Abstract
The uppermost aspect of the hair follicle, known as the infundibulum or hair canal, provides a passageway for hair shaft egress and sebum secretion. Recent studies have indicated that the infundibulum and sebaceous ducts are lined by molecularly distinct differentiated cells expressing markers including Keratin 79 and Gata6. Here, we ablated Gata6 from the skin, and observed dilation of both the hair canal and sebaceous ducts, independent of gender and hair cycle stage. Constitutive loss of Gata6 yielded only a mild delay in depilation-induced entry into anagen, while unperturbed mutant mice possessed overtly normal skin and hair. Furthermore, we noted that Keratin 79 and Gata6 expression and localization did not depend upon each other. Our findings implicate Gata6 in maintaining the upper hair follicle, and suggest that regulation of this transcription factor may be compromised in pathologies such as acne or infundibular cystic diseases that are characterized by abnormal expansion of this follicular domain.
Keywords: Gata6, K79, Krt79, skin pore, infundibulum, pilosebaceous unit, hair cycling
Background
The hair follicle canal serves a structural function, linking the surface epidermis with the mid-region of the follicle, while in the process providing an opening through which the hair shaft and sebum can exit (1). Although seemingly nondescript at first glance, this domain is colonized by a rich microflora (2, 3), is immunologically and molecularly distinct (4–8), and is perturbed in a variety of common skin diseases including acne, epidermoid cysts, hidradenitis suppurativa, keratosis pilaris and milia, among others (1, 9–11). Importantly, the unique structural features of the hair canal, also known as the infundibulum, likely modulate topical drug delivery through the skin (12, 13). Conversely, clogging of the hair canal can lead to enlarged follicular ostia or facial pores—a problem that remains a major focus of the multi-billion dollar cosmetics industry (14).
Studies in mice over the past few years have shown that the infundibulum is maintained by Lrig1+ stem cells located near the mid-section, or junctional zone region, of the hair follicle (7, 15). These stem cells also maintain the sebaceous glands, whose main function is to secrete sebum into the hair canal via the sebaceous duct (16, 17). Our previous studies have shown that the differentiated suprabasal cells that line both the hair canal and sebaceous duct can be identified by Keratin 79 (K79) (6). Gene expression experiments further showed that Lrig1 and K79 expression correlate with that of Gata6, which encodes a zinc finger transcription factor that plays multiple key roles during development (7, 18). Deletion of Gata6 has been reported to stifle hair regeneration by causing replicative stress in fast-cycling matrix progenitor cells (19), and can also lead to a reduction in upper hair follicle cells that ordinarily express this protein (20). Morphological defects in the upper hair follicle, however, have not been described in these mutants. Here, we show that both constitutive and inducible deletion of Gata6 cause expansion of the hair follicle infundibulum and sebaceous duct. Surprisingly, constitutive Gata6 mutants did not exhibit major defects in hair growth, suggesting that other Gata factors may compensate.
Question Addressed
Does Gata6 serve a role in maintaining the upper hair follicle?
Results
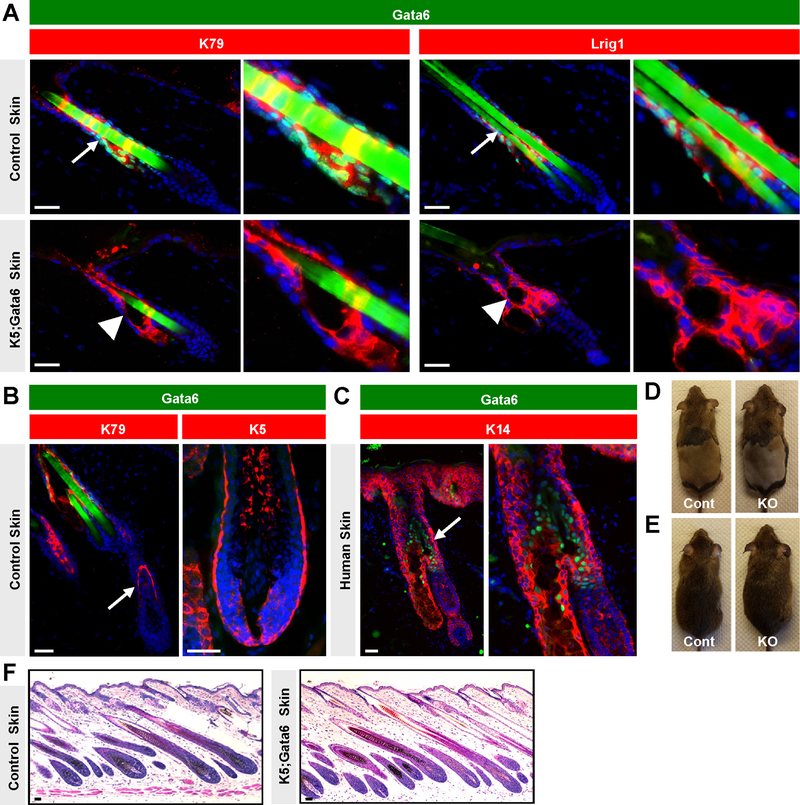
We began by assessing the localization of nuclear Gata6 in telogen hair follicles in mice and observed substantial overlap with K79 expression in suprabasal cells of the infundibulum and sebaceous ducts (Figure 1A), confirming previous findings (20). While most Lrig1+ stem cells also exhibited nuclear Gata6, expression of Lrig1 was stronger in basal cells, whereas Gata6 was elevated in suprabasal cells (Figure 1A and S1). We further noted that nuclear Gata6 was not detected in the epidermis or lower anagen follicle. This included early anagen, when matrix progenitors initially give rise to K79+ cells that form the companion layer (21), as well as later stages, when matrix cells surround the mesenchymal dermal papilla (Figure 1B). We confirmed these results using a second independent antibody against Gata6 and similarly did not detect nuclear Gata6 in matrix cells or their differentiated progeny (Figure S2). In human skin, nuclear Gata6 was also localized to suprabasal cells in the upper hair follicle, but not in the lower anagen bulb (Figure 1C and S3).
Figure 1. Gata6 localization and effects on hair cycling.
(A) Localization of nuclear Gata6 (green) in control (top) or K5;Gata6 mutant skin (bottom). Arrows, Gata6 in upper follicle. Arrowheads, dilated sebaceous ducts. Magnified views also shown. (B) Lack of Gata6 (green) in early- (left) or mid-anagen (right) control follicles. On left, K79 (red) identifies early companion layer (arrow) (21). (C) Nuclear Gata6 (green, arrow) in upper domain of human hair follicle. Magnified view on right. (D) Control (left) and K5;Gata6 male mice (right), 2 weeks post-depilation. (E) The same mice at ~20 weeks of age. (F) Histology of untreated control and K5;Gata6 anagen skin at 4 weeks of age. Scale bars, 50 μm.
To assess the function of Gata6, we generated mice expressing Keratin 5 promoter-driven Cre recombinase coupled with homozygous floxed alleles of Gata6 (K5;Gata6). K5;Gata6 mice were born in the expected Mendelian ratios and did not exhibit overt phenotypes, with the exception of a single hindlimb supernumerary preaxial digit in ~50% of mutant animals (Figure S4A), as previously reported (22). We confirmed loss of Gata6 from the upper hair follicle by immunohistochemistry, and noted that K79 and Lrig1 were both properly expressed in the absence of Gata6 (Figure 1A). Conversely, Gata6 expression was not dependent on K79 (Figure S4B). Finally, although nerves can regulate expression of stem cell markers such as Gli1 and Lgr6 in the skin (23, 24), we further noted that the domain of Gata6 expression was unchanged in denervated hair follicles (Figure S4C–D).
We next assessed hair cycling by shaving K5;Gata6 and littermate control animals at ~3.5 weeks of age to directly observe synchronized entry into anagen. In 10 gender-matched cohorts, we did not observe any major changes in hair cycling kinetics between mutant and control animals (Figure S5A). To determine whether Gata6 affects experimentally-induced hair regeneration, we depilated adult K5;Gata6 and littermate control animals at 8 weeks of age. In 5 gender-matched cohorts, we observed that mutant males displayed a mild 2–7 day delay in anagen re-entry, whereas mutant females did not differ from controls (Figure 1D and Figure S5B). All mice, irrespective of genotype, eventually regenerated and maintained a full coat of hair up to 20 weeks of age (Figure 1E). Histological analysis further revealed that untreated K5;Gata6 mutants properly entered postnatal anagen at 4 weeks of age (Figure 1F), while an independent cohort of 5 completely unperturbed K5;Gata6 mutants also did not exhibit overt hair phenotypes between 18–45 weeks of age (Figure S5C).
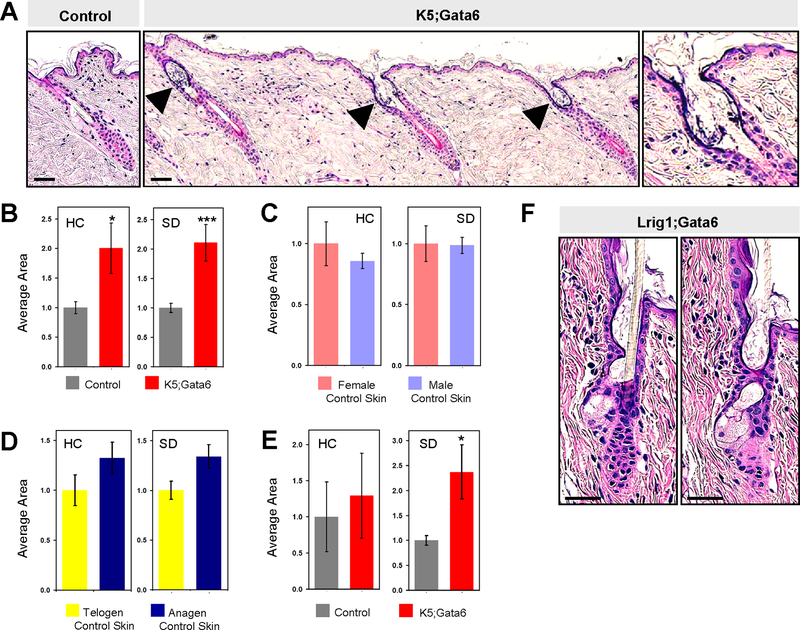
Although indistinguishable by eye, K5;Gata6 mice possessed microscopic structural defects in the upper hair follicle. We observed that both the sebaceous duct and infundibulum were dilated, with hair canals resembling early cystic lesions containing keratotic material (Figure 1A, 2A–B). These aberrent domains expressed markers of epidermal and infundibular differentiation (Figure S6), did not upregulate Gata3 (Figure S7), and were not associated with increased skin inflammation (Figure S8). In control animals, hair canal and sebaceous duct size did not vary by gender but increased slightly during anagen (Figure 2C–D); nonetheless, sub-group analysis revealed that the differences seen in K5;Gata6 mice largely persisted even when controlling for gender and overall hair cycle stage (Figure S9). In immature 4 week old animals, K5;Gata6 mutants already exhibited enlarged sebaceous ducts (Figure 2E). Finally, we generated mice harboring tamoxifen-inducible Lrig1 promoter-driven Cre recombinase coupled with Gata6 floxed alleles (Lrig1;Gata6 mice) to induce deletion in adults. As before, we confirmed loss of Gata6 (Figure S1), and observed that Lrig1;Gata6 mice recapitulated the appearance of dilated hair canal and sebaceous ducts, ~13 weeks after deletion (Figure 2F). Altogether, these findings support a role for Gata6 in maintaining proper upper hair follicle morphology.
Figure 2. Loss of Gata6 causes upper hair follicle expansion.
(A) Dorsal skin from control (left) or K5;Gata6 (right) littermate mice at ~20 weeks of age. Arrowheads, dilated hair canals, magnified in right panel. (B) Hair canal (HC) or sebaceous duct (SD) area in control or K5;Gata6 mice at ~20 weeks of age. (C) HC and SD area in control mice at ~20 weeks of age, sub-divided by gender or hair cycle stage (D). (E) HC and SD area in K5;Gata6 and control mice at 4 weeks of age. (F) Consecutive sections from Lrig1;Gata6 follicle, 13 weeks post-tamoxifen. *, p < 0.05; ***, p < 0.001. Scale bars, 50 μm.
Conclusions and Discussion
Our findings differ from those of Wang et al., who previously reported that nuclear Gata6 is present in the epidermis, infundibulum, matrix and inner root sheath, and plays a crucial role during anagen (19). Recent gene expression studies have also shown that Gata6 mRNA is expressed in hair follicle stem cells and matrix progenitors (25, 26). Although these discrepancies in Gata6 mRNA and protein localization are difficult to reconcile, it should be noted that our hair cycle studies utilized mice where Gata6 was constitutively deleted from the skin, whereas Wang et al., inducibly and acutely deleted Gata6. Thus, it is conceivable that chronic loss of Gata6 enabled other Gata factors to compensate during hair growth, yielding a milder phenotype. Indeed, Gata4 can play overlapping roles with Gata6 during pancreas and cardiovascular development (27–29). At the same time, the lack of a major hair cycling defect in Gata6 mutants is also consistent with our observation that Gata6 protein is not readily detected in the lower anagen follicle.
As the mechanism underlying hair canal dilation in Gata6 mutants remains unclear, we cannot formally rule out the possibility that loss of Gata6 affects the structural integrity of the upper follicle, possibly leading to histological artifacts. However, consistent with our findings, loss of Gata6 has previously been associated with cyst formation in other organs such as the pancreas and ovary (30, 31). In the skin, the dilated hair canals are reminiscent of early utriculi-like structures that appear in hairless (hr) mice or when Notch signaling is suppressed in Lrig1+ stem cells (6, 32). Disruption of Notch pathway components causes cyst formation in the hair follicle resembling nevus comedonicus (33), while mutations in γ-secretase, which activates Notch, have been associated with hidradenitis suppurativa (34). Whether a functional link exists between Notch and Gata6 is tantalizing but currently remains unclear and will require further investigation.
Experimental Design
Mouse strains included Gata6tm2.1Sad/J, K79tm2a, Tg(KRT5-cre)5132Jl and Lrig1tm1.1(cre/ERT2)Rjc /J (21, 35–37). Gata6 antibodies were from Cell Signaling (D61E4) or provided by Dr. Xiang-Xi Xu (38). Denervation was described previously (39). For additional details, see Supplementary Materials.
Supplementary Material
Figure S1. Localization of Gata6 and deletion in Lrig1;Gata6 mutant hair follicles. Top panels, co-localization of nuclear Gata6 (green) with Lrig1 (red) in control skin. Bottom, loss of Gata6 in Lrig1;Gata6 mice, 3 days after tamoxifen-induced gene deletion. Arrows point to the mid-section of the telogen hair follicle, magnified in the middle panels. Right panels depict the same view without DAPI staining. Note that nuclear Gata6 is enriched in inner, suprabasal cells, whereas Lrig1 is also expressed in the basal cell layer (dotted line). Scale bars, 50 μm.
Figure S2. Validation of Gata6 localization using an independent antibody. Upper panels, nuclear Gata6 (green) is detected in the upper hair follicle, but not in the lower anagen bulb (bottom panels). Right panels are magnified views of the boxed areas, with DAPI omitted for clarity. Asterisk, hair shaft autofluorescence. Arrows, nuclear Gata6. Bottom images were taken after long exposure to confirm lack of nuclear Gata6 in the anagen bulb. These stainings were performed using a Gata6 antibody generated by Dr. Xiang-Xi (Mike) Xu (38). Scale bars, 50 μm.
Figure S3. Gata6 is localized to the upper hair follicle in human skin. Nuclear Gata6 (green, arrows) is localized to the upper follicle in normal facial skin. Hair follicles in telogen (left panel), early anagen (middle panel) and more advanced anagen (right panel) are depicted. Note the absence of staining in the lower anagen bulb and epidermis. The middle panel is also shown in Figure 1C. Scale bars, 50 μm.
Figure S4. Gata6 localization is unaffected in the absence of K79 and hair follicle innervation. (A) A subset of K5;Gata6 mutant mice exhibit a single supernumerary digit in the hindlimb (arrow, right), with the other hindlimb unaffected (left). (B) Gata6 expression (green) in control (top) and K79-deficient (bottom) skin. (C) Sham-operated (top panels) and denervated skin (bottom panels) stained for neurofilament (NF, green) or K5 (red). (D) Sham-operated (top panels) and denervated skin (bottom panels) stained for Gata6 (green) and Lrig1 (red). Arrows in (B) and (D) point to the mid-section of the telogen hair follicle, magnified in the adjoining right panels. Arrow in (C) points to innervation of the upper bulge region, which is lost upon denervation. Scale bars, 50 μm.
Figure S5. K5;Gata6 mutant male mice exhibit a mild delay in experimentally-induced hair regeneration. (A) Visual observations of hair cycling in K5;Gata6 (KO) or control littermate animals, sub-divided by gender, after shaving at ~3.5 weeks of age. (B) Visual observations of experimentally-induced anagen re-entry in K5;Gata6 or control littermate animals, after depilation at 8 weeks of age. (C) Visual observations of 5 unperturbed K5;Gata6 mutant mice at the indicated ages.
Figure S6. Dilated hair canals in K5;Gata6 mutant mice express epidermal and infundibular differentiation markers. Top, control skin showing expression of K14 (red) in the basal cell layer, and differentiation markers (green) in the suprabasal layer, as indicated. Note that expression of these differentiation markers is found in suprabasal cells of both the interfollicular epidermis and hair canal (arrows). Bottom, these same markers are expressed in dilated hair canals (arrows) from K5;Gata6 mutant mice at 20 weeks of age. Scale bars, 50 μm.
Figure S7. K5;Gata6 mutant hair follicles do not exhibit ectopic Gata3 expression. Control (top) and K5;Gata6 mutant (bottom) anagen skin at 4 weeks of age display proper expression of Gata3 (green) in the inner root sheath (arrows) and not elsewhere in the follicle. Surrounding staining in the dermis is background staining. Scale bars, 50 μm.
Figure S8. K5;Gata6 mutant skin does not exhibit an overall increase in inflammation. (A) Control (top) and K5;Gata6 mutant (bottom) telogen skin at 20 weeks of age possess similar numbers of inflammatory cells, as assessed by staining for the pan-leukocyte marker CD45 (green). Two typical fields are shown for both control and mutant skin. (B) Quantitation for (A), where the average number of CD45+ leukocytes per low-power field in control skin was normalized to ‘1’. Scale bars, 50 μm.
Figure S9. K5;Gata6 mutant mice possess expanded upper hair follicle domains. (A) Average hair canal (HC) or sebaceous duct (SD) area in control or K5;Gata6 mice at ~20 weeks of age, sub-divided by gender, or hair cycle stage (B). Most comparisons approached, but did not reach, statistical significance due to smaller numbers of independent samples in sub-group analyses. *, p < 0.05; **, p < 0.01.
Acknowledgements
We thank Drs. Michele Battle for Gata6 mice and Mike Xu for Gata6 antibody. These studies were supported by the NIH (R01AR065409), the University of Michigan Department of Dermatology, the Biological Sciences Scholars Program, and the Center for Organogenesis.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res 2014: 358: 697–704. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, Christoph T, Müller-Röver S. Immunology of the hair follicle: a short journey into terra incognita. J Investig Dermatol Symp Proc 1999: 4: 226–234. [DOI] [PubMed] [Google Scholar]
- 3.Chronnell CM, Ghali LR, Ali RS, et al. Human beta defensin-1 and −2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol 2001: 117: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 4.Bertolini M, Meyer KC, Slominski R, et al. The immune system of mouse vibrissae follicles: cellular composition and indications of immune privilege. Exp Dermatol 2013: 22: 593–598. [DOI] [PubMed] [Google Scholar]
- 5.Nagao K, Kobayashi T, Moro K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 2012: 13: 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veniaminova NA, Vagnozzi AN, Kopinke D, et al. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development 2013: 140: 4870–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page ME, Lombard P, Ng F, et al. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 2013: 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joost S, Zeisel A, Jacob T, et al. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst 2016: 3: 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol 2009: 18: 821–832. [DOI] [PubMed] [Google Scholar]
- 10.Zaenglein AL, Graber EM, Thiboutot DM. Acne vulgaris and acneiform eruptions. In: Goldsmith LA, Katz SI, Gilchrest BA, et al. , eds. Fitzpatrick’s dermatology in general medicine Chicago: McGraw Hill Medical, 2012: 897–917. [Google Scholar]
- 11.Thomas VD, Snavely NR, Lee KK, et al. Cysts of epidermal origin. In: Goldsmith LA, Katz SI, Gilchrest BA, et al. , eds. Fitzpatrick’s dermatology in general medicine Chicago: McGraw Hill Medical, 2012: 1333–1336. [Google Scholar]
- 12.Lademann J, Richter H, Schaefer UF, et al. Hair follicles - a long-term reservoir for drug delivery. Skin Pharmacol Physiol 2006: 19: 232–236. [DOI] [PubMed] [Google Scholar]
- 13.Illel B, Schaefer H, Wepierre J, et al. Follicles play an important role in percutaneous absorption. J Pharm Sci 1991: 80: 424–427. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Seok J, Jeong SY, et al. Facial Pores: Definition, Causes, and Treatment Options. Dermatol Surg 2016: 42: 277–285. [DOI] [PubMed] [Google Scholar]
- 15.Jensen KB, Collins CA, Nascimento E, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009: 4: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 2012: 23: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinde E, Haslam IS, Schneider MR, et al. A practical guide for the study of human and murine sebaceous glands in situ. Exp Dermatol 2013: 22: 631–637. [DOI] [PubMed] [Google Scholar]
- 18.Santegoets LA, Seters M v, Helmerhorst TJ, et al. HPV related VIN: highly proliferative and diminished responsiveness to extracellular signals. Int J Cancer 2007: 121: 759–766. [DOI] [PubMed] [Google Scholar]
- 19.Wang AB, Zhang YV, Tumbar T. Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J 2017: 36: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donati G, Rognoni E, Hiratsuka T, et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat Cell Biol 2017: 19: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesler AL, Veniaminova NA, Lull MV, et al. Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep 2017: 19: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozhemyakina E, Ionescu A, Lassar AB. GATA6 is a crucial regulator of Shh in the limb bud. PLoS Genet 2014: 10: e1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao XH, Nguyen H. Epidermal expression of Lgr6 is dependent on nerve endings and Schwann cells. Exp Dermatol 2014: 23: 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brownell I, Guevara E, Bai CB, et al. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011: 8: 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezza A, Wang Z, Sennett R, et al. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep 2016: 14: 3001–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Adams RC, Ge Y, et al. Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell 2017: 169: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin M, Davis CA, Molkentin JD, et al. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA 2006: 103: 11189–11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Watt AJ, Battle MA, et al. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol 2008: 317: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xuan S, Sussel L. GATA4 and GATA6 regulate pancreatic endoderm identity through inhibition of hedgehog signaling. Development 2016: 143: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xuan S, Borok MJ, Decker KJ, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest 2012: 122: 3516–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai KQ, Caslini C, Capo-Chichi CD, et al. Loss of GATA4 and GATA6 expression specifies ovarian cancer histological subtypes and precedes neoplastic transformation of ovarian surface epithelia. PLoS One 2009: 4: e6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panteleyev AA, van der Veen C, Rosenbach T, et al. Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol 1998: 110: 902–907. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Lin MH, Tian X, et al. gamma-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell 2004: 7: 731–743. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Yang W, Wen W, et al. gamma-secretase gene mutations in familial acne inversa. Science 2010: 330: 1065. [DOI] [PubMed] [Google Scholar]
- 35.Powell AE, Wang Y, Li Y, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012: 149: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez A, Page A, Gandarillas A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis 2004: 39: 52–57. [DOI] [PubMed] [Google Scholar]
- 37.Sodhi CP, Li J, Duncan SA. Generation of mice harbouring a conditional loss-of-function allele of Gata6. BMC Dev Biol 2006: 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai KQ, Capo-Chichi CD, Rula ME, et al. Dynamic GATA6 expression in primitive endoderm formation and maturation in early mouse embryogenesis. Dev Dyn 2008: 237: 2820–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson SC, Brownell I, Wong SY. Cutaneous surgical denervation: a method for testing the requirement for nerves in mouse models of skin disease. J Vis Exp 2016: doi: 10.3791/54050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Localization of Gata6 and deletion in Lrig1;Gata6 mutant hair follicles. Top panels, co-localization of nuclear Gata6 (green) with Lrig1 (red) in control skin. Bottom, loss of Gata6 in Lrig1;Gata6 mice, 3 days after tamoxifen-induced gene deletion. Arrows point to the mid-section of the telogen hair follicle, magnified in the middle panels. Right panels depict the same view without DAPI staining. Note that nuclear Gata6 is enriched in inner, suprabasal cells, whereas Lrig1 is also expressed in the basal cell layer (dotted line). Scale bars, 50 μm.
Figure S2. Validation of Gata6 localization using an independent antibody. Upper panels, nuclear Gata6 (green) is detected in the upper hair follicle, but not in the lower anagen bulb (bottom panels). Right panels are magnified views of the boxed areas, with DAPI omitted for clarity. Asterisk, hair shaft autofluorescence. Arrows, nuclear Gata6. Bottom images were taken after long exposure to confirm lack of nuclear Gata6 in the anagen bulb. These stainings were performed using a Gata6 antibody generated by Dr. Xiang-Xi (Mike) Xu (38). Scale bars, 50 μm.
Figure S3. Gata6 is localized to the upper hair follicle in human skin. Nuclear Gata6 (green, arrows) is localized to the upper follicle in normal facial skin. Hair follicles in telogen (left panel), early anagen (middle panel) and more advanced anagen (right panel) are depicted. Note the absence of staining in the lower anagen bulb and epidermis. The middle panel is also shown in Figure 1C. Scale bars, 50 μm.
Figure S4. Gata6 localization is unaffected in the absence of K79 and hair follicle innervation. (A) A subset of K5;Gata6 mutant mice exhibit a single supernumerary digit in the hindlimb (arrow, right), with the other hindlimb unaffected (left). (B) Gata6 expression (green) in control (top) and K79-deficient (bottom) skin. (C) Sham-operated (top panels) and denervated skin (bottom panels) stained for neurofilament (NF, green) or K5 (red). (D) Sham-operated (top panels) and denervated skin (bottom panels) stained for Gata6 (green) and Lrig1 (red). Arrows in (B) and (D) point to the mid-section of the telogen hair follicle, magnified in the adjoining right panels. Arrow in (C) points to innervation of the upper bulge region, which is lost upon denervation. Scale bars, 50 μm.
Figure S5. K5;Gata6 mutant male mice exhibit a mild delay in experimentally-induced hair regeneration. (A) Visual observations of hair cycling in K5;Gata6 (KO) or control littermate animals, sub-divided by gender, after shaving at ~3.5 weeks of age. (B) Visual observations of experimentally-induced anagen re-entry in K5;Gata6 or control littermate animals, after depilation at 8 weeks of age. (C) Visual observations of 5 unperturbed K5;Gata6 mutant mice at the indicated ages.
Figure S6. Dilated hair canals in K5;Gata6 mutant mice express epidermal and infundibular differentiation markers. Top, control skin showing expression of K14 (red) in the basal cell layer, and differentiation markers (green) in the suprabasal layer, as indicated. Note that expression of these differentiation markers is found in suprabasal cells of both the interfollicular epidermis and hair canal (arrows). Bottom, these same markers are expressed in dilated hair canals (arrows) from K5;Gata6 mutant mice at 20 weeks of age. Scale bars, 50 μm.
Figure S7. K5;Gata6 mutant hair follicles do not exhibit ectopic Gata3 expression. Control (top) and K5;Gata6 mutant (bottom) anagen skin at 4 weeks of age display proper expression of Gata3 (green) in the inner root sheath (arrows) and not elsewhere in the follicle. Surrounding staining in the dermis is background staining. Scale bars, 50 μm.
Figure S8. K5;Gata6 mutant skin does not exhibit an overall increase in inflammation. (A) Control (top) and K5;Gata6 mutant (bottom) telogen skin at 20 weeks of age possess similar numbers of inflammatory cells, as assessed by staining for the pan-leukocyte marker CD45 (green). Two typical fields are shown for both control and mutant skin. (B) Quantitation for (A), where the average number of CD45+ leukocytes per low-power field in control skin was normalized to ‘1’. Scale bars, 50 μm.
Figure S9. K5;Gata6 mutant mice possess expanded upper hair follicle domains. (A) Average hair canal (HC) or sebaceous duct (SD) area in control or K5;Gata6 mice at ~20 weeks of age, sub-divided by gender, or hair cycle stage (B). Most comparisons approached, but did not reach, statistical significance due to smaller numbers of independent samples in sub-group analyses. *, p < 0.05; **, p < 0.01.