Schisandrin B down‐regulated the expression of BCYRN1, and miR‐150 inhibitor reversed this effect. C, Viability of ASMCs was decreased after stimulated by IgE/anti‐IgE+Schisandrin B+NC, and miR‐150 inhibitor reversed this effect. D, Proliferation of ASMCs was decreased after stimulated by IgE/anti‐IgE+Schisandrin B+NC, and miR‐150 inhibitor reversed this effect. E, The number of ASMCs was decreased after stimulated by IgE/anti‐IgE+Schisandrin B+NC, and miR‐150 inhibitor reversed this effect.** P < .01 compared to control; ## P < .01 compared to IgE/anti‐IgE+Schisandrin B+NC
4. DISCUSSION
Airway smooth muscle, as a producer of pro‐ and anti‐inflammatory mediators, can affect apoptosis, proliferation and migration of ASMCs.27 Abnormal ASMCs proliferation and migration in the airway cause increasing thickness of airway wall, participating in the development of airway remodelling of asthma.19 Hence, it is necessary to study medicals that can effectively inhibit ASMC proliferation and migration in asthma research. In our previous study, we demonstrated that Schisandrin B attenuated the inspiratory and expiratory resistances in sensitized rats, and could inhibit ASMC proliferation and migration.14 To clarify the molecular mechanism of Schisandrin B inhibiting ASMC proliferation and migration, we detected the expression of miR‐150 and BCYRN1 in MCs and ASMCs in asthmatic rats treated with or without Schisandrin B. We found the expression of miR‐150 was decreased in ASMCs, while the expression of BCYRN1 was increased in ASMCs, and Schisandrin B reversed these effects. These data indicated that Schisandrin B could regulate BCYRN1 through miR‐150, and inhibit ASMC proliferation and migration.
There are a number of reports about miR‐150. It can play up‐regulation or down‐regulation role in different diseases. Cao et al proved that miR‐150 promoted the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1.28 Zhou et al found that miR‐150 promoted renal fibrosis by increasing profibrotic molecules through down‐regulation of SOCS1.29 Honda et al reported that down‐regulation of miR‐150 contributed to the constitutive Type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3.30 In this study, miR‐150 expression changed in asthma model rats compared with normal rats, which suggested that miR‐150 might also play an important role in asthma. To further clarify this mechanism, MCs were stimulated with IgE/anti‐IgE, and MVs were isolated from these MCs to treat ASMCs. We found the expression of miR‐150 was decreased in ASMCs, and Schisandrin B reversed the effect. The data were consistent with that from animal experiment we performed. It also suggested the inhibition effect of Schisandrin B on ASMC proliferation and migration was positively related with MVs secreted by MCs.
Previously study indicated that BCYRN1 promoted the proliferation and migration of ASMCs in asthma rats.22 As Schisandrin B could inhibit proliferation and migration of ASMCs, here, we hypothesized that Schisandrin B could inhibit BCYRN1 expression. But why Schisandrin B could inhibit BCYRN1 expression? In 2014, Perry21 showed that many miRNAs could mediate ASM cell functions, and lnc BCYRN1 acted as miRNA ‘sponges’ for miR‐150. Hence, we assumed that Schisandrin B inhibited BCYRN1 expression via miR‐150. To verify this hypothesis and explore the specific mechanism, we performed a series of experiment to figure out the relationship between miR‐150 and BCYRN1. Finally, we proved that miR‐150 negatively regulated the expression of BCYRN1. This is the first study of regulatory mechanism of lnc BCYRN1 and miR‐150 in asthmatic rats. At the same time, we reveal that Schisandrin B down‐regulates lncRNA BCYRN1 expression of ASMCs to inhibit the proliferation and migration of ASMC in asthmatic rats.
In summary, these findings suggest that Schisandrin B up‐regulated miR‐150 expression and down‐regulated BCYRN1, and BCYRN1 expression was inhibited by miR‐150, which indicated that Schisandrin B could regulate BCYRN1 through miR‐150. Although further investigations were needed for clinical applications, this study has provided basis for the important role of BCYRN1 in abnormal ASMCs proliferation and migration in asthma.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest.
Zhang X‐Y, Tang X‐Y, Ma L‐J, et al. Schisandrin B down‐regulated lncRNA BCYRN1 expression of airway smooth muscle cells by improving miR‐150 expression to inhibit the proliferation and migration of ASMC in asthmatic rats. Cell Prolif. 2017;50:e12382 10.1111/cpr.12382
Funding informationThis work was supported by a grant from the National Natural Science Foundation of China(No. U1304801) and Scientific and Technological Foundation of Henan Province of China(142102310423).
Contributor Information
Zhuo‐chang Chen, Email: 18603710696@163.com.
Luo‐xian Zhang, Email: 642732963@qq.com.
REFERENCES
- 1. Saunders R, Sutcliffe A, Woodman L, et al. The airway smooth muscle CCR3/CCL11 axis is inhibited by mast cells. Allergy. 2008;63:1148‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926‐938. [DOI] [PubMed] [Google Scholar]
- 3. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001‐2010. Nchs Data Brief. 2012;94:1‐8. [PubMed] [Google Scholar]
- 4. Radhakrishna N, Tay TR, Hore‐Lacy F, Hoy R, Dabscheck E, Hew M. Profile of difficult to treat asthma patients referred for systematic assessment. Respiratory Med. 2016;117:166‐173. [DOI] [PubMed] [Google Scholar]
- 5. Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by MicroRNA‐221 in severe asthma. Am J Respiratory Cell Mol Biol. 2014;50:7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandt A. Mast cells promote airway smooth muscle cell differentiation via autocrine up‐regulation of TGF‐β1. J Immunol. 2008;181:5001‐5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast‐cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2009;346:1699‐1705. [DOI] [PubMed] [Google Scholar]
- 8. Bradding P. Mast cell regulation of airway smooth muscle function in asthma. Eur Respiratory J. 2007;29:827‐830. [DOI] [PubMed] [Google Scholar]
- 9. Amin K, Janson C, Boman G, Venge P. The extracellular deposition of mast cell products is increased in hypertrophic airways smooth muscles in allergic asthma but not in nonallergic asthma. Allergy. 2005;60:1241‐1247. [DOI] [PubMed] [Google Scholar]
- 10. El‐Shazly A, Berger P, Girodet PO, et al. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J Immunol. 2006;176:1860‐1868. [DOI] [PubMed] [Google Scholar]
- 11. Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunondelara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu GT. Pharmacological actions and clinical use of fructus schizandrae. Chin Med J. 1989;102:740‐749. [PubMed] [Google Scholar]
- 13. Lee MY, Seo CS, Lee NH, et al. Anti‐asthmatic effect of schizandrin on OVA‐induced airway inflammation in a murine asthma model. Int Immunopharmacol. 2010;10:1374‐1379. [DOI] [PubMed] [Google Scholar]
- 14. Zhang XY, Zhang LX, Guo YL, Zhao LM, Tang XY, Tian CJ, et al. Schisandrin B inhibits the proliferation of airway smooth muscle cells via microRNA‐135a suppressing the expression of transient receptor potential channel 1. Cell Biol Int. 2016;40(7):742‐749. [DOI] [PubMed] [Google Scholar]
- 15. Lv XJ, Zhao LJ, Hao YQ, et al. Schisandrin B inhibits the proliferation of human lung adenocarcinoma A549 cells by inducing cycle arrest and apoptosis. Int J Clin Exp Med. 2015;8:6926‐6936. [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang Y, Zhang Q, Bao J, et al. Schisandrin B inhibits the proliferation and invasion of glioma cells by regulating the HOTAIR‐micoRNA‐125a‐mTOR pathway. NeuroReport. 2017;28:93‐100. [DOI] [PubMed] [Google Scholar]
- 17. Hassan T, Mckiernan PJ, Mcelvaney NG, Cryan SA, Greene CM. Therapeutic modulation of miRNA for the treatment of proinflammatory lung diseases. Expert Rev Anti‐Infect Ther. 2014;10:359‐368. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA‐155 for Normal Immune Function. Science. 2007;316:608‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR‐150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104:7080‐7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clifford RL, Singer CA, John AE. Epigenetics and miRNA emerge as key regulators of smooth muscle cell phenotype and function. Pulm Pharmacol Ther. 2013;26:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perry MM, Tsitsiou E, Austin PJ, et al. Role of non‐coding RNAs in maintaining primary airway smooth muscle cells. Respiratory Res. 2014;15:63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang XY, Zhang LX, Tian CJ, et al. LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am J Transl Res. 2016;8:3409‐3418. [PMC free article] [PubMed] [Google Scholar]
- 23. Chang PJ, Bhavsar PK, Michaeloudes C, Khorasani N, Chung KF. Corticosteroid insensitivity of chemokine expression in airway smooth muscle of patients with severe asthma. J Allergy Clin Immunol. 2012;130:877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brightling CE, Kaur DP, Morgan AJ, Wardlaw AJ, Bradding P. Differential expression of CCR3 and CXCR3 by human lung and bone marrow‐derived mast cells: implications for tissue mast cell migration. J Leukoc Biol. 2005;77:759‐766. [DOI] [PubMed] [Google Scholar]
- 25. Alkhouri H, Cha V, Tong K, Moir LM, Armour CL, Hughes JM. Human lung mast cell products regulate airway smooth muscle CXCL10 levels. J Allergy. 2014;2014:875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bustin SA. Absolute quantification of mRNA using real‐time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169‐193. [DOI] [PubMed] [Google Scholar]
- 27. Koopmans T, Anaparti V, Castro‐Piedras I, et al. Ca2+ handling and sensitivity in airway smooth muscle: emerging concepts for mechanistic understanding and therapeutic targeting. Pulm Pharmacol Ther. 2014;29:108‐120. [DOI] [PubMed] [Google Scholar]
- 28. Cao M, Hou D, Liang H, et al. miR‐150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur J Cancer. 2014;50:1013‐1024. [DOI] [PubMed] [Google Scholar]
- 29. Zhou H, Hasni SA, Perez P, et al. miR‐150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J Am Soc Nephrol. 2013;24:1073‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Honda N, Jinnin M, Kira‐Etoh T, Makino K, Kajihara I, Makino T, et al. miR‐150 down‐regulation contributes to the constitutive type i collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am J Pathol. 2013;182:206‐216. [DOI] [PubMed] [Google Scholar]


