Abstract
Introduction
This study aimed to investigate the functions of delta‐like homologue 1 (DLK1) in the proliferation and differentiation of human dental pulp stem cells (hDPSCs).
Methods
Immunohistochemical analysis was used to determine the expression of alkaline phosphatase (ALP), dentin sialophosphoprotein (DSPP), DLK1, NOTCH1 and p‐ERK1/2 in the mouse first maxillary molar. Recombinant lentivirus was constructed to overexpress DLK1 stably in hDPSCs. The cell viability and proliferation of hDPSCs were examined by CCK8 and EdU incorporation assay respectively. The odontoblastic differentiation of hDPSCs was determined by detection of ALPase activity assay, ALP and alizarin red staining and the expression of mineralization‐related genes including ALP, DSPP and dental matrix protein. The mRNA and protein levels of DLK1 and p‐ERK1/2 protein expression were detected. ERK inhibitor was used to test the differentiation effect of DLK1 on hDPSCs.
Results
Delta‐like homologue 1 was highly expressed on the odontoblasts and dental pulp cells on the first maxillary molar; the expression of p‐ERK1/2 is similar with the DLK1 in the same process. The expression level of DLK1 increased significantly after the odontoblastic induction of hDPSCs. DLK1 overexpression increased the proliferation ability of hDPSCs and inhibited odontoblastic differentiation of hDPSCs. The protein level of p‐ERK1/2 significantly increased in hDPSCs/dlk1‐oe group. ERK signalling pathway inhibitor reversed the odontoblastic differentiation effects of DLK1 on hDPSCs.
Conclusions
The proliferation of hDPSCs was promoted after DLK1 overexpression. DLK1 inhibited the odontoblastic differentiation of hDPSCs, which maybe through ERK signalling pathway.
1. Introduction
The dental pulp contains undifferentiated mesenchymal cells and plays roles in dentinogenesis.1 Dental pulp stem cells (DPSCs) can proliferate and differentiate into odontoblasts (OD) to create reparative dentin in response to the appropriate stimuli.2, 3 A number of factors are involved in regulating reparative dentin formation, such as BMP2,4 HMGB1,5 NOTCH6 and so on. Understanding the genetic and molecular events that regulate the reparative dentin formation will be applied to prevent and treat the heritable and acquired loss.
Delta‐like homologue 1 (DLK1), also named preadipocyte factor 1, pG2, or foetal antigen 1, imprinted cluster located on human chromosome 14q32, is a transmembrane and secreted protein.7 It is highly expressed in diverse embryonic tissues during early organogenesis and adulthood, and it maintains precursor cell populations and inhibits differentiation. DLK1 has also been proven to play important roles in development and differentiation of many tissues for it is a feature of stem cell niches.8, 9 Moreover, DLK1 is also found locally overexpressed in adult tissues after injury, which may be associated with stem cell activation and tissue repair responses.10, 11 Therefore, we hypothesized that DLK1 may exert effects on odontoblastic differentiation during tooth development and reparative dentin formation in caries.
In the present study, the expression of DLK1 in mouse tooth OD and mouse DPCs was detected by immunohistochemical analysis. Meanwhile, the expression of DLK1 during human dental pulp stem cells (hDPSCs) odontoblastic differentiation was detected by quantitative real‐time polymerase chain reaction (RT‐PCR) and Western blot analysis, then DLK1 was stably overexpressed in hDPSCs to determine its effects on proliferation and odontoblastic differentiation of hDPSCs, and the molecular mechanism was analysed.
2. Materials and methods
The entire study was performed according to an informed protocol approved by the Ethics Committee of Shanghai Tenth People's Hospital, Tongji University School of Medicine.
2.1. Immunohistochemical analysis
The adult C57BL/6J mice were mated overnight. The day that a new born mouse observed was designated as postnatal 0 (PN0). The PN mice at tooth developmental stage (PN2, PN6 and PN20) were used in the study. For immunohistochemistry, maxillaries of each stage were fixed in 4% paraformaldehyde overnight at 4°C and demineralized with 10% ethylenediamine tetra‐acetic acid (pH 7.4) from 2 to 14 days depending on stages at 4°C. After being dehydrated and embedded in paraffin, these samples were sectioned at a thickness of 5 μm. Sections were dipped into xylene to remove the paraffin, rehydrated through a series of alcohol. Sections were incubated in 3% hydrogen peroxide for 10 minutes at room temperature to prevent endogenous peroxidase activity, and then were incubated in 0.01 mol/L citrate for 10 minutes at 100°C followed by cooling down at room temperature for 20 minutes. Then slides were blocked in 5% bovine serum albumin in phosphate‐buffered saline (PBS). Subsequently, slides were incubated with primary antibodies against alkaline phosphatase (ALP) (1:300, rabbit monoclonal antibody; Abcam Inc., Cambridge, MA, USA), dentin sialophosphoprotein (DSPP) (1:50, rabbit polyclonal antibody; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA), DLK1 (1:300, rabbit polyclonal antibody; Santa Cruz Biotechnology, Inc), NOTCH1 (1:150, rabbit monoclonal antibody; Abcam, Inc) and p‐ERK1/2 (1:100, rabbit monoclonal antibody; Abcam, Inc) overnight at 4°C, while for negative control, slides were incubated with PBS. Then, the slides were washed with PBS and incubated with the polymer helper and poly‐HRP‐anti‐Rabbit IgG (Zhong Shan Golden Bridge Biotechnology, Beijing, China) for 1 hour at 37°C. After being counterstained with haematoxylin, the samples were visualized under a light microscope (Carl Zeiss, Hamburg, Germany).
2.2. Cell culture and differentiation
The primary cultured hDPCs were isolated from healthy human premolars undergoing tooth extraction for orthodontic treatment (12‐14 years old) with informed consent obtained before tooth extraction, and were cultured in high‐glucose Dulbecco's modified Eagle's medium (DMEM; Gibco‐BRL, Grand Island, NY, USA) supplemented with 20% foetal bovine serum (FBS; Gibco‐BRL Life Technologies, Paisley, UK, USA) and antibiotics (100 U/mL penicillin and 100 U/mL streptomycin; Gibco‐BRL, USA) in a humidified atmosphere of 5% CO2 at 37°C. The isolation of hDPSCs was performed according to previously described methods.12 When primary cells reached 80% confluence, the cells were digested to isolate single cell clones by limiting dilution in 96‐well plates. The cells from these clones were observed as hDPSCs and were designed as passage 0. hDPSCs were expanded for the following experiments. Cell cultures between the third and sixth passages were used.
DMEM supplemented with 10% FBS, antibiotics, 50 μg/mL ascorbic acid (Sigma, St. Louis, MO, USA), 10 mmol/L sodium β‐glycerophosphate (Sigma), and 10 nmol/L dexamethasone (Sigma) was used as odontoblastic induction medium. The cultured hDPSCs were randomly divided into two groups: wild‐type hDPSCs without infection were cultured in odontoblastic induction medium for 0, 3, 7, 11 and 14 days and hDPSCs infected by lentivirus were cultured in odontoblastic induction medium for 0, 7 and 14 days. The RNA and protein of these two groups were extracted for real‐time reverse transcription‐polymerase chain reaction (RT‐PCR), Western blot analysis and ALPase activity assay.
2.3. DLK1 lentivirus transfection
For the lentivirus transfection, lentivirus harbouring human Dlk1 gene was constructed (GeneChem, Shanghai, China) (The structure of lentivirus vectors is provided in supplement.) hDPSCs were seeded in 6 cm plates, and reached about 50% confluence before transfection, which was performed according to the manufacturer's recommendations. After hDPSCs were infected using polybrene (Sigma) for 24 hours, 8 μg/mL Puromycin (Sigma) was added to select the positive transfected cells for 1 week at 37°C, 5% CO2. Stably infected cells (termed as hDPSC/control or hDPSC/dlk1‐oe) were chosen and used for further experiments. The transfected cells were analysed by fluorescent microscope, verified by RT‐PCR and Western blots.
2.4. Cell viability and proliferation assay
The Cell Counting Kit‐8 (CCK‐8; Dojindo Kagaku Co, Kumamoto, Japan) was used to analyse the effects of DLK1 on hDPSCs viability according to the manufacturer's protocols. Briefly, the hDPSC/wt, hDPSC/control or hDPSC/dlk1‐oe were seeded at a density of 5 × 103 cells/well in four 96‐well plates (Corning Inc, Corning, NY, USA) and then were cultured overnight. Subsequently, fresh medium with 10% FBS was changed every 3 days. After the cells were cultured for 1, 3, 5 and 7 days, the number of cells was assessed using a cell counting kit. Absorbance was measured using a microplate reader at 450 nm to determine the number of vital cells in each well. The well with medium and CCK‐8 solution but without cells was used as a blank control. Cell proliferation was represented as the mean ± standard deviation (SD) of absorbance for five wells from each group.
Meanwhile, the newly synthesized DNA of replicating cells were detected using a Cell‐Light 5‐ethynyl‐2′‐deoxyuridine (EdU) DNA cell proliferation kit according to the manufacturer's instructions (Guangzhou RiboBio, Guangzhou, China). Briefly, adherent hDPSCs in a 96‐well plate (Falcon) plated at a density of 2 × 104 cells/well were serum starved for 12 hours, incubated with EdU for 3 hours and fixed in 4% paraformaldehyde. Nuclei were stained with Hoechst 33342 before mounting by fluorescent microscopy. Data were obtained from the counts of EdU‐positive cells. Statistical analysis of the EdU‐positive cells between groups was performed.
2.5. Alizarin red staining and alkaline phosphatase staining
The hDPSC/wt, hDPSC/control or hDPSC/dlk1‐oe cells were incubated under odontoblastic induction for 14 days. The induction medium was replaced every 3 days. Mineralization was assessed by alizarin red staining. Briefly, 1% alizarin red (pH=4.3) (Sigma) was prepared in distilled water, and then applied to the cells in six‐well plates for 30 minutes at 37°C. For ALP staining, the plates were harvested at 7 days. The ALP colour development kit (Beyotime Institute of Biotechnology, Shanghai, China) was used in the study according to the manufacturer's protocols. Moreover, for determining whether DLK1 inhibited the odontoblastic differentiation through ERK signalling pathway. hDPSC/control or hDPSC/dlk1‐oe groups were incubated under odontoblastic induction containing the special ERK signalling pathway inhibitor U0126 (10 nmol/L) for 7 days, then alizarin red staining and ALP staining were conducted. All the cells were washed thrice with distilled water and then observed by phase‐contrast microscopy.
2.6. ALPase activity assay
ALPase activity was determined using cell lysates. Briefly, 220 μL of 0.9 mmol/L 2‐amino‐2‐methyl‐1‐propanol buffer was added to 5 μL of samples or standards. Subsequently, 25 μL of 15 mmol/L p‐nitrophenyl phosphate was added and mixtures were incubated at 37°C for 30 minutes. ALP activity was measured at 405 nm using an ELISA microplate reader (Bio‐Tek Instruments Inc, Winooski, Vermont, USA). ALPase activity (U/mg) was defined as the release of 1 mol p‐nitrophenol per mg total cellular protein.
2.7. RNA isolation and quantitative RT‐PCR analysis
Total RNA of the hDPSC/wt, hDPSC/control or hDPSC/dlk1‐oe cultured in odontoblastic induction for different times was isolated with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol. cDNA synthesized by a PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio, Shiga, Japan) was used as a template in PCR. The odontoblastic differentiation of the cells was monitored by analysing the OD‐related markers DSPP, dental matrix protein (DMP1) and ALP. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used to normalize the RNA expression. The sequences of the specific primers used in this study were as follows: DSPP (forward: 5′‐TCG GTT ACC GGT TGA CAT GG‐3′, reverse:5′‐ TCA CAA GGG AGA AGG GAA TGG‐3′); DMP1 (forward: 5′‐CCC TTG GAG AGC AGT GAG TC‐3′, reverse: 5′‐CTC CTT TTC CTG TGC TCC TG‐3′); ALP (forward: 5′‐CCA CAA GCC CGT GAC AGA‐3′, reverse: 5′‐GCG GCA GAC TTT GGT TTC‐3′); DLK1 (forward: 5′‐CTC CCT GAC TCT TGT TTG G‐3′, reverse: 5′‐AAC GGT GAC AAT GAC TTG C‐3′); GAPDH (forward: 5′‐TCA TGG GTG TGA ACC ATG AGA A‐3′, reverse: 5′‐ GGC ATG GAC TGT GGT CAT GAG‐3′). Real‐time PCR reaction was amplified with HieffTM qPCR SYBR® Green Master Mix (Yeasen Bio, Shanghai, China) in an ABI 7500 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). Amplification and detection were performed at the following conditions: 5 minutes hot start at 95°C in the holding stage; 40 cycles of 10 seconds at 95°C, 30 seconds at 60°C in the cycling stage; and 15 seconds at 95°C, 1 minute at 60°C, and 15 seconds at 60°C in the melt curve stage. Relative gene expression was calculated using the comparative 2−ΔΔCt method. The mean Ct value of target gene was normalized to its averaged Ct values of GAPDH to obtain a ΔCt value, which was then normalized to control samples to obtain a ΔΔCt value. Each measurement was assessed in triplicate. The gene expression ratio was shown as mean ± SD from three independent experiments.
2.8. Western blot analysis
The hDPSC/wt, hDPSC/control or hDPSC/dlk1‐oe cultured in odontoblastic induction for different days (0, 7 and 14 days) was lysed in a protein extraction kit (Piece, Rockford, IL, USA). Protein concentrations were determined using a bicinchoninic acid protein assay kit (Piece). An equal amount of proteins was separated and then transferred onto the nitrocellulose membranes (Millipore Corporation, Billerica, MA, USA). After blocking, the primary antibodies of rabbit anti‐mouse DLK1 (1:1000; Santa Cruz Biotechnology, Inc.), rabbit anti‐mouse DMP1 (1:1000; Bio Vision Research Products, Mountain View, CA, USA), rabbit anti‐mouse DSPP (1:500; Santa Cruz Biotechnology, Inc.), phosphorylated ERK1/2 (p‐ERK1/2) (1:1000, rabbit monoclonal antibody; Abcam, Inc) or total ERK1/2 (1:1000; Santa Cruz Biotechnology, Inc.) and rabbit anti‐mouse β‐actin (1:5000; Santa Cruz Biotechnology, Inc.) were used. After washes, membranes were incubated with goat anti‐mouse IRDye800 or goat anti‐rabbit IRDye680 (1:10 000; Invitrogen). After the final wash, the membranes were visualized using the Odyssey LI‐CDR system.
2.9. ERK signalling pathway inhibitor
ERK signalling pathway special inhibitor U0126 was chosen in the study. The different concentrations of U0126 (0, 2.5, 5, 10 and 20 nmol/L) were chosen to treat the hDPSC/control or hDPSC/dlk1‐oe for 7 days. Cells were lysed using a protein extraction kit (Piece), and the protein level of p‐ERK1/2 and total ERK1/2 was evaluated using Western blot analysis. The appropriate concentration of U0126 was chosen and used in the next experiment according to the results of Western blot analysis.
2.10. Statistical analysis
Experiments were performed in triplicate, and data were presented as mean ± SD. Data were evaluated by one‐way ANOVA using the spss software (version 10.0; SPSS, Chicago, IL, USA). P values <.05 were considered statistically significant.
3. Results
3.1. Location of DLK1 protein during mouse tooth development
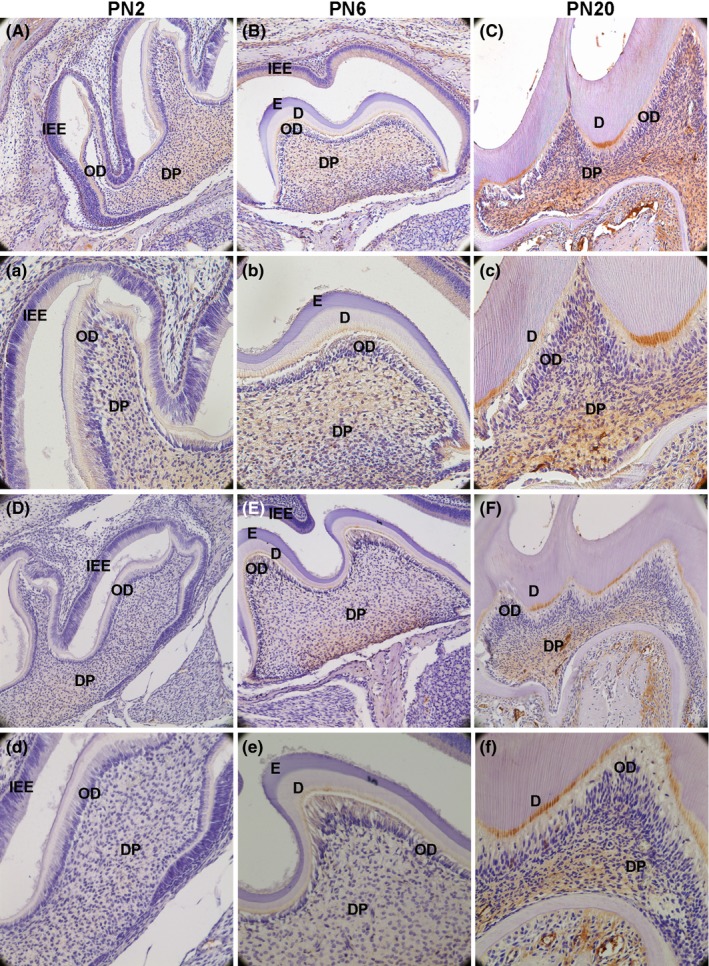
To detect the expression and location of ALP, DSPP and DLK1, immunohistochemical analysis was used in the front sections of mouse first maxillary molar at PN2, PN6 and PN20. ALP and DSPP were increased during the dentin development (Figure 1). Dentin began formation at PN2, DLK1 was located in the ameloblasts and odontoblasts (OD), and also mildly expressed in dental pulp cells (DPCs) near the OD (Figure 2A,a). Dentin was formed at PN6, DLK1 was strongly expressed in OD, and detected in some DPCs (Figure 2B,b). After dentin development finished at PN20, DLK1 was not only highly expressed in ODs but also strongly located in a large number of DPCs (Figure 2C,c). During the dentin development, DLK1 positive expression in ODs and DPCs near the ODs was observed. Moreover, DLK1 expression was up‐regulated gradually in ODs and DPCs from PN2 to PN20 (Figure 2), which showed the same trend with ALP and DSPP (Figure 1).
Figure 1.
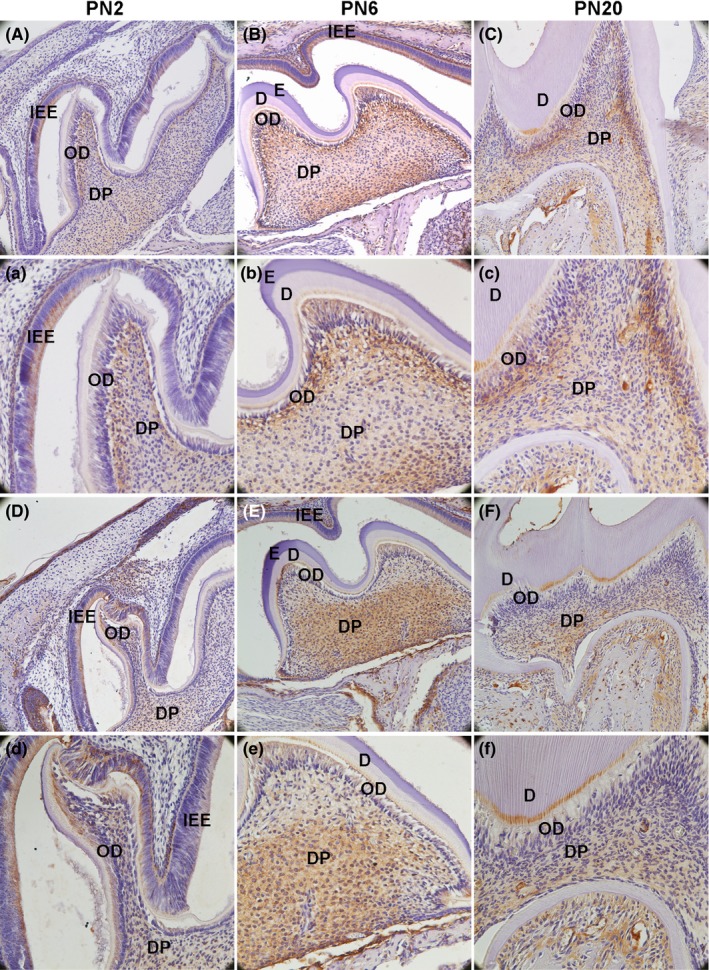
Alkaline phosphatase (ALP) and dentin sialophosphoprotein (DSPP) protein expression in the first maxillary molar in mice were detected by immunohistochemical analysis. A(a), B(b) and C(c) show the result of ALP. D(d), E(e) and F(f) show the result of DSPP. Both of them were increased during the development (IEE: inner enamel epithelium; OD: odontoblast; DP: dental pulp; E: enamel; D: dentin; A‐F: 200×; a‐f: 400×)
Figure 2.
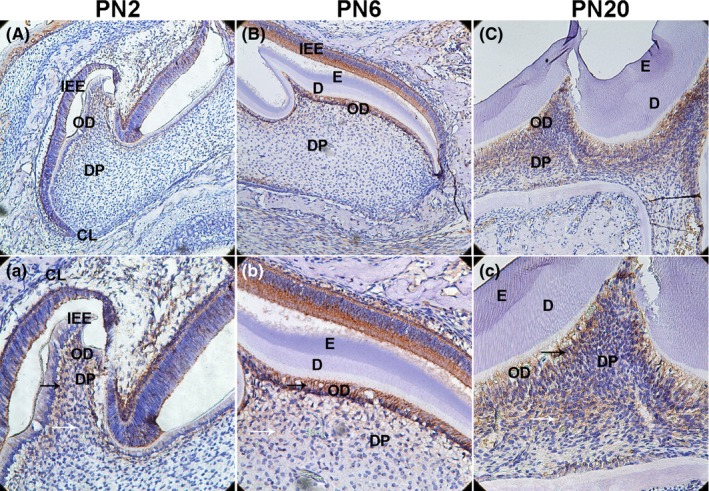
Delta‐like homologue 1 (DLK1) protein expression in the first maxillary molar in mice was detected by immunohistochemical analysis. A(a), At PN2,DLK1 was expressed in the ODs, and mildly expressed in a few dental pulp cells near ODs layer. B(b): At PN6, strong positive immunolabelling was observed in the whole ODs layer, and DLK1 was much higher in some DPCs near ODs layer. C(c): At PN6, DLK was not only highly expressed in ODs but also strongly located in a large number of DPCs in DP. Primarily, DLK1 was increasingly expressed in the ODs and DPCs. (IEE: inner enamel epithelium; OD: odontoblast; DP: dental pulp; CL: cervical loop; E: enamel; D: dentin; DPCs: dental pulp cells; white arrows mean DPCs; black arrows mean ODs; A‐C: 200×; a‐c: 400×)
3.2. Expression of DLK1 during odontoblastic differentiation of hDPSCs
To detect the expression of DLK1 during odontoblastic induction, the mRNA and protein levels of DMP1, DSPP, ALP and DLK1 were measured by real‐time PCR and Western blot analysis during the odontoblastic induction of hDPSCs (Figure 3). The mRNA and protein levels of DMP‐1, DSPP and ALP increased during the induction (Figure 3A‐C, F‐G, and I). ALPase activity exhibited the same trend as ALP mRNA expression (Figure 3D). Meanwhile, the mRNA and protein levels of DLK1 were up‐regulated during the odontoblastic differentiation (Figure 3E, H and I).
Figure 3.
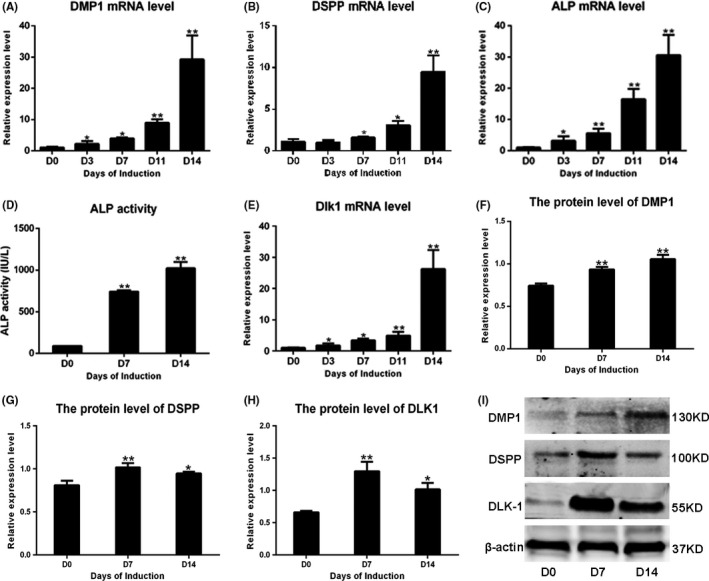
The expression of delta‐like homologue 1 (DLK1) during odontoblastic differentiation of hDPSCs. A, B, C and E represent the up‐regulation of mRNA levels of DMP1, DSPP, ALP and DLK1 during the induction respectively. D shows the increased ALP activity in the process. I shows the Western blots results of DMP1, DSPP and DLK1 during the induction. F‐H show the quantification protein level of DMP1, DSPP and DLK1 relative to β‐actin according to the Western blots results, which showed the same trend with mRNA. The data represent the mean ± SD of three independent experiments
3.3. Stable overexpression of DLK1 in hDPSCs
To construct stably transfected hDPSC/control and hDPSC/dlk1‐oe, hDPSCs were transfected with either lentiviral vector expressing green fluorescent protein (GFP) alone or lentiviral vector expressing DLK1 and GFP in vitro. The results demonstrated GFP expression in almost all cells after selecting the positive transfected cells using puromycin (Sigma) for 1 week (Figure 4A). The mRNA and protein expression of DLK1 were obviously higher in hDPSC/dlk1‐oe than those in hDPSC/control (Figure 4B‐D). These results showed that DLK1 was stably overexpressed in hDPSC/dlk1‐oe group.
Figure 4.
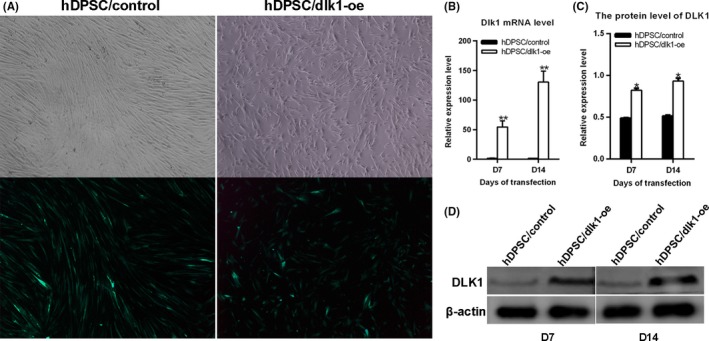
Stable overexpression of DLK1 in hDPSCs. A: hDPSCs transfected with lentiviral vector in vitro demonstrated GFP expression after being infected and selected for 1 wk (200×). B and D show the mRNA and protein levels of DLK1 after hDPSCs transfected with lentiviral vector in vitro for 7 and 14 d. C presents the quantification result of D
3.4. DLK1 promotes proliferation of hDPSCs
To measure cell proliferation, EdU labelling was performed. In the hDPSC/dlk1‐oe group, cells showed red fluorescence indicating EdU labelling were much more than those in groups of hDPSC/control (Figure 5A,B). Meanwhile, we measured cell viability using CCK8 according to the manufacturer's instructions. The results indicated that the cell viability of hDPSC/dlk1‐oe groups were much higher than the hDPSC/control groups (P<.05) on days 1, 3, 5 and 7 (P>.05) (Figure 5C). These results indicated that DLK1 overexpression up‐regulated the proliferation and viability of hDPSCs.
Figure 5.
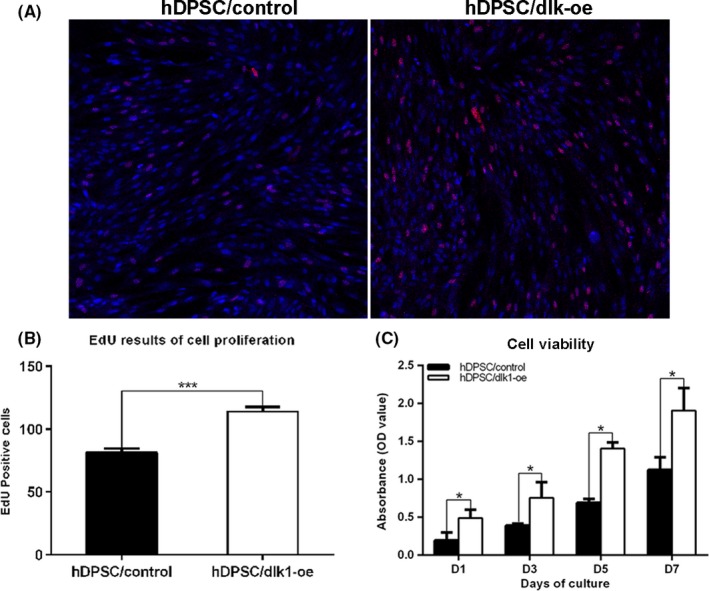
Delta‐like homologue 1 (DLK1) promotes the proliferation of hDPSCs. A: shows the EdU labelling in hDPSC/dlk1‐oe and hDPSC/control group (red: EdU labelling; blue: DAPI; 200×). B: shows the quantified results of EdU analyses (by measuring the positive cells); there were much more EdU‐positive cells in hDPSC/dlk1‐oe group than hDPSC/control groups (P<.0001). C: CCK‐8 assay indicated that the cell viability of hDPSC/dlk1‐oe groups was much higher than the hDPSC/control groups (P<.05) on days 1, 3, 5 and 7, and the data represent the mean ± SD of three independent experiments
3.5. Inhibition of odontoblastic differentiation of hDPSCs after overexpression of DLK1
To directly address a functional role for DLK1 in odontoblastic differentiation, mineralization assay, ALPase activity and mineralization‐related genes were measured. After 14 days of culture in the odontoblastic induction medium, mineralized nodule formation was observed by scanner and phase‐contrast microscopy. A significantly lower ALP staining in the hDPSC/dlk1‐oe group on 7 days compared with in the hDPSC/wt and hDPSC/control groups was found (Figure 6A,B). The amount of mineralized nodules formed in the hDPSC/dlk1‐oe group on 14 days showed the same trend as the ALP staining in the three groups (Figure 6C,D). Overexpression of DLK1 also inhibited mRNA levels of DMP‐1, DSPP and ALP (P<.05; Figure 7A‐C). Also ALPase activity, DMP‐1 and DSPP protein levels in the DLK1 overexpression groups were lower than control groups (Figure 7D‐G). These results indicated that DLK1 overexpression inhibited the odontoblastic differentiation of hDPSCs.
Figure 6.
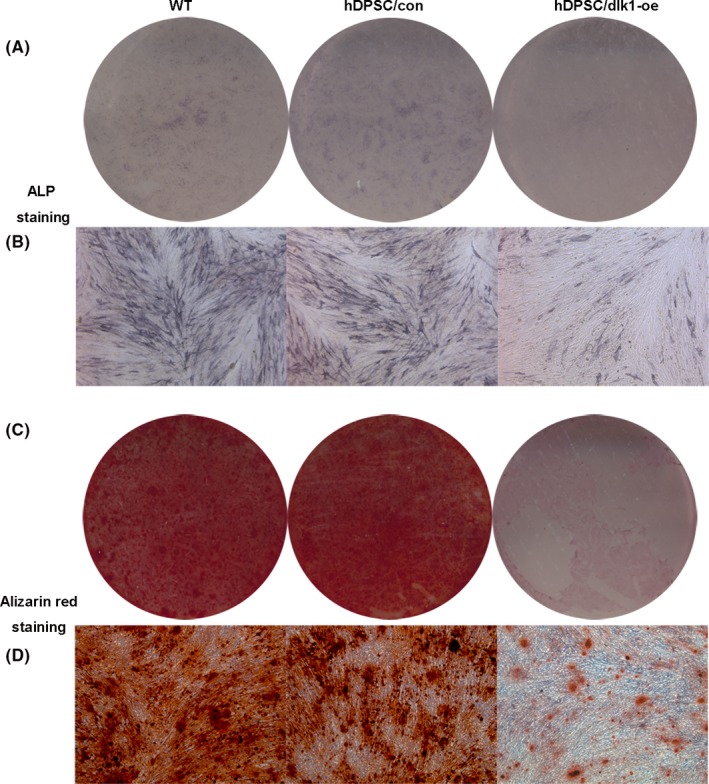
ALP staining and mineralized nodule formation results. (A and B) A significantly lower ALP staining in the hDPSC/dlk1‐oe group on 7 d compared with in the hDPSC/wt and hDPSC/control groups was found. (C and D) The amount of mineralized nodules formed in the hDPSC/dlk1‐oe group was much less than control groups (A, C: scanner; B and D: phase‐contrast microscopy, 50×)
Figure 7.
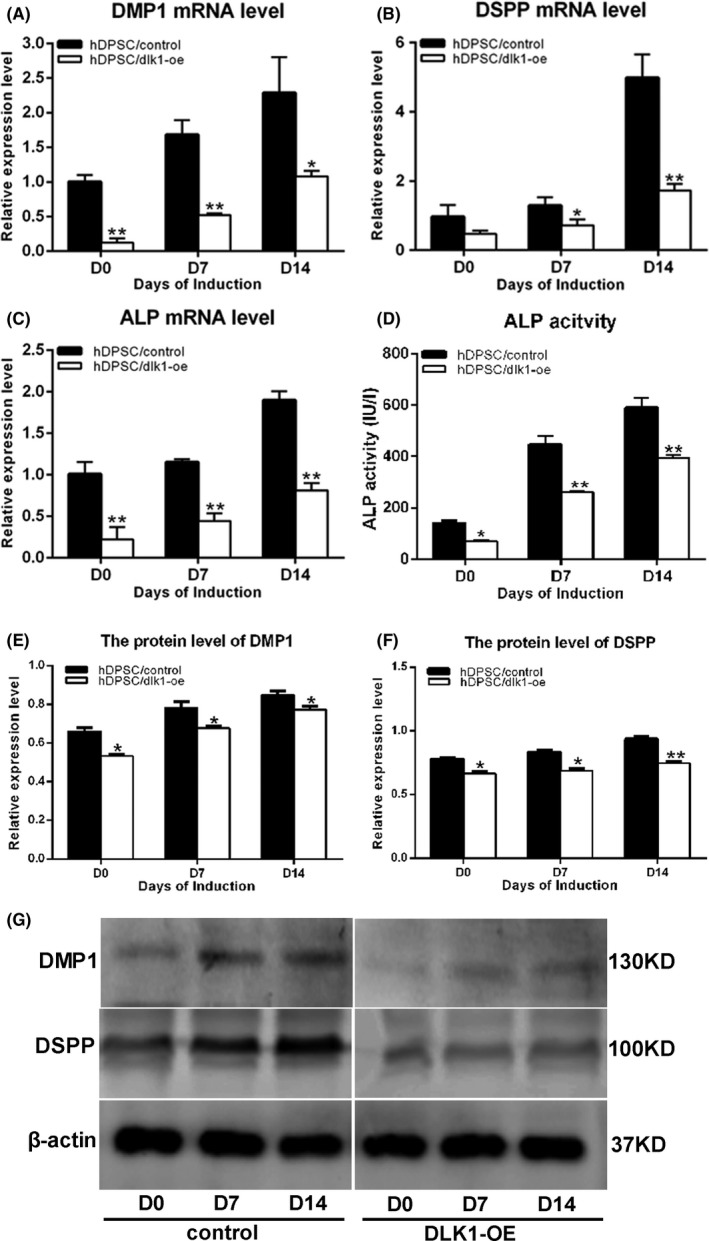
The inhibition effect of DLK1 on the odontoblastic differentiation of hDPSCs. A, B and C represent the mRNA level of DMP1, DSPP and ALP after DLK1 overexpression in hDPSCs during the odontoblastic differentiation. D shows the ALP activity after DLK1 overexpression. G shows the protein level of DMP1 and DSPP by Western blot analysis. E and F show the quantified protein level of DMP1 and DSPP relative to β‐actin during the induction according to Western blots. All the data represent the mean±SD of three independent experiments
3.6. DLK1 regulates the differentiation through ERK signalling pathway
To clarify the mechanism of DLK1 on the differentiation of hDPSCs, the protein of total ERK1/2 and p‐ERK1/2 were detected after DLK1 was overexpressed in hDPSCs for 1 and 7 days. Western blot analysis showed that there were two bands for the protein of ERK: p‐ERK1/2 relative to total ERK1/2 was up‐regulated in hDPSC/dlk1‐oe group, which suggested that ERK signalling pathway was activated after DLK1 was overexpressed (Figure 8A,C). Meanwhile, the expression and location of p‐ERK1/2 was detected using immunohistochemical analysis in the front sections of mouse first maxillary molar at PN2, PN6 and PN20. p‐ERK1/2 protein level was increased in DPCs during the process (Figure 9), which showed the similar trend with DLK1 (Figure 2).
Figure 8.
p‐ERK1/2 and NOTCH1 protein expression in the first maxillary molar in mice were detected by immunohistochemical analysis. A(a), B(b) and C(c) show the result of p‐ERK1/2. D(d), E(e) and F(f) show the result of NOTCH1. p‐ERK1/2 expression pattern was much more similar with the DLK1 than NOTCH1 during the tooth development. (IEE: inner enamel epithelium; OD: odontoblast; DP: dental pulp; E: enamel; D: dentin; A, B, C, D, E and F: 200×; a, b, c, d, e and f:400×)
Figure 9.
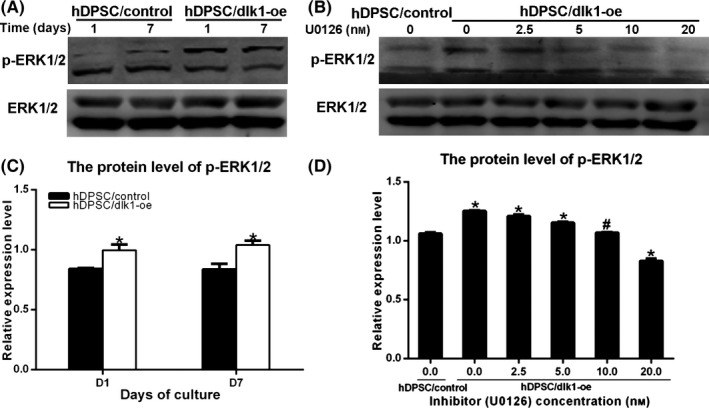
DLK1 regulates the differentiation through ERK signalling pathway. A shows activation of p‐ERK1/2 after DLK1 overexpression. B shows that the appropriate concentration of U0126 was 10 nmol/L, which shows the similar level with the control group. C and D show the quantification protein level of p‐ERK1/2 relative to total ERK1/2 of A and B respectively
Furthermore, different concentrations of ERK‐specific inhibitor U0126 were used in hDPSC/dlk1‐oe group for 7 days. The p‐ERK1/2 protein level was down‐regulated after treatment with different concentrations of the inhibitor U0126 (Figure 8B). After quantification of protein level, 10 nmol/L of U0126 was chosen as the appropriate concentration (Figure 8D) and was used in the next experiment (Figure 10). Mineralized nodule formation and ALP staining were significantly higher in U0126‐treated hDPSC/dlk1‐oe groups than hDPSC/dlk1‐oe without U0126 groups (Figure 10), which suggested the inhibitor effects of DLK1 on odontoblastic differentiation were reversed. These results suggested that DLK1 may regulate the odontoblastic differentiation of hDPSCs via the ERK signalling pathway.
Figure 10.
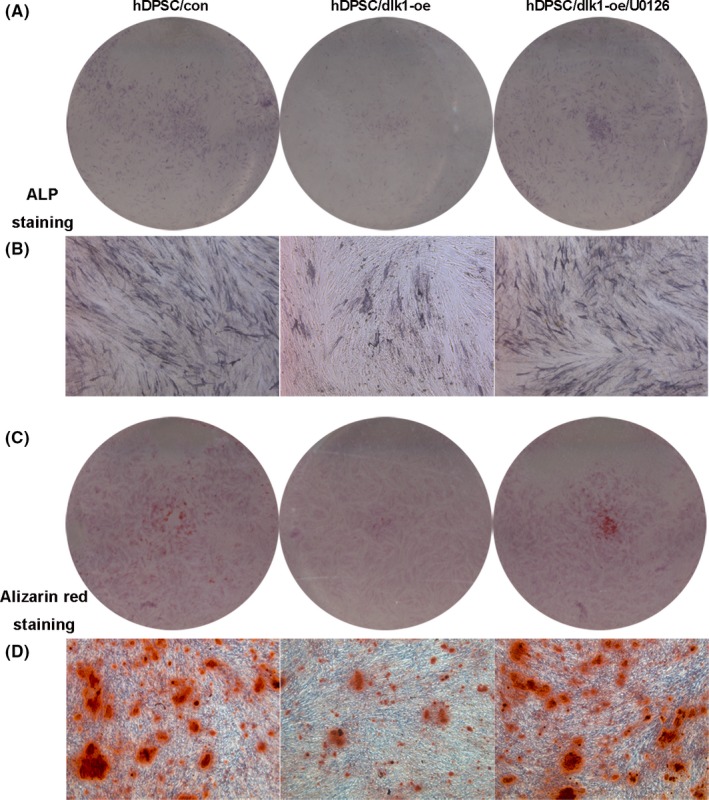
A and B present the ALP staining results; C and D show mineralized nodule formation results, all of which suggested that the inhibitor effects of DLK1 on odontoblastic differentiation were reversed by U0126 (10 nmol/L)
4. Discussion
Human dental pulp cells contain progenitor/stem cells of OD lineage which has the ability to proliferate and differentiate into OD. hDPSCs can form primary, secondary and reactionary tertiary dentin which are very important for dentin repair.13, 14 Tooth development and dentin repair involve a variety of growth factors and signalling molecules.14 For example, HMGB1 promotes the proliferation and odontoblastic differentiation of hDPCs.5 Leukaemia inhibitory factor promotes the proliferation of DPCs, and inhibits the odontoblastic differentiation via the Jak2‐Stat3 signalling pathway. NRAGE regulates the odontoblastic differentiation of hDPCs through NF‐kB signalling pathway.15 The ERK signalling pathway plays important roles in odontogenic differentiation of hDPSCs and repair after dental pulp injury.
In most previous studies, DLK1 as a Notch regulator, exerts regulatory effects on cell differentiation fate and regulates variety of signalling pathway during development.16 Variety of defects is observed in DLK1‐null mice: growth retardation, obesity and skeletal malformations.17 DLK1 has a more restricted expression pattern in the adult, where it is mainly found in undifferentiated cells in fat, liver and brain. While the functions of DLK1 in vivo and vitro on proliferation and OD differentiation of hDPSCs is unclear. This study would appear to be the first one to analyse the function of DLK1 during the proliferation and odontoblastic differentiation of hDPSCs.
During embryonic development, DLK1 is highly expressed in many tissues including the chondroblasts18 and skeletal myotubes.19 DLK1 is a negative regulator of multiple differentiation processes including neuroendocrine differentiation19 and osteogenesis, as well as chondrogenesis18 and muscle regeneration.20 Meanwhile, DLK1 plays as a niche in bone marrow to control osteoblast differentiation.21Moreover, DLK1‐positive skeletal cells contribute at different levels and time points during skeletal muscle remodelling in response to myositis, myopathies and acute injure. DLK1 is uniquely found in a condensation of cells within the regenerating tissue, and may contribute to the regenerative capacity of tissue wound. In our study, DLK1 was highly expressed on OD and DPCs on PN2, PN6 and PN20 and gradually increased during the dentin development (Figure 2). These results suggested that DLK1 may play a key role in regulating dentin development and repair.
Dental matrix protein‐1, alkaline phosphatase (ALP) and DSPP are used as mineralization markers for the odontoblastic differentiation of DPSCs.22 DSP/DSPP was reported to be OD‐specific markers.23 In this study, ALP expression and ALPase activity were increased during the odontoblastic induction of hDPSCs. DMP1 and DSPP were up‐regulated during the same process (Figure 3). These results manifest that the cells were differentiated into OD‐like cells. Meanwhile, the mRNA and protein levels of DLK1 were significantly up‐regulated in this process (Figure 3).
To further prove the functions of DLK1 on odontoblastic differentiation and proliferation of hDPSCs in vitro, hDPSCs were cultured as previous methods.12 Lentiviral vectors provided efficient gene overexpression and infected hDPSCs in vitro (Figure 4). The results of CCK8 and EdU demonstrated that DLK1 overexpression increased the hDPSCs cell viability and proliferation (Figure 5), which showed the same trend as in chondroblasts,18 skeletal myotubes19 and osteoblasts.21
In this study, ALP staining and mineralization assays proved that DLK1 overexpression inhibited the mineralization potential of hDPSCs (Figure 6). To confirm this result, the mRNA and protein levels of the odontoblastic differentiation markers, which includes DMP1, DSPP and ALP, were also down‐regulated in hDPSC/dlk1‐oe group (Figure 7). All of these results suggested that DLK1 inhibit odontoblastic differentiation, which may prevent excessive dentin formation thereby regulating the dentin formation during tooth development. As we mentioned previously, the immunohistochemical assay showed that DLK1 immunoreactivity within OD was stronger than that in dental pulp core. The DLK1 expression was also up‐regulated during the odontoblastic differentiation of hDPSCs. These phenomena seems somewhat contradictory with DLK1 inhibited the odontoblastic differentiation. From the results, we hypothesized that DLK1 up‐regulation was not the reason, but the results of dentin development in mouse molar and odontoblastic differentiation, which will play a negative feedback regulating the odontoblastic differentiation. Actually, none of the single factor can control the effect or function of DPSCs, which was controlled by the balance of promotion and inhibition by cytokines, growth factors and hormones. For example, transforming growth factor‐β and epidermal growth factor also inhibit the odontoblastic differentiation, whereas they are highly expressed in OD and up‐regulated during the induction. Therefore, the eventual effects may depend on the balance between promotion and inhibition.
DLK1 activate the NOTCH signalling, which plays key roles in differentiation of OD and osteoblasts, calcification of tooth hard tissue, formation of cusp patterns and generation of tooth roots.24 Meanwhile, DLK1 activates human stem cell through interruption of Shh signalling pathway,25 and DLK1 regulates osteogenesis and chondrogenesis through PI3K/AKT signalling.18 DLK1 also activates the MAPK pathways to inhibit the adipogenesis and chondrogenesis.26 DLK1 strongly inhibits cell differentiation into mature adipocytes by binding to fibronectin.27 The ERK signalling pathway plays important roles in odontogenic differentiation of hDPCs and repair after dental pulp injury.28, 29, 30 Most importantly, most studies focused on the role of DLK1 through NOTCH signalling pathway, rarely focused on the ERK signalling pathway. Therefore, this study mainly declares the communication of DLK1 and ERK signalling pathway during hDPSCs differentiation. Furthermore, NOTCH1 and p‐ERK1/2 protein level were up‐regulated during the dentin development in vivo (Figure 8), p‐ERK1/2 expression was much stronger than NOTCH1, and p‐ERK1/2 expression pattern was much more similar with DLK1, especially on ODs at PN20 in our study (Figure 8C,c). DLK1 also inhibited the hDPSCs differentiation, and the ERK signalling pathway was activated after DLK1 overexpression in hDPSCs (Figure 9A,C). Therefore, we hypothesized that DLK1 regulated the hDPSCs differentiation via the ERK signalling pathway. The ERK signalling pathway special inhibitor U0126 was used in the study. The p‐ERK1/2 protein level was down‐regulated after treatment with the inhibitor (Figure 9B,D). Considering the functions of ERK signalling pathway in odontoblastic differentiation of hDPSCs, the p‐ERK1/2 protein level being similar to the expression in the hDPSC/control group was designed as standard to choose the appropriate concentration after treatment with different concentrations of U0126. That meant the up‐regulation of p‐ERK1/2 protein level resulting from DLK1 overexpression was inhibited by the appropriate concentration of U0126. Therefore, the appropriate concentration was 10 nmol/L (Figure 9B,D), which was used in ALP staining and mineralization assays. The results showed that the inhibitor effects of DLK1 on odontoblastic differentiation were reversed, which suggested that DLK1 may regulate the odontoblastic differentiation of hDPSCs via the ERK signalling pathway (Figure 10). More intensive studies are still required to explicit the relationship between DLK1 and the ERK signalling pathway in the odontoblastic differentiation.
In summary, this study demonstrated that hDPSCs exhibit increased proliferation ability, decreased mineralization and expression of mineralization‐associated genes and proteins by DLK1 overexpression, which maybe through ERK signalling pathway.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of China (no. 81500806) and China Postdoctoral Science Foundation (no. 2015M581633) and Science Foundation of Shanghai health and family planning commission (no. 20144Y0257) and Fundamental Research Funds for the central Universities (no. 2014KJ090 and no. 2014KJ077). All the authors denied any conflicts of interest for the study.
Qi S, Yan Y, Wen Y, et al. The effect of delta‐like 1 homologue on the proliferation and odontoblastic differentiation in human dental pulp stem cells. Cell Prolif. 2017;50:e12335 10.1111/cpr.12335
Contributor Information
Yuanzhi Xu, Email: amyxyz01@sina.com.
Raorao Wang, Email: raoraowang@tongji.edu.cn.
References
- 1. Sinanan AC, Hunt NP, Lewis MP. Human adult craniofacial muscle‐derived cells: neural‐cell adhesion‐molecule (NCAM; CD56)‐expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2004;40:25–34. [DOI] [PubMed] [Google Scholar]
- 2. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Y, Sun H, Song F, Fu D, Wang J. DDIT3 overexpression increases odontoblastic potential of human dental pulp cells. Cell Prolif. 2014;47:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo F, Feng J, Wang F, et al. Bmp2 deletion causes an amelogenesis imperfecta phenotype via regulating enamel gene expression. J Cell Physiol. 2015;230:1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi SC, Cui C, Yan YH, Sun GH, Zhu SR. Effects of high‐mobility group box 1 on the proliferation and odontoblastic differentiation of human dental pulp cells. Int Endod J. 2013;46:1153–1163. [DOI] [PubMed] [Google Scholar]
- 6. Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development. 2010;137:3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Traustadottir GA, Jensen CH, Thomassen M, et al. Evidence of non‐canonical NOTCH signaling: delta‐like 1 homolog (DLK1) directly interacts with the NOTCH1 receptor in mammals. Cell Signal. 2016;28:246–254. [DOI] [PubMed] [Google Scholar]
- 8. Yevtodiyenko A, Schmidt JV. Dlk1 expression marks developing endothelium and sites of branching morphogenesis in the mouse embryo and placenta. Dev Dyn. 2006;235:1115–1123. [DOI] [PubMed] [Google Scholar]
- 9. Ferron SR, Charalambous M, Radford E, et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersen DC, Jensen L, Schroder HD, Jensen CH. “The preadipocyte factor” DLK1 marks adult mouse adipose tissue residing vascular cells that lack in vitro adipogenic differentiation potential. FEBS Lett. 2009;583:2947–2953. [DOI] [PubMed] [Google Scholar]
- 11. Andersen DC, Petersson SJ, Jorgensen LH, et al. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009;27:898–908. [DOI] [PubMed] [Google Scholar]
- 12. Zhu L, Yang J, Zhang J, et al. In vitro and in vivo evaluation of a nanoparticulate bioceramic paste for dental pulp repair. Acta Biomater. 2014;10:5156–5168. [DOI] [PubMed] [Google Scholar]
- 13. Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. [DOI] [PubMed] [Google Scholar]
- 14. Lin LM, Rosenberg PA. Repair and regeneration in endodontics. Int Endod J. 2011;44:889–906. [DOI] [PubMed] [Google Scholar]
- 15. Qi S, Wu Q, Ma J, et al. Effects of neurotrophin receptor‐mediated MAGE homology on proliferation and odontoblastic differentiation of mouse dental pulp cells. Cell Prolif. 2015;48:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai X, Gong P, Huang Y, Lin Y. Notch signalling pathway in tooth development and adult dental cells. Cell Prolif. 2011;44:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheung LY, Rizzoti K, Lovell‐Badge R, Le Tissier PR. Pituitary phenotypes of mice lacking the notch signalling ligand delta‐like 1 homologue. J Neuroendocrinol. 2013;25:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen L, Qanie D, Jafari A, et al. Delta‐like 1/fetal antigen‐1 (Dlk1/FA1) is a novel regulator of chondrogenic cell differentiation via inhibition of the Akt kinase‐dependent pathway. J Biol Chem. 2011;286:32140–32149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Floridon C, Jensen CH, Thorsen P, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. [DOI] [PubMed] [Google Scholar]
- 20. Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref‐1. J Bone Miner Res. 2004;19:841–852. [DOI] [PubMed] [Google Scholar]
- 21. Benetatos L, Hatzimichael E. Delta‐like homologue 1 and its role in the bone marrow niche and hematologic malignancies. Clin Lymphoma Myeloma Leuk. 2014;14:451–455. [DOI] [PubMed] [Google Scholar]
- 22. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iejima D, Sumita Y, Kagami H, Ando Y, Ueda M. Odontoblast marker gene expression is enhanced by a CC‐chemokine family protein MIP‐3alpha in human mesenchymal stem cells. Arch Oral Biol. 2007;52:924–931. [DOI] [PubMed] [Google Scholar]
- 24. Cai X, Gong P, Huang Y, Lin Y. Notch signalling pathway in tooth development and adult dental cells. Cell Prolif. 2011;44:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han Z, Yu C, Tian Y, et al. Expression patterns of long noncoding RNAs from Dlk1‐Dio3 imprinted region and the potential mechanisms of Gtl2 activation during blastocyst development. Biochem Biophys Res Commun. 2015;463:167–173. [DOI] [PubMed] [Google Scholar]
- 26. Kim SW, Muise AM, Lyons PJ, Ro HS. Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J Biol Chem. 2001;276:10199–10206. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Zhao L, Smas C, Sul HS. Pref‐1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol. 2010;30:3480–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woo SM, Lim HS, Jeong KY, Kim SM, Kim WJ, Jung JY. Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Mol Cells. 2015;38:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattei V, Santacroce C, Tasciotti V, et al. Role of lipid rafts in neuronal differentiation of dental pulp‐derived stem cells. Exp Cell Res. 2015;339:231–240. [DOI] [PubMed] [Google Scholar]
- 30. Chen I, Salhab I, Setzer FC, Kim S, Nah HD. A new calcium silicate‐based bioceramic material promotes human osteo‐ and odontogenic stem cell proliferation and survival via the extracellular signal‐regulated kinase signaling pathway. J Endod. 2016;42:480–486. [DOI] [PubMed] [Google Scholar]


