Abstract
Objectives
Non–small‐cell lung cancer (NSCLC) is one of the leading causes of cancer deaths worldwide. Increasing levels of visfatin are correlated with worse clinical prognosis of NSCLC. However, the effects of visfatin on drug resistant are still not well illustrated.
Materials and methods
Effects of visfatin on drug resistant cells were checked by CCK‐8 kit. Gene and protein variations were measured by real‐time PCR and western blot analysis, respectively.
Results
Our present data confirmed that expression of visfatin was significantly increased in NSCLC cells and tissues. In addition, protein and mRNA expression of visfatin were significantly elevated in doxorubicin (Dox) resistance of NSCLC cells when compared with their corresponding sensitivity parental cells. Overexpression of visfatin can down‐regulate the Dox sensitivity of NSCLC cells and up‐regulate the mRNA and protein expression of ABCC1, while has no effect on ABCB1. Knockdown of visfatin can down‐regulate the expression of ABCC1 in Dox‐resistant NSCLC cells. Visfatin can increase the phosphorylation and nuclear localization of Akt in NSCLC cells. LY294002 can decrease the expression of multidrug resistance protein‐1 (MRP1) in NSCLC Dox‐resistant cells. Chromatin immunoprecipitation assays showed that overexpression of visfatin can significantly increase the binding of Akt with the promoter of ABCC1 in both A549 and H1793 cells.
Conclusions
These data showed that visfatin can decrease Dox sensitivity of NSCLC cells via activation of Akt/MRP1. It indicated that inhibition of visfatin signals might be a promising therapeutic strategy for the management of chemoresistance of NSCLC patients.
1. INTRODUCTION
Non–small‐cell lung cancer (NSCLC), which accounts for about 70%‐80% of lung cancers, is one of the major causes of cancer‐related deaths worldwide.1 Chemotherapy has become the important therapy approach for NSCLC patients, especially for patients who have aggressive tumours by the time of diagnosis.2 However, the multidrug resistance (MDR) seriously hampers the treatment efficiency of chemotherapy. Doxorubicin (Dox), which belongs to the anthracycline family of anti‐cancer drugs, has been widely used for various cancers including NSCLC.3 It can enter the nucleus and then interrupt the binding and function of topoisomerase II to inhibit the NDA synthesis and cell growth.4 It had been clinically used to treat aggressive and metastatic cancers including lung cancer. However, the drug resistance of NSCLC cells limited the clinical use of Dox for clinical treatment.5 Therefore, the investigation of this limitation is important for clinical application of Dox on NSCLC treatments.
Various cytokines have been reported to regulate the progression of NSCLC cells.6 Among identified cytokines, recent study revealed visfatin is critical for the growth and metastasis of NSCLC cells.7, 8 It was reported that visfatin can trigger the migration and invasion of NSCLC cells via up‐regulation of matrix metalloproteinases (MMPs).7 While targeted inhibition of visfatin can induce apoptotic cell death of NSCLC cells.8 Okumura et al.9 found that visfatin is a potent therapeutic target for the treatment of epidermal growth factor receptor (EGFR)‐gene‐mutated NSCLC. Furthermore, numerous studies revealed that visfatin can trigger the progression of several cancers including colon, stomach, pancreas, liver, prostate, and breast cancers.10 Visfatin is also involved in lymphocyte development and cellular resistance to genotoxic stress.11 However, there are very limited data concerning the effects of visfatin on chemoresistance. One recent study revealed that inhibition of visfatin can sensitize glioblastoma cells to temozolomide via activation of ROS/JNK signals.12 Considering that visfatin can significantly promote the development of NSCLC, we, therefore, hypothesized that visfatin might also be involved in the Dox resistance of NSCLC cells.
It was reported that overexpression of ATP‐binding cassette (ABC) transporters is the major cause for chemoresistance.13 Among the 49 ABC transporters encountered in human, ABCB1/MDR1/P‐gp and ABCC1/MRP1 are reported to be the mostly implicated in the MDR of NSCLC and other cancers.14 Considering that understanding the mechanisms of therapy resistance is important for improving prognosis, the roles of visfatin in Dox resistant of NSCLC cells were investigated. Its effects on ABC transporters and the related mechanisms were further illustrated.
2. MATERIALS AND METHODS
2.1. Cell line and cell culture
Human NSCLC A549, H1793, H1395, and H1299 cells purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle medium or RPMI 1640 at 37°C in a humidified atmosphere (90%) containing 5% CO2. BEAS‐2B cells derived from normal bronchial epithelial human cells were cultured in bronchial epithelial cell basal medium (BEBM, LONZA, Wokingham, UK) supplemented with 0.1% (v/v) human recombinant epidermal growth factor and 0.1% (v/v) insulin. Cells at passage 10‐20 were used in this study. The Dox‐resistant A549/Dox and H1793/Dox cells were selected from A549 and H1793 cells, respectively, over 6 months by selecting cells stepwise increasing concentrations of Dox (0.01‐1 μmol/L). Then, both A549/Dox and H1793/Dox cells were exposed to 1 μmol/L Dox to maintain their drug resistance. The addition of Dox was omitted 1 week before experiment.
2.2. Human tissue sample collection
All experiments were proved by the ethical committee of our hospital. A group of 10 NSCLC breast cancer patients with histologically confirmed in our hospital participated during 2014‐2016. The NSCLC tissues and adjacent normal tissues were collected by experienced pathologists. All samples were immediately frozen by liquid nitrogen and kept at −80°C until use. The mRNA levels of visfatin were analysed by real‐time qPCR.
2.3. siRNAs transfection
Two siRNAs for visfatin were synthesized by RiboBio (Guangzhou, China) and transfected cells buy use of lipofectamine 2000 according to the instructions of manufactures. After transfection, cells were exposed to increasing concentrations of Dox to evaluate the sensitivity.
2.4. Cell proliferation assay
The cell proliferation assay was performed by sue of cell count kit‐8 (CCK‐8; Dojindo Molecular Technologies, Kumamoto, Japan) according to the instructions of manufacturer. Cell number was correlated to optical density measured by microplate reader (Bio‐Rad, Hercules, CA, USA) at 450 nm. The experiments were conducted in triplicates.
2.5. Real‐time qPCR
The total RNA was extracted by use of Trizol according to the manufacturer's instructions. Then, 1 μg total RNA was used to generate cDNA by use of the Transcriptor First Strand cDNA synthesis kit (Takara, Takara Bio Inc., Otsu, Shiga, Japan). The mRNA expression of targeted genes was measured by using SYBR Green PCR Master Mix kit in triplicate in ABI StepOne Plus Real‐Time PCR System (Applied Biosystems Life Technologies, Foster City, CA, USA). The primers were as follows: visfatin forward, 5′‐TCA CCC TGT GGA AAG TGC GAA GAT‐3′ and reverse, 5′‐TTC CCT GCT GGG GTC CTA TGT AAA‐3′; ABCC1 forward, 5′‐CCG TGT ACT CCA ACG CTG ACA T‐3′ and reverse, 5′‐ATG CTG TGC GTG ACC AAG ATC C‐3′; ABCB1 forward, 5′‐GCT GTC AAG GAA GCC AAT GCC T‐3′ and reverse, 5′‐TGC AAT GGC GAT CCT CTG CTT C‐3′; Glyceraldehyde‐3‐Phosphate Dehydrogenase (GAPDH) forward, 5′‐GTC TCC TCT GAC TTC AAC AGC G‐3′ and reverse, 5′‐ACC ACC CTG TTG CTG TAG CCA A‐3′. All data were normalized to GAPDH and expressed as relative levels.
2.6. Western blot assay
After treatment, cells were washed three times with phosphate‐buffered saline (PBS) and then lysed with lysis buffer (30 mmol/L HEPES, 1% Triton X‐100, 10% glycerol, 5 mmol/L MgCl2, 25 mmol/L NaF, 1 mmol/L EDTA, and 10 mmol/L NaCl). Protein was centrifuged (14 000 g) at 4°C for 15 minutes, and then the concentrations were measured by a Varioskan multimode microplate spectrophotometer (Thermo, Waltham, MA, USA). Proteins (20 μg) were separated by 10% sodium dodecyl sulphate‐polyacrylamide gels and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA) 100 V for 30 minutes at room temperature. The membranes were blocked by 5% milk in PBST, incubated with primary antibody for overnight at 4°C followed by goat anti‐rabbit/mouse labelled with horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All blots were visualized by use of a chemiluminescence detection system using ECL detection kit (Engreen Biosystem, Beijing, China). GAPDH was used as the loading internal control.
2.7. Subcellular fractionation
The cytosolic and nuclear fractions of cells were isolated to investigate the localization of STAT3 after visfatin treatment. The nuclear and cytosolic fractions were isolated by use of Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA) according to the manufacture's instructions. Histone protein H2A and GAPDH were used as the marker of nucleus and cytoplasm, respectively.
2.8. Immunofluorescence staining
Cells were cultured on glass slides in 12‐well plates and treated with or without visfatin for the indicated times. After fixed with 4% paraformaldehyde for 20 minutes, washed with PBS three times, and permeabilized in 0.1% Triton X‐100, cells were incubated with primary antibody overnight at 4°C. After washed for three times with PBS, cells were further incubated with secondary antibody FITC‐conjugated goat anti‐mouse IgG (1:500, Chemicon, Inc., Temecula, CA, USA) and 4′,6‐diamidino‐2‐phenylindole (for nuclear staining) for 2 hours at room temperature. After three times wash with PBS, cells were visualized under a Leica system DM 4000B microscope (Leica Microsystems, Wetzlar, Germany).
2.9. ChIP
Cells were washed with PBS for three times, cross‐linked and lysed by use of ChIP‐IT Express Kit (Active Motif). Then, the chromatin was sonicated to 200‐600 bp fragments and incubated with Akt antibody or IgG control (M‐7023; Sigma‐Aldrich, St. Louis, MO, USA) as control overnight at 4°C. After immunoprecipitated by use of protein A beads (Millipore), DNA was purified and amplified using real‐time PCR with the primers of promoters of ABCC1: 5′‐TGC AAG TGA TTA GCC AGG TG‐3′ (forward) and 5′‐CCA GGG GAA AAT AAC GTG AA‐3′(reverse).
2.10. Statistical analysis
All data were expressed as the means and standard deviation and analysed by SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Student's t test was used to identify significant differences. P<.05 was considered as statistically significant difference.
3. RESULTS
3.1. Visfatin is increased in NSCLC cells and tissues
We evaluated the expression of visfatin in NSCLC cells and compared with BEAS‐2B cells. The results showed that mRNA levels of visfatin in A549, H1793 and H1395 cells were more than 2‐fold higher than that in BEAS‐2B cells (Figure 1A). This is also confirmed by the western blot analysis that higher protein levels of visfatin were also observed in A549, H1793 and H1395 cells (Figure 1B). We further compared the mRNA expression of visfatin in 10 paired NSCLC tissues and their corresponding adjacent normal tissues. The results showed that mRNA levels of visfatin are significantly increased in 80% (8/10) NSCLC tissues (Figure 1C). These data suggested that visfatin is increased in both NSCLC cells and tissues.
Figure 1.
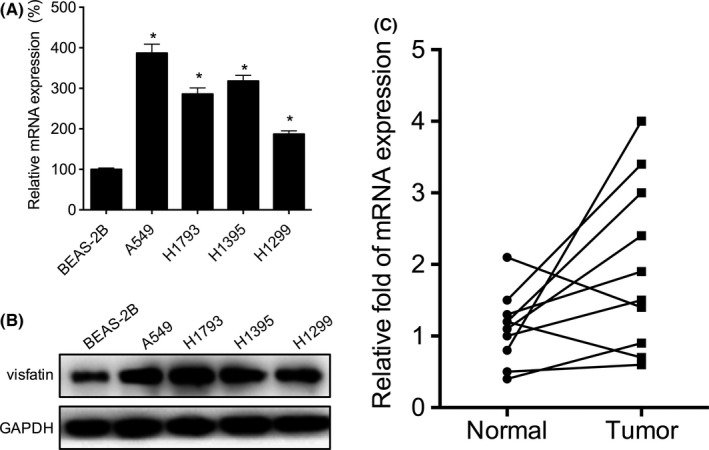
Visfatin is up‐regulated in non–small‐cell lung cancer (NSCLC) cells and tissues. The mRNA (A) or protein (B) levels of visfatin in NSCLC and BEAS‐2B cells were measured by qRT‐PCR and western blot analysis, respectively. (C) The relative mRNA levels of visfatin in 10 NSCLC tissues and paired adjacent normal tissues. *P<.05 compared with BEAS‐2B
3.2. Visfatin is increased in NSCLC Dox‐resistant cells
The Dox resistance of A549/Dox and H1793/Dox cells were evaluated and compared with their corresponding parental cells. The results showed that both A549/Dox (Figure 2A) and H1793/Dox (Figure 2B) cells were much more resistant than A549 and H1793 cells, respectively. We then tested the expression of visfatin in both Dox‐resistant and sensitive NSCLC cells. The results of qRT‐PCR showed that mRNA levels of visfatin in A549/Dox and H1793/Dox were significantly (P<.05) greater than that in their corresponding Dox‐sensitive cells (Figure 2C). This is confirmed by the results of western blot analysis that protein of visfatin in A549/Dox and H1793/Dox were higher than that in A549 and H1793 cells, respectively (Figure 2D). Collectively, our data showed that visfatin level is increased in NSCLC Dox‐resistant cells.
Figure 2.
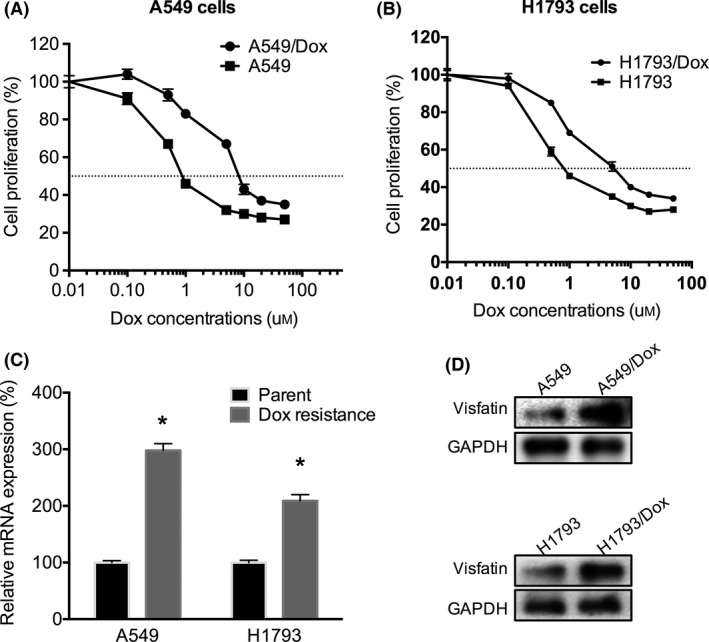
Visfatin is up‐regulated in doxorubicin (Dox) resistant of non–small‐cell lung cancer (NSCLC) cells. The cell proliferation of A549/Dox (A) or H1793/Dox (B) cells and their corresponding sensitive cells exposed to increasing concentrations of Dox were measured by cell count kit‐8 (CCK‐8) kit. The mRNA (C) or protein (D) levels of visfatin in A549/Dox or H1793/Dox cells and their corresponding sensitive cells were measured by qRT‐PCR and western blot analysis, respectively. *P<.05 compared with the control
3.3. Visfatin is involved in Dox resistance of NSCLC cells
To test whether visfatin is involved in Dox resistance of NSCLC cells, we used siRNA‐targeted visfatin to knockdown its expression. Since si‐visfatin‐1 was more efficient than that of si‐visfatin‐2 (Figure 3A), therefore si‐visfatin‐1 was chosen for further studies. The results showed that si‐visfatin can significantly increase the Dox sensitivity of both A549/Dox (Figure 3B) and H1793/Dox (Figure 3C) cells. In addition, we overexpressed visfatin in both A549 and H1793 cells (Figure 3D). The results showed that overexpression of visfatin in both A549 (Figure 3E) and H1793 (Figure 3F) can significantly decrease the Dox sensitivity. These data showed that visfatin is involved in Dox resistance of NSCLC cells.
Figure 3.
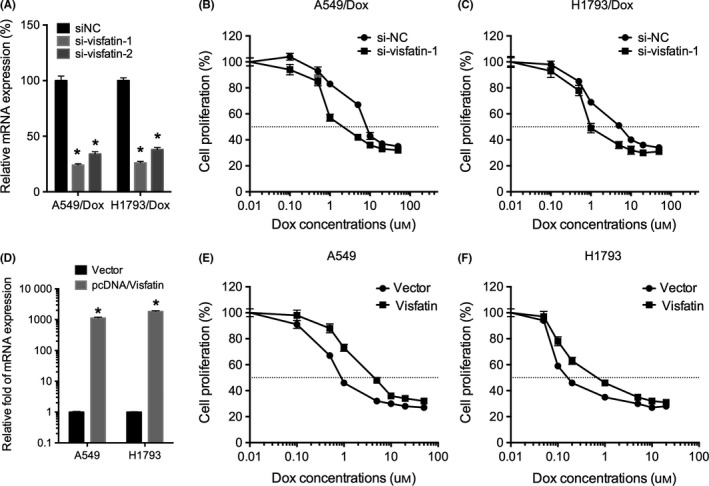
Visfatin is involved in doxorubicin (Dox) resistance of non–small‐cell lung cancer (NSCLC) cells. (A) A549/Dox and H1793/Dox cells were transfected with siRNA negative control (siNC) or si‐visfatin‐1/‐2 for 24 hours, the expression of visfatin was measured by qRT‐PCR; A549/Dox (B) and H1793/Dox (C) cells were transfected with si‐visfatin‐1 for 24 hours and then exposed to increasing concentrations of Dox for 24 hours; (D) A549 or H1793 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the mRNA expression of visfatin was measured by qRT‐PCR; A549 (E) and H1793 (F) cells were transfected with pcDNA or pcDNA/visfatin for 24 hours and then exposed to increasing concentrations of Dox for 24 hours. *P<.05 compared with the control
3.4. The up‐regulation of ABCC1 is involved in visfatin‐induced NSCLC Dox resistance
ABCB1/MDR1/P‐gp and ABCC1/MRP1 are reported to be the mostly implicated in the MDR of NSCLC and other cancers.14 We, therefore, evaluated the expression of ABCB1 and ABCC1 in NSCLC cells treated with visfatin. The results showed overexpression of visfatin can increase the protein levels of MRP1, while not P‐gp, in both A549 and H1793 cells (Figure 4A). This was confirmed by the results that overexpression of visfatin can also increase the mRNA levels of ABCC1, while not ABCB1, in both A549 and H1793 cells (Figure 4B). In addition, the knockdown of visfatin can down‐regulate the protein expression of MRP1 in both A549/Dox and H1793/Dox cells (Figure 4C). These data suggested that ABCC1 is involved in visfatin‐induced Dox resistance of NSCLC cells.
Figure 4.
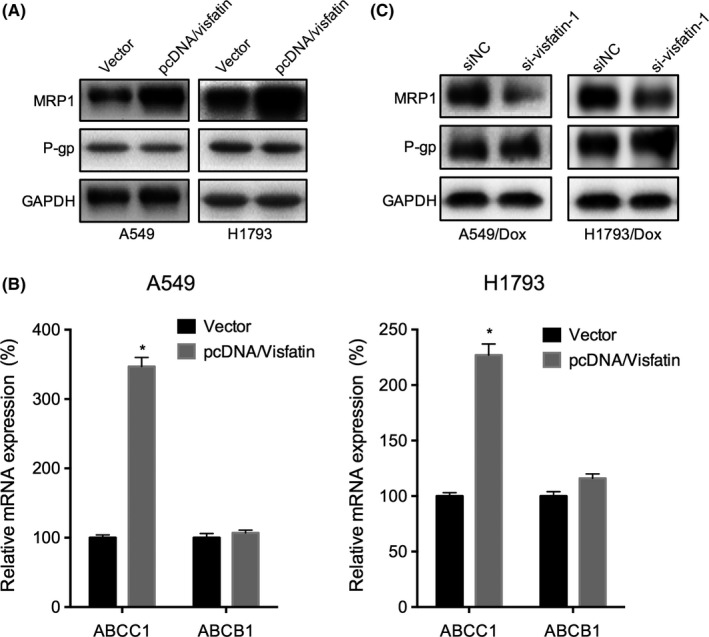
The up‐regulation of ABCC1 is involved in visfatin‐induced non–small‐cell lung cancer (NSCLC) doxorubicin (Dox) resistance. (A) Cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the expression of MRP1 and P‐gp was measured by western blot analysis; (B) A549 or H1793 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the mRNA expression of ABCC1 and ABCB1 was measured by qRT‐PCR; (C) A549/Dox and H1793/Dox cells were transfected with siRNA negative control (siNC) or si‐visfatin‐1 for 24 hours, the mRNA expression of ABCC1 and ABCB1 was measured by qRT‐PCR. *P<.05 compared with the control
3.5. Activation of Akt mediates visfatin‐induced up‐regulation of MRP1
Various signal molecules such as ERK1/2, PI3K/Akt and NF‐κB (p65) can regulate the expression of ABCC1.15, 16 We, therefore, tested that the effects of visfatin on the phosphorylation of ERK1/2, Akt and p65 in A549 cells. The result showed that visfatin can significantly increase the phosphorylation of ERK1/2 and Akt, while not p65, in A549 cells (Figure 5A). While only PI3K/Akt inhibitor (LY294002, LY), while not ERK1/2 inhibitor (PD98059, PD), abolished visfatin‐induced overexpression of MRP1 in A549 and H1793 cells (Figure 5B). Furthermore, LY, while not PD, can decrease the expression of MRP1 in both A549/Dox and H1793/Dox (Figure 5C) cells. In addition, the phosphorylation of Akt was also increased in A549/Dox and H1793/Dox cells as compared with their corresponding parent cells (Figure 5D). Furthermore, the treatment of LY can reverse visfatin induced decreased sensitivity of Dox to A549 cells (Figure 5E). Collectively, our data showed that activation of Akt is involved in visfatin‐induced up‐regulation of ABCC1 and down‐regulation of Dox sensitivity.
Figure 5.
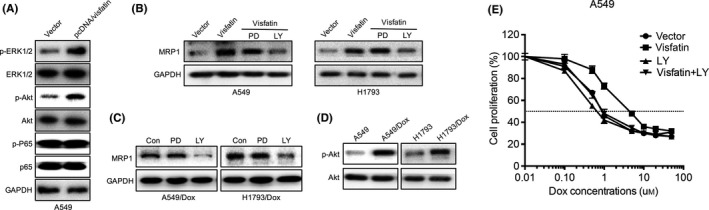
Activation of Akt is involved in visfatin induced up‐regulation of MRP1. (A) A549 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the phosphorylation and total expression of proteins were measured by western blot analysis. (B) A549 or H1793 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours and then further treated with PI3K/Akt inhibitor (LY294002, LY, 10 μmol/L) or ERK1/2 inhibitor (PD98059, PD, 10 μmol/L) for another 24 hours. (C) A549/Dox and H1793/Dox were treated with LY (10 μmol/L) or PD (10 μmol/L) for 24 hours. (D) The phosphorylation of Akt was measured in A549/Dox and H1793/Dox cells and compared with their corresponding parent cells. (E) A549 cells transfected with pcDNA or pcDNA/visfatin for 24 hours were further treated with PI3K/Akt inhibitor (LY294002, LY, 10 μmol/L) and increasing concentrations of doxorubicin (Dox) for 24 hours
3.6. Visfatin increases the nuclear translocation of Akt and its binding with ABCC1
We, therefore, investigated the effects of visfatin on the activation and cellular localization of Akt. The results showed that overexpression of visfatin can obviously increase the nucleus translocation of Akt in A549 cells after transfection for 24 hours (Figure 6A). This is confirmed by the results of immunofluorescence staining (Figure 6B). Furthermore, the ChIP assay showed that overexpression of visfatin can significantly increase the binding of Akt with the promoter of ABCC1 in both A549 and H1793 cells (Figure 6C). These data confirmed that visfatin can increase the activity and nuclear translocation of Akt and its transcriptional activity on ABCC1.
Figure 6.
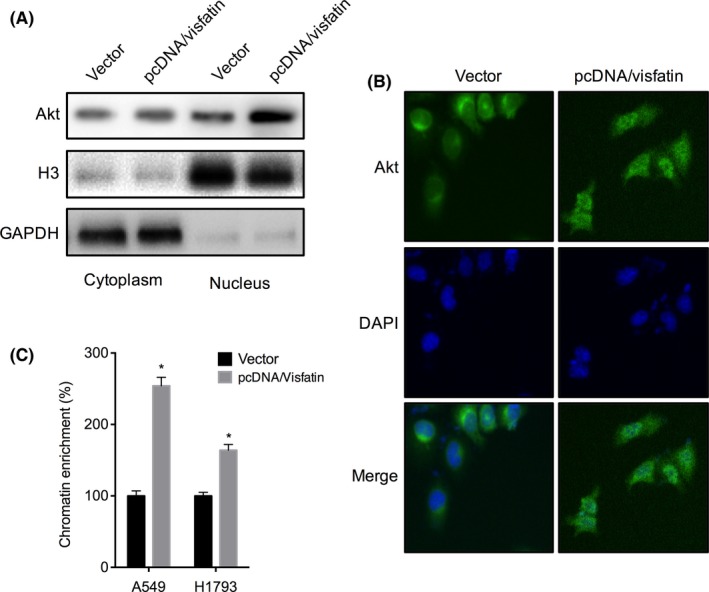
Visfatin increases the nuclear translocation of Akt and its binding with ABCC1. (A) A549 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the nucleus and cytoplasm levels of Akt were measured by western blot analysis. (B) A549 cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the cellular Akt was detected by immunofluorescence staining. (C) A549 or H1793cells were transfected with pcDNA3.1 (vector) or pcDNA/Visfatin for 24 hours, the recruitment of Akt to ABCC1 promoter was determined by chromatin immunoprecipitation (ChIP). *P<.05 as compared with the control
4. DISCUSSION
Although numerous studies indicated that visfatin can trigger the progression of cancers including NSCLC,10 the roles and related mechanisms of visfatin on chemoresistance are still not well investigated. Our present study revealed that the expression of visfatin was significantly increased in NSCLC Dox‐resistant cells. The expression of visfatin in NSCLC cells and tissues were also increased. Knockdown of visfatin by siRNAs can increase the Dox sensitivity of NSCLC Dox‐resistant cells. However, overexpression of visfatin can significantly decrease the Dox sensitivity. Visfatin can positively regulate the expression of ABCC1, which is the mostly implicated in the MDR of NSCLC. The activation of Akt, while not ERK1/2, mediated visfatin induced up‐regulation of ABCC1. This is confirmed by the result that visfatin can increase the nuclear translocation of Akt and its binding with ABCC1.
Our results revealed that visfatin is up‐regulated in NSCLC cells and positively regulate the Dox resistance of NSCLC cells. The elevated expression of visfatin in NSCLC cells was confirmed by recent study that plasma levels of visfatin were significantly higher in NSCLC patients and positively correlated with Tumor Node Metastasis (TNM) stage and node and distant metastasis.7 In addition, higher levels of visfatin were also observed in the serum of gastric,17 colorectal10 and endometrial11 cancer patients as compared with the healthy controls. Targeted inhibition of visfatin can sensitize glioblastoma cells to temozolomide treatment.12 Laboratory studies also indicated that visfatin can trigger the in vitro migration and invasion of NSCLC cancers via up‐regulation of MMP‐2 and MMP‐9.7 Considering that migration and invasion also contribute to drug resistance,18 our present study, together with the published data, confirmed that visfatin might be a therapy target for NSCLC to suppress its progression.
Our study suggested that up‐regulation of ABCC1 is involved in visfatin‐induced Dox resistant of NSCLC cells. ABCC1 was identified as the cause of multidrug resistance in a human small‐cell lung carcinoma cell line.19 An increased ABCC1 expression level was observed in 37% of the NSCLC tumour samples compared with the respective normal lung cancer samples.20 In addition, the expression of MRP1 in the NSCLC cell lines was higher than that in SCLC cell lines.21 Doxorubicin is one of the substances transported by MRP1.22 The expression of MRP1 in lung cancer cells was correlated with the resistance of cells to Dox.21 Our data revealed that visfatin can positively regulate the expression of MPR1/ABCC1, while not P‐gp. It revealed that ABCC1 is involved in visfatin‐induced Dox resistance of NSCLC cells.
This study also suggested that Akt, while not ERK1/2 or NF‐κB (p65), mediated visfatin‐induced Dox resistance and up‐regulation of ABCC1 in NSCLC cells. It was reported that visfatin can activate various downstream signal pathways including MAPK, Akt, and NF‐κB (p65).23 Akt has been reported to mediate visfatin‐induced human endothelial VEGF and MMP‐2/9 production.17 However, targeted inhibition of PI3K/Akt via its inhibitor LY294002 can sensitize drug‐resistant colon carcinoma cells to Dox‐induced apoptosis.24 Our data showed that visfatin can trigger the nucleus translocation and phosphorylation of Akt and increase its transcriptional activity on ABCC1. It was reported that LY294002 treatment did not up‐regulate MRP1 levels in colon cancer cells.24 This is not consistent with our present study that LY294002 can down‐regulate the MRP1 in Dox‐resistant NSCLC cells. This might be due to the difference of cell lines and needs further studies.
Take together, our present study revealed that visfatin mediates Dox resistance in human NSCLC via Akt mediated up‐regulation of ABCC1. Therefore, targeted inhibition of visfatin might be helpful to elevate the Dox sensitivity of NSCLC patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Cao Z, Liang N, Yang H, Li S. Visfatin mediates doxorubicin resistance in human non–small‐cell lung cancer via Akt‐mediated up‐regulation of ABCC1. Cell Prolif. 2017;50:e12366 10.1111/cpr.12366
Funding Information
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (No.20131106120063), Capital Special Project for Featured Clinical Application (Z151100004015157), Fund for Peking Union Medical College Hospital Youth Researcher (PUMCH‐2016‐2.25), Fund for Peking Union Medical College Youth Teacher (14‐2014zlgc0717), and Beijing Municipal Science and Technology Project (Z171100002017013).
Zhili Cao, Naixin Liang and Huaxia Yang these authors contributed equally to this work.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 2. Gaspar LE, Chansky K, Albain KS, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non‐small‐cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23:2955‐2961. [DOI] [PubMed] [Google Scholar]
- 3. Vatsyayan R, Chaudhary P, Lelsani PCR, et al. Role of RLIP76 in doxorubicin resistance in lung cancer. Int J Oncol. 2009;34:1505‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin‐induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234‐235:119‐124. [PubMed] [Google Scholar]
- 5. Ranek MJ, Wang X. Activation of the ubiquitin‐proteasome system in doxorubicin cardiomyopathy. Curr Hypertens Rep. 2009;11:389‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Enewold L, Mechanic LE, Bowman ED, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18:215‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang G, Tian W, Liu Y, et al. Visfatin triggers the cell motility of non‐small cell lung cancer via up‐regulation of matrix metalloproteinases. Basic Clin Pharmacol Toxicol. 2016;119:548‐554. [DOI] [PubMed] [Google Scholar]
- 8. Liu H‐Y, Li Q‐R, Cheng X‐F, Wang G‐J, Hao H‐P. NAMPT inhibition synergizes with NQO1‐targeting agents in inducing apoptotic cell death in non‐small cell lung cancer cells. Chin J Nat Med. 2016;14:582‐589. [DOI] [PubMed] [Google Scholar]
- 9. Okumura S, Sasaki T, Minami Y, Ohsaki Y. Nicotinamide phosphoribosyltransferase: a potent therapeutic target in non‐small cell lung cancer with epidermal growth factor receptor‐gene mutation. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2012;7:49‐56. [DOI] [PubMed] [Google Scholar]
- 10. Grolla AA, Travelli C, Genazzani AA, Sethi JK. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br J Pharmacol. 2016;173:2182‐2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rongvaux A, Galli M, Denanglaire S, et al. Nicotinamide phosphoribosyl transferase/pre‐B cell colony‐enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol Baltim Md. 1950;2008:4685‐4695. [DOI] [PubMed] [Google Scholar]
- 12. Feng J, Yan P‐F, Zhao H, Zhang F‐C, Zhao W‐H, Feng M. Inhibitor of nicotinamide phosphoribosyltransferase sensitizes glioblastoma cells to temozolomide via activating ROS/JNK signaling pathway. BioMed Res Int [Internet]. 2016:1450843 10.1155/2016/1450843. Epub 2016 Dec 20. [DOI] [PMC free article] [PubMed]
- 13. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714‐726. [DOI] [PubMed] [Google Scholar]
- 14. Szakács G, Paterson JK, Ludwig JA, Booth‐Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219‐234. [DOI] [PubMed] [Google Scholar]
- 15. Pajic M, Norris MD, Cohn SL, Haber M. The role of the multidrug resistance‐associated protein 1 gene in neuroblastoma biology and clinical outcome. Cancer Lett. 2005;228:241‐246. [DOI] [PubMed] [Google Scholar]
- 16. Chang X. Molecular mechanism of ATP‐dependent solute transport by multidrug resistance‐associated protein 1. Methods Mol Biol (Clifton, NJ). 2010;596:223‐249. [DOI] [PubMed] [Google Scholar]
- 17. Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP‐2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin‐induced angiogenesis. Cardiovasc Res. 2008;78:356‐365. [DOI] [PubMed] [Google Scholar]
- 18. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741‐4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science. 1992;258:1650‐1654. [DOI] [PubMed] [Google Scholar]
- 20. Dragoj M, Milosevic Z, Bankovic J, Tanic N, Pesic M, Stankovic T. Targeting CXCR4 and FAK reverses doxorubicin resistance and suppresses invasion in non‐small cell lung carcinoma. Cell Oncol. 2017;40:47‐62. [DOI] [PubMed] [Google Scholar]
- 21. Young LC, Campling BG, Cole SPC, Deeley RG, Gerlach JH. Multidrug Resistance Proteins MRP3, MRP1, and MRP2 in Lung Cancer. Clin Cancer Res. 2001;7:1798‐1804. [PubMed] [Google Scholar]
- 22. Deeley RG, Cole SPC. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006;580:1103‐1111. [DOI] [PubMed] [Google Scholar]
- 23. Oita RC, Ferdinando D, Wilson S, Bunce C, Mazzatti DJ. Visfatin induces oxidative stress in differentiated C2C12 myotubes in an Akt‐ and MAPK‐independent, NFĸB‐dependent manner. Pflüg Arch – Eur J Physiol. 2010;459:619‐630. [DOI] [PubMed] [Google Scholar]
- 24. Abdul‐Ghani R, Serra V, Györffy B, et al. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25:1743‐1752. [DOI] [PubMed] [Google Scholar]


