Summary
Background:
Untreated opioid addiction in persons with HIV is associated with poor outcome. Slow release, long-acting, implantable naltrexone may improve outcomes.
Methods:
We conducted a 48-week outcome study beween July 2011 and April 2015 of opioid addicted males and females starting antiretroviral treatment (ART) for HIV whose viral loadwas ≥1000 copies per mL and were seeking treatment at HIV and Narcology programs in St. Petersburg and the surrounding Leningrad Region, Russian Federation. We stratified participants according to gender, viral load (VL) and CD4 cells per μL, and randomized them to addiction treatment with a naltrexone implant and oral naltrexone placebo (NI), or oral naltrexone and placebo implant (ON). The primary outcome was plasma viral load of ≤400 copies per mL at 24 and 48 weeks. We included all randomized participants in outcome analyses. Treatment staff was blinded to group assignment. The study is complete and registered at ClinicalTrials.gov, NCT01101815
Findings:
238 potential subjects were screened, 35 excluded for not meeting inclusion criteria, 3 declined to participate and 200 were randomized. There was no difference between NI and ON in the number of participants with VL≤400 copies per mL at week 24 (38 [38%] vs 35 [35%] p=0·77) but more NI than ON participants had a VL≤400 copies per mL at week 48 (66 [66%] vs 50 [50%] RR: 1·32 [95% CI: 1·04−1·68] p=0·0451). There were seven serious adverse events: three deaths in NI (one heart disease, one trauma, one AIDS), and four in ON (two overdoses, one pancreatic cancer, one AIDS). The overdose deaths occurred 9–10 months after the last naltrexone dose.
Interpretation:
The longer the blockade, the more protection from missed doses and the impulsive behaviors that lead to relapse and poor, even fatal outcomes. Commercial development of implants could result in a meaningful addition to current addiction treatment options.
Introduction
Untreated opioid dependence (e.g. “addiction”) is associated with suboptimal adherence to HIV treatment and poor outcomes (1). Methadone and buprenorphine maintenance improve these outcomes (2,3) but are not always available (4), illegal under Russian law even if used for detoxification, and some opioid addicted individuals prefer non-agonist treatment (5,6). Naltrexone is another option as it blocks opioid effects, is approved for preventing relapse to opioid, and alcohol dependence, does not cause tolerance or withdrawal, has no abuse potential or known interactions with HIV medications, and is free of the regulations that limit access to agonist treatment. It has been available since the 1970’s as a 50 mg tablet that blocks opioids for up to 24 hours but its efficacy has been limited by non-adherence in all but narrow categories of highly motivated individuals such as medical professionals or persons on probation or parole (7,8). Slow release formulations block opioids for one to three months, depending on the formulation, and improve addiction outcomes (9,10,11), and a recent study showed that extended release injectable naltrexone improved six-month HIV outcomes when offered to prisoners with HIV and opioid use disorders (12).
Here we report the results of a study evaluating the impact of a slow release naltrexone implant vs oral naltrexone on HIV and addiction treatment outcomes. The implant (Prodetoxon®) was developed in the Russian Federation, approved by the Ministry of Health in 2005, and provides stable plasma levels of naltrexone and its active metabolite 6β-naltrexol for about three months. We hypothesized that it would also improve HIV treatment outcomes in opioid addicted individuals and conducted the study we report here to test it.
Methods
Study design and participants
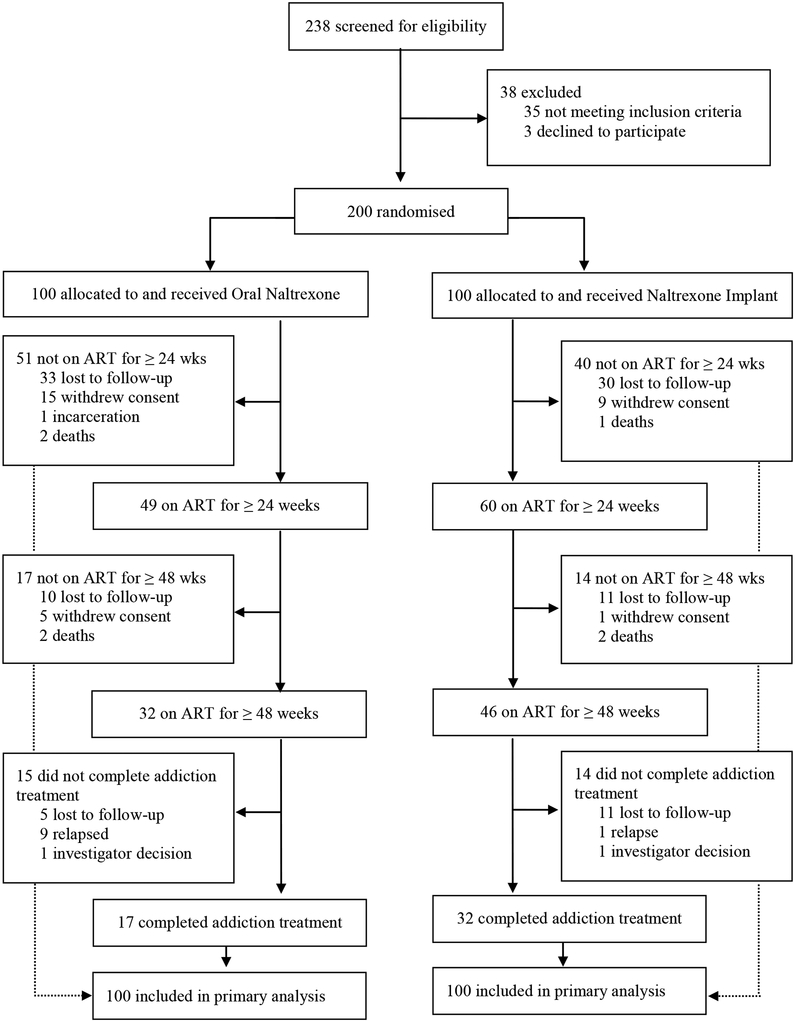
The study was a 48-week double-blind, double-dummy trial conducted between July 2011 and April 2015 in St. Petersburg, Russia, and the surrounding Leningrad Region. We randomized HIV-infected, treatment-seeking, consenting, opioid addicted males and females aged 18 or above who were never treated with ART or had not been treated for the last year or more to receive a naltrexone implant (NI) every 12 weeks with oral naltrexone placebo, or a placebo implant with 50 mg/day oral naltrexone (ON), each with drug counseling and an offer of additional doses over the next year. All participants met DSM-IV criteria for opioid dependence (“addiction”); were recently detoxified with no evidence of current physiologic dependence by self-report, physical examination, urine testing and a naloxone challenge; had a viral load of 1000 or more copies per mL; liver enzymes not greater than 5 times the upper limit of normal; not pregnant; able to provide a phone number and contact information of three or more persons who might know where to reach them; and were free of psychiatric, medical, or legal problems that might interfere with their ability to participate or provide informed consent. Initially, a CD4 count of 350 cells per μL or less was required to start ART but participants with higher counts were enrolled as the study progressed due to changes in treatment guidelines and judgments of providers; 238 potential candidates were screened, 35 excluded for not meeting inclusion criteria, 3 declined to participate, and 200 were randomized (Figure 1). The prococol can be accessed at: psom-ocr@pobox.upenn.edu.
Figure 1:
Trial profile
We used a plasma HIV RNA threshold of ≤400 copies per mL to define “undetectable” viral load because it was the lower limit of quantification for HIV assays clinically available in Russia at initiation of the study and because viral loads in ranges below this value have unclear clinical significance with respect to emergence of resistance and HIV transmission.
The implant is an 8.5×18 mm cylinder that contains 1000 mg naltrexone and 10 mg of triamcinolone to prevent inflammation (http://en.prodetoxon.info/). It is inserted subcutaneously through a 2 to 3 cm incision under the skin of the abdominal wall, closed with 2–3 sutures, and dissolves over 2–3 months. Drug counseling was provided every two weeks for the first 24 weeks and then every 4 weeks through week 48. Research assistants did urine testing, brief assessments of progress, recorded adverse events, and downloaded data from a medication event monitoring system (MEMS) at the time of counseling sessions, with more detailed assessments every 12 weeks. Research staff provided a supply of oral medication lasting until the next appointment at each counseling session unless the participant had relapsed. The protocol and consents were written in English, translated into Russian, reviewed by bilingual staff in Russia and the U.S, and approved by human subjects’ committees at First Pavlov State Medical University and the University of Pennsylvania. The sponsor and the two committes did annual progress reviews and evaluated reports of adverse events.
Randomisation and blinding
Treatment sites depended on the participant’s primary residence - the Pavlov/Botkin Hospital and City AIDS Center for those living in St. Petersburg, and the Leningrad Addiction and AIDS Center for those in the surrounding Leningrad Region. The Pavlov Statistical Department created a table with stratification on gender distribution of 1:4 females to males based on the expected ratio with 1:1 random blocks and randomisation to the two addiction treatment conditions at each site. VL stratification was based on >/< 100·000 copies per mL and CD4 >/< 50 cells per μL. Placebo implants of identical appearance to the active product were provided by the manufacturer (Fidelity Capital, location, Russian Federation). Identically-appearing naltrexone or placebo capsules with riboflavin added as a check on adherence were prepared by the Pavlov research pharmacist. Medications were dispensed in blinded boxes with numbers matched to a table kept by the research pharmacist and delivered to the clinical sites. Only the research pharmacist had a record of group assignments. Participants, investigators, and research staff were masked to treatment allocation. The blinding could be broken in case of emergency but it was never required.
Procedures
Staff at the HIV and addiction treatment sites, social contacts, and members of community organizations working with non-governmental organizations identified potential study candidates and referred them to the local addiction or HIV treatment program where a research technician provided an overview of the study. Those who expressed interest were scheduled for testing including viral load and CD4 count at the AIDS Center serving their area followed by an appointment with a psychiatrist who provided more detailed information about the study and answered questions. Those who remained interested were asked to sign a consent to be evaluated by an addiction psychiatrist who reviewed medical and psychosocial information from the AIDS Center and assessed the individual for study inclusion and exclusion criteria. Those who appeared eligible and remained interested were encouraged to ask questions, read the consent, and correctly answer at least 8 of a 10-item quiz to test understanding of the study before signing the consent (consent and quiz on pp 2 and 3 of Appendix).
Those who passed the quiz and signed the consent met with a research assistant who did the baseline assessment and made an appointment with an addiction psychiatrist to rule out current residual physiologic opioid dependence. If self-report, a urine drug test, and the absence of signs or symptoms of recent opioid use suggested no current dependence, the psychiatrist administered 0·8 mg naloxone IM or by slow IV infusion. Those who had withdrawal were treated with clonidine and/or benzodiazepines, observed until symptoms resolved, referred to usual addiction treatment, and not randomised or enrolled in the study. If no signs or symptoms of withdrawal emerged within 20 minutes, the participant was randomised, study medication ordered from the research pharmacist, and a surgeon working with research staff was contacted and inserted the implant in a room set aside for that purpose. The participant was introduced to his/her counselor, given a 4week supply of oral study medication (the extra 2 weeks to avoid running out if there were transportation problems), an appointment for wound check and suture removal in 5−7 days, and a schedule of future appointments, assessments, oral medication refills, and implantations.
Staff renewed supplies of oral addiction medication at counseling sessions every two weeks in the first 24 weeks and monthly through week 48, and implants were reinserted every 12 weeks. Procedures to rule out physiologic dependence were repeated each time addiction treatment medication was renewed. Staff repeated the naloxone challenge only when relapse was suspected but uncertain. Staff referred those who relapsed to usual addiction treatment, encouraged them to continue study assessments and HIV treatment, and counted them as addiction treatment non-completers. Participants were paid 1500 rubles (~US$49) for more detailed assessments at week48, 1000 rubles (~US$33) for the assessment at week 24, and 500 rubles (~US$16) for completing less detailed assessments at biweekly and monthly counseling visits through week 48.
Psychiatrists and psychologists who participated in prior naltrexone studies provided drug counseling and were guided by a manual modeled after one on the NIDA web site that had been translated into Russian did the drug counseling (http://www.INda.INh.gov/TXManuals/IDCA/IDCA1.html). Counseling focused on providing support; encouraging adherence to prescribed medication; dealing with craving; avoiding situations associated with drug use and behaviors that spread HIV; providing help for psychiatric and psychosocial problems; and documenting adverse effects. The Ministry of Health provided information on the importance of medication adherence and avoiding behaviors that spread HIV to all study participants. AIDS Center physicians prescribed and managed ART according to clinical preferences and drug availability and AIDS Center pharmacists dispensed it. Viral load and CD4 testing were done at baseline and repeat tests scheduled for weeks 24 and 48. Follow-up (24 and 48 weeks) VL and CD4 data were available for 70 ON participants and 82 from NI. Antiretroviral therapy could be started before or after the first dose of addiction treatment medication but addiction and HIV treatments always began within 3 weeks of each other.
Medical personnel at the addiction programs administered the Global Assessment of Function (GAF) (13) and the Clinical Global Impressions Scale (CGI) at baseline and weeks 24 and 48; research assistants administered all other measures including the HIV Risk Assessment Battery Brief Psychiatric Rating Scale (BPRS), and Beck Depression Inventory (BDI). Retention in HIV and addiction treatment was documented by records of AIDS Center visits and addiction counseling appointments kept; remission was documented by self-reports, urine testing, and a naloxone challenge (if indicated) prior to receipt of study medication. Adherence to ART was assessed by the Medication Event Monitoring System (MEMS). It consists of a cap placed on a bottle that electronically records the time and date the bottle is opened (Aardex, Zug, Switzerland). Research staff instructed participants to bring the bottle with the cap to counseling sessions where they downloaded the data using MEMS software. Adherence to addiction treatment medication was documented by records of medications dispensed and implanted.
ART regimens were two nucleoside reverse transcript inhibitors (NRTI) + a protease inhibitor (PI,101 participants); two NRTI’s + a non-nucleoside reverse transcriptase inhibitor (61 participants);a NRTI + a protease inhibitor (16 participants); and two NRTI’s + an integrase strand inhibitor; all equally divided between NI and ON (Table 1). Drugs monitored were: zidovudine/lamivudine fixed dose combination (n=94); lamivudine (n=52); abacavir/lamivudine fixed dose combination (n=10); and eight others (n=44), also equally distributed between groups. Only one ART medication per participant was monitored. Continued access to ART was available to all study participants as part of national health care in Russia. List of all medications used is in the Appendix, p.12
Table 1:
Distribution of medication regimens
| Regimens | NI | ON | Total |
|---|---|---|---|
| 2 NRTI + PI | 51 | 50 | 101 |
| 2 NRTI + NNRTI | 28 | 33 | 61 |
| 1 NRTI + PI | 9 | 7 | 16 |
| 2 NRTI + INSTI | 5 | 5 | 10 |
| Other combinations | 7 | 5 | 12 |
ART regimens: NRTI = nucleos(t)ide analog reverse transcriptase inhibitor; PI = protease inhibitor; NNRTI = non-nucleoside analog reverse transcriptase inhibitor; INSTI = integrase strand transferase inhibitor
Outcomes
The primary outcome was viral load (VL) <400 copies per mL at 24 and 48 weeks. Secondary outcomes were adherence to ART; time to relapse; change in CD4 cells per μL from baseline to week 48; HIV risk behavior; opioid positive urine tests; number of days kept scheduled appointments; psychiatric symptoms, other substance use, and clinical global impression of outcome.
Statistical Analysis
The statistical analisys was conducted using SAS 9.4 softwear. The sample size was calculated (an alpha level of 0·025 was used) according to Cohen (13) for the hypothesis that there would be a difference in the proportion of individuals with a VL≤400 copies per mL between the NI and ON groups at 24 and 48 weeks. Longitudinal data, a 1:1 allocation of participants to the two groups, and a 10% attrition rate for 200 subjects (100/group) provided >85% power to detect a difference of at least 22% with a significance of 0·05. The primary and all secondary analyses followed intention to treat principles.
AIDS Center records documented weeks in HIV treatment; Narcology Center records documented weeks in addiction treatment. Self-report, an opioid negative urine test, and a naloxone challenge (if clinically indicated) documented non-relapse before each study medication prescription refill and implantation. Missing VL’s were counted as >400 copies per mL and missing urines were counted opioid positive. Primary outcomes were compared using Fisher’s Exact tests and an OR with 95%CI. Kaplan-Meier survival analysis with a Log-rank test calculated the proportion retained in HIV and addiction treatments. Analyses of CD count, VL, alcohol TLFB, and ART adherence used mixed ANOVA (after log transformation and with subject as random factor) with the Tukey-Kramer adjustment for multiple comparisons. Numbers of ART and addiction treatment appointments kept, AE’s and drug use were analyzed with chi-square or Fisher’s Exact test, as appropriate. All comparisons were two-sided with a p value of <0·05 as a cut-off for significance; the proportion of participants with each type of AE was compared between groups using Fisher’s Exact test.
Role of the funding source
The National Institute on Drug Abuse and programs at the Penn Centers for AIDS Research and Mental Health funded by the National Institute of Health sponsored the study. Funders had no role in data collection, analysis, interpretation, or writing the manuscript. Drs. Krupitsky, Blokhina, and Verbitskaya had direct access to the data and Drs. Krupitsky and Woody were responsible for the decision to submit for publication.
Results
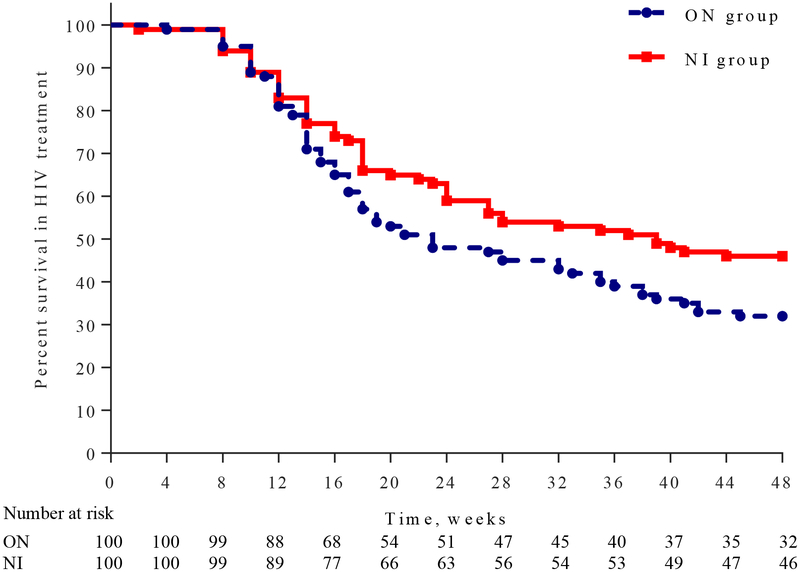
Research staff screened 238 candidates for eligibility between July 14, 2011 and April 14, 2014 and 200 were randomly assigned to Oral Naltrexone or Naltrexone Implant. Two candidates had a positive naloxone challenge prior to randomisation and referred to usual treatment; 35 did not pass the study quiz on the first try but passed on the second. Lack of education was not a limiting factor to passing the quiz. There were no significant baseline differences among study participants (Table 2). At week 24 there was no difference in the number of participants with VL≤400 copies per mL in NI(38 [38%] vs ON 35 [35%] p=0·77). At week 48, more NI than ON participants had a VL≤400 copies per mL (66 [66%] vs 50 [50%] RR: 1·32 [95% CI: 1·04−1·68] p=0·0451). The proportion of those who completed 48-weeks of HIV treatment was higher in NI than ON: 46 (46%) vs. 32 (32%), р=0·0423, OR=1·8 (95% CI: 1·02−3·22, Figure 2) and there were more weeks of prescribed ART in NI than ON (Median [LQ−HQ] 40 weeks [16−48]) vs. (Median [LQ−HQ] 21 weeks [14−42]; Log-Rank; р=0·0462). There was no difference in ART adherence between groups based on total MEMS cap openings (Mean ± SD): 70·2% ± 29·0% for ON vs. 66·6% ± 28·5% for NI (ANOVA F1·198 =0·79; p=0·3767), or the overall percent of openings that would have occurred if the participant removed the cap for each scheduled dose (Mean ± SD): 22·1% ± 19·4% for ON vs. 19·3% ± 18·0% for NI (ANOVA F1·198 =0·44; p=0·51). There was a difference in openings between participants who continued addiction treatment without relapsing and those who dropped out (Mean ± SD): 73·3 ± 22% vs. 65·1% ±32·1% (ANOVA F1·198 =4·37; p=0·0382), and in the proportion of openings the participant was supposed to take if he/she remained in addiction treatment (Mean ± SD): 38·45% ± 16·2% vs. 9·22% ± 8·52% (ANOVA F1·174 =268·46; p<0·0001).
Table 2:
Baseline characteristics of the intention-to-treat population
| ON (N=100) | NI (N=100) | |
|---|---|---|
| Age (years) | 32·3 (4·5) | 33·2 (4·6) |
| Female gender | 28 (28%) | 29 (29%) |
| Education (years) | 11·4(2) | 11·1 (2·1) |
| Employment (yes) | 37 (37%) | 39 (39%) |
| Hepatitis B Surface Antigen + | 17 (17%) | 17 (17%) |
| Hepatitis C Antibody + | 85 (85%) | 88 (88%) |
| Years since HIV infection diagnosed | 8·5 (3·6) | 8·5 (3·7) |
| Years of opioid addiction | 12·9 (4·2) | 13·0 (4·5) |
| Alcohol, grams/day | 11·6 (24·9) | 10·0 (33·2) |
| Log HIV RNA, copies | 4·1 (1·1) | 4·2(1·2) |
| CD4+ lymphocyte count, cells/mm3 | 220·4 (106·3) | 248·5 (189·0) |
| ALT (IU/l) | 56·4 (33·9) | 57·2 (34·6) |
| AST (IU/l) | 52·1 (24·1) | 56·0 (36·2) |
Age, years of education, years since HIV diagnosed, years of opioid addiction, CD4 count, ALT, and AST are mean (SD), other data are n (%).
Figure 2:
Weeks in HIV Treatment
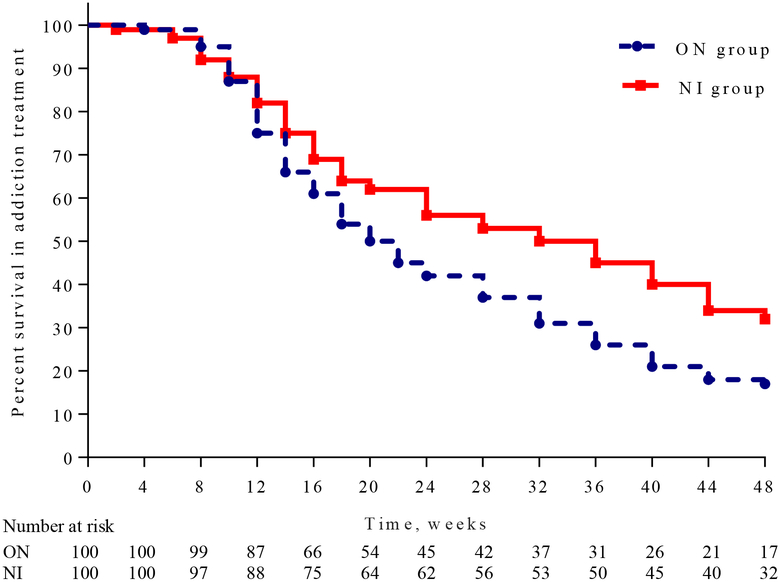
NI participants had more weeks of addiction treatment without relapse (Median [LQ−HQ]), more time before they relapsed (32 [16−48] weeks vs 20 weeks [14−40]; Log-rank test p=0·0107, Figure 3), and more NI than ON participants remained in addiction treatment to the end of the study (32 vs.17); Fisher Exact Test p=0·0208. The odds of achieving a VL≤400 copies per mL among those who completed addiction treatment were greater if they were in the NI group: (OR=3·03 [95% CI: 1·66−5·52]) and there was a correlation between time to relapse and time to withdraw from ART (Spearman Ro=0·891; p<0·0001). Regardless of group assignment, more participants who continued naltrexone treatment for 24 weeks had a VL≤400 copies per mL than those who did not continue (51 [70 %] vs 22 [30%] RR: 2·89 [95% CI: 1·91−4·38] p<0·0001). Findings were similar at week 48 for those who continued naltrexone (VL≤400 copies per mL for 64 [55%] vs those who did not continue: 52 [44%] RR: 1·53 [95% CI: 1·21−1·95] p=0·0005).
Figure 3:
Weeks in Addiction Treatment
CD4 cells per μL were higher in those who continued naltrexone without relapsing than those who dropped out: Median [LQ-HQ] (429 [265−560] cells per μL vs. 165 [121−276] cells per μL [Mixed ANOVA F1,195=12·4; p=0·0005]), and the CD4 increase from baseline to 48 weeks was greater in NI than ON (151 [−97−1010] cells per μL vs. 0 [−370−535] cells per μL) [Mixed ANOVA F1·195=28·45; p<0·0001]). Based on the HIV Risk Assessment Battery among patients that were retained in the study, drug risk behavior gradually decreased in both groups with a significant group effect favoring NI (Mixed ANOVA F1·357=9·5; p=0·0023) and this difference was not modified by time (group by time interaction p=0·19). No significant difference between groups in HIV sex risk behavior were noted.
The number of scheduled appointments kept was higher in NI than ON 1211 (60·6%) vs 1067 (53·4%); OR 1·64 [95%CI: 1·48−1·88]; Fisher Exact Test p<0·0001), as was the number of opioid negative urine tests NI 1063 (59·1%) vs ON 859 (47·7%); OR 1·58 [95%CI: 1·39−1·80]; Fisher Exact Test p<0·0001). The mean quantity of alcohol consumed per day during each week of addiction treatment was lower in NI than ON (Median [LQ-HQ] 6·25 [2·64−18·30] grams vs 9·90 [3·72−20·00] grams; p=0·0473. Timeline follow-back and urine testing showed that about 15% of participants reporting using marijuana; amphetamine, benzodiazepine, and methadone use was 8%or less and there were no differences between groups and no evidence of cocaine use.
Among patients retained in the study, psychiatric symptoms gradually declined with no significant differences between groups (BPRS total score [Mixed ANOVA Main Time effect F4·164=4·43; p=0·0017]; BDI score [Mixed ANOVA Main Time effect F4·164=24·48; p<0·0001]). The GAF was not significantly different between groups (Mixed ANOVA Main Time effect F4·164=24·48; p<0·0001) while the CGI showed more positive change in NI than ON (Appendix, p.12). Craving scores declined dramatically among NI participants throughout the study but not among ON participants (Appendix, p.13).
Implantable and oral naltrexone doses were generally well tolerated with only one participant in the NI group discontinuing due to adverse events; 61 (30·5%) of 200 participants experienced at least one adverse event with equal distribution between the groups except for high blood pressure (Table 3). All registered non-serious adverse events were judged mild or moderate by investigators. The seven wound infections were localized to the implant site and responded to antibiotic treatment within a few days. Four NI participants had high systolic blood pressure ranging from 150−177 mm on 8 occasions; one of them had 3 high readings with return to normal twice; 2 had two high readings and one returned to normal; one had a single high reading that returned to normal; no diastolic readings were greater than 90 mm. The overdose deaths occurred 9−10 months after the last naltrexone dose.
Table 3.
Clinical adverse events
| ON (N=100) | NI (N=100) | p value | |
|---|---|---|---|
| CNS (headache, insomnia, depression etc.) | 15 (24%) | 16 (23%) | >0·99 |
| Fatigue | 8 (13%) | 7 (10%) | >0·99 |
| Gastrointestinal (nausea, vomiting, diarrhea etc.) | 13 (21%) | 16 (23%) | 0·69 |
| High blood pressure | ‥ | 8 (11%) | 0·0021 |
| Increase of liver enzymes | 5 (8%) | 6 (9%) | >0·99 |
| Respiratory (cough, bronchitis, pneumonia etc.) | 10 (16%) | 8 (11%) | 0·81 |
| Surgical (wound infections) | 4 (6%) | 3 (4%) | >0·99 |
| Other | 8 (13%) | 7 (10%) | >0·99 |
| ≥1 adverse event | 32 (32%) | 29 (29%) | 0·76 |
| ≥1 serious adverse event* | 4 (4%) | 3 (3%) | >0·99 |
| Discontinued owing to adverse events | ‥ | 1 (1%) | >0·99 |
Data are number (%). CNS – central nervous system.
Four serious adverse events were registered for ON (two deaths due to overdose, one from AIDS and one death due to pancreatic cancer) and three serious adverse events were registered for NI (two deaths due to heart disease and one death from trauma).
Discussion
Study findings show that an extended release naltrexone implant can not only alter the course of the opioid addiction, but also improves HIV treatment outcomes. Extended release medications have been used for many years in other areas of medicine. Examples are birth control implants, insulin pumps, and injectable antipsychotics. The idea of using them in addiction medicine is not new since NIDA funded contracts to develop naltrexone implants in the 1970’s but no products were registered in any country until Russia approved Prodetoxon® in 2005. To the best of our knowledge, this study is the first to evaluate a naltrexone implant on HIV treatment outcome. Participants were of similar age, gender, and educational level as others seeking HIV and opioid addiction treatment in St. Petersburg and enrollment methods were similar to those generally used. We doubt that financial incentives led to an unrepresentative sample since payments were provided only for completing follow-ups and not for the baseline assessment or enrolling in the study. Participants may have been more representative of the target population, and more likely to continue taking naltrexone than those with HIV and opioid addiction in the UK or EU because agonist maintenance is against the law in Russia and naltrexone is the only effective medication. It was not difficult to recruit study participants and though dropout was significant, there were few complaints about the implants. Two participants admitted trying to remove their implant (both were NI) and we suspect that a few of the other infections were caused the same way. We interpret these events as proof the implant blocks opioid effects but does not always reduce craving. Implants cost the ruble equivalent of about $720 U.S. and are not provided free of charge by the national health service, thus likely used by more affluent individuals who are often treated in private practice settings.
The higher proportion of relapse free weeks and weeks in HIV treatment among NI participants is the most likely contributor to their better outcomes. As seen in the survival curves, the proportions remaining in HIV and addiction treatment did not begin to separate widely until week 16, which likely contributed to the absence of significant differences in viral load at week 24. The MEMS data were mixed with no difference in openings between groups but there was a significant difference favoring those who continued addiction treatment vs. those who did not, findings that likely reflect the complexity of getting measurements of ART adherence (19). Though self-reported alcohol use was low overall, the finding of less use in NI participants is consistent with studies showing that naltrexone suppresses use of alcohol as well as opioids (20) and that it impairs ART adherence (21). Though the NI improved outcomes, it achieved a level of VL suppression well below that seen in persons with no history of injecting drug use (22), but was similar to findings from three observational studies of opioid addicted individuals receiving agonist maintenance and being treated for HIV. One of these studies used data that is routinely collected during HIV treatment in France and showed that only 29% of 89 opioid addicted patients who had been on methadone or buprenorphine for at least 6 months had undetectable VL (<500 copies per mL). However as in the study reported here, there was a positive correlation between continuing addiction treatment and undetectable VL (23). A second study assessed the relationship between undetectable VL (<50 copies per mL) and cocaine use among 304 HIV-infected, methadone maintained opioid/cocaine users in Vancouver, Canada and found that undetectable VL’s ranged from 58–70% depending on whether the patient was injecting cocaine less than daily or using crack once or more a day (24). A third study recruited 295 HIV-infected opioid addicted individuals, 176 on ART and 119 not on ART, and started them on sublingual buprenorphine/naloxone (bup/nal). Assessments over the next year found that staying on bup/nal for three or more quarters was significantly associated with VL suppression (<400 copies per mL) for 64 of the 119 participants (54%) who were not on ART at baseline (2).
Though the participants and methods in these studies vary, all found that many did not achieve a level of VL suppression that maximizes the chance for favorable HIV outcome and a reduced risk of HIV transmission to sexual partners (25), and that continuing addiction treatment medication, whether agonist or antagonist, is associated with better HIV outcomes. Though these findings show that relapse prevention medication is an important component of HIV treatment for opioid addicted individuals, they do not show what can be done to achieve VL levels that minimize HIV spread and maximize chances for favorable outcomes among a high proportion of the target population. Neither do these or other studies show when it is safe to stop addiction treatment medication, a particularly difficult thing to predict in view of the remitting and relapsing course of the disorder.
This study has many limitations, the most import being missing data. Of the 200 participants, 48 (18 NI; 30 in ON) had missing VL data at week 48, however none of these 48 had been prescribed ART for a month or more before the VL test and studies showing that the VL rebounds within a month after stopping ART (26) support the validity of imputing these missing data as >400 copies per mL. Drop out is a common problem in addiction treatment studies and likely due to a combination of craving to resume opioid use and poor impulse control. Consequences were that self-reported data and urine test results were available only when patients kept appointments. Another limitation is uncertainty about the generalizability of these findings to settings where ethnic diversity is greater than in St. Petersburg and where amphetamines, cocaine, benzodiazepines, and other abuseable substance are more readily available than what appears to be the case in St. Petersburg. The purity of illicit opioids could also influence outcome, particularly in the US where fentanyl, carfentanil and other highly potent opioids are available. On the other hand, these concerns are not supported by the similarity in results of extended-release formulations that have been tested in the U.S., Norway, Finland and the UK (27−30). Another limitation is the degree to which implants will be used in other settings. The absence of information on this point is largely a function of the absence of registered implants in countries other than Russia. This situation may change over the next several years since the Australian implant recently received an IND from the FDA (personal communication from Dr. Hulse to Dr. Woody) and BioCor, a small U.S.-based pharmaceutical company with which Dr. Woody has consulted, met with the FDA in January 2018 and submitted a grant application to NIDA to develop a version of the Russian implant that can be submitted to the FDA for approval.
In summary, a naltrexone implant that blocks opioid effects for three months caused no serious adverse events and resulted in better HIV and addiction treatment outcomes than an active control, likely due to longer periods of remission among NI participants which in turn allowed them to focus more on HIV treatment than on purchasing and using opioids. Agonist maintenance is helpful but, as seen this study, not free of ART adherence problems. Implantable naltrexone was helpful for more than a few participants and has the potential to be a meaningful option for opioid addicted individuals who live in settings where agonist therapy is unavailable, difficult to access, or prefer non-agonist treatment. The degree to which naltrexone implants are used in a wider range of settings will likely depend on the results of commercial development. The findings presented here show that they have potential to become a meaningful treatment option for persons that do not have access to agonist maintenance or do not want it.
Supplementary Material
Research in Context.
Evidence before this study
We searched the PubMed database for the ten years prior to June 30, 2018 using the terms “naltrexone for HIV treatment” and “naltrexone for opioid addiction and HIV treatment”. We found two recent studies by Springer et al showing that extended-release injectable naltrexone improved 6-month outcomes when offered to HIV-infected prisoners with opioid or alcohol dependence prior to and after reentry, and an Australian study of showing that naltrexone implants improved hepatitis C treatment outcome in opioid addicted individuals.
Added value of this study
This study is the first to show that a naltrexone implant can improve HIV and opioid addiction treatment outcomes in persons with these disorders. Adherence was a problem, as in earlier naltrexone studies, but it was less with the implant than the oral naltrexone controls.
Implications of all the available evidence
Naltrexone implants can improve outcomes of opioid addicted persons with HIV and may fill an important niche for persons that do not want or have ready access to agonist maintenance or long-term residential treatment. The implant used in this study is registered in Russia and the only one currently approved by any regulatory agency. Development and registration of similarly-acting implants in other cultural settings would likely provide a meaningful addition to currently available treatments for opioid addiction and its comorbidities.
Acknowledgments:
David Metzger provided valuable input into the study design. Gary Hulse provided information on the current regulatory status of the Australian (Go Medical) implant. The study would not have been possible without NIDA and NIH funding, and the collaboration and work of staff at the AIDS and Addiction treatment programs in St. Petersburg and the Leningrad Region.
Funding NIDA Grants: R01 DA026336; K05 DA17009; U10 DA013043; UG1 DA013034; Penn Center for AIDS Research P30 A1045008; Penn Mental Health AIDS Research Center P30 MH097488
Declaration of Interests: Fidelity Capital provided Prodetoxon®) at a reduced price and Prodetoxon placebo at no charge. Dr. Woody is a consultant to BICX102, a company that submitted a grant to NIDA requesting funds to develop and test a similarly-designed implant that could be submitted to the FDA for approval in the U.S. Dr. Krupitsky was a consultant to Alkermes in 2008–2011 and was a consultant for Prodetoxon LTD in 2017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Evgeny Krupitsky, First Pavlov State Medical University of St. Petersburg, Russian Federation; V.M. Bekhterev National Medical Research Center for Psychiatry and Neurology, Saint Petersburg, Russian Federation.
Elena Blokhina, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Edwin Zvartau, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Elena Verbitskaya, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Dmitri Lioznov, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Tatiana Yaroslavtseva, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Vladimir Palatkin, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Marina Vetrova, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Natalia Bushara, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Andrei Burakov, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Dmitri Masalov, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Olga Mamontova, First Pavlov State Medical University of St. Petersburg, Russian Federation.
Daniel Langleben, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
Sabrina Poole, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
Robert Gross, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
George Woody, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
References
- (1).Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST). Int J Drug Policy 2007; 18: 262–70. [DOI] [PubMed] [Google Scholar]
- (2).Altice F, Bruce RD, Lucas GM, et al. HIV Treatment Outcomes Among HIV-Infected, Opioid-Dependent Patients Receiving Buprenorphine/Naloxone Treatment within HIV Clinical Care Settings: Results from a Multisite Study. J Acquir Immune Defic Syndr 2011; 56: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Palepu A, Tyndall MW, Joy R, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend 2006; 84:188–94. [DOI] [PubMed] [Google Scholar]
- (4).Burns RM, Pacula RL, Bauhoff S, et al. Policies related to opioid agonist therapy for opioid use disorders: The evolution of state policies from 2004 to 2013. Subst Abus 2016; 37: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Marcus R, Makarenko I, Mazhnaya A, et al. Patient preferences and extended-release naltrexone: A new opportunity to treat opioid use disorders in Ukraine. Drug Alcohol Depend 2017; 179: 213–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. Journal of Substance Abuse Treatment 2016; 66: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ling W, Wesson DR. Naltrexone treatment for addicted health care professionals: A collaborative private practice experiment. J Clin Psychiatry 1984; 45: 46–48. [PubMed] [Google Scholar]
- (8).Cornish JW, Metzger D, Woody GE, et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Substance Abuse Treatment 1997; 14: 529–34. [DOI] [PubMed] [Google Scholar]
- (9).Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1-month) relapse after opiate detoxification: a pilot study of two cohorts totalling 101 patients with a note on naltrexone blood levels. Addict Biol 2003; 8: 211–17. [DOI] [PubMed] [Google Scholar]
- (10).Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011; 377: 1506–13. [DOI] [PubMed] [Google Scholar]
- (11).Krupitsky EM, Zvartau EE, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs. oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry 2012; 69: 973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Springer SA, Di Paola A, Azar MM, et al. Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: Results of a double-blind, placebo-controlled randomized trial. J Acquir Immune Defic Syndr 2018; 78: 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Frances A, Pincus H, First MB (Eds.). Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition (DSM-IV). Substance Related Disorders; 1994: 175–272. [Google Scholar]
- (14).Guy W. Clinical Global Impressions (CGI) Scale, Modified In Rush JA. Task force for the Handbook of Psychiatric Measures. 2000; First Edition American Psychiatric Association, Washington, DC. [Google Scholar]
- (15).Navaline HA, Snider EC, Petro C, Tobin D, Metzger D, Alterman A, Woody GE Preparations for AIDS vaccine trials. An automated version for the risk assessment battery (RAB): Enhancing the assessment of risk behaviors. AIDS Res Human Retroviruses 1994. [PubMed] [Google Scholar]
- (16).Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962; 10: 799–812. [Google Scholar]
- (17).Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–71. [DOI] [PubMed] [Google Scholar]
- (18).Cohen J Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Assoc; Hillsdale, N.J. 1988; Second Edition. [Google Scholar]
- (19).Berg KM, Arnsten JH. Practical and Conceptual Challenges in Measuring Antiretroviral Adherence. Journal of Acquired Immune Deficiency Syndromes 2006; 43: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Volpicelli RG, Fenton M. Sustained-release naltrexone formulations for the treatment of alcohol and opioid dependence. Future Neurology 2006; 1: 389–98. [Google Scholar]
- (21).Samet JH, Horton NJ, Meli J, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res 2004; 28: 572–77. [DOI] [PubMed] [Google Scholar]
- (22).Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GSUS-380–1489): a double-blind, multicentre, phase 3, randomized controlled non-inferiority trial. Lancet 2017; 390: 2063–72. [DOI] [PubMed] [Google Scholar]
- (23).Roux P, Carrieri MP, Cohen J, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected drug users receiving antiretroviral treatment. Clinical Infectious Diseases 2009; 49: 1433–40. [DOI] [PubMed] [Google Scholar]
- (24).Socias ME, Wood E, Small W, et al. Methadone maintenance therapy and viral suppression among HIV-infected opioid users: The impacts of crack and injection cocaine. Drug Alcohol Depend 2016; 168: 211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 2016. November 24; 375(21): 2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Papasavvas E, Kostman JR, Mounzer K, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med 2004; 1: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lee JD, Nunes EV, Novo P, et al. Comparative effectivenes of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X-BOT): a multicentre, open-label, randomised controlled trial. Lancet 2017; 391: 309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kunøe N, Lobmaier P, Ngo H, Hulse G. Injectable and implantable sustained release naltrexone in the treatment of opioid addiction. Br J Clin Pharmacol 2014; 77: 264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Tihonen J, Krupitsky E, Verbitskaya E, et al. Naltrexone implant for the treatment of polydrug dependence: A randomized controlled trial. Am J Psychiatry 2012; 169: 531–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.