Abstract
Objectives
Neural stem cells (NSCs) are self‐renewing, undifferentiated and multipotent precursors that can generate neuronal and glial lineages. MicroRNAs (miRNAs) are small non‐coding RNAs that act crucial roles in cell proliferation, differentiation and migration. However, the role of miR‐1297 in the development of NSCs is still unknown.
Materials and methods
Primary NSCs were isolated from rat's embryos. The expression of miR‐1297 and Hes1 were measured by qRT‐PCR. Western blot was performed to detect the protein expression of Hes1, β‐tubulin‐III and GFAP.
Results
We showed that miR‐1297 expression was upregulated during NSC differentiation, while the expression of Hes1 was decreased during NSC differentiation. Elevated expression of miR‐1297 promoted the NSCs viability and increased the formation of NSCs to neurospheres. Ecoptic expression of miR‐1297 promoted β‐tubulin‐III expression in the NSCs. Overexpression of miR‐1297 decreased GFAP expression in the NSCs. Furthermore, we demonstrated that miR‐1297 regulated NSCs viability and differentiation by directly targeting Hes1. Overexpression of miR‐1297 suppressed Hes1 expression in the NSCs.
Conclusions
These results suggested that miR‐1297 played an important role in NSCs viability and differentiation through inhibiting Hes1 expression.
1. Introduction
Neural stem cells (NSCs) are multipotent and self‐renewing cells that present in the adult and developing central nervous system (CNS).1, 2, 3, 4 Neural stem cells have the capability to differentiate into glia (astrocytes and oligodendrocytes) and neurons.5, 6, 7 The transplantation of NSCs may provide a therapeutic strategy for several neurological disorders including Alzheimer's disease, Parkinson's disease, spinal cord injuries and Huntington's disease.1, 8, 9, 10 However, there are still various challenges to complete before the clinical application of NSCs.11, 12, 13 Therefore, it is important to understand the molecular signal pathway regulating NSCs proliferation and differentiation.
MicroRNAs (miRNAs) are a class of small, endogenous, non‐coding RNAs with less than 22 nucleotides.14, 15, 16, 17, 18 miRNAs regulate the target gene expression through binding to the 3′UTR (3′ untranslated region) of mRNA and leading to the translational repression or mRNA degradation.19, 20, 21, 22 Previous studies showed that miRNAs played crucial roles in various cellular processes such as cell differentiation, proliferation, apoptosis, migration and metastasis.11, 13, 23, 24, 25 A lot of evidences implicated that deregulated expression of miRNAs were found in various cancers, highlighting their potential tools as prognostic, therapeutic and diagnostic in the most human tumours.26, 27, 28, 29 Recently, studies have showed that miRNAs play an important role in neural stem cell development, proliferation and differentiation.7, 9, 11, 30
In this study, we showed a novel role for miR‐1297 during NSC viability and differentiation. We showed that the expression of miR‐1297 was upregulated during NSC differentiation. Elevated expression of miR‐1297 promoted NSCs viability. In line with this, overexpression of miR‐1297 increased the formation of neurospheres from NSCs.
2. Materials and Methods
2.1. NSC culture and transfection
Primary neural stem cells (NSCs) were isolated and cultured using the modified method of the previous published protocol.12, 31 NSCs were from rat's embryos and cultured in the growth medium with N2, EGF and bFGF (Gibco) supplement. miR‐1297 and scramble, Hes1 vector and control vector were purchased from the GenePharma (Shanghai, China) and transfected to the NSCs using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following to the manufacturer’s instructions. This study was agreed by the ethical board of the First Hospital of Harbin Medical University and complied with Declaration of Helsinki.
2.2. qRT‐PCR
Total RNA from cells was isolated by using TRIzol reagent and reverse transcribed by using the reverse transcription kit following the manufacturer's description. qRT‐PCR was performed and determined using the ABI 7300 system (Applied Biosystems, Foster City, CA, USA). Details of the primers were as follows: Hes1 (forward 5′‐ TGAAGGATTCCAAAAATAAAATTCTCTGGG‐3′ and reverse 5′‐ CGCCTCTTCTCCATGATAGGCTTTGATGAC‐3′); β‐tubulin‐III (forward 5′‐AGCAAGGTGCGTGAGGAGTA‐3′ and reverse 5′‐AAGCCGGGCATGAAGAAGT‐3′); Nestin (forward 5′‐GATCTAAACAGGAAGGAAATCCAGG‐3′ and (reverse 5′‐TCTAGTGTCTCATGGCTCTGGTTTT‐3′); GFAP (forward 5′‐CAACGTTAAGCTAGCCCTGGACAT‐3′ and (reverse: 5′‐CTCACCATCCCGCATCTCCACAGT‐3′); and GAPDH (forward 5′‐ATTCCATGGCACCGTCAAGGCTGA‐3′ and (reverse 5′‐TTCTCCATGGTGGTGAAGACGCCA‐3′). The expression of Hes1 and miR‐1297 was normalized to that of GAPDH or U6 by the 2−ΔΔCt cycle respectively.
2.3. Cell viability
The NSCs were plated in the 96‐well plate and the cell growth was detected with the MTT kit (Sigma St. Louis, MO, USA) following the manufacturer's information. The optical density at 490 nm was determined with the microplate reader (Bio‐Rad, CA, USA).
2.4. Dual luciferase assay
The mutant (Mut) or wild‐type (WT) 3′UTR of Hes1 was cloned into the pHSA‐MIR‐REPORT (Ambion Waltham, MA USA) cloned into pHSA‐MIR‐REPORT (Ambion). The NSCs were transfected with the vector carrying Mut 3′UTR or WT 3′UTR of Hes1 and miR‐1297 or scramble using the Lipofectamine 2000 (Invitrogen) according to the instructions. Cells were harvested to measure the relative activities of luciferase by using the Dual‐Luciferase Assay after 48‐hour incubation.
2.5. Western blot analysis
Total protein was isolated from cell, and protein concentration was determined using the BCA (Sigma‐Aldrich, Oakville, ON, Canada) assay kit. Total protein was resolved on the 12% SDS‐PAGE gel and then transferred to the PVDF membranes. After blocking with non‐fat dry milk for 2 hours, membrane was inoculated with the primary antibodies (Hes1 and GAPDH, Abcam, USA) overnight. The membranes were subsequently blotted with secondary peroxidase‐conjugated antibodies for 1 hour at the room temperature and the signal was measured by the chemiluminescent reagents.
2.6. Immunohistochemistry
Primary neural stem cells and their differentiation cells were fixed with the 4% paraformaldehyde and were blocked using the FBS and Triton™X‐100 (Sigma, Oakville, ON, Canada) for 1 hour. Then, the cells were incubated with the primary antibodies (GFAP, β‐tubulin‐III and Nestin, Sigma) at 4°C overnight. Cells were incubated with the fluorescence‐labelled antibody. Nuclei were stained with DAPI (Sigma).
2.7. Statistical analysis
Data are shown as the mean ± SD. Difference from groups was compared by using the two‐way ANOVA and Student's t test. A value of P<.05 was considered as the statistically significant.
3. Results
3.1. NSCs could self‐proliferate and differentiate into neuron and astrocyte
Isolated primary NSCs could self‐proliferate and form neurospheres, which was positive for Nestin (neural stem cell specific marker) (Figure 1A,B). After withdrawal of EGF and bFGF and FBS, NSCs could differentiate into neuron and astrocyte. Nucleus of the neuron and astrocyte cells was detected by DAPI immunostaining (Figure 1C). These cells were positive for β‐tubulin‐III (neuron‐specific marker) and GFAP (astrocyte‐specific marker) (Figure 1D,E).
Figure 1.
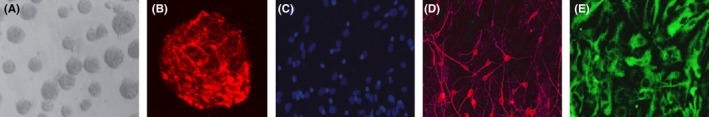
Neural stem cells (NSCs) can self‐proliferate and differentiate into neuron and astrocyte. A, Representative neurospheres are shown. B, Immunocytochemical staining of purified neural stem cell with Nestin. C, Immunocytochemical staining of nucleus with DAPI. D, Immunocytochemical staining of neuron with β‐tubulin‐III. E, Immunocytochemical staining of astrocyte with GFAP
3.2. miR‐1297 was upregulated and Hes1 expression was downregulated during NSC differentiation
The expression of miR‐1297 was upregulated during NSC differentiation by using the qRT‐PCR method (Figure 2A). The mRNA and protein expression of Hes1 was decreased during NSC differentiation by using the qRT‐PCR method (Figure 2B) and Western blot (Figure 2C).
Figure 2.
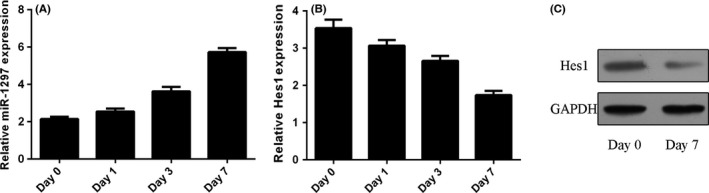
miR‐1297 was upregulated and Hes1 expression was downregulated during Neural stem cells (NSC) differentiation. A, The expression of miR‐1297 was detected by using the qRT‐PCR method. B, The mRNA expression of Hes1 was detected by using the qRT‐PCR method. C, The protein expression of Hes1 was measured by Western blot
3.3. Hes1 was a direct gene of miR‐1297 in the NSCs
miR‐1297 included a potential target site in the 3′UTR of Hes1 (Figure 3A). The expression of miR‐1297 was significantly upregulated in NSCs after treating with miR‐1297 mimic (Figure. 3B). Overexpression of miR‐1297 suppressed Hes1 expression in the NSCs (Figure. 3C,D). The luciferase activity was inhibited in the WT 3′UTR of Hes1, while it was not changed in the mutation putative (MT) miR‐1297 target site (Figure. 3E).
Figure 3.
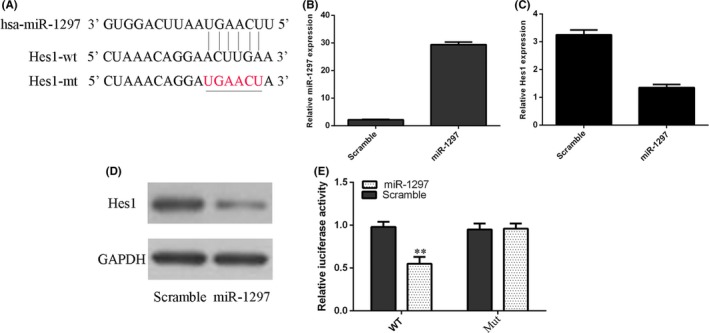
Hes1 was a direct gene of miR‐1297 in the neural stem cells (NSCs). A, miR‐1297 has a potential target site in the 3′UTR of Hes1 by using the Targetscan bioinformatics software. B, The expression of miR‐1297 was detected by using the qRT‐PCR method. C, Overexpression of miR‐1297 suppressed the Hes1 mRNA expression in the NSCs. D, The protein expression of Hes1 was measured by Western blot. E, The luciferase activity was inhibited in the wild‐type (WT) 3′UTR of Hes1, and the luciferase activity of the mutation putative (MuT) miR‐1297 target site was not changed **p<0.01.
3.4. miR‐1297 regulated NSCs viability and differentiation
As shown in Figure 4A, elevated expression of miR‐1297 promoted the NSCs viability. In line with this, overexpression of miR‐1297 increased neurospheres formation from NSCs (Figure 4B,C). Furthermore, ecoptic expression of miR‐1297 promoted β‐tubulin‐III expression in the NSCs (Figure 4D,E). In addition, overexpression of miR‐1297 decreased GFAP expression in the NSCs (Figure 4F,G).
Figure 4.
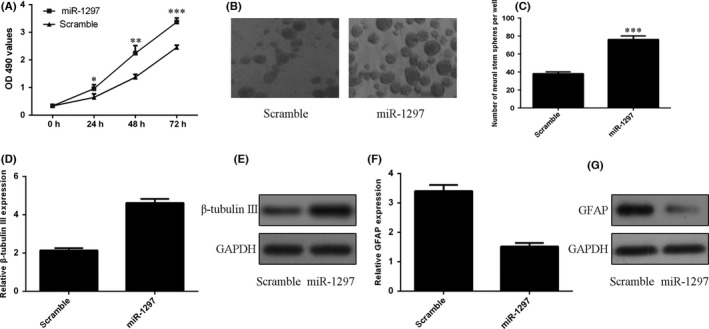
miR‐1297 regulated NSCs proliferation and differentiation. A, Elevated expression of miR‐1297 promoted the NSCs proliferation. B, Representative images of neurospheres in response to miR‐1297 mimic were shown. C, The relative neurosphere number was shown. D, Ecoptic expression of miR‐1297 promoted the β‐tubulin‐III mRNA expression. E, The protein expression of β‐tubulin‐III was measured by Western blot. F, Ecoptic expression of miR‐1297 suppressed the GFAP mRNA expression. G, The protein expression of GFAP was measured by Western blot *p<0.05, **p<0.01 and ***p<0.001.
3.5. miR‐1297 regulated NSCs viability and differentiation by regulating Hes1 expression
The expression of Hes1 was significantly upregulated in the NSCs after treating with Hes1 vector (Figure 5A,B). Ectopic expression of Hes1 decreased miR‐1297‐induced viability in NSCs (Figure 5C). Moreover, overexpression of Hes1 suppressed miR‐1297‐induced NSCs formation to neurospheres (Figure 5D,E). Furthermore, ecoptic expression of Hes1 suppressed β‐tubulin‐III expression in the miR‐1297 mimic‐treated NSCs (Figure. 5F,G). In addition, overexpression of Hes1 enhanced GFAP expression in the miR‐1297 mimic‐treated NSCs (Figure 5H,I).
Figure 5.
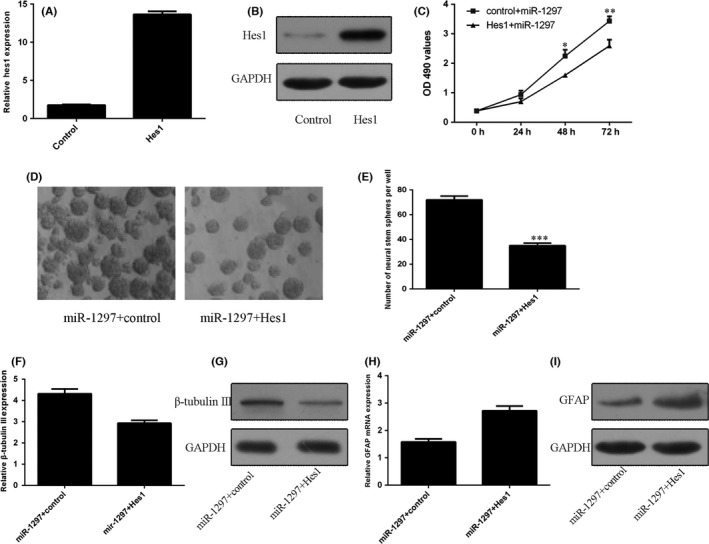
miR‐1297 regulated NSCs proliferation and differentiation by regulating Hes1 expression. A, The mRNA expression of Hes1 was detected by using the qRT‐PCR method. B, The protein expression of Hes1 was measured by Western blot. C, The cell proliferation was determined by CCK‐8 assay. D, Representative images of neurospheres in different groups were shown. E, The relative neurosphere number was shown. F, The mRNA expression of β‐tubulin‐III was determined by qRT‐PCR. G, The protein expression of β‐tubulin‐III was measured by Western blot. H, The mRNA expression of GFAP was determined by qRT‐PCR. I, The protein expression of GFAP was measured by Western blot *p<0.05, **p<0.01 and ***p<0.001.
4. Discussion
NSCs are one type of precursor cells that are located in the subgranular zone and subventricular zone of brain with capacity to self‐renew and generate multiple neuronal cell types such as neurons and glial cells, oligodendrocytes and astrocytes.32, 33, 34 The proliferation, maintenance and differentiation of NSCs are regulated by several molecular networks.12, 35, 36 Recently, increasing evidences have showed that miRNAs play an important role in the proliferation and differentiation of NSCs. For example, Wang et al.31 demonstrated that miR‐506‐3p played a crucial role in regulating the NSC proliferation and differentiation through inhibiting TCF3 expression. Li et al.37 showed that miR‐765 played an important role in NSCs proliferation and differentiation through inhibiting Hes1 expression. In our study, we demonstrated a novel role of miR‐1297 during NSC proliferation and differentiation. We showed that the expression of miR‐1297 was upregulated while the expression of Hes1 was decreased during NSC differentiation. Elevated expression of miR‐1297 promoted NSCs proliferation. In line with this, overexpression of miR‐1297 increased NSCs formation to neurospheres. Furthermore, ecoptic expression of miR‐1297 promoted β‐tubulin‐III expression in the NSCs. Overexpression of miR‐1297 decreased GFAP expression in the NSCs. We identified Hes1 as a direct target gene of miR‐1297 in the NSCs. Overexpression of miR‐1297 suppressed Hes1 expression in the NSCs. The luciferase activity was inhibited in the WT 3′UTR of Hes1, and the luciferase activity of the MT miR‐1297 target site was not changed. Furthermore, we demonstrated that miR‐1297 regulated NSCs proliferation and differentiation by regulating Hes1 expression. These results suggested that miR‐1297 played an important role in NSCs proliferation and differentiation through inhibiting Hes1 expression.
Previous studies showed that miR‐1297 played important roles in cell proliferation and tumour development.38, 39, 40, 41 For example, Liang et al.38 demonstrated that miR‐1297 expression was downregulated in prostate cancer tissues and cell lines. Overexpression of miR‐1297 suppressed prostate cancer cell proliferation and invasion through regulating AEG‐1 expression. Wang et al.39 showed that miR‐1297 expression was downregulated in the glioma tissues and cell lines. Overexpression of miR‐1297 inhibited the glioma cell colony formation and proliferation by inhibiting high mobility group protein A1 (HMGA1) expression. However, Li et al.42 found that overexpression of miR‐1297 increased the laryngeal squamous cell carcinoma cell migration, proliferation and tumour genesis through targeting PTEN. In our study, we first measured the miR‐1297 expression during NSC differentiation. The expression of miR‐1297 was upregulated during NSC differentiation. In addition, elevated expression of miR‐1297 promoted NSCs proliferation. In line with this, overexpression of miR‐1297 increased NSCs formation to neurospheres. Furthermore, ecoptic expression of miR‐1297 promoted β‐tubulin‐III expression in the NSCs. Overexpression of miR‐1297 decreased GFAP expression in the NSCs.
Hes gene was a mammalian homologue of the Drosophila hairy which encodes bHLH (basic helix‐loop‐helix) transcriptional regulators.43 Hes1 is one downstream modulator of Notch signal pathway and is expressed in central nervous system.44, 45 Hes1 has important roles in regulating neurogenesis.46, 47 Previous studies showed that Hes1 played an important role in the central nervous system growth and it regulated the NSCs differentiation and proliferation.45, 48 Shi et al.45 demonstrated that miR‐381 regulated NSCs differentiation and proliferation by inhibiting Hes1 expression. Li et al.37 also demonstrated that miR‐765 played an important role in NSCs proliferation and differentiation through inhibiting Hes1 expression. In our study, we demonstrated that Hes1 expression was decreased during NSC differentiation. Overexpression of miR‐1297 suppressed Hes1 expression in the NSCs. The luciferase activity was inhibited in the WT 3′UTR of Hes1, and the luciferase activity of the MT miR‐1297 target site was not changed. These results suggested that Hes1 was a direct target gene of miR‐1297 in the NSCs. Moreover, we demonstrated that miR‐1297 regulated NSCs proliferation and differentiation by regulating Hes1 expression.
In conclusion, our observations showed that miR‐1297 was novel miRNA that regulated NSCs proliferation and differentiation and could decrease Hes1 expression. These data help us to understand the mechanism of Hes1 in NSCs and suggest that miR‐1297 may be a new therapeutic strategy for neurological disorders.
Zheng J, Yi D, Shi X, Shi H. miR‐1297 regulates neural stem cell differentiation and viability through controlling Hes1 expression. Cell Prolif. 2017;50:e12347 10.1111/cpr.12347
References
- 1. Rolando C, Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014;107:183‐206. doi: 10.1016/B978-0-12-416022-4.00007-X. [DOI] [PubMed] [Google Scholar]
- 2. Goustard‐Langelier B, Koch M, Lavialle M, Heberden C. Rat neural stem cell proliferation and differentiation are durably altered by the in utero polyunsaturated fatty acid supply. J Nutr Biochem. 2013;24:380‐387. doi: 10.1016/j.jnutbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3. Germano I, Swiss V, Casaccia P. Primary brain tumors, neural stem cell, and brain tumor cancer cells: where is the link? Neuropharmacology. 2010;58:903‐910. doi: 10.1016/j.neuropharm.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao C, Sun G, Li S, et al. MicroRNA let‐7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107:1876‐1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Githinji J, McLaughlin B, Wilczek K, Nolta J. Role of miRNAs in neuronal differentiation from human embryonic stem cell‐derived neural stem cells. Stem Cell Rev. 2012;8:1129‐1137. doi: 10.1007/s12015-012-9411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juliandi B, Abematsu M, Nakashima K. Chromatin remodeling in neural stem cell differentiation. Curr Opin Neurobiol. 2010;20:408‐415. doi: 10.1016/j.conb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7. Cui Y, Xiao Z, Han J, et al. MiR‐125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci. 2012;13:116. doi: 10.1186/1471-2202-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pham JT, Gallicano GI. Specification of neural cell fate and regulation of neural stem cell proliferation by microRNAs. Am J Stem Cells. 2012;1:182‐195. [PMC free article] [PubMed] [Google Scholar]
- 9. Bian S, Hong J, Li Q, et al. MicroRNA cluster miR‐17‐92 regulates neural stem cell expansion and transition to intermediate progenitors in the developing mouse neocortex. Cell Rep. 2013;3:1398‐1406. doi: 10.1016/j.celrep.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roese‐Koerner B, Stappert L, Koch P, Brustle O, Borghese L. Pluripotent stem cell‐derived somatic stem cells as tool to study the role of microRNAs in early human neural development. Curr Mol Med. 2013;13:707‐722. [DOI] [PubMed] [Google Scholar]
- 11. Morgado AL, Xavier JM, Dionisio PA, et al. MicroRNA‐34a modulates neural stem cell differentiation by regulating expression of synaptic and autophagic proteins. Mol Neurobiol. 2014;51:1168‐1183. doi: 10.1007/s12035-014-8794-6. [DOI] [PubMed] [Google Scholar]
- 12. Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR‐34a regulates mouse neural stem cell differentiation. PLoS ONE. 2011;6:e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gioia U, Di Carlo V, Caramanica P, et al. Mir‐23a and mir‐125b regulate neural stem/progenitor cell proliferation by targeting Musashi1. RNA Biol. 2014;11:1105‐1112. doi: 10.4161/rna.35508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Y, Xue Q, Wang D, Du M, Zhang Y, Gao S. miR‐873 induces lung adenocarcinoma cell proliferation and migration by targeting SRCIN1. Am J Transl Res. 2015;7:2519‐2526. [PMC free article] [PubMed] [Google Scholar]
- 15. Yu X, Li Z, Chan MT, Wu WK. microRNA deregulation in keloids: an opportunity for clinical intervention? Cell Prolif. 2015;48:626‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu X, Li Z, Shen J, Chan MT, Wu WK. Role of microRNAs in primary central nervous system lymphomas. Cell Prolif. 2016;49:147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu Z, Zhang Y, Gao N, Wang X. Overexpression of miR‐506 inhibits growth of osteosarcoma through Snail2. Am J Transl Res. 2015;7:2716‐2723. [PMC free article] [PubMed] [Google Scholar]
- 18. Shen L, Chen XD, Zhang YH. MicroRNA‐128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069‐2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 19. Huang YZ, Zhang J, Shao HY, Chen JP, Zhao HY. MicroRNA‐191 promotes osteosarcoma cells proliferation by targeting checkpoint kinase 2. Tumour Biol. 2015;36:6095‐6101. doi: 10.1007/s13277-015-3290-9. [DOI] [PubMed] [Google Scholar]
- 20. Sun XH, Geng XL, Zhang J, Zhang C. miRNA‐646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2). Tumour Biol. 2015;36:2127‐2134. doi: 10.1007/s13277-014-2822-z. [DOI] [PubMed] [Google Scholar]
- 21. Shen L, Wang P, Yang J, Li X. MicroRNA‐217 regulates WASF3 expression and suppresses tumor growth and metastasis in osteosarcoma. PLoS ONE. 2014;9:e109138. doi: 10.1371/journal.pone.0109138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Salah Z, Arafeh R, Maximov V, et al. miR‐27a and miR‐27a* contribute to metastatic properties of osteosarcoma cells. Oncotarget. 2015;6:4920‐4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao D, Jia P, Wang W, Zhang G. VEGF‐mediated suppression of cell proliferation and invasion by miR‐410 in osteosarcoma. Mol Cell Biochem. 2015;400:87‐95. doi: 10.1007/s11010-014-2265-2. [DOI] [PubMed] [Google Scholar]
- 24. Zhang S, Zhao Y, Wang L. MicroRNA‐198 inhibited tumorous behaviors of human osteosarcoma through directly targeting ROCK1. Biochem Biophys Res Commun. 2016;472:557‐565. doi: 10.1016/j.bbrc.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 25. Chen G, Fang T, Huang Z, et al. MicroRNA‐133a inhibits osteosarcoma cells proliferation and invasion via targeting IGF‐1R. Cell Physiol Biochem. 2016;38:598‐608. doi: 10.1159/000438653. [DOI] [PubMed] [Google Scholar]
- 26. Pu Y, Zhao F, Cai W, Meng X, Li Y, Cai S. MiR‐193a‐3p and miR‐193a‐5p suppress the metastasis of human osteosarcoma cells by down‐regulating Rab27B and SRR, respectively. Clin Exp Metastasis. 2016;33:359‐372. doi: 10.1007/s10585-016-9783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, He R, Xia H, Wei YU, Wu S. MicroRNA‐101 has a suppressive role in osteosarcoma cells through the targeting of c‐FOS. Exp Ther Med. 2016;11:1293‐1299. doi: 10.3892/etm.2016.3085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Wu S, Du X, Wu M, Du H, Shi X, Zhang T. MicroRNA‐409‐3p inhibits osteosarcoma cell migration and invasion by targeting catenin‐delta1. Gene. 2016;584:83‐89. doi: 10.1016/j.gene.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 29. Wang GC, He QY, Tong DK, et al. MiR‐367 negatively regulates apoptosis induced by adriamycin in osteosarcoma cells by targeting KLF4. J Bone Oncol. 2016;5:51‐56. doi: 10.1016/j.jbo.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garg N, Po A, Miele E, et al. microRNA‐17‐92 cluster is a direct Nanog target and controls neural stem cell through Trp53inp1. EMBO J. 2013;32:2819‐2832. doi: 10.1038/emboj.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan W, Chen JQ, Chen ZY, Chen HM. MicroRNA‐506‐3p regulates neural stem cell proliferation and differentiation through targeting TCF3. Gene. 2016;593:193‐200. doi: 10.1016/j.gene.2016.08.026.. [DOI] [PubMed] [Google Scholar]
- 32. Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self‐renewal. Crit Rev Oncol Hematol. 2008;65:43‐53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR‐106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging. 2011;3:108‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935‐945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin‐28 and let‐7 controls pre‐let‐7 maturation during neural stem‐cell commitment. Nat Cell Biol. 2008;10:987‐993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 36. Palm T, Hemmer K, Winter J, et al. A systemic transcriptome analysis reveals the regulation of neural stem cell maintenance by an E2F1‐miRNA feedback loop. Nucleic Acids Res. 2013;41:3699‐3712. doi: 10.1093/nar/gkt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li SO, Zhao WN, Xu Q, Yu Y, Yin CH. MicroRNA‐765 regulates neural stem cell proliferation and differentiation by modulating Hes1 expression. Am J Transl Res. 2016;8:3115‐3123. [PMC free article] [PubMed] [Google Scholar]
- 38. Liang X, Li HC, Fu DL, Chong T, Wang ZM, Li ZL. MicroRNA‐1297 inhibits prostate cancer cell proliferation and invasion by targeting the AEG‐1/Wnt signaling pathway. Biochem Biophys Res Commun. 2016;480:208‐214. doi: 10.1016/j.bbrc.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 39. Wang JC, Xu XY, Mo SW, et al. Involvement of microRNA‐1297, a new regulator of HMGA1, in the regulation of glioma cell growth in vivo and in vitro. Am J Transl Res. 2016;8:2149‐2158. [PMC free article] [PubMed] [Google Scholar]
- 40. Liu FH, He YK, Shu RH, Wang SK. MicroRNA‐1297 regulates hepatocellular carcinoma cell proliferation and apoptosis by targeting EZH2. Int J Clin Exp Pathol. 2015;8:4972‐4980. [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang C, Chi YL, Wang PY, et al. miR‐511 and miR‐1297 inhibit human lung adenocarcinoma cell proliferation by targeting oncogene TRIB2. PLoS ONE. 2012;7:e46090. doi: 10.1371/journal.pone.0046090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li X, Wang HL, Peng X, Zhou HF, Wang X. miR‐1297 mediates PTEN expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427:254‐260. doi: 10.1016/j.bbrc.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 43. Katoh M. Human FOX gene family (Review). Int J Oncol. 2004;25:1495‐1500. [PubMed] [Google Scholar]
- 44. Garzia L, Andolfo I, Cusanelli E, et al. MicroRNA‐199b‐5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS ONE. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi X, Yan C, Liu B, et al. miR‐381 regulates neural stem cell proliferation and differentiation via regulating Hes1 expression. PLoS ONE. 2015;10:e0138973. doi: 10.1371/journal.pone.0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Andolfo I, Liguori L, De Antonellis P, et al. The micro‐RNA 199b‐5p regulatory circuit involves Hes1, CD15, and epigenetic modifications in medulloblastoma. Neuro‐oncol. 2012;14:596‐612. doi: 10.1093/neuonc/nos002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hughes DP. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res. 2009;152:479‐496. doi: 10.1007/978-1-4419-0284-9_28. [DOI] [PubMed] [Google Scholar]
- 48. Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor‐alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: role of Hes1. Mol Cell Neurosci. 2010;43:127‐135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]


