Abstract
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous, synthetic anthropogenic chemicals known to infiltrate and persist in biological systems as a result of their stability and bioaccumulation potential. This study investigated 15 PFAS, including short-chain carboxylic and sulfonic acids, and their presence in a threatened herbivore, the West Indian manatee (Trichechus manatus). Seven of the 15 PFAS examined were detected in manatee plasma. Perfluorooctanesulfonic acid (PFOS) (ranging from 0.13 to 166 ng/g ww) and perfluorononanoic acid (PFNA) (ranging from 0.038 to 3.52 ng/g ww) were detected in every manatee plasma sample examined (n = 69), with differing medians across sampling sites in Florida, Crystal River (n = 39), Brevard County (n = 18), Everglades National Park (n = 8), and four samples (n = 4) from Puerto Rico. With an herbivorous diet and long life-span, the manatee provides a new perspective to monitoring PFAS contamination.
Keywords: PFAS, PFOS, West Indian manatee, health parameters, Florida, Puerto Rico
INTRODUCTION:
Marine mammals have been utilized as sentinel species to assess biological responses to environmental stressors, such as exposure to contaminants, climate changes and diseases (1–6). Likewise, to humans, wild marine mammals are exposed to a highly variable environment and therefore can act as models to study the exposure and effects of complex chemical burdens. In addition, chemical bioaccumulation through trophic levels, especially in the aquatic environment, allows the employment of fish-eating predators as sentinels to offer additional insight for translation to environmental and ultimately human health (7, 8). For example, exogenous chemical burdens, inclusive of perfluorinated compounds, organohalogens, and flame retardant materials, have been studied in marine mammals such as bottlenose dolphins (Tursiops truncatus), killer whales (Orcinus orca), and northern fur seals (Callorhinus ursinus) (9–12). Attention has been placed on monitoring perfluoroalkyl substances (PFAS) due to an increased presence in the environment, wildlife, and humans (13–15). These commonly utilized synthetic and toxic chemicals are noted to have long half-lives, are resistant to environmental degradation, and bioaccumulate (16). Most notably, they are known to be used in products such as some cookware, paints, and firefighting foams due to their water-repellant properties (17, 18). An increasing number of adverse health outcomes have been associated with PFAS exposure, in both wildlife and humans (15–16, 19–23). A primary focus of PFAS analyses on marine wildlife has been placed on examining predatory species, due to the route of exposure (fish consumption) and concern over bioaccumulation and biomagnification (9, 24–27). However, to date, no studies have examined the PFAS burden in large herbivorous marine mammals, which represents a current gap in PFAS exposure research.
Population and foraging ecology, as well as the habitat status, husbandry, health and morphology of the West Indian manatee (Trichechus manatus) have been studied extensively as a result of its wavering status under the Endangered Species Act (ESA) (28–38). However, despite these efforts to learn more about and protect the manatee, there have only been a few studies analyzing their chemical contaminant burden (1, 37–40). The Florida and Antillean manatees (subspecies of the West Indian manatee) live in warm waters, close to the shores of Florida and Puerto Rico (29). Previous studies suggest the resilience of manatees in regard to disease, with little record of widespread outbreaks in the past and a relatively long life-span of about sixty years (41). However, the manatee’s history of endangerment is universally acknowledged as a result of human related impacts, such as boat strikes and entanglement in discarded fishing gear (42). In addition, with their close proximity to human populated areas, manatees can ingest, inhale, or come in dermal contact with anthropogenic compounds introduced into coastal waterways leading to increased opportunities of exposure. Because PFAS are ubiquitous, the need to expand efforts describing their presence are necessary to completely determine their biological impact (43).
This study assessed PFAS burden in the West Indian manatee in an effort to determine the presence of these chemicals in a lower trophic mammal. Manatees inhabiting three coastal sites in Florida were examined (Brevard County, Crystal River, and Everglades National Park). The difference among sites provided an opportunity to investigate PFAS burden within differing habitats. Each of the three sites have been identified as common locations for manatees year-round as a result of the relatively warm water temperatures. Crystal River and locations within Brevard County (such as the Banana and Indian rivers) are known designated critical habitats for the West Indian manatee (44). A fourth site consisted of a pilot-size sample set (n = 4) from two places in Puerto Rico, Guayanilla and Cabo Rojo. Considered the most endangered marine mammal in Puerto Rico (45), research concerning manatee health is essential in protecting the current population of manatees. The entire manatee plasma cohort (n = 69) was assessed for the presence and concentration of 15 different PFAS. The PFAS concentrations obtained were then related to the location of the sampling site. Correlations between PFAS values and physical morphology (e.g. sex) were also investigated.
MATERIALS AND METHODS:
Sample collection
Plasma samples from West Indian manatees (n = 69) were collected by researchers from the U.S. Geological Survey (USGS) Sirenia Project between 2003 and 2017 (USFWS Research permit: MA-791721, USGS IACUC permit: USGS-WARC-2016–03, and previous permits). Samples were collected in Florida (n = 65), among the following three sites (Supplemental Fig 1 and Supplemental Fig 2), Crystal River (CR) (n = 39), Brevard County (BC) (n = 18), and Everglades National Park (EP) (n = 8). Four additional samples were collected in Puerto Rico (PR, Supplemental Fig 1 and Supplemental Fig 3) in Guayanilla (n = 3) and Cabo Rojo (n = 1). Each manatee was captured and sampled utilizing established sampling procedures (40) and then released. Hematology and morphometrics (length, weight, sex, and body condition indices (BCI); Supplemental Table 1) were obtained by research biologists and veterinarians referring to previously outlined methods (31, 46).
PFAS Standards and SRM 1950
Samples were analyzed for the following 15 PFAS by using a previously published protocol: perfluorobutyric acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTriA), perfluorotetradecanoic acid (PFTA), perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS), perfluorooctanesulfonic acid (PFOS), and perfluorooctanesulfonamide (PFOSA) (47, 48). An internal standard (IS) solution was made (Cambridge Isotope Laboratories, RTI International, and Wellington Laboratories), and consisted of 13C4-PFBA, 13C2-PFHxA, 18O2-PFHxS, 13C8-PFOA, 13C9-PFNA, 13C9-PFDA, 13C2-PFUnA, 13C2-PFDoA, 18O2-PFBS, 13C4-PFOS, and 18O2-PFOSA (48). In order to identify the PFAS within the plasma, samples were extracted using methodology described in a prior publication (48). The National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1950 Metabolites in Human Plasma (n = 3), that provides concentration values for six PFAS as reference values, was prepared and analyzed along with samples, functioning as a quality control sample (http://srm1950.nist.gov/).
Sample preparation
In brief, the method consisted of utilizing either 1.0 g of each manatee plasma sample, 1.0 g of deionized water (blank) for each analysis (n = 3), or 1.0 g of SRM 1950 for each analysis (n = 3). The IS solution was gravimetrically added in 600 μL increments to the samples, blanks, and SRMs before extraction (49). After equilibrating for 1.5 h, 4 mL of acetonitrile was added to each tube, sonicated for 30 min, and centrifuged for 5 min at 2500 rpm. The supernatant was then pipetted out of the Falcon tubes and transferred into glass vials. A TurboVap LV (Biotage, Charlotte, NC) was used to exchange the acetonitrile solvent to methanol and was evaporated to 2 mL with nitrogen at 35 °C. The sample in the 2 mL vials was purified using a Supelco Supelclean ENVI-Carb (Supelco, Bellefonte, PA) solid phase extraction cartridge. The extracts were then evaporated to 1 mL under nitrogen gas in the TurboVap LV. At 1 mL, the samples were added to autosampler vials and analyzed for PFAS using LC-MS/MS. The analysis of samples was conducted using an Agilent 1100 HPLC (Santa Clara, CA) coupled with an Applied Biosystems API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Each sample was injected onto a Kinetex PFP LC column (50 mm x 3 mm, 2.6 μm; Phenomenex, Torrance, CA). The autosampler temperature was 18 °C. The PFAS were separated using a flow rate of 150 μL/min and a gradient elution scheme (Supplemental Table 2) with mobile phase [A] 20 mmol ammonium acetate in methanol and [B] 20 mmol ammonium acetate in water. Multiple-reaction monitoring (MRM) transitions were used to detect and quantify PFAS, one transition for the quantitation of each PFAS and one transition for validation to confirm identification (49).
Quantification
The data produced by the LC-MS/MS was processed for quantitation using Analyst 1.6.2 software. Each PFAS was quantified using a linear equation of the calibration curve. The method detection limits (MDL) were calculated as either the maximum value of the average mass in the extract (ng) plus three times the standard deviation of blanks, divided by the mass of the sample (g) or the lowest calibrant detected, divided by the mass of the sample. The concentrations determined include branched and linear isomers.
Statistical Analysis
IBM SPSS statistical software (version 23) and JMP (12.1.0) were used to identify and investigate correlations between PFAS levels and site differences. Normality was tested in the data by utilizing the Kolmogorov-Smirnov test, in which the Lilliefors correction was implemented as provided by SPSS. Both Pearson and Spearman methods were used to determine correlations in normal (PFNA, PFOS, PFDA, PFUnA, PFOSA, and PFDoA) and non-normal data (PFHxS values), respectively. Kruskal-Wallis testing was utilized to compare differences in PFAS levels among sites that had detections in more than 60 % of the samples, and a Dunn all pairs posthoc was conducted on identified significant relationships. For the Kruskal-Wallis, samples that were below the limit of detection were reassigned a value of zero to ensure that they would be tied in ranking statistical tests.
RESULTS AND DISCUSSION:
Each manatee plasma sample analyzed (n = 69) contained at least two PFAS of detectable abundance (PFNA and PFOS), consistent with a previous study done with American alligators from the Southeast US (47). Beyond the PFNA and PFOS burden, a number of individuals also had PFHxS, PFUnA and PFDA (percent frequency of detection, 94 %, 88 %, and 81 %, respectively, as shown in Table 1). Several PFAS, such as PFOSA and PFDoA, were infrequently measured, only detected in 6 % (n = 4 samples) and 19 % (n = 13 samples) of the total number of samples (n = 69) respectively (Table 1). The following PFAS were not detected in any of the manatee plasma samples: PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFTriA, PFTA, and PFBS. Many of these congeners are characterized as short-chain perfluoroalkyl acids and have been found to be less bioaccumulative than long-chain analogues such as PFOS (14). Thus, their infrequency of detection in this particular study supports these prior results. However, the lack of PFOA in the manatee samples presents an interesting point of further investigation, due to its categorization as a long-chain perfluoroalkyl acid with a high tendency to bioaccumulate in other species (14). PFAS concentration values for each manatee are shown in Supplemental Table 3
Table 1.
Summary statistics of PFAS detected above method detection limit (MDL). Concentrations are in ng/g (wet weight).
| PFHxS | PFNA | PFOS | PFDA | PFUnA | PFOSA | PFDoA | |
|---|---|---|---|---|---|---|---|
| Detected | 65 | 69 | 69 | 56 | 61 | 4.0 | 13 |
| % > MDL | 94 | 100 | 100 | 81 | 88 | 6.0 | 19 |
| Median | 0.105 | 0.233 | 5.41 | 0.228 | 0.123 | 0.0985 | 0.0370 |
| Min | < 0.012 | 0.038 | 0.13 | < 0.009 | < 0.010 | < 0.011 | < 0.009 |
| Max | 3.40 | 3.52 | 166 | 3.08 | 1.49 | 0.123 | 0.0950 |
The total number of samples, n = 69. “Detected” indicates the number of manatees with a concentration for the PFAS above the method detection limit (MDL). “<” signifies the MDL. Wet weight refers to the weight of material inclusive of the presence of water.
PFOS was determined to be the highest PFAS in manatee plasma by concentration among all sites sampled, reaching a maximum of 166 ng/g wet mass for one manatee in Brevard County (Table 2 and Supplemental Table 3). Median PFAS values were also compared by site in which PFHxS, PFDA, PFOSA, and PFDoA display a median less than the method detection limit or zero (Table 2). Throughout all of the manatee samples, PFOS was the most abundant PFAS detected, comprising at least 75 % of each sites’ overall PFAS burden (Supplemental Fig 4).
Table 2.
PFAS median (ng/g ww) and range for all PFAS measured in manatees across sites
| Location | PFHxS | PFNA | PFOS | PFDA | PFUnA | PFOSA | PFDoA | |
|---|---|---|---|---|---|---|---|---|
| BC (n = 18) | % of samples detected in | 100 | 100 | 100 | 100 | 100 | 0.05 | 0.22 |
| Median | 0.852 | 0.453 | 29.3 | 0.336 | 0.176 | 0.0320 | 0.0235 | |
| Range | 3.04 | 0.950 | 162 | 0.690 | 0.330 | 0 | 0.0400 | |
| CR (n = 39) | % of samples detected in | 100 | 100 | 100 | 100 | 100 | 0.02 | 0.18 |
| Median | 0.0920 | 0.185 | 5.05 | 0.159 | 0.0930 | 0.0990 | 0.0370 | |
| Range | 0.490 | 3.48 | 135 | 3.08 | 1.49 | 0 | 0.130 | |
| EP (n = 8) | % of samples detected in | 100 | 100 | 100 | 100 | 100 | 0.25 | 0.25 |
| Median | 0.0405 | 0.325 | 1.49 | 0.188 | 0.187 | 0.111 | 0.0660 | |
| Range | 0.420 | 1.29 | 12.8 | 1.55 | 1.03 | 0.0200 | 0.0600 | |
| PR (n = 4) | % of samples detected in | 100 | 100 | 100 | 100 | 100 | 0 | 0 |
| Median | 0 | 0.0845 | 0.698 | 0 | 0.0755 | - | - | |
| Range | 0.020 | 0.080 | 0.56 | 0.020 | 0.050 | - | - |
(Brevard County- BC, Crystal River- CR, Everglades National Park- EP, Puerto Rico- PR), n = number of samples the specific PFAS was detected in for each site. Total sample sizes for each site were BC (n = 18), CR (n = 39), EP (n = 8), and PR (n = 4).
Surprisingly, PFAS burden in BC manatees is comparable to predatory mammals, despite their low status in the trophic hierarchy (Supplemental Table 4). Manatees were hypothesized to contain low concentrations of PFAS due to their lower trophic status and the fact they are primarily herbivorous (35). In comparison to another marine herbivore, the green sea turtle (Chelonia mydas), the BC manatees had a maximum PFOS concentration approximately 43 times greater ((50), Supplemental Table 4). In comparison to a marine omnivore, the leatherback sea turtle (Dermochelys coriacea), the BC manatees had a maximum PFOS level approximately 21 times larger (50). The maximum PFOS level of BC manatees were within the range (minimum and maximum range) of reports for predatory marine species, including juvenile Kemp’s ridley sea turtles (Lepidochelys kempii), minke whales (Balaenoptera acutorostrata), and both adult and juvenile bottlenose dolphins (Tursiops truncatus), as shown in Supplemental Table 4 (9, 12, 49–51).
As this is the first study examining the PFAS burden in manatees, nothing is known about the health implications of the high PFAS levels in the BC manatees. Further, the exposure route of PFAS for manatees is also unclear, with current hypotheses centered on PFAS accumulation via diet (e.g., select vegetation through sediment) (52, 53). It has been suggested that certain PFAS, like PFOS and PFDoA, have an ability to bind readily to sediment in aquatic environments and accumulate within underwater vegetation (54). This proposed pathway, along with our findings and the fact that manatees have a long-life span and high-volume diet of vegetation (consuming up to 10% of their body weight a day), suggests that this could be a previously unexplored route of PFAS exposure (29). Future investigations could examine the levels of PFAS in aquatic plant species, such as those consistent with manatee diets (e.g., seagrass), in order to fully understand the level at which herbivorous organisms are exposed via these dietary routes (36).
Site Correlations
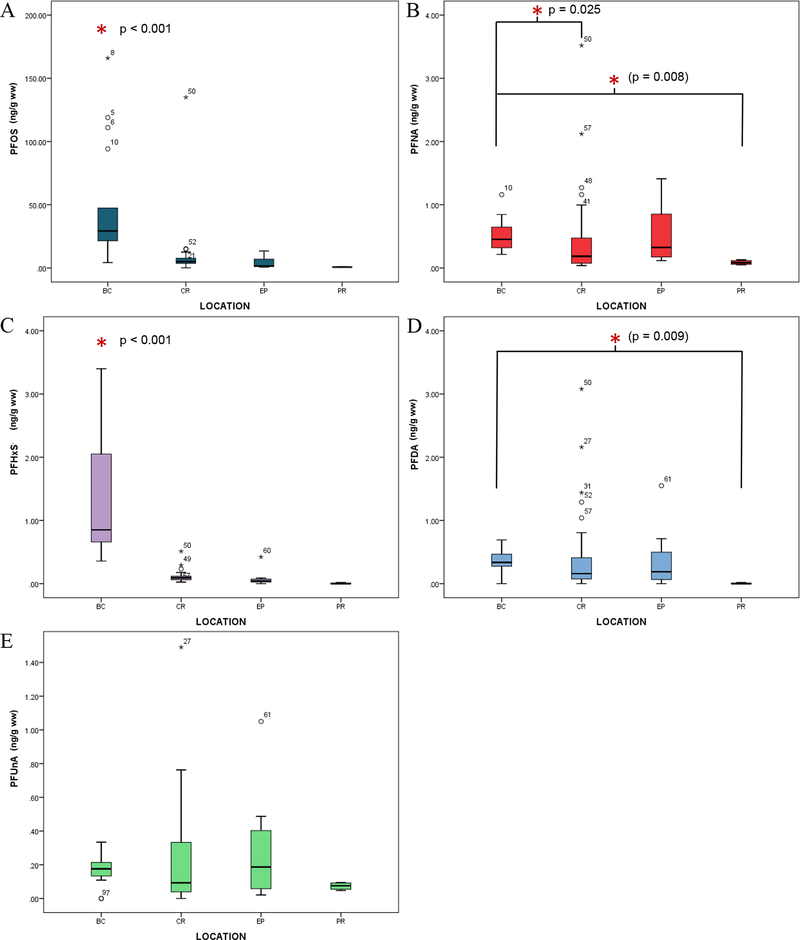
As a result of their rather solitary living style, manatee movements tend to be individual based, with general migration patterns dependent on seasonal water temperature. However, once an individual range is established, the animal usually demonstrates high site fidelity and returns to sites previously visited during winter/summer seasons (30). With that knowledge, site-based differences were examined, with BC manatees demonstrating significantly higher levels of PFAS in comparison to the other sites sampled (Fig 1 and Table 2). Specifically, BC manatees had a statistically significant difference in PFOS and PFHxS concentration compared to all locations (p < 0.001 for both analytes), suggesting that the high concentrations observed at this site were most likely a reflection of a high-level site-specific point source of PFAS contamination. The BC manatees had approximately 6, 20, and 42 times more PFOS, when compared to CR, EP, and PR manatees, respectively. Additionally, PFNA was significantly higher in manatees from BC than CR or PR (p = 0.025 and p = 0.008 respectively). The BC manatees had approximately 2, 1.3, and 5 times more PFNA, when compared to CR, EP, and PR manatees, respectively. PFDA, though not detected in every sample, was significantly higher in BC manatees compared to PR manatees (p = 0.009). However, this difference in PFDA concentrations was not significant among the other sites and given the small sample size for the PR population, further investigation into site specific PFDA concentrations is warranted. There were no site specific significant differences in PFUnA. Interestingly, high levels of PFAS detected in American alligator (Alligator mississippiensis) plasma (compared to other Southeast US sites) have been previously reported in Brevard County (47, 55). A potential source of these chemicals is aqueous film-forming foam (AFFF) usage, which are commonly utilized in locations of fire-training and contain PFAS, such as PFOS and PFHxS (56), which notably are the two compounds at significantly higher concentrations among the manatees at BC compared to all other sites. Thus, the BC manatee population could be investigated further in relation to the potential health effects associated with this PFAS burden.
Fig 1.
Site differences in concentrations (ng/g ww) of PFAS in manatee blood. Results of Kruskal-Wallis. Asterisk represents significant differences from Dunn all pairs posthoc with significant p-value adjacent. (A) PFOS (p < 0.001); (B) PFNA (p = 0.002); (C) PFHxS (p < 0.001); (D) PFDA (p = 0.013); (E) PFUnA (p = 0.361).
Morphometrics
Multiple studies have provided evidence for species-based differences in the correlation of sex and PFAS concentration among marine mammals and reptiles (8, 55, 57). The analysis of the manatee samples did not produce any significant correlation of PFAS concentration and sex. One possible reason why our study did not yield any sex-based differences with the manatee could be due to our smaller sample sizes distributed among the four locations, thus limiting the sample numbers needed to determine whether sex was a contributor to differences in PFAS abundance throughout the entire manatee sub-populations.
Other hematology and morphometric measurements were considered when analyzing the PFAS data, and included physical morphology (Supplemental Table 1), BCI, TP, and ALT (31–46, 58–60). Examining multiple measurements (straight and curved length, weight, and axial/max girth) with PFAS concentrations provided no significant correlations. Further, BCI was calculated for each manatee, using a previously outlined method, and also was found to contain no statistically significant relationship to PFAS burden (31). Hematology reports were not further investigated in this study, due to the small sample set and immediate focus on determining the presence of these chemicals in manatee species. Additional work should be conducted in order to investigate potential biomarkers for high PFAS burden.
CONCLUSIONS:
This study is the first to highlight the presence of PFAS in an herbivorous marine mammal, the West Indian manatee. Correlations between specific hematology profiles and other health metrics may be investigated further to determine potential biomarkers of health effects from PFAS exposure in manatees. Sites, such as those within Brevard County, Florida, can also be investigated further, due to the elevated levels of PFAS detected in the manatees and other reported species. Additional research about the prevalence and bioaccumulation mechanisms of these chemicals in water, sediments, and plants could also be useful for obtaining a better understanding of their environmental impacts. Knowledge of chemical contaminant impact on manatees, inclusive of PFAS, may have the potential to be used to help improve the protection of sirenian species and further the biochemical understanding of PFAS toxicity mechanisms in wildlife.
Supplementary Material
ACKNOWLEDGMENTS:
We are indebted to the manatee capture teams assembled by the USGS, Florida Fish and Wildlife Conservation Commission, the University of Florida (UF), Everglades National Park, and the Inter American University of Puerto Rico, Bayamon Campus who provided opportunities to collect data and archive samples from wild manatees. Special thanks to Drs. Martine deWit, Margaret Hunter, Antonio Mignucci-Giannoni, and Michael Walsh for health assessment assistance during manatee captures. Manatee blood analyses were conducted by the UF College of Veterinary Medicine. The material presented here is based on work supported by the U.S. National Science Foundation under Award No. DBI-1359079. The study was completed during the 2017 Research Experience for Undergraduates program with the College of Charleston’s Grice Marine Laboratory, directed by Robert Podolsky.
Footnotes
Publisher's Disclaimer: DISCLAIMER:
Publisher's Disclaimer: Certain commercial equipment or instruments are identified in the paper to specify adequately the experimental procedures. Such identification does not imply recommendations or endorsement by the NIST; nor does it imply that the equipment or instruments are the best available for the purpose. Any use of trade, firm, or product names is for descriptive purposes only and does not constitute endorsement by the U.S. Government.
REFERENCES:
- 1.Ross PS. Marine mammals as sentinels in ecological risk assessment. Human and Ecological Risk Assessment. 2000;6(1):29–46. [Google Scholar]
- 2.Moore SE. Marine mammals as ecosystem sentinels. Journal of Mammalogy. 2008;89(3):534–40. [Google Scholar]
- 3.Reif JS. Animal sentinels for environmental and public health. Public Health Reports. 2011;126(1_suppl):50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossart GD. Marine mammals as sentinel species for oceans and human health. Oceanography. 2006;19(2):134–7. [DOI] [PubMed] [Google Scholar]
- 5.Baily JL, Foster G, Brown D, Davison NJ, Coia JE, Watson E, et al. Salmonella infection in grey seals (Halichoerus grypus), a marine mammal sentinel species: Pathogenicity and molecular typing of Salmonella strains compared with human and livestock isolates. Environmental Microbiology. 2016;18(3):1078–87. [DOI] [PubMed] [Google Scholar]
- 6.Randhawa N, Gulland F, Ylitalo GM, DeLong R, Mazet JA. Sentinel California sea lions provide insight into legacy organochlorine exposure trends and their association with cancer and infectious disease. One Health. 2015;1:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahrens L, Bundschuh M. Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: A review. Environmental Toxicology and Chemistry. 2014;33(9):1921–9. [DOI] [PubMed] [Google Scholar]
- 8.Fair PA, Romano T, Schaefer AM, Reif JS, Bossart GD, Houde M, et al. Associations between perfluoroalkyl compounds and immune and clinical chemistry parameters in highly exposed bottlenose dolphins (Tursiops truncatus). Environmental Toxicology and Chemistry. 2013;32(4):736–46. [DOI] [PubMed] [Google Scholar]
- 9.Fair PA, Houde M, Hulsey TC, Bossart GD, Adams J, Balthis L, et al. Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex and geographic locations. Marine Pollution Bulletin. 2012;64(1):66–74. [DOI] [PubMed] [Google Scholar]
- 10.Ross PS. Fireproof killer whales (Orcinus orca): flame-retardant chemicals and the conservation imperative in the charismatic icon of British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63(1):224–34. [Google Scholar]
- 11.Reiner JL, Becker PR, Gribble MO, Lynch JM, Moors AJ, Ness J, et al. Organohalogen contaminants and vitamins in northern fur seals (Callorhinus ursinus) collected during subsistence hunts in Alaska. Archives of Environmental Contamination and Toxicology. 2016;70(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fair and Houde. Poly- and perfluoroalkyl substances in marine mammals In Marine mammal ecotoxicology: Impacts of multiple stressors on population health, Panti C and Fossi C, Editors. Academic Press; 2018:1–512. [Google Scholar]
- 13.Houde M, De Silva AO, Muir DC, Letcher RJ. Monitoring of perfluorinated compounds in aquatic biota: an updated review: PFCs in aquatic biota. Environmental Science & Technology. 2011;45(19):7962–73. [DOI] [PubMed] [Google Scholar]
- 14.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environmental Assessment and Management. 2011;7(4):513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicological Sciences. 2007;99(2):366–94. [DOI] [PubMed] [Google Scholar]
- 16.Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environmental Sciences Europe. 2011;23(1):38. [Google Scholar]
- 17.Herzke D, Olsson E, Posner S Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway – A pilot study. Chemosphere. 2012;88(8):980–987. [DOI] [PubMed] [Google Scholar]
- 18.Posner S Perfluorinated Compounds: Occurrence and Uses in Products In: Knepper T., Lange F. Editors. Polyfluorinated Chemicals and Transformation Products. The Handbook of Environmental Chemistry. 2012;17 Springer, Berlin, Heidelberg. [Google Scholar]
- 19.Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ. Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks. Environment International. 2017;99:43–54. [DOI] [PubMed] [Google Scholar]
- 20.Andersen ME, Butenhoff JL, Chang S-C, Farrar DG, Kennedy GL Jr, Lau C, et al. Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicological Sciences. 2007;102(1):3–14. [DOI] [PubMed] [Google Scholar]
- 21.Vélez M, Arbuckle T, Fraser W. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Human Reproduction. 2015;30(3):701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du G, Hu J, Huang H, Qin Y, Han X, Wu D, et al. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine‐related genes in vitro and in vivo. Environmental Toxicology and Chemistry. 2013;32(2):353–60. [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen LS, Bonefeld-Jørgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environmental Science and Pollution Research. 2013;20(11):8031–44. [DOI] [PubMed] [Google Scholar]
- 24.Law RJ, Bersuder P, Mead LK, Jepson PD. PFOS and PFOA in the livers of harbour porpoises (Phocoena phocoena) stranded or bycaught around the UK. Marine Pollution Bulletin. 2008;56(4):792–7. [DOI] [PubMed] [Google Scholar]
- 25.Bossi R, Riget FF, Dietz R, Sonne C, Fauser P, Dam M, et al. Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environmental Pollution. 2005;136(2):323–9. [DOI] [PubMed] [Google Scholar]
- 26.Van de Vijver KI, Hoff PT, Das K, Van Dongen W, Esmans EL, Jauniaux T, et al. Perfluorinated Chemicals Infiltrate Ocean Waters: Link between Exposure Levels and Stable Isotope Ratios in Marine Mammals. Environmental Science & Technology. 2003;37(24):5545–50. [DOI] [PubMed] [Google Scholar]
- 27.Kannan K, Koistinen J, Beckmen K, Evans T, Gorzelany JF, Hansen KJ, et al. Accumulation of Perfluorooctane Sulfonate in Marine Mammals. Environmental Science & Technology. 2001;35(8):1593–8. [DOI] [PubMed] [Google Scholar]
- 28.West Indian manatee. https://www.fws.gov/southeast/wildlife/mammals/manatee/ (accessed 23 June 2017).
- 29.Bonde RK, Aguirre AA, Powell J. Manatees as sentinels of marine ecosystem health: are they the 2000-pound canaries? EcoHealth. 2004;1(3):255–62. [Google Scholar]
- 30.Deutsch CJ, Reid JP, Bonde RK, Easton DE, Kochman HI, O’Shea TJ. Seasonal movements, migratory behavior, and site fidelity of West Indian manatees along the Atlantic coast of the United States. Wildlife Monographs. 2003:1–77. [Google Scholar]
- 31.Harshaw LT, Larkin IV, Bonde RK, Deutsch CJ, Hill RC. Morphometric body condition indices of wild Florida manatees (Trichechus manatus latirostris). Aquatic Mammals. 2016;42(4):428. [Google Scholar]
- 32.Harvey JW, Harr KE, Murphy D, Walsh MT, Chittick EJ, Bonde RK, et al. Clinical biochemistry in healthy manatees (Trichechus manatus latirostris). Journal of Zoo and Wildlife Medicine. 2007;38(2):269–79. [DOI] [PubMed] [Google Scholar]
- 33.Harvey JW, Harr KE, Murphy D, Walsh MT, Nolan EC, Bonde RK, et al. Hematology of healthy Florida manatees (Trichechus manatus). Veterinary Clinical Pathology. 2009;38(2):183–93. [DOI] [PubMed] [Google Scholar]
- 34.Kellogg ME, Burkett S, Dennis TR, Stone G, Gray BA, McGuire PM, et al. Chromosome painting in the manatee supports Afrotheria and Paenungulata. BMC Evolutionary Biology. 2007;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Shea TJ. 11 Toxicology of sirenians. Toxicology of Marine Mammals. 2003:270. [Google Scholar]
- 36.Reep RL, Bonde RK. The Florida manatee: Biology and Conservation. 2006.
- 37.O’Shea TJ, Moore JF, Kochman HI. Contaminant concentrations in manatees in Florida. The Journal of Wildlife Management. 1984:741–8. [Google Scholar]
- 38.Anzolin D, Sarkis J, Diaz E, Soares D, Serrano I, Borges J, et al. Contaminant concentrations, biochemical and hematological biomarkers in blood of West Indian manatees Trichechus manatus from Brazil. Marine Pollution Bulletin. 2012;64(7):1402–8. [DOI] [PubMed] [Google Scholar]
- 39.Stavros H-CW, Bonde RK, Fair PA. Concentrations of trace elements in blood and skin of Florida manatees (Trichechus manatus latirostris). Marine Pollution Bulletin. 2008;56(6):1221–5. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi NY, Walsh MT, Bonde RK, Powell JA, Bass DA, Gaspard JC III, et al. Baseline Reference Range for Trace Metal Concentrations in Whole Blood of Wild and Managed West Indian Manatees (Trichechus manatus) in Florida and Belize. Aquatic Mammals. 2016;42(4):440. [Google Scholar]
- 41.Cairns K, Miami-Dade D, Haag K. 4.1.4.4 Florida Manatee (Trichechus manatus latirostris). Everglades Habitats: Knowledge Gained. 2011;Section 4:191–6. [Google Scholar]
- 42.Adimey NM, Hudak CA, Powell JR, Bassos-Hull K, Foley A, Farmer NA, White L, Minch K. Fishery gear interactions from strnaded bottlenose dolphins, Florida manatees, and sea turtles in Florida U.S.A. Marine Pollution Bulletin. 2014; 81: 103–115. [DOI] [PubMed] [Google Scholar]
- 43.Betts KS. Perfluoroalkyl acids: what is the evidence telling us? Environmental Health Perspectives. 2007;115(5):A250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefebvre LW, Reid JP, Kenworthy WJ, Powell JA. Characterizing Manatee habitat use and seagrass grazing in Florida and Puerto Rico: implications for conservation and management. Pacific Conservation Biology. 2000; 5: 289–298. [Google Scholar]
- 45.Mignucci-Giannoni AA, Montoya-Ospina RA, Jimenez-Marrero NM, Rodriguez-Lopez MA, Williams EH, Bonde RK. Manatee mortality in Puerto Rico. Environmental Management. 2000, 25(2): 189–198. [DOI] [PubMed] [Google Scholar]
- 46.Bonde RK, Garrett A, Belanger M, Askin N, Tan L, Wittnich C. Biomedical health assessments of the Florida manatee in Crystal River—providing opportunities for training during the capture, handling, and processing of this endangered aquatic mammal. Journal of Marine Animals and Their Ecology. 2012;5(2):17–28. [Google Scholar]
- 47.Bangma JT, Bowden JA, Brunell AM, Christie I, Finnell B, Guillette MP, et al. Perfluorinated alkyl acids in plasma of American alligators (Alligator mississippiensis) from Florida and South Carolina. Environmental Toxicology and Chemistry. 2017;36(4):917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiner JL, Phinney KW, Keller JM. Determination of perfluorinated compounds in human plasma and serum Standard Reference Materials using independent analytical methods. Analytical and Bioanalytical Chemistry. 2011;401(9):2899. [DOI] [PubMed] [Google Scholar]
- 49.Reiner JL, O’Connell SG, Butt CM, Mabury SA, Small JM, De Silva AO, et al. Determination of perfluorinated alkyl acid concentrations in biological standard reference materials. Analytical and Bioanalytical Chemistry. 2012;404(9):2683–92. [DOI] [PubMed] [Google Scholar]
- 50.Keller JM, Ngai L, McNeill JB, Wood LD, Stewart KR, O’Connell SG, et al. Perfluoroalkyl contaminants in plasma of five sea turtle species: Comparisons in concentration and potential health risks. Environmental Toxicology and Chemistry. 2012;31(6):1223–30. [DOI] [PubMed] [Google Scholar]
- 51.Moon H, Kannan K, Yun S, An Y, Choi S, Park J, et al. Perfluorinated compounds in minke whales (Balaenoptera acutorostrata) and long-beaked common dolphins (Delphinus capensis) from coastal Korean waters. Marine Pollution Bulletin. 2010:60(7):1130. [DOI] [PubMed] [Google Scholar]
- 52.Lewis MA, Devereux R. Nonnutrient anthropogenic chemicals in seagrass ecosystems: fate and effects. Environmental Toxicology and Chemistry. 2009;28(3):644–61. [DOI] [PubMed] [Google Scholar]
- 53.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: a review. Environmental Science & Technology. 2006;40(11):3463–73. [DOI] [PubMed] [Google Scholar]
- 54.Lin AY-C, Panchangam SC, Tsai Y-T, Yu T-H. Occurrence of perfluorinated compounds in the aquatic environment as found in science park effluent, river water, rainwater, sediments, and biotissues. Environmental Monitoring and Assessment. 2014;186(5):3265–75. [DOI] [PubMed] [Google Scholar]
- 55.Bangma JT, Reiner JL, Jones M, Lowers RH, Nilsen F, Rainwater TR, et al. Variation in perfluoroalkyl acids in the American alligator (Alligator mississippiensis) at Merritt Island National Wildlife Refuge. Chemosphere. 2017;166:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at US Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere. 2016;150:678–85. [DOI] [PubMed] [Google Scholar]
- 57.Christie I, Reiner JL, Bowden JA, Botha H, Cantu TM, Govender D, et al. Perfluorinated alkyl acids in the plasma of South African crocodiles (Crocodylus niloticus). Chemosphere. 2016;154:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bangma JT, Reiner JL, Botha H, Cantu TM, Gouws MA, Guillette MP, et al. Tissue distribution of perfluoroalkyl acids and health status in wild Mozambique tilapia (Oreochromis mossambicus) from Loskop Dam, Mpumalanga, South Africa. Journal of Environmental Sciences. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rylander C, Dumeaux V, Olsen KS, Waaseth M, Sandanger TM, Lund E. Using blood gene signatures for assessing effects of exposure to perfluoroalkyl acids (PFAAs) in humans: the NOWAC postgenome study. International Journal of Molecular Epidemiology and Genetics. 2011;2(3):207. [PMC free article] [PubMed] [Google Scholar]
- 60.Harr KE, Allison K, Bonde RK, Murphy D, & Harvey JW. Comparison of Blood Aminotransferase Methods for Assessment of Myopathy and Hepatopathy in Florida Manatees (Trichechus manatus latirostris). Journal of Zoo and Wildlife Medicine, 2008:39(2):180–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.