Abstract
Objectives
Recent studies have reported the existence of stem cells in ovarian tissue that show enhanced proliferative and differentiation potential compared to other adult tissues. Based on this evidence, we hypothesized that ovarian tissue contained mesenchymal‐like stem cells (MSC) that could be isolated using a novel rapid plastic adhesion technique.
Materials and methods
We established MSC lines derived from ovarian and adipose tissue based on their ability to rapidly adhere to plastic culture dishes in the first 3 hours after plating and studied their potentiality in terms of molecular markers and differentiation capacity.
Results
Morphological and kinetic properties of in vitro cultured ovarian MSC were similar to adipose‐derived MSC, and both reached senescence after similar passage numbers. Ovarian‐derived MSC expressed mesenchymal (CD90 and CD44) but not haematopoietic markers (CD34 and CD45), indicating similarity to adipose‐derived MSC. Moreover, ovarian‐derived MSC expressed NANOG, TERT, SOX2, OCT4 and showed extensive capacity to differentiate not only into adipogenic, osteogenic and chondrogenic tissue but also towards neurogenic and endodermal lineages and even precursors of primordial germ cells.
Conclusion
These results show for the first time the derivation of ovarian cells with the molecular properties of MSC as well as wide differentiation potential. Canine ovarian tissue is accessible, expandable, multipotent and has high plasticity, holding promise for applications in regenerative medicine.
1. INTRODUCTION
Two classes of stem cells have been identified in animals: embryonic (ESC) and adult somatic cells.1 ESC hold a great advantage across various applications due to their pluripotency, but their use is clouded by ethical issues, immunogenicity and neoplasic formation.2 The adult somatic cells, known as mesenchymal stem cells (MSC), are multipotent stromal cells responsible for regular tissue turnover that reside in various tissues.1 MSC are easy to isolate compared to ESC, and their isolation from adults makes autologous treatments possible. MSC have been the target of public discussion involving questions about the veracity of these cells, where the central question has been whether or not they can be differentiated into non‐mesenchymal cell lineages.1 Here we investigated the ability of ovarian‐derived MSC to differentiate into ectodermal, mesodermal, endodermal, and primordial germ lineages.
Replacement of cells after death due to age, injury or shedding by new healthy cells is a normal biological process. This process of homeostasis in adult tissues is possible due to the subpopulation of stem cells residing within the tissues.3 In the ovary, substantial physical injury occurs during the process of follicular rupture and ovulation, triggering a rapid repair mechanism that prepares the ovarian surface epithelium for the subsequent ovulatory cycle.4 In human ovaries, MSC have been found to play a role in the mesenchymal‐epithelial transition of ovarian tunica albuginea.5, 6 It was suggested that multipotent or pluripotent cells might be present in ovarian tissue.5, 7 Nonetheless, even though the ovary constantly undergoes substantial tissue remodelling, limited studies have focused on the presence of mesenchymal stem cells in the mammalian ovary.
Large animal species have several advantages with regard to understanding the basic biology of stem cells and in developing protocols for stem cell‐based regenerative medicine.9 Beyond their importance in the field of veterinary medicine,10 dogs have been widely accepted as an animal model for preclinical research on transplantation due to their many similarities to humans, including disease presentation, physiology, clinical responses, and the fact that more than half of canine hereditary diseases have counterparts in humans.11, 12, 13 In the dog, stem cell kinetics are more similar to those of humans than are those of mice or other laboratory animals, making the dog highly relevant for cell biology studies.14
Finally, since ovarian tissue is frequently available in veterinary clinics that perform elective castrations by ovary‐salpingo‐hysterectomy surgery and the ovary is often discarded as biological waste, the dog is an interesting research model to advance our understanding of ovarian stem cell biology and to develop potential clinical applications for cell‐based therapies and banking of stem cells for regenerative medicine. Therefore, this study aims to verify whether mesenchymal‐like stem cells are present in canine ovarian tissue and to investigate their molecular and differentiation characteristics. Adipose‐derived MSC are a well‐characterized source of adult stem cells, and for this reason they were used as controls for the various characterizations performed on ovarian‐derived MSC.
2. MATERIALS AND METHODS
2.1. Derivation of mesenchymal‐like stem cells from ovarian and adipose tissue
Ovaries and visceral adipose tissue samples were collected from female dogs undergoing elective ovariohysterectomy at the local veterinary hospital with owner consent. We used 4 mongrel bitches ranging in age from 6 months to 1 year. This experiment was approved by the Ethics Committee on the use of animals of UNESP‐FCAV (CEUA; protocol no. 026991/13), certifying its compliance with the Ethical Principles on Animal Experimentation adopted by the Brazilian College of Animal Experimentation (COBEA). Tissues were weighed and then washed in phosphate‐buffered saline (PBS; Thermo Fisher Scientific, Grand Island, NY, USA) to remove blood. Samples were sliced and incubated with collagenase I (Thermo Fisher Scientific) for 3 hours at 37.5°C. After two washings with PBS, the cell pellet was placed in a 75 cm2 culture flask (Corning T75, Corning, NY, USA) with 8 mL of Dulbecco's modified Eagle's medium low glucose (DMEM; Thermo Fisher Scientific) with 10% foetal bovine serum (FBS; Thermo Fisher Scientific). After 3 hours of incubation at 37.5°C at 5% CO2, non‐attached cells were discarded by washing and replacing with fresh medium for expansion. After reaching confluence, cells were trypsinized (TrypLE; Thermo Fisher Scientific) and re‐seeded at a density of 1.5 × 105 cells in a 25 cm2 tissue culture flask (Corning T25) or 3.0 × 105 cells per T75 flask. The primary culture was defined as passage zero (P0), and successive passages used the similar denomination, for example passage one (P1), etc.
Some canine ovarian‐ (cO‐MSC) and adipose‐derived (cA‐MSC) putative MSC were frozen at P1 and P2 to keep a bank of cells at early passages. At each passage, some cells were collected and resuspended in cryopreservation medium made of 80% DMEM, 10% dimethyl sulfoxide (DMSO; Sigma‐Aldrich, St. Louis, MO, USA) and 10% FBS. Temperatures were lowered slowly to −80°C using a styrofoam box (Mr. Frosty; Thermo Fisher Scientific) for 24 hours and then stored at −80°C. Thawing of cells was performed by placing cell vials into a 37°C water‐bath for 1‐2 minutes and washed in expansion medium to eliminate DMSO. After 72 hours in culture, P1 cells were counted to determine cell yield in relation to the initial amount of tissue digested.
2.2. Measurement of population‐doubling time
Population‐doubling time was determined by cultures in triplicates of cA‐MSC and cO‐MSC harvested at subconfluence at P1 and P9. After trypsinization, cells were counted and re‐seeded at a density of 1.0 × 103 cells per well, using a 24‐well plate (Nunc Cell Culture; Sigma). Cells were trypsinized and counted at 48, 72, 96 and 120 hours. Calculations of cell growth parameters were obtained according to the following formulas: CD = log2(Nf)−log2(Ni) and DT = CT/CD, where DT is the cell‐doubling time, CT the cell culture time, CD the cell‐doubling number, Nf the final number of cells and Ni the initial number of cells.
2.3. Colony‐forming unit assay
Colony‐forming unit assays were performed by seeding 500 cells in 35 mm‐diameter well and incubated for 7 days with medium replacement every 3 days. After 7 days, cells were fixed using 100% methanol for 15 minutes and stained with Giemsa (Sigma). All colonies with at least 50 cells were counted using inverted microscopy (Nikon, Mississauga, ON, Canada).
2.4. RNA isolation and transcript analysis
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Toronto, ON, Canada) and treated with DNase I (Qiagen). 500 ng of total RNA was reverse‐transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). Gene expression was quantified by RT‐PCR. Samples were run in duplicate and fluorescence was assessed with the Rotor‐gene SYBR Green kit (Qiagen). The expression of each gene was calculated as a ratio with three housekeeping genes. Primers were designed (Table 1) using Oligo 6 software (Molecular Biology Insights, Colorado Springs, CO, USA). PCR products were sequenced to confirm the specificity of all primers utilized
Table 1.
Sequences of primers used for gene expression analysis
| Gene | Primer sequences | Gene reference |
|---|---|---|
| GAPDH | Forward CCATCTTCCAGGAGCGAGAT | NC_006609.3 |
| Reverse TTCTCCATGGTGGTGAAGAC | ||
| RPL13 | Forward GGAGAAGGCCAGAGTCATCACA | NC_006587.3 |
| Reverse TTTGCCCTGATGCCAAAAAG | ||
| HPRT | Forward CACTGGGAAAACAATGCAGA | NC_006621.3 |
| Reverse ACAAAGTCAGGTTTATAGCCAACA | ||
| CD44 | Forward GCCCTGAGCGTGGGCTTTGA | NC_006600.3 |
| Reverse TCTGGCTGTAGCGGGTGCCA | ||
| CD90 | Forward CAGAACACCTCATGGCTGCTGT | NC_006587.3 |
| Reverse GGAGAAACCAGACAGAAGCGA | ||
| CD105 | Forward GGTTCACTGCATCAACATGG | NC_006591.3 |
| Reverse AAGCTGAAGCGCACATCACC | ||
| CD184 | Forward GCGGGCGAGCGGTTACCAT | NC_006601.3 |
| Reverse CCTCCCGGAAGCAGGGTTCCTT | ||
| NANOG | Forward GAATAACCCGAATTGGAGCAG | NC_006609.3 |
| Reverse AGCGATTCCTCTTCACAGTTG | ||
| TELOMERASE | Forward GCCGTGGTGATCGAGCAGA | AF380351.1 |
| Reverse GAAACAACCTGCGCTCCATGT | ||
| OCT4 | Forward GAGTGAGAGGCAACCTGGAG | NC_006594.3 |
| Reverse GTGAAGTGAGGGCTCCCATA | ||
| SOX2 | Forward CCCACCTACAGCATGTCCTA | NC_006616.3 |
| Reverse GGAGTGGGAGGAGGTAA |
2.5. Immunohistochemistry and confocal imaging
Cells from adipose and ovarian tissue at P2 were grown on coverslips in expansion culture medium. After 48 hours, the cells were washed twice in PBS with 0.01% Tween‐20 (TBS), fixed for 30 minutes in 4% paraformaldehyde (Sigma) and permeabilized with 0.1% Triton X‐100 (Sigma). After blocking with 5% goat serum, cells were incubated overnight at 4°C with primary antibodies (Table 2) diluted in 5% goat serum (Sigma). After washing the cells three times in TBS, fluorescein isothiocyanate (FITC)‐conjugated antibodies (secondary antibody) were added, and the cells were incubated for 40 minutes at room temperature. As a control, cells were incubated only with secondary antibody. Subsequently, cells were washed and observed by confocal microscopy (FV‐1000; Olympus, Richmond Hill, ON, Canada).
Table 2.
Antibodies and dilutions used for FACS and immunofluorescence analysis
| Antibody | Dilution | Catalog number—supplier |
|---|---|---|
| CD45 | 1:100 | MCA2035S—AbD Serotec, Hercules, CA, USA |
| CD34 | 1:100 | MCA2411GA—AbD Serotec |
| CD90 | 1:100 | MCA1036G—AbD Serotec |
| CD44 | 1:100 | MCA1041—AbD Serotec |
| Nestin | 1:100 | MAB353—Millipore, Toronto, ON, Canada |
| β‐tubulin | 1:100 | MAB1637—Millipore |
| DDX4 | 1:200 | PA5‐23378—Invitrogen, Carlsbad, CA, USA |
| IgG‐FITC | 1:100 | STAR80F—AbD Serotec |
| IgG‐FITC | 1:100 | STAR120F—AbD Serotec |
2.6. Flow cytometry assay (FACS)
Adipose and ovarian‐derived MSC derived from 4 animals were analysed at P1 using FACS. For surface markers, cells were collected, washed with PBS and incubated for 30 minutes in goat serum. Subsequently, the cell suspension was incubated with the primary antibodies directed against the specific cell surface markers (Table 2) followed by incubation with FITC‐conjugated secondary antibody. As a control, we used cells with no antibody incubation (Figure 2). Each FACS sample comprised 104 cells suspended in 200 μL of PBS.
Figure 2.
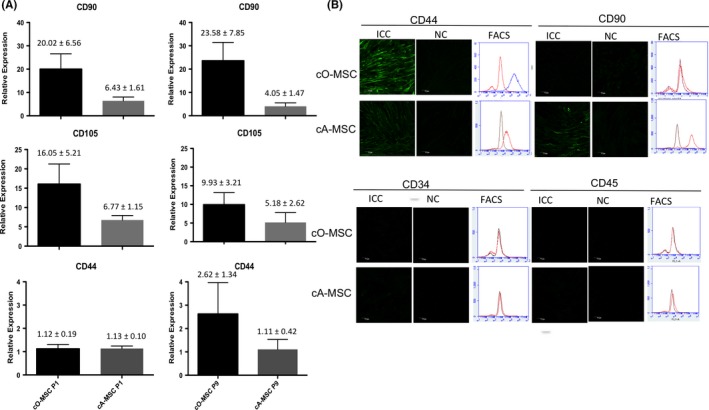
Mesenchymal markers in ovarian‐ and adipose‐derived mesenchymal stem cell (MSC). (A) Relative expression of MSC‐specific marker genes CD44,CD90 and CD105 in ovarian (cO‐MSC)‐ and adipose (cA‐MSC)‐derived cells at first (P1) and ninth (P9) passages. (B) Protein analysis by immunocytochemistry (ICC), negative control (NC) and flow cytometry (FACS) in cO‐MSC and cA‐MSC of mesenchymal cell markers (CD44 and CD90) and hematopoietic markers (CD34 and CD45). Scale bars = 70 μm
2.7. Mesodermal differentiation assay and cytochemical staining
Differentiation assay was performed by seeding 1.0 × 104 cells per 2 cm2 well in triplicate using a 24‐well culture dish and incubated for 30 days, with media replacement every 4 days. Specific differentiation kits were used in assays for osteogenic, adipogenic and chondrogenic differentiation (StemPro; Thermo Fisher Scientific). For cytochemical staining, differentiated cells were washed twice with PBS, fixed with 4% formalin for 10 minutes and washed again with PBS. Fixed cells were subjected to tissue‐specific cytochemical staining protocols according to the manufacturer's guidelines. Adipogenic differentiation was visualized by red‐coloured lipid vacuoles accumulated in differentiated cells after Oil red‐O (Sigma‐Aldrich), osteogenic differentiation by staining with Von Kossa (Sigma‐Aldrich) to visualize brown‐red colour deposited calcium and chondrogenic differentiation by staining with 0.1% Safranin‐O to detect proteoglycan‐stained cells in red.
2.8. Ectodermal (neurogenic) lineage differentiation
Cells were seeded at a density of 1.0 × 104 cells per well in triplicate in a 24‐well culture dish and incubated for 10 days, with media replacement every 4 days. The media used contained DMEM low glucose (Thermo Fisher Scientific), 2 mmol/L valproic acid, 1 μmol/L hydrocortisone, 10 μm forskolin, 5 mmol/L potassium chloride, 5 μg/mL insulin and 200 μmol/L butylated hydroxyanisole. All reagents were purchased from Sigma. To evaluate differentiation, some wells were used for qRT‐PCR analysis and for immunofluorescence for Nestin and β‐tubulin antibodies (Table 2).
2.9. Endodermal lineage differentiation
Cells were seeded at a density of 1.0 × 104 cells per well in triplicate in a 24‐well culture dish and incubated for 5 days in STEMdiff™ Definitive Endoderm media (Stem Cell TM®), according to the manufacturer's instructions. The expression of CD184 and SOX17 was evaluated to confirm differentiation to endodermal precursors.
2.10. Primordial germ cell lineage differentiation
Cells were plated onto a cover slip dish at a density of 1.0 × 104 cells per well in triplicate in a 24‐well culture dish. Cultures were performed for 2 weeks in the media used for culture of female germ cells derived from the ovary of neonatal mice.15, 16 MEM‐α, 10% FBS, 1 mmol/L sodium pyruvate, 1 mmol/L non‐essential amino acids (Life Technologies, Grand Island, NY, USA), 2 mmol/L glutamine (Sigma‐Aldrich), 0.1 mmol/L β‐mercaptoethanol (Sigma‐Aldrich), 10 ng/mL LIF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 20 μg/mL transferrin, 5 μg/mL insulin (Sigma‐Aldrich), 60 μmol/L putrescine (Sigma‐Aldrich), 10 ng/mL EGF (mouse epidermal growth factor; Sigma‐Aldrich), 40 ng/mL human GDNF (glial cell line‐derived neurotrophic factor; R&D systems, Minneapolis, MN, USA), 1 ng/mL human bFGF (basic fibroblast growth factor; BD Biosciences Clontech, Franklin Lakes, NJ, USA) and 15 mg/L penicillin (Sigma). Expression analysis of DDX4, and OCT4 by immunocytochemistry (ICC) was used to verify differentiation towards primordial germ cell lineages.
2.11. Statistical analysis
The statistical comparisons were performed using parametric t test (GraphPad Prism 5) with a level of significance set at 5% (P ≤ .05). Data in the graphs are presented as the mean ± standard deviation.
3. RESULTS
3.1. Morphological and growth characteristics of ovarian‐ and adipose‐derived cells
Initial experiments were aimed at determining whether MSC were present in the canine ovary and how they compared to adipose‐derived MSC. The average weight of the ovaries of the 4 females was 0.41 ± 0.11 g (range of 0.29‐0.85), and the average weight of the adipose tissue dissected from the gonadal region was 0.45 ± 0.14 g (range of 0.22‐0.71). Putative MSC with rapid plastic‐adhesive properties were successfully isolated from canine ovarian (cO‐MSC) and adipose (cA‐MSC) tissue of all animals. Cells harvested from both tissues adhered rapidly to the plastic surface of the culture dish and grew into homogeneous monolayer cultures with typical MSC‐like morphology (Figure 1A). Both cO‐MSC and cA‐MSC maintained stable morphology and expanded in culture for 15 passages. Adipose tissue yielded significantly more cells per gram of tissue compared to ovarian tissue (1771 ± 262 vs 1937 ± 593, P ≤ .05). Growth curve analysis (Figure 1B) indicated that cA‐MSC initiated exponential growth after 48 hours of initial culture while cO‐MSC began exponential growth 24 hours later. However, cell numbers at 120 hours after seeding were similar between ovarian and adipose‐derived MSC and the population‐doubling time measurements during the 48‐hour period following initial exponential growth indicated that both cell types showed similar growth patterns (P ≥ .05), indicating that ovarian‐ and adipose‐derived MSC showed similar overall in vitro growth characteristics. Moreover, we assessed the growth parameters at later passages (P9) and showed that no differences in growth curves or doubling were present between ovarian‐ and adipose‐derived cells, further confirming the similarity in growth characteristics of ovarian‐ and adipose‐derived MSC. Nonetheless, although cells from both tissues could form colonies at low‐density seeding (Figure 1C), more colonies were identified in adipose‐derived than in ovarian‐derived MSC (37.08 ± 2.10 vs 27.08 ± 1.45; P ≤ .05). Together, these results indicate that although adipose‐derived MSC adapt more promptly to in vitro culture conditions, MSC are present in large quantities in the canine ovary and show similar growth characteristics to adipose‐derived MSC.
Figure 1.
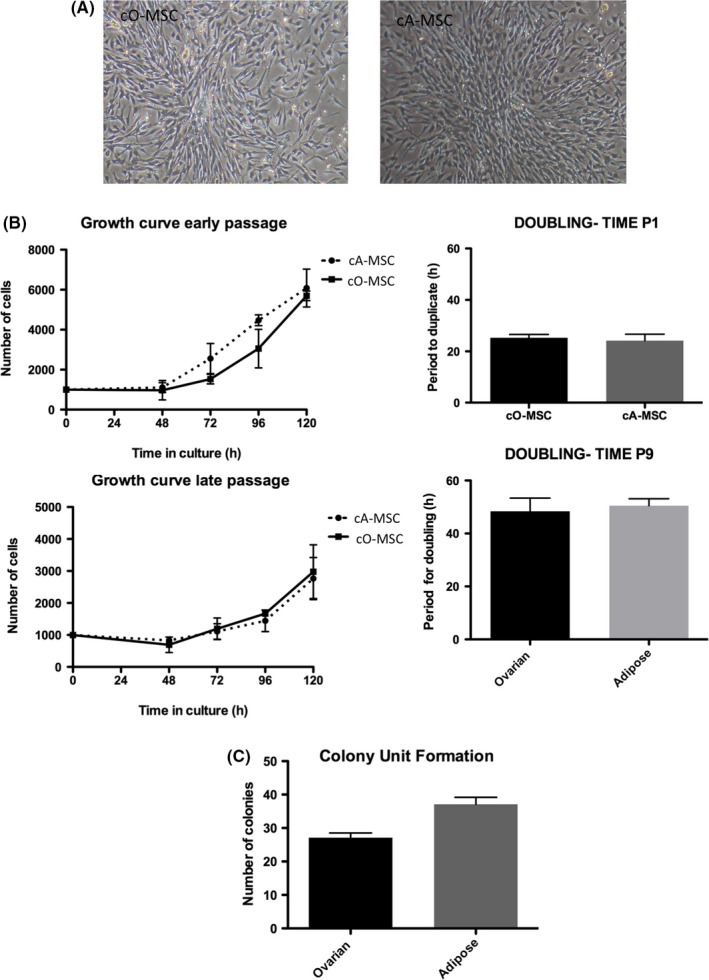
Morphological and growth characteristics of canine ovarian‐ and adipose‐derived mesenchymal stem cell (MSC). (A) Light photographs (10× and 40× objectives) showing fibroblast‐like morphology of MSC derived from ovarian tissue (cO‐MSC) and adipose tissue (cA‐MSC) at first passage. (B) Growth curve (line graph) and doubling time (histogram) of cO‐MSC and cA‐MSC at first (early; P1) and ninth (late; P9) passages. (C) Colony unit formation (histogram) and colony morphology (H&E stain; 10X objective) of cO‐MSC and cA‐MSC at initial passages
3.2. MSC‐specific markers in ovarian MSC
Having confirmed the presence and analysed the growth characteristics of ovarian MSC, we next performed a molecular validation of MSC‐specific transcripts and cell surface antigens commonly found in MSC populations. Using qRT‐PCR, we showed that the transcripts for the canine MSC markers CD44, CD90 and CD105 were present in both ovarian‐ and adipose‐derived cells (Figure 2A). Although differences were not significant between tissues, transcripts for CD90 tended to be higher in ovarian cells both at early (P = 2) and late (P = 9) passages. Moreover, ICC and FACS analysis in early passage cells confirmed the presence of the MSC‐specific cell surface antigens CD44 (cO‐MSCs, 57 ± 11%; cA‐MSCs, 78 ± 4%) and CD90 (cO‐MSCs, 38 ± 10%; cA‐MSCs, 62 ± 8%) and absence of the hematopoietic‐specific markers CD34 and CD45, further confirming that the ovary contains cells expressing an MSC‐specific phenotype‐like adipose‐derived MSC.
3.3. Pluripotency‐specific markers in ovarian MS
Having shown that the ovary contains cells that resemble MSC and express MSC‐specific markers, we next verified whether they also expressed pluripotency‐specific markers by qRT‐PCR and FACS. Transcripts for NANOG, SOX2 and TERT were present and expressed at similar levels in cO‐MSC and cA‐MSC, both at P1 and at P9 (Figure 3A). A tendency for elevated OCT4 transcripts (P = .006) was observed, with a 5‐fold higher level in cO‐MSC. This coincided with results obtained by FACS, in which a tendency to have higher levels of OCT4 expression was observed in cO‐MSC with 49.47 ± 15.17 vs 9.7 ± 2.63 for adipose MSC (Figure 3B). Together, these results indicate that MSC derived from the ovary express pluripotency markers possibly at slightly higher levels than MSC derived from adipose tissue.
Figure 3.
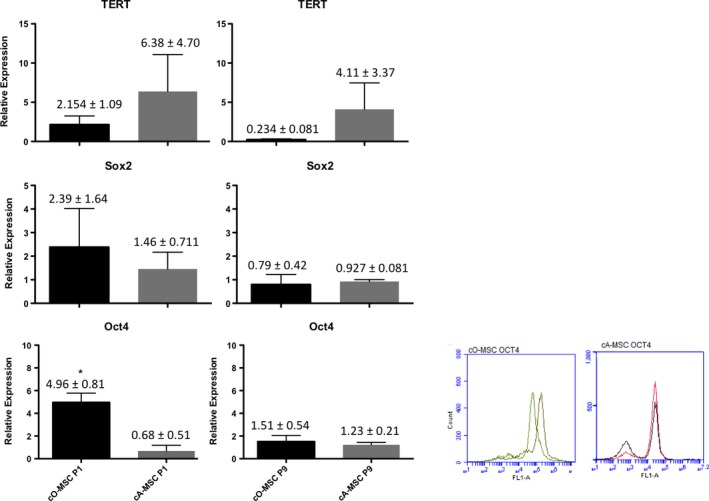
Pluripotency markers in ovarian‐ and adipose‐derived mesenchymal stem cell (MSC). (A) Relative expression of pluripotent genes OCT4,NANOG,TERT and SOX2 in canine ovarian (cO‐MSC)‐ and adipose (cA‐MSC) derived MSC at passage one (P1) and nine (P9). (B) OCT4 analysis of P1 ovarian and adipose MSC by FACS
3.4. Differentiation properties of ovarian MS‐like cells
To validate our findings from the molecular characterization, functional evaluations were performed with ovarian‐ and adipose‐derived MSC to examine their capability of differentiation into mesodermal, ectodermal, endodermal and primordial germ derivatives. Mesodermal lineage differentiation was demonstrated for MSC derived from both ovarian and adipose tissues (Figure 4A). After 30 days of exposure to specific differentiation protocols, osteogenic commitment was confirmed by the presence of calcium deposits by Von Kossa staining, chondrogenic commitment by proteoglycan staining with Safranin‐O and adipogenic commitment by lipid staining with Oil Red O. Ectodermic lineage differentiation was evidenced by immunostaining for the neuroectodermal stem cell marker Nestin and β‐tubulin after exposure of ovarian‐ and adipose‐derived MSC for 10 days to a neuronal lineage differentiation protocol. Endodermal‐specific cell morphologies and CD184 and SOX17 transcripts were observed in both ovarian‐ and adipose‐derived MSC after 5 days of exposure to specific differentiation protocols (Figure 4C). Finally, germ cell‐specific proteins DDX4 and OCT4 were identified in both ovarian‐ and adipose‐derived MSC exposed for 14 days to in vitro conditions used for culture of female germ cells derived from of neonatal mouse ovaries. Together, these results indicate that MSC derived from ovarian tissue can differentiate into a wide range of cell derivatives after exposure to specific differentiation protocols.
Figure 4.
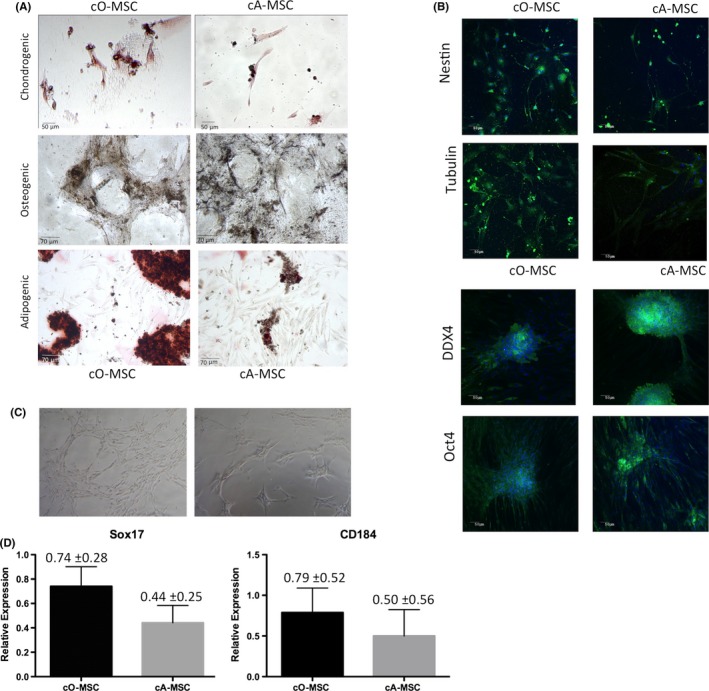
Differentiation properties of ovarian (cO‐MSC)‐ and adipose (cA‐MSC) derived MSC. (A) Mesodermal trilineage differentiation of cO‐MSC and cA‐MSC after 30 days of differentiation. Chondrogenic differentiation observed by proteoglycan staining (red). More cells differentiated (stained red) towards chondrocytes in cO‐MSC than cA‐MSC groups. Osteogenic differentiation (calcium deposits detected by Von Kossa staining) was observed after differentiation in both cO‐MSC and cA‐MSC. Adipogenic differentiation (Oil Red O staining) was observed in both cO‐MSC and cA‐MSC. More lipid droplets were present after differentiation of cO‐MSC than cA‐MSC. (B) Immunofluorescence staining of cA‐MSC and cO‐MSC for the neuronal markers Nestin and β‐tubulin. (C) Endodermal morphological characteristics and marker gene (CD184 and SOX17) expression levels in cO‐MSC and cA‐MSC exposed for 5 days to endoderm induction media. (D) Immunofluorescence staining for the primordial germ cell markers DDX4 and OCT4 of ovarian and adipose‐derived MSC exposed for 14 days to primordial germ cell culture medium
4. DISCUSSION
Stem cells in the ovary have been of interest in many studies in the past years, particularly regarding their origin and ability to undergo neo‐oogenesis. Mesenchymal stem cells are present in various tissues and play an essential role in regular tissue turnover.1 Suggestions that multipotent or pluripotent cells might be present in ovarian stromal cells7, 8 and, moreover, that germ stem cells may reside in the ovary of mice and adult humans5, 6, 7, 8, 15, 16, 17, 18 prompted our interest to study the differentiation properties of mesenchymal‐like stem cell populations from the ovary.
By exclusively selecting cells that adhered rapidly to the plastic surface of the culture dish, our ovarian‐ and adipose‐derived cell populations were indirectly selected for cell adhesion molecules, a known characteristic of MSC populations.19 To our knowledge this is the first characterization of ovarian cell populations based on their rapid adhesive capacity to plastic. Similarly to adipose‐derived MSC, ovarian‐derived MSC populations are morphologically like fibroblasts, forming colonies when cultured at low concentrations, and showing rapid proliferation that can be maintained in culture for at least fourteen passages. We demonstrated their mesenchymal nature through testing for the presence of MSC‐specific molecular markers and in vitro differentiation capacity, according to the criteria established for MSC in 2006.19
As for bone marrow,20 we demonstrate herein that ovarian‐derived MSC are 8% less abundant per tissue unit and form 30% fewer colonies after primary plating when compared to adipose‐derived MSC. However, ovarian‐derived MSC showed similar proliferation rates after standardizing the primary cultures, confirming previous reports that the doubling time depends not only on the tissue origin but also the density of the initial plating.21 Moreover, senescence signs were observed at the same passage number in both cell types, indicating that adipose‐ and ovarian‐derived MSC possess similar lifetimes in culture.22
Apart from the ability to adhere to plastic, the presence/absence of specific surface markers and differentiation properties are critical parameters for validating novel MSC populations.19 Ovarian‐derived MSC showed presence of transcripts and antigens of several markers identified in adipose canine tissues, such as CD105, CD90 and CD44, suggesting overall similarities between MSC originating from the ovary and elsewhere. In addition, both ovarian‐ and adipose‐derived MSC were negative for the hematopoietic markers CD34 and CD45. The expression of mesenchymal markers in the ovary can be explained physiologically by the fact that such cells are involved in the tissue repair process after ovulation.4 This process of wounding and repair over the course of the female reproductive life confers plasticity to the ovary, and promotes the expression of both epithelial and mesenchymal genes needed for tissue remodelling. Additionally, mesenchymal progenitor cells could possibly contribute to the development of granulosa cell‐like epithelial cells.23 CD44 is highly expressed in ovarian surface epithelium24 and CD90 plays a role in intracellular adhesion25 and was also expressed in porcine theca stem cells, which showed the ability to differentiate into osteocytes and adipocytes.26 In the mouse, CD90 was dominantly expressed in somatic compartments of ovarian follicles, suggesting that CD90 might have different functions.27 Finally, CD105 is highly expressed in germ cell compartments in the ovary, suggesting its expression might be associated with the germline stem cells from which oocytes originate.27
The presence of germ stem cells (GSC) in the mammalian ovary has been discussed extensively in recent years. It has been postulated (i) that functional GSC are present in the ovary of mammalian adults,8, 15, 16, 17, 35, 36 (ii) that the cells found in mammalian adult ovaries may be bipotent and able to give rise to granulosa and GSC,23 and (iii) that a population of very small embryonic‐like cells are present in different locations of the organism, including the ovary.37 Our results reflect an undifferentiated phenotype of ovarian cells with high differentiation potential. However, since our derivation protocol selected for adherent cells, it is likely that we excluded the less adhering pluripotent subpopulations identified in previous ovarian studies. However, although our protocol selected for cells with rapid adhesion properties, pluripotency markers OCT4, SOX2, NANOG and TERT were present both in ovarian‐ and adipose‐derived canine cells. OCT4 and NANOG have been shown to modulate the regulation of multipotentiality and proliferative and differentiation potential of MSC.28 In dogs, adipose MSC have been shown to express OCT4, NANOG, SOX2 and SSEA‐4.10, 29, 30, 31, 32, 33, 34 Moreover, human ovarian‐derived cells express OCT4, SOX2, NANOG and TERT.15, 35 We also observed TERT expression in MSC in ovarian MSC, concurring with studies in other species that showed that at least a subpopulation of MSC is telomerase‐active.22
To continue the characterization of the ovarian‐derived MSC, we performed differentiation assays as an essential step for determining whether ovarian‐derived cells met another essential standard for identification as MSC.19 Confirming previous reports showing the multipotential properties of canine MSC\ adipose tissue‐derived cells were able to differentiate into all three mesodermal lineages, i.e. able to undergo adipogenic, chondrogenic and osteogeneic differentiation.10, 31, 32, 33, 34, 38, 39, 40, 41, 42, 43 Interestingly, ovarian‐derived MSC were similarly able to differentiate into all three mesodermal cell types, indicating multipotential properties similar to adipose‐derived MSC. Moreover, since previous studies have reported neuronal differentiation from MSC33, 34, 39, 44, 45, 46 we performed neuronal differentiation assays and showed that both ovarian‐ and adipose‐derived cells express the neural‐specific markers nestin and β‐tubulin. A subpopulation of mural granulosa cells that expressed OCT4 were able to differentiate into a neurogenic lineage when cultured and expanded in media containing LIF,47 supporting the role of OCT4 in the differentiation of pluripotent stem cells into neural cells.48 We also demonstrated that ovarian‐ and adipose‐derived MSC expressed endodermal precursor markers after differentiation induction. A previous study with human ovarian MSC also demonstrated the ability of those cells to differentiate into mesenchymal, ectodermal and endodermal lineages, concurring with our results describing the high differentiation potential of the cells from the ovary.7 Canine adipose‐derived stem cells have already been shown to differentiate into germ cells,49 so our next step was to verify if the cells derived in the present paper also possessed the capability to differentiate into putative germ cells by using the culture conditions for female germ cells derived from the ovary of neonatal mice.15, 16 After 2 weeks in this media, the cells changed their morphology from fibroblast‐like to colonies of rounded cells that were positive for OCT4 and DDX4 by immunocytochemistry. This differentiation potential could eventually provide recourse for castrated dogs that owners later desire to breed.
5. CONCLUSION
The present study derived ovarian cells possessing the main characteristics of MSC by selecting for cells with rapid plastic adhesion capability. Moreover, ovarian cells demonstrated a wide range of differentiation, representing a promising tool for regenerative medicine, but further studies are necessary to verify the safety of cell transplantation before regenerative medicine therapies can be attempted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Amanda Baracho Trindade Hill made contributions to conception, design, acquisition of data, analysis and interpretation of data and drafting the manuscript.
Jacinthe Therrien made contributions to design and data acquisition.
Joaquim Mansano Garcia made contributions to conception and design.
Lawrence Charles Smith made contributions to conception and design, interpretation of data and revising for important intellectual content.
ACKNOWLEDGEMENTS
This work was supported by grants from FAPESP, NSERC and NSERC (Canada Research Chair).
Trindade Hill AB, Therrien J, Garcia JM, Smith LC. Mesenchymal‐like stem cells in canine ovary show high differentiation potential. Cell Prolif. 2017;50:e12391 10.1111/cpr.12391
Funding Information
FAPESP (process nº.2013/14293‐0), NSERC(RGPIN/06228) and NSERC (Canada Research Chair, 950‐206864)
[Correction added on 1 February 2018 after first online publication: The Corresponding Author's name has now been updated in this version.]
REFERENCES
- 1. Gazit Z, Pelled G, Sheyn D, Kimelman N, Gazit D. Mesenchymal stem cells In: Atala A, Lanza R, Thompson JA, Nerem RM, eds. Principles of Regenerative Medicine. London, UK: Academic Press, 2011:285‐304. [Google Scholar]
- 2. Del Carlo RJ, Monteiro BS, Argolo Neto NM. Células tronco e fatores de crescimento na reparação tecidual. Ciência Veterinária nos Trópicos. 2008;11:167‐169. [Google Scholar]
- 3. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003‐1007. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed N, Thompson EW, Quinn MA. Epithelial‐mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581‐588. [DOI] [PubMed] [Google Scholar]
- 5. Virant‐Klun I, Skutella T, Stimpfel M, Sinkovec J. Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells. J Biomed Biotechnol. 2011;2011: 381928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parte S, Patel H, Sriraman K, Bhartiya D. Isolation and characterization of stem cells in the adult mammalian ovary. Methods Mol Biol. 2015;1235:203‐229. [DOI] [PubMed] [Google Scholar]
- 7. Stimpfel M, Cerkovnik P, Novakovic S, Maver A, Virant‐Klun I. Putative mesenchymal stem cells isolated from adult human ovaries. J Assist Reprod Genet. 2014;31:959‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145‐150. [DOI] [PubMed] [Google Scholar]
- 9. Cibelli J, Emborg ME, Prockop DJ, et al. Strategies for improving animal models for regenerative medicine. Cell Stem Cell. 2013;12:271‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinello T, Bronzini I, Maccatrozzo L, et al. Canine adipose‐derived‐mesenchymal stem cells do not lose stem features after a long‐term cryopreservation. Res Vet Sci. 2011;91:18‐24. [DOI] [PubMed] [Google Scholar]
- 11. Guercio A, Di Marco P, Casella S, et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189‐194. [DOI] [PubMed] [Google Scholar]
- 12. Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 2003;196:176‐196. [DOI] [PubMed] [Google Scholar]
- 13. Schneider MR, Wolf E, Braun J, Kolb HJ, Adler H. Canine embryonic stem cells: state of the art. Theriogenology. 2010;74:492‐497. [DOI] [PubMed] [Google Scholar]
- 14. Horn PA, Morris JC, Neff T, Kiem HP. Stem cell gene transfer–efficacy and safety in large animal studies. Mol Ther. 2004;10:417‐431. [DOI] [PubMed] [Google Scholar]
- 15. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631‐636. [DOI] [PubMed] [Google Scholar]
- 16. Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis‐based magnetic bead sorting. Stem Cells Dev. 2011;20:2197‐2204. [DOI] [PubMed] [Google Scholar]
- 17. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive‐age women. Nat Med. 2012;18:413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pacchiarotti J, Maki C, Ramos T, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159‐170. [DOI] [PubMed] [Google Scholar]
- 19. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 20. Neupane M, Chang CC, Kiupel M, Yuzbasiyan‐Gurkan V. Isolation and characterization of canine adipose‐derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007‐1015. [DOI] [PubMed] [Google Scholar]
- 21. Fossett E, Khan WS. Optimising human mesenchymal stem cell numbers for clinical application: a literature review. Stem Cells Int. 2012;2012:465259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91‐116. [DOI] [PubMed] [Google Scholar]
- 23. Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol. 2005;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowen NJ, Walker LD, Matyunina LV, et al. Gene expression profiling supports the hypothesis that human ovarian surface epithelia are multipotent and capable of serving as ovarian cancer initiating cells. BMC Med Genomics. 2009;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saalbach A, Haustein UF, Anderegg U. A ligand of human thy‐1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy‐1‐positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882‐888. [DOI] [PubMed] [Google Scholar]
- 26. Lee YM, Kumar BM, Lee JH, et al. Characterisation and differentiation of porcine ovarian theca‐derived multipotent stem cells. Vet J. 2013;197:761‐768. [DOI] [PubMed] [Google Scholar]
- 27. Tepekoy F, Ozturk S, Sozen B, Ozay RS, Akkoyunlu G, Demir N. CD90 and CD105 expression in the mouse ovary and testis at different stages of postnatal development. Reprod Biol. 2015;15:195‐204. [DOI] [PubMed] [Google Scholar]
- 28. Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self‐renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169‐182. [DOI] [PubMed] [Google Scholar]
- 29. Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue‐derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jun C, Zhihui Z, Lu W, et al. Canine bone marrow mesenchymal stromal cells with lentiviral mHCN4 gene transfer create cardiac pacemakers. Cytotherapy. 2012;14:529‐539. [DOI] [PubMed] [Google Scholar]
- 31. Chung DJ, Hayashi K, Toupadakis CA, Wong A, Yellowley CE. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012;92:66‐75. [DOI] [PubMed] [Google Scholar]
- 32. Spencer ND, Chun R, Vidal MA, Gimble JM, Lopez MJ. In vitro expansion and differentiation of fresh and revitalized adult canine bone marrow‐derived and adipose tissue‐derived stromal cells. Vet J. 2012;191:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee KS, Nah JJ, Lee BC, et al. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013;94:144‐151. [DOI] [PubMed] [Google Scholar]
- 34. Fernandes RA, Wenceslau CV, Reginato AL, Kerkis I, Miglino MA. Derivation and characterization of progenitor stem cells from canine allantois and amniotic fluids at the third trimester of gestation. Placenta. 2012;33:640‐644. [DOI] [PubMed] [Google Scholar]
- 35. Virant‐Klun I, Zech N, Rozman P, et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76:843‐856. [DOI] [PubMed] [Google Scholar]
- 36. Johnson J, Bagley J, Skaznik‐Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Felici M, Barrios F. Seeking the origin of female germline stem cells in the mammalian ovary. Reproduction. 2013;146:R125‐R130. [DOI] [PubMed] [Google Scholar]
- 38. Zucconi E, Vieira NM, Bueno DF, et al. Mesenchymal stem cells derived from canine umbilical cord vein–a novel source for cell therapy studies. Stem Cells Dev. 2010;19:395‐402. [DOI] [PubMed] [Google Scholar]
- 39. Dissanayaka WL, Zhu X, Zhang C, Jin L. Characterization of dental pulp stem cells isolated from canine premolars. J Endod. 2011;37:1074‐1080. [DOI] [PubMed] [Google Scholar]
- 40. Schwarz C, Leicht U, Rothe C, et al. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Research in veterinary science. 2013;93:457‐462. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Zhao Y, Yang WJJ, Ge L. Preliminary Study on Dental Pulp Stem Cell–mediated Pulp Regeneration in Canine Immature Permanent Teeth. J Endod. 2013;39:195‐201. [DOI] [PubMed] [Google Scholar]
- 42. Hodgkiss‐Geere HM, Argyle DJ, Corcoran BM, et al. Characterisation and cardiac directed differentiation of canine adult cardiac stem cells. Vet J. 2012;191:176‐182. [DOI] [PubMed] [Google Scholar]
- 43. Hodgkiss‐Geere HM, Argyle DJ, Corcoran BM, et al. Characterisation and differentiation potential of bone marrow derived canine mesenchymal stem cells. Vet J. 2012;194:361‐368. [DOI] [PubMed] [Google Scholar]
- 44. Oh HJ, Park J E, Kim MJ, et al. Recloned dogs derived from adipose stem cells of a transgenic cloned beagle. Theriogenology 2011; 75; 1221‐1231. [DOI] [PubMed] [Google Scholar]
- 45. Park SS, Lee YJ, Lee SH, et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural‐induced adipose‐derived mesenchymal Stem cells. Cytotherapy. 2012;14:584‐597. [DOI] [PubMed] [Google Scholar]
- 46. Kim EY, Lee KB, Yu J, et al. Neuronal cell differentiation of mesenchymal stem cells originating from canine amniotic fluid. Hum Cell. 2014;27:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kossowska‐Tomaszczuk K, De Geyter C, De Geyter, et al. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 2009; 27.1: 210‐219. [DOI] [PubMed] [Google Scholar]
- 48. Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei Y, Fang J, Cai S, Lv C, Zhang S, Hua J. Primordial germ cell–like cells derived from canine adipose mesenchymal stem cells. Cell Prolif. 2016;49:503‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]


