Abstract
Purpose
To compare transplant outcomes in patients with advanced myelodysplastic syndrome (MDS) after CD34+ selected or unmodified allografts.
Patients and Methods
This analysis included initially 181 patients; 60 underwent CD34+ selected transplant and 121 had an unmodified transplant. Due to significant differences in disease characteristics, the analysis was limited to patients who had <10% blasts prior to transplant (N=145). Two groups were defined: (1) low risk: good and intermediate risk cytogenetics (CD34+, N=39; unmodified, N=46), and (2) high risk: poor and very poor risk cytogenetic (CD34+, N=19; unmodified, N=41).
Results
In the low risk group, grade II-IV acute GVHD at 1 year was 18% in the CD34+ subgroup vs. 41.3% in the unmodified subgroup, p=0.015. There were no differences in the incidence of grade III-IV acute GVHD. Chronic GVHD at 3 years was 5.3% vs. 56%, p<0.001, respectively. At 3 years, relapse, overall survival (OS) and relapse-free survival (RFS) for the CD34+ and unmodified subgroups were similar: 8.1% vs 19.4%, p=0.187; 58.5% vs 53.7%, p=0.51; 59.5% vs 52.4%, p=0.448. However, the composite outcome combining chronic extensive GVHD free status and relapse free status (CRFS) at 3 years was 59.5% in the CD34+ selected group vs 19.2% in the unmodified group, p<0.001.
In the high risk group, CD34+ vs. unmodified, grade II-IV acute GVHD at 1 year was 31.6% vs 24.4%, p=0.752. There were no differences in the incidence of grade III-IV acute GVHD. Chronic GVHD at 3 years was 0% vs. 27.6%, p=0.013. At 3 years, relapse, OS, RFS, and CRFS were 31.6% vs. 69.3%, p=0.007; 35.5% vs 14.5%, p=0.068; 31.6% vs 10.7% p=0.045; and 31.6% vs 6.1%, p=0.001, respectively. Cytogenetic abnormalities at diagnosis and transplant type had a significant univariate association with RFS in the high-risk cohort. Only cytogenetics (p=0.03) remained associated with this outcome in a multivariate model.
Conclusions
Overall survival was similar between the two types of transplant, however CRFS was superior in CD34+ selected transplants.
Keywords: myelodysplastic syndrome, allogeneic transplantation, T cell depletion, unmodified
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment available for patients with myelodysplastic syndrome (MDS)1,2. Despite major improvements in transplant outcomes, mostly due to decrease in transplant related mortality (TRM) 3, 4, 5, graft versus host disease (GVHD) and relapse remain the major challenges affecting quality of life and survival post-transplant.
Acute and chronic GVHD are significant post-transplant complications with cumulative incidence ranging from 30 to 60%6,7. A very effective method to prevent GVHD is depletion of T lymphocytes from the allograft before infusion 8,9, 10, 11, 12, 13, 14,15. Although several different methods were used in the past to deplete T cells ex-vivo 16,17,18 more recently the only method available is positive selection of CD34+ stem cells. The efficacy of ex-vivo CD34+ selection in reducing the risk of acute and chronic GVHD without higher relapse rates has been reported in previous publications19, 12,14. These findings were confirmed in a prospective multicenter phase 2 trial sponsored by the Bone Marrow Transplant Clinical Trial Network in patients with acute myeloid leukemia in complete hematologic remission 7. To date, results from prospective trials comparing CD34+ selected to unmodified transplants have not been reported. However, retrospective comparison studies have been published in patients with acute myelogenous leukemia (AML) in first complete remission20 and acute lymphoblastic leukemia (ALL)21 in first or second complete remission and have shown similar survival although with much less GVHD in the CD34+ selected transplants. This study compares the standard transplant outcomes and also the composite end point of chronic GVHD free and relapse free survival (CRFS)22 which is currently being studied in a prospective manner through a BMT CTN study (BMT CTN 1301, NCT02345850) in patients with advanced MDS who underwent CD34+ selected allo-HSCT at MSKCC with those who received unmodified allo-HSCT at MDACC between 2001–2012.
Patients and Methods
Patients
Adult patients (18 and older) who underwent allo-HSCT for advanced MDS (RAEB-1&2), between 2001 and 2012 were included in this retrospective analysis after approval by each institutional Review Board. Demographics, disease characteristics, treatment, GVHD and survival data were retrieved from databases at the respective institutions. The MDS subtypes and prognostic classification at diagnosis and before transplantation were determined according to the 2008 World Health Organization (WHO)23 and the Revised International Prognostic Scoring (IPSS-R) criteria24. Donor-recipient human leukocyte antigen (HLA) matching was established by DNA sequence-specific oligonucleotide typing for HLA-A,-B,-Cw,-DQB1, and –DRB1 loci, in both institutions.
Transplant Procedure and Supportive Care
Sixty patients who received CD34+ selected grafts at MSKCC and 121 patients who received unmodified grafts at MDACC were identified initially for this retrospective analysis. All recipients of CD34+ selected grafts underwent myeloablative conditioning regimen (MAC): 55 (92%) had a chemotherapy-based regimen and 5 (8%) had a TBI-based regimen. T cell depletion of granulocyte colony-stimulated factor (G-CSF) -mobilized PBSCs was accomplished by positive selection of CD34+ stem cells using the ISOLEX 300i Magnetic Cell Separator (Baxter, Deerfield, IL) and then sheep RBC rosette depletion (N= 30)25, and since October 2010 the CliniMACS CD34 Reagent System (N=25)26 (Miltenyi Biotech, Gladbach, Germany). Five patients received bone marrow (BM) graft wherein T cell depletion was achieved by sequential soybean lectin agglutination and sRBC-rosetting11, 14, which provides level of T cell depletion similar to CD34+ selection. The T cell depleted allograft was infused within 24–48 hours after completion of cytoreduction. All patients received antithymocyte globulin (ATG) to prevent graft rejection. No pharmacologic post transplant GVHD prophylaxis was given.
Of the 121 patients who received unmodified graft at MDACC, 81 (67%) after a MAC and 40 (33%) after a reduced intensity conditioning regimen (RIC). All patients received a chemotherapy based regimen; the backbone for the MAC regimen was a combination of busulfan and fludarabine (91.3%) and the backbone of the RIC regimen was melphalan and fludarabine (65%). The GVHD prophylaxis consisted of tacrolimus and mini-dose methotrexate (5 mg/m2 on days 1, 3, 6 and 11) in the majority of patients (N=118). ATG was given to all recipients of matched unrelated donor (MUD) (N=59). Patients were managed at both institutions according to each institution’s standard guidelines.
GVHD was diagnosed clinically, confirmed pathologically whenever possible, and classified according to standard criteria for acute GVHD 27 and for chronic GVHD28. Only patients who engrafted were evaluable for GVHD assessment. Cause of death was determined using a NMDP algorithm29. CRFS was defined as the combined outcome of being alive and free of chronic extensive GVHD and relapse state22.
Statistical methods
Patient characteristics were compared between CD34+ selected and unmodified transplant groups using the Wilcoxon Rank-Sum test for continuous covariates and Chi-square or Fisher’s exact test for categorical covariates as appropriate. OS and RFS were defined as the time from transplant until death, and relapse or death, respectively. CRFS was defined as the time from transplant until death, relapse, or extensive cGVHD. Estimates and 95% confidence intervals for OS, RFS, and CRFS were calculated using the Kaplan-Meier method, with comparisons across groups based on the log-rank test. The cumulative incidence of relapse, non-relapse mortality, aGVHD, and cGVHD were estimated using the cumulative incidence method for competing risks, with differences across groups based on Gray’s test. Death in the absence of relapse was considered a competing risk for relapse, while relapse was considered a competing risk for non-relapse mortality. For acute and chronic GVHD, death and relapse were both considered to be competing risks. Cox proportional hazards regression was used to investigate the joint effects of patient characteristics on RFS in multivariate analysis.
Results
Patient Characteristics
Patient demographics and clinical characteristics of all patients in both groups are summarized in table 1. Patients in the unmodified cohort had higher proportion of therapy-related MDS (31.4% vs. 11.7%, p=0.007), very poor risk cytogenetics at diagnosis (34.7% vs. 13.6%, overall p=0.016) and BM blast count >10% at time of transplant (24.3% vs. 0%, overall p<0.001). The CD34+ selected cohort had a higher number of patients receiving PB grafts (91.7% vs. 67.8%, p=0.001), HLA-mismatched donors (N = 14, 23.3%), and all patients were conditioned with a myeloablative regimen. The median (range) time from diagnosis to transplant was 8.2 (1.8, 161.1) months in the CD34+ selected cohort and 7.4 (0.9, 53.0) months in the unmodified cohort (p=0.314).
Table 1:
*Patients and transplant characteristics
| Characteristics | CD34+ SELECTED (N=60), MSKCC cohort | Unmodified (N=121) MDACC cohort | p-value |
|---|---|---|---|
| Median follow-up, months (range) | 43.4 (3.8–119.5) | 50.2 (12.2–136.3) | |
| Age, years (range) | 57.1 (21.9–72) | 57 (19–72) | 0.585 |
| Female gender | 34 (56.7%) | 40 (33.1%) | 0.004 |
| MDS-t | 7 (11.7%) | 38 (31.4%) | 0.007 |
| Cytogenetic risk at diagnosis (IPSS-R) | 0.016 | ||
| Good | 25 (42.4%) | 47 (38.8%) | |
| Intermediate | 15 (25.4%) | 18 (14.9%) | |
| Poor | 11 (18.6%) | 14 (11.6%) | |
| Very poor | 8 (13.6%) | 42 (34.7%) | |
| Missing | 1 | ||
| Blasts at transplant | < 0.001 | ||
| < 5% | 48 (81.4%) | 53 (46.1%) | |
| 5–9% | 11 (18.6%) | 34 (29.6%) | |
| 10–19% | 0 | 28 (24.3%) | |
| Missing | 1 | 6 | |
| Donor type | < 0.001 | ||
| MRD | 21 (35.0%) | 62 (51.2%) | |
| MUD | 25 (41.7%) | 59 (48.8%) | |
| MMD | 14 (23.3%) | 0 | |
| Stem cell source | 0.001 | ||
| BM | 5 (8.3%) | 39 (32.2%) | |
| PB | 55 (91.7%) | 82 (67.8%) | |
| Conditioning regimen | <0.001 | ||
| MAC | 60 (100%) | 81 (66.9%) | |
| RIC | 0 | 40 (33.1%) | |
| ATG in conditioning regimen | 60 (100%) | 59 (48.8%) | |
| Time from diagnosis to transplant (months) | 8.2 (1.8–161.1) | 7.4 (0.9–53) | 0.314 |
The initial cohort included 181 patients. Due to large differences between the MSKCC (CD34+ selected group) and MDACC (unmodified group) the analysis was limited to 145 patients (table 2). MDS-t= therapy related MDS, MRD=matched related donor, MUD= matched unrelated donor, MMD= mismatched donor, BM= bone marrow, PB= peripheral blood, MAC= myeloablative, RIC= reduced intensity
Considering the significant differences between the 2 cohorts, patients with ≥ 10% blasts at the time of transplant were excluded and the analysis was limited to two groups that had similar characteristics: (1) Low risk group: patients with <10% blasts at time of transplant and good and intermediate risk cytogenetics at diagnosis (CD34+ selected, N=39, unmodified, N=46). (2) High risk group: patients with <10% blasts at time of transplant and poor and very poor risk cytogenetics at diagnosis (CD34+ selected, N=19, unmodified, N=41). The patient’s characteristics of these two groups are summarized in table 2 and all comparison results are summarized in table 3.
Table 2:
Patients and transplant characteristics in the ‘low’ and ‘high’ risk subgroups
| Good or intermediated cytogenetic risk at diagnosis&<10% blasts | Poor or very poor cytogenetic risk at diagnosis&<10% blasts | |||||
|---|---|---|---|---|---|---|
| CD34 SELECTED (N=39) MSKCC cohort | Unmodified (N=46) MDACC cohort | P value | CD34 SELECTED (N=19) MSKCC cohort | Unmodified (N=41) MDACC cohort | P value | |
| Median follow-up, month (range) | 43.4 (3.8–109.2) | 44.9 (12.9–115.6) | - | 52.0 (16.2–119.5) | 53.3 (14.2–93.9) | - |
| Age, years (range) | 56.9 (21.9–72) | 55.5 (25–69) | 0.357 | 59.2 (26.7–69) | 58 (32–72) | 0.994 |
| Female gender | 23 (59.0) | 17 (37.0) | 0.071 | 9 (47.4) | 13 (31.7) | 0.377 |
| MDS-t | 3 (7.7) | 8 (17.4) | 0.316 | 4 (21.1) | 16 (39.0) | 0.280 |
| Cytogenetic risk at diagnosis | 0.148 | 0.042 | ||||
| Good | 24 (61.5) | 36 (78.3) | - | - | ||
| Intermediate | 15 (38.5) | 10 (21.7) | - | - | ||
| Poor | - | - | 11 (57.9) | 11 (26.8) | ||
| Very poor | - | - | 8 (42.1) | 30 (73.2) | ||
| Blasts at transplant | 0.465 | 0.016 | ||||
| < 5% | 30 (76.9) | 31 (67.4) | 17 (89.5) | 22 (53.7) | ||
| 5–9% | 9 (23.1) | 15 (32.6) | 2 (10.5) | 19 (46.3) | ||
| Donor type | 0.001 | 0.008 | ||||
| Match related | 15 (38.5) | 25 (54.3) | 5 (26.3) | 21 (51.2) | ||
| Match unrelated | 14 (35.9) | 21 (45.7) | 10 (52.6) | 20 (48.8) | ||
| Mismatch | 10 (25.6) | 0 | 4 (21.1) | 0 | ||
| Graft type | 0.010 | 0.012 | ||||
| Bone marrow | 4 (10.3) | 16 (34.8) | 1 (5.3) | 15 (36.6) | ||
| Peripheral blood | 35 (89.7) | 30 (65.2) | 18 (94.7) | 26 (63.4) | ||
| Conditioning intensity | < 0.001 | 0.010 | ||||
| Myeloablative | 39 (100.0) | 31 (67.4) | 19 (100.0) | 27 (65.9) | ||
| Reduced intensity | 0 | 15 (32.6) | 0 | 14 (34.1) | ||
| ATG in conditioning regimen | 39 (100.0) | 21 (45.7) | 19 (100.0) | 20 (48.8) | ||
| Time from diagnosis to transplant, month | 12.1 (2.5–161.1) | 9.5 (2.3–53) | 0.884 | 7.9 (1.8–28.8) | 5.9 (1.5–42.9) | 0.499 |
MDS-t= therapy related MDS, MRD=matched related donor, MUD= matched unrelated donor, MMD= mismatched donor, BM= bone marrow, PB= peripheral blood, MAC= myeloablative, RIC= reduced intensity
Table 3:
Transplant outcomes in ‘low’ and ‘high’ risk subgroups
| Good or intermediated cytogenetic risk at diagnosis & <10% blasts | Poor or very poor cytogenetic risk at diagnosis & <10% blasts | |||||
|---|---|---|---|---|---|---|
| CD34 SELECTED (N=39) MSKCC cohort | Unmodified (N=46) MDACC cohort | P value | CD34 SELECTED(N=19) MSKCC cohort | Unmodified (N=41) MDACC cohort | P value | |
| AGVHD (grade II–IV) | 0.015 | 0.752 | ||||
| day 100 | 12.8% (4.6–25.4) | 41.3% (26.9–55.1) | 15.8 %(3.7–35.6) | 22% (10.7–35.7) | ||
| 1 year | 18.0% (7.8–31.7) | 41.3% (26.9–55.1) | 31.6% (12.3–53) | 24.4%(12.5–38.4) | ||
| AGVHD (grade III–IV) | 0.349 | |||||
| day 100 | 12.8% (4.6–25.4) | 13.0% (5.2–24.5) | 5.3%(0.3–22.1) | 7.3% (1.9–18) | ||
| 1 year | 15.4% (6.1–28.5) | 15.2% (6.6–27.1) | 15.8%(3.7–35.6) | 7.3% (1.9–18) | ||
| CGVHD | < 0.001 | 0.013 | ||||
| 1 year | 5.3% (0.9–15.8) | 47.8% (32.6–61.5) | 0% | 24.4%(12.2–38.8) | ||
| 3 year | 5.3% (0.9–15.8) | 56% (39.3–69.8) | 0% | 27.6%(14.2–42.9) | ||
| NRM | 0.939 | 0.091 | ||||
| 100 day | 10.3% (3.2–22.2) | 10.9 %(3.9–21.8) | 5.3%(0.3–22) | 4.9%(0.9–14.7) | ||
| 1 year | 18.1%(7.8–31.7) | 17.4% (8.1–29.7) | 26.3%(9.1–47.5) | 14.6%(5.8–27.4) | ||
| 3 year | 32.4%(17.8–47.9) | 28.2% (15.3–42.6) | 36.8%(15.7–58.3) | 20%(9–34.1) | ||
| Relapse | 0.187 | 0.007 | ||||
| 1 year | 2.6% (0.2–12.1) | 8.7% (2.7–19.1) | 21.1%(6.2–41.8) | 58.5%(41.6–72.1) | ||
| 3 year | 8.1%(2.0–19.9) | 19.4% (8.8–33.0) | 31.6%(12.2–53.2) | 69.3%(51.6–81.6) | ||
| RFS | 0.448 | 0.045 | ||||
| 1 year | 79.3% (62.8–89) | 73.9% (58.7–84.3) | 52.6%(28.7–71.9) | 26.8%(14.5–40.8) | ||
| 3 year | 59.5 %(41.7–73.4) | 52.4% (35.8–66.5) | 31.6%(12.9–52.2) | 10.7%(3.5–22.7) | ||
| OS | 0.51 | 0.068 | ||||
| 1 year | 79.3 %(62.8–89) | 78.3%(63.4–87.7) | 57.9% (33.2–76.3) | 34.1%(20.3–48.5) | ||
| 3 year | 58.5% (40.5–72.8) | 53.7%(36.9–67.9) | 35.5%(15.2–56.6) | 14.5%(5.5–27.7) | ||
| CRFS | <0.001 | 0.001 | ||||
| 1 year | 79.3% (62.8–89) | 32.6%(19.7–46.1) | 52.6% (28.7–71.9) | 12.2% (4.5–24.1) | ||
| 3 year | 59.5% (41.7–73.4) | 19.2% (8.8–32.6) | 31.6% (12.9–52.2) | 6.1% (1.2–17.0) | ||
The median follow-up among survivors in the low risk CD34+ selected and unmodified subgroups was 43.4 months (range: 3.8–109.2) and 44.9 months (range: 12.9–115.6). In the high risk CD34+ selected subgroup, the follow-up was 52 months (16.2–119.5) and 53.3 months (14.2–93.9) in the unmodified subgroup.
Acute graft-versus-host disease
In the low risk group, the day-100 cumulative incidence (CI) of grade II-IV aGVHD was 12.8% (95% CI 4.6–25.4%) in the CD34+ selected subgroup and 41.3% (26.9–55.1%) in the unmodified subgroup. The 1-year CI of grade II-IV aGVHD was 18.0% (7.8–31.7%) and 41.3 % (26.9–55.1%), respectively (p=0.015). Grade III-IV aGVHD was 12.8% (4.6–25.4%) in the CD34+ selected subgroup and 13.0% (5.2–24.5%) in the unmodified subgroup at day 100 and 15.4% (6.1–28.5%) and 15.2% (6.6%−27.1%) at 1 year post-transplant (p=0.98).
In the high-risk group, the day-100 CI of grade II-IV aGVHD was 15.8% (3.7–35.6%) in the CD34+ selected subgroup and 22% (10.7–35.7%) in the unmodified subgroup. At 1-year, it was 31.6% (12.3–53%) and 24.4% (12.5–38.4%), (p=0.752). Grade III-IV aGVHD was 5.3% (0.3–22.1%) in the CD34+ selected subgroup and 7.3% (1.9–18%) in the unmodified subgroup at day 100 and 15.8% (3.7–35.6%) and 7.3% (1.9%−18%) at 1 year post-transplant (p=0.349).
Chronic graft-versus host disease
The incidence of cGVHD was significantly lower in the CD34+ selected recipients. In the low risk group, the 1-year CI of cGVHD was 5.3% (0.9%−15.8%) in the CD34+ selected subgroup and 47.8% (32.6–61.5%) in the unmodified subgroup, while the 3-year CI was 5.3% (0.9%−15.8%) and 56% (39.3%−69.8%), respectively (p<0.001).
In the high-risk group, none of the patients in the CD34+ selected subgroup had cGVHD. The 1-year and 3-year CI of cGVHD in the unmodified subgroup were 24.4% (12.2–38.8%) and 27.6% (14.2–42.9%). Of note, none of the CD34 selected recipients in either risk group had extensive cGVHD.
Non-relapse mortality
There were no significant differences in NRM between the CD34+ selected and unmodified groups. In the low risk group, the CI of NRM at day-100 was 10.3% (3.2–22.2%) in the CD34+ selected subgroup and 10.9% (3.9–21.8%) in the unmodified subgroup. At 1-year the CI of NRM was 18.1% (7.8–31.7%) and 17.4% (8.1–29.7%), and at 3-years 32.4% (17.8–47.9%) and 28.2% (15.3–42.6%), respectively (p=0.939).
In the high-risk group, the CI of NRM at day-100 was 5.3% (0.3–22%) in the CD34+ selected subgroup and 4.9% (0.9–14.7%) in the unmodified subgroup. At 1- year the CI was 26.3% (9.1–47.5%) and 14.6% (5.8–27.4%) and at 3-years 36.8% (15.7–58.3%) and 20% (9–34.1%), respectively (p=0.091).
The causes of death in the CD34+ selected group were infections (41%), relapse (21%), graft failure (6%), GVHD (6%), toxicity (19%) and other causes (16%); and in the unmodified group: relapse (36%), GVHD (36%), infections (18%), graft failure (2.4%), toxicity (3.6%) and other causes (4%) (Table 5).
Table 5:
Causes of death in the two cohorts
| Cause Of Death | CD34+ Selected (N=31) MSKCC cohort | Unmodified (N=57) MDACC cohort |
|---|---|---|
| Relapse | 8 (25%) | 36 (63%) |
| Infection | 10 (32%) | 8 (14%) |
| Bacterial | 5 | 2 |
| Viral | 2 | 4 |
| Fungal | 2 | |
| PCP | 1 | |
| Unspecified | 2 | |
| GVHD | 4 (13%) | 8 (14%) |
| Graft failure | 1 (3.0%) | 1 (2%) |
| Toxicity | 3 (10%) | 3 (5%) |
| Other | 5 (17%) | 1 (2%) |
Relapse
In the low risk group, the relapse rate was similar between the CD34+ selected and unmodified subgroups. The 1-year CI of relapse was 2.6% (0.2–12.1%) in the CD34+ selected subgroup and 8.7% (2.7%−19.1%) in the unmodified subgroup, while the 3-year CI was 8.1% (2.0–19.9%) and 19.4% (8.8–33%), (p=0.187).
In the high-risk group, the relapse rate was lower in recipients of CD34+ selected transplants. The 1-year CI of relapse was 21.1% (6.2–41.8%) in the CD34+ selected subgroup and 58.5% (41.6–72.1%) in the unmodified subgroup, while the 3-year CI of relapse was 31.6% (12.2–53.2%) and 69.3% (51.6–81.6%), (p=0.007).
Overall survival, relapse-free survival and extensive chronic GVHD-Relapse-free survival (CRFS)
There were no significant differences in OS between both groups; however RFS was higher in the high-risk subgroup who had received CD34+ selected transplants.
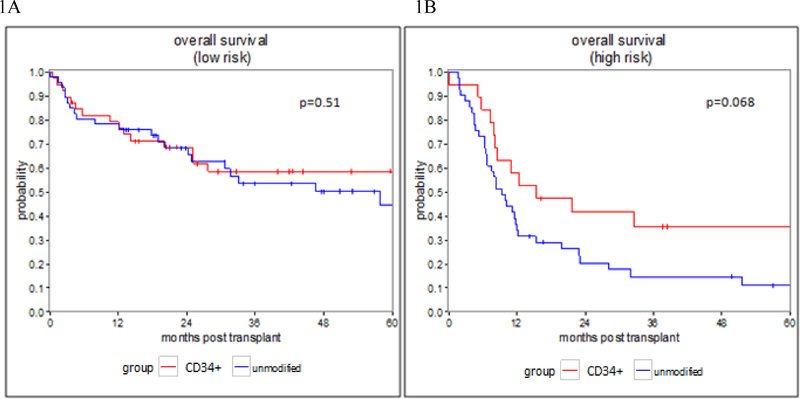
In the low risk group, the 1-year OS was 79.3% (62.8–89%) for the CD34+ selected subgroup, and 78.3% (63.4–87.7%) in the unmodified subgroup, while the 3-year OS was 58.5% (40.5–72.8%) and 53.7% (36.9–67.9%) respectively (p=0.51) (Figure 1A). The 1-year RFS was 79.3% (62.8–89%) in the CD34+ selected subgroup and 73.9% (58.7–84.3%) in the unmodified subgroup, while the 3-year RFS was 59.5% (41.7–73.4%) and 52.4% (35.8–66.5%),(p=0.448).
Figure 1: Overall Survival.
Figure 1: overall survival in patients who underwent CD34+ selected and unmodified allo-HSCT.
1A: OS in the low risk group, 1B: OS in the high risk group
In the high-risk group, the 1-year OS was 57.9% (33.2–76.3%) for the CD34+ selected subgroup and 34.1% (20.3–48.5%) in the unmodified subgroup, while the 3-year OS was 35.5% (15.2–56.6%) and 14.5% (5.5–27.7%) respectively (p=0.068) (Figure 1B). The 1-year RFS was 52.6% (28.7–71.9%) in the CD34+ selected subgroup and 26.8% (14.5–40.8%) in the unmodified subgroup, while the 3-year RFS was 31.6% (12.9–52.2%) and 10.7% (3.5–22.7%), (p= 0.045).
Due to the differences in RFS between CD34+ selected and unmodified patients in the high risk group, Cox proportional hazard regression models were fit to evaluate potential differences in RFS due to cytogenetic risk at diagnosis (poor vs. very poor), blasts at transplant (<5% vs. 5–9%), donor type (match vs. mismatch), conditioning intensity (MAC vs. RIC) and type of transplant (CD34 selected vs. unmodified) (table 4). In univariate analysis, patients with very poor cytogenetic risk at diagnosis (HR = 2.18, 95% CI 1.21–3.92, p=0.009) and patients receiving unmodified transplants (HR = 1.84, 95% CI 1.00–3.37, p=0.049) were at significantly greater risk of relapse or death. However, in a multivariate model adjusting for both cytogenetic risk and transplant type, cytogenetic risk remained the only significant covariate predicting for worse RFS (HR = 1.95, 95% CI 1.07–3.58, p=0.030) while type of transplant was non significant (p=0.176).
Table 4:
Factors Relating to RFS and CRFS in the ‘high risk’ subgroup
| univariate | multivariate | |||
|---|---|---|---|---|
| RFS | ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| cytogenetic risk at diagnosis | 0.009 | 0.030 | ||
| Poor | reference | reference | ||
| very poor | 2.18 (1.21, 3.92) | 1.95 (1.07, 3.58) | ||
| blasts at transplant | 0.877 | |||
| < 5 % | reference | |||
| 5–9 % | 1.05 (0.59,1.85) | |||
| donor type | 0.250 | |||
| Match | reference | |||
| Mismatch | 0.50 (0.16,1.62) | |||
| conditioning intensity | 0.102 | |||
| Myeloablative | reference | |||
| RIC | 1.72 (0.90, 3.30) | |||
| transplant type | 0.049 | 0.176 | ||
| CD34 SELECTED | reference | reference | ||
| Unmodified | 1.84 (1.00, 3.37) | 1.54 (0.82, 2.88) | ||
| CRFS | ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| cytogenetic risk at diagnosis | 0.007 | 0.048 | ||
| Poor | reference | reference | ||
| very poor | 2.21 (1.24, 3.93) | 1.82 (1.01, 3.29) | ||
| blasts at transplant | 0.455 | |||
| < 5 % | reference | |||
| 5–9 % | 1.24 (0.71, 2.15) | |||
| donor type | 0.138 | |||
| Match | reference | |||
| Mismatch | 0.41 (0.13, 1.33) | |||
| conditioning intensity | 0.147 | |||
| Myeloablative | reference | |||
| RIC | 1.61 (0.85, 3.08) | |||
| transplant type | 0.002 | 0.010 | ||
| CD34 SELECTED | reference | reference | ||
| Unmodified | 2.68 (1.43, 5.01) | 2.31 (1.22, 4.40) | ||
| Graft source | 0.363 | |||
| BM | reference | |||
| PBSC | 0.76 (0.42, 1.38) | |||
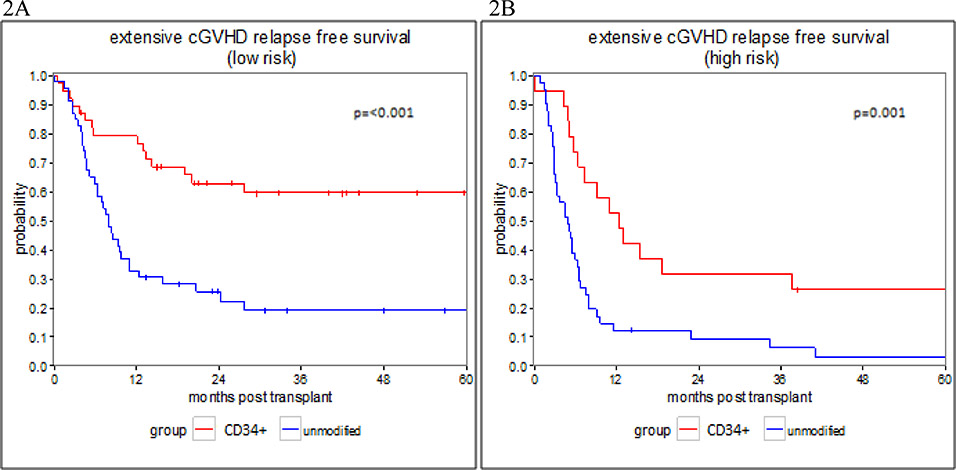
The CRFS was significantly higher in the recipients of CD34+ selected transplant. In the low risk group, the 1-year CRFS was 79.3 % (62.8–89%) for the CD34 selected subgroup and 32.6% (19.7–46.1%) in the unmodified subgroup, while the 3-years CRFS was 59.5% (41.7–73.4%) and 19.2% (8.8–32.6%) respectively (p<0.001) (Figure 2A). In the high-risk group, the 1-year CRFS was 52.6% (28.7–71.9%) for the CD34+ selected subgroup and 12.2% (4.5–24.1%) in the unmodified subgroup, while the 3-year CRFS was 31.6% (12.9–52.2%) and 6.1% (1.2–17%), (p=0.001) (Figure 2B). Cox proportional hazard regression models were fit to evaluate potential differences in CRFS in a similar fashion described above for RFS (table 4). In univariate and multivariate models, both cytogenetic risk (MV HR = 1.82, 95% CI 1.01–3.29, p=0.048) and transplant type (MV HR = 2.31, 95% CI 1.22–4.40, p=0.010), were significant covariate predicting for worse CRFS.
Figure 2: CRFS- extensive chronic GVHD and relapse free survival.
Figure 2: Extensive chronic GVHD and relapse free survival in patients who underwent CD34+ selected and unmodified allo-HSCT.
2A: CRFS in the low risk group, 2B: CRFS in the high risk group
Discussion
This retrospective study comparing CD34+ selected and unmodified transplants, demonstrates that in patients with advanced MDS, the OS was similar between the two transplant methods, though with a significantly lower incidences of chronic GVHD and without an increased relapse rate, and as a result, a better composite outcome of CRFS after CD34+ selected transplants. We attempted to compare two similar cohorts and to include in the analysis all the factors that may contribute to differences in outcomes. However, we recognize the limitation of a retrospective analysis and the use of cohorts from two different institutions. We specifically acknowledge that conditioning intensity and the use of ATG in the unmodified cohort may be factors that can affect outcomes, though in this analysis they were found not to be significant.
This study confirms previous observations that the incidence of acute and chronic GVHD are lower in recipients of CD34+ selected transplants20,21, despite a higher proportion (24%) of mismatch donors in this cohort. An unexpectedly low CI of aGVHD was seen in the high-risk subgroup of recipients of unmodified transplants; similar to that of CD34+ selected recipients, but lower than the CI in recipients of unmodified transplants in the low risk subgroup. This lower incidence of aGVHD in this unmodified subgroup was seen despite similar proportion of related and unrelated donors, use of ATG and use of MAC regimens. A likely explanation is that the lower incidence of GVHD seen in this group was secondary to a higher relapse rates since relapse and GVHD were competing risks. Although the incidence of grade II-IV acute GvHD was lower in the CD34-selected group, the incidence of grade III - IV was similar between the two cohorts, suggesting that when acute GVHD occurs after a CD34+ selected graft it tends to present in a more aggressive way.
Chronic GVHD was markedly low after CD34 selected transplant in both the low risk and high risk-groups despite using peripheral blood stem cells in nearly all patients. The largest phase 3 randomized multicenter trial comparing PB to BM graft showed a high incidence of extensive chronic GVHD of 53% in patients who received unmodified PB grafts30 and of 41% in those who received BM graft. Both are still about one log higher than the cGVHD seen after CD34+ selected graft (5%), despite using a PBSC graft source in almost all patients.
NRM was similar in recipients of CD34+ selected and unmodified graft regardless of the disease risk subgroup; however the distribution of causes of death was different. Infections were the most common primary cause of NRM in the CD34+ selected group, while GVHD was the most common primary cause of NRM in the unmodified group. Infections were also a major complication after unmodified transplant as they were the most common secondary cause of death in recipients of unmodified graft developing GVHD. The basis for this difference after CD34+ selection is the delayed immune reconstitution since this method eliminates all subsets of mature T cells. The process of generation of a new functional immune system de- novo takes several months and, at times, up to 1 to 2 years post transplant 31,32,33. Therefore, infections and particularly viral reactivation, such as CMV, EBV, adenovirus and others remain a major challenge after CD34+ transplants. Adoptive immunotherapy with donor derived or third-party viral specific cytotoxic T cells has been used both after unmodified and CD34+ selected allo-HSCT with varying level of success34, 35, 36,37,38,39,40, however, the low incidence of GVHD and lack of immune suppressive medications post CD34+ selected allo-HSCT make it an ideal setting for this type of therapy.
Early studies comparing ex vivo T cell depleted with unmodified transplants reported significantly higher relapse rates in CD34+ selected transplant41. However, the intensity of the preparative regimen in some of these studies was non-myeloablative and the T cell depletion techniques were different from CD34+ selection. More recently, the only devices licensed for T cell depletion are based on CD34+ selection. A recent prospective study7 and several retrospective analysis using myeloablative conditioning regimen and CD34+ cell selection with the Miltenyi CliniMACS device and without pharmacological GVHD prophylaxis15,13 reported relapse rates of 20.6% at 1 year for AML in CR1, 11.8% for MDS, and 23% for ALL, which are similar to relapse rates reported for unmodified transplants 42, 43,44. In this comparative analysis, there was no difference in relapse rate among the low risk patients but there was a higher incidence of relapse among the poor risk patients receiving unmodified transplants. Further, in a multivariate RFS model including cytogenetic abnormalities at diagnosis and transplant type for the high risk cohort, only cytogenetics remained significantly associated with the outcome. This is in agreement with other reports emphasizing the importance of cytogenetic abnormalities on transplant outcome 15, 45,46,47. Post-transplant Interventions to reduce the incidence of relapse are being explored both after unmodified and CD34+ selected transplants48, 49, this type of intervention has the potential to also affect GVHD incidence by affecting different subsets of T cells.
Overall survival was similar for recipients of CD34+ selected and unmodified grafts as reported in similar comparison studies in patients with AML and ALL20,21.However, the composite end point of survival without relapse and without chronic GVHD was significantly higher for recipients of CD34+ selected transplants. In recent years it became evident that the routinely used parameters to assess transplant outcomes, i.e. survival, relapse, GVHD and NRM lack the ability to assess cure without ongoing morbidity. Composite end points acknowledge that both survival and rates of other critical events are important when testing new therapies50 and this composite endpoint has been incorporated into outcomes reports also in the field of BMT more recently51, 52. We believe that the ongoing multicenter study sponsored by the BMT CTN (BMT CTN 1301, NCT 02345850) will have the ability to address the issues raised in this analysis in a prospective manner. This study is comparing unmodified allo-HSCT with GVHD prophylaxis using tacrolimus and methotrexate vs. CD34+ selected allo-HSCT vs. allo-HSCT with post-transplant cyclophosphamide, using only myeloablative conditioning regimen, with the primary end point being CRFS.
Highlights.
A retrospective analysis comparing unmodified to CD34+ selected allo-HCT in MDS.
The composite end-point of survival without relapse and without chronic GVHD was higher in the CD34+ selected group.
Relapse is affected by high risk cytogenetic and not by type of transplant.
Acknowledgments
Financial disclosures: This research was supported in part by National Institutes of Health award number P01 CA23766 (MSKCC investigators) and NIH/NCI Cancer Center Support Grant P30 CA008748 (MSKCC investigators), The MDS Research Fund, The Phyllis Dunn Fund and the Bergstein Family Research Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation formyelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia. 1998;12(Suppl 1):S25–S29. [PubMed] [Google Scholar]
- 2.Warlick ED, Cioc A, Defor T, et al. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–38. [DOI] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA et al. , Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010. November 25;363(22):2091–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remberger M, Ackefors M, Berglund S et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study Biol Blood Marrow Transplant. 2011. November;17(11):1688–97 [DOI] [PubMed] [Google Scholar]
- 5.Horan JT, Logan BR, Agovi-Johnson MA et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011. March 1;29(7):805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. [DOI] [PubMed] [Google Scholar]
- 7.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champlin RE, Passweg JR, Zhang MJ, et al. T-cell depletion of bone marrow transplants for leukemia from donors other than HLA-identical siblings: advantage of T-cell antibodies with narrow specificities. Blood. 2000. June 15;95(12):3996–4003. [PubMed] [Google Scholar]
- 9.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001. December 1;98(12):3192–204. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos EB, Carabasi MH, Castro-Malaspina H et al. , T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998. February 1;91(3):1083–90. [PubMed] [Google Scholar]
- 12.Jakubowski AA, Small TN, Kernan NA et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011. September;17(9):1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013. February;19(2):208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB et al. , Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008. April;14(4):458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamari R, Chung SS, Papadopoulos EB et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015. December;21(12):2106–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisner Y, Kapoor N, O’Reilly RJ et al. Allogeneic bone marrow transplantation using stem cells fractionated by lectins: VI, in vitro analysis of human and monkey bone marrow cells fractionated by sheep red blood cells and soybean agglutinin. Lancet. 1980. December 20–27;2(8208–8209):1320–4. [DOI] [PubMed] [Google Scholar]
- 17.de Witte T, Hoogenhout J, de Pauw B, et al. Depletion of donor lymphocytes by counterflow centrifugation successfully prevents acute graft-versus-host disease in matched allogeneic marrow transplantation. Blood. 1986. May;67(5):1302–8. [PubMed] [Google Scholar]
- 18.Wagner JE, Donnenberg AD, Noga SJ, et al. Lymphocyte depletion of donor bone marrow by counterflow centrifugal elutriation: results of a phase I clinical trial. Blood. 1988. October;72(4):1168–76. [PubMed] [Google Scholar]
- 19.Urbano-Ispizua A, Brunet S, Solano C, et al. Allogeneic transplantation of CD34+-selected cells from peripheral blood in patients with myeloid malignancies in early phase: a case control comparison with unmodified peripheral blood transplantation. Bone Marrow Transplant. 2001;28(4):349–54. [DOI] [PubMed] [Google Scholar]
- 20.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013. June;19(6):898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015. April;50(4):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barba P, Hilden P, Devlin SM, et al. Ex Vivo CD34+-Selected T Cell-Depleted Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome Is Associated with Low Incidence of Acute and Chronic Graft-versus-Host Disease and High Treatment Response. Biol Blood Marrow Transplant. 2017. March;23(3):452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009. July 30;114(5):937–51. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012. September 20;120(12):2454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007. December 15;110(13):4552–9. Epub 2007 Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS® System CD34- enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012. May;18(5):690–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995. June;15(6):825–8. [PubMed] [Google Scholar]
- 28.1990;50:79–98. Chronic graft-versus-host disease. Sullivan KM. [DOI] [PubMed] [Google Scholar]
- 29.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007. December;13(12):1469–76. [DOI] [PubMed] [Google Scholar]
- 30.Anasetti C, Logan BR, Lee SJ et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012. October 18;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keever CA, Small TN, Flomenberg N et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989. April;73(5):1340–50. [PubMed] [Google Scholar]
- 32.Lewin SR, Heller G, Zhang L et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood 2002. September 15;100(6):2235–42. [PubMed] [Google Scholar]
- 33.Castermans E, Hannon M, Dutrieux J et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011. February;96(2):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012. March 15;119(11):2644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koehne G, Hasan A, Doubrovina E, et al. , Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia. Biol Blood Marrow Transplant. 2015. September;21(9):1663–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Reilly RJ, Doubrovina E, Trivedi D, et al. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants., Immunol Res. 2007;38(1–3):237–50. [DOI] [PubMed] [Google Scholar]
- 37.Heslop HE, Slobod KS, Pule MA, et al. , Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010. February 4;115(5):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackinnon S, Thomson K, Verfuerth S, et al. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008. J;40(1):63–7. [DOI] [PubMed] [Google Scholar]
- 39.Clancy LE, Blyth E, Simms RM, et al. , Cytomegalovirus-specific cytotoxic T lymphocytes can be efficiently expanded from granulocyte colony-stimulating factor-mobilized hemopoietic progenitor cell products ex vivo and safely transferred to stem cell transplantation recipients to facilitate immune reconstitution. Biol Blood Marrow Transplant. 2013. May;19(5):725–34. [DOI] [PubMed] [Google Scholar]
- 40.Ma CK, Blyth E, Clancy L, et al. , Addition of varicella zoster virus-specific T cells to cytomegalovirus, Epstein-Barr virus and adenovirus tri-specific T cells as adoptive immunotherapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Cytotherapy. 2015. October;17(10):1406–20. [DOI] [PubMed] [Google Scholar]
- 41.Marmont AM, Horowitz MM, Gale RP et al. , T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991. October 15;78(8):2120–30. [PubMed] [Google Scholar]
- 42.Deol A, Sengsayadeth S, Ahn KW et al. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis. Cancer. 2016. June 17. doi: 10.1002/cncr.30140. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010. April 10;28(11):1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachanova V, Marks DI, Zhang MJ, et al. , Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2014. March;28(3):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gauthier J, Damaj G, Langlois C et al. Contribution of Revised International Prognostic Scoring System Cytogenetics to Predict Outcome After Allogeneic Stem Cell Transplantation for Myelodysplastic Syndromes: A Study From the French Society of Bone Marrow Transplantation and Cellular Therapy. Transplantation. 2015. August;99(8):1672–80. [DOI] [PubMed] [Google Scholar]
- 46.Oran B, Kongtim P, Popat U, et al. Cytogenetics, donor type, and use of hypomethylating agents in myelodysplastic syndrome with allogeneic stem cell transplantation. Marrow Transplant. 2014. October;20(10):1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ustun C, Trottier BJ, Sachs Z, et al. Monosomal karyotype at the time of diagnosis or transplantation predicts outcomes of allogeneic hematopoietic cell transplantation in myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015. May;21(5):866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2015. October;21(10):1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010. December 1;116(23):5420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankoh AJ, Li H, D’Agostino RB Sr. et al. Use of composite endpoints in clinical trials.Stat Med. 2014. November 30;33(27):4709–14. [DOI] [PubMed] [Google Scholar]
- 51.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015. February 19;125(8):1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inamoto Y, Kimura F, Kanda J, et Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016. December;101(12):1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]