Abstract
Respiratory syncytial virus (RSV) is the leading cause of severe respiratory tract infection in infants and young children, but no vaccine is currently available. Live-attenuated vaccines represent an attractive immunization approach, however balancing attenuation while retaining sufficient immunogenicity and efficacy has prevented the successful development of such a vaccine. Recently, a recombinant RSV strain lacking the gene that encodes the matrix (M) protein (RSV M-null) was developed. The M protein is required for virion assembly following infection of a host cell but is not necessary for either genome replication or gene expression. Therefore, infection with RSV M-null produces all viral proteins except M but does not generate infectious virus progeny, resulting in a single-cycle infection. We evaluated RSV M-null as a potential vaccine candidate by determining its pathogenicity, immunogenicity, and protective capacity in BALB/c mice compared to its recombinant wild-type control virus (RSV recWT). RSV M-null-infected mice exhibited significantly reduced lung viral titers, weight loss, and pulmonary dysfunction compared to mice infected with RSV recWT. Despite its attenuation, RSV M-null infection induced robust immune responses of similar magnitude to that elicited by RSV recWT. Additionally, RSV M-null infection generated serum antibody and memory T cell responses that were similar to those induced by RSV recWT. Importantly, RSV M-null immunization provided protection against secondary viral challenge by reducing lung viral titers as efficiently as immunization with RSV recWT. Overall, our results indicate that RSV M-null combines attenuation with high immunogenicity and efficacy and represents a promising novel live-attenuated RSV vaccine candidate.
Introduction
Respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory tract infection in infants and young children (1). An estimated 33 million episodes of RSV-associated lower respiratory tract infection occur annually in children under five years of age, with over three million episodes requiring hospitalization (2). RSV infection is responsible for up to 150,000 deaths in young children each year (2). Nearly all children become infected with RSV at least once by two years of age (3). Additionally, approximately half of all children are infected with RSV multiple times by the age of two, although the most severe disease is observed in children under one year of age experiencing a primary RSV infection (3). Despite the immense healthcare burden attributed to RSV infection, there remains no licensed RSV vaccine. Given the high risk of severe disease associated with a primary RSV infection, RSV sero-negative infants are an important target population for RSV vaccine development.
The challenges of developing an efficacious RSV vaccine are highlighted by the failure of a formalin-inactivated RSV (FI-RSV) vaccine in the 1960s. FI-RSV vaccination failed to induce sterilizing immunity against a subsequent natural RSV infection (4–7). Surprisingly, FI-RSV-vaccinated children exhibited severe respiratory disease following a natural RSV infection with approximately 80 percent requiring hospitalization at one of the trial sites, compared to five percent of a control vaccine group (4–7). Furthermore, two of the FI-RSV-vaccinated children died as a result of a subsequent natural RSV infection (7). Given the disastrous results of the FI-RSV vaccine trial, the development of a non-live viral vaccine for use in the RSV sero-negative infant population has been hampered due to lingering safety concerns. Live-attenuated RSV vaccines are a particularly attractive vaccine platform due to their opportunity to be administered through the intranasal route allowing for direct stimulation of the immune response within the respiratory tract (8, 9). Importantly, live-attenuated RSV vaccines have been tested in sero-negative infants for many years without any evidence of enhanced respiratory disease following a natural RSV infection (10, 11). However, achieving an appropriate balance between sufficient attenuation and retention of immunogenicity has remained a primary obstacle in the successful development of a live-attenuated RSV vaccine.
Single-cycle viruses have been developed as potential live-attenuated vaccine candidates for a variety of viruses, including influenza A virus (IAV) (12–24). Single-cycle viruses are attenuated through a genetic modification that prevents the formation of mature virions capable of spreading to neighboring uninfected cells. This approach is advantageous because an anti-viral immune response may be initiated in the absence of the corresponding pathology that occurs following a wild-type (WT) virus infection. A novel single-cycle recombinant RSV strain lacking the gene encoding the matrix (M) protein (RSV M-null) was recently created (25). While not required for either genome replication or gene expression, the M protein is essential for assembly of the mature RSV virion following infection of a host cell (25). Therefore, RSV M-null is not able to form infectious viral particles following the initial infection of a host cell and undergoes only a single round of infection. Indeed, RSV M-null infection in vitro elicited robust expression of multiple RSV proteins in the absence of virus spread beyond the initially infected cell, confirming that RSV M-null behaves as a single-cycle virus in vitro (25). Because M is poorly tolerated, RSV M-null could not be sufficiently amplified in M-expressing cells to enable in vivo studies. Therefore, a second generation RSV M-null virus strain and production cell line were generated in which RSV M-null contains a Tet transactivator gene in place of the M protein open-reading frame (ORF), and the M gene in the M-expressing cell line is preceded by a Tet-responsive promoter. In this manner, RSV M-null viral titers can be readily generated at similar levels to WT virus and utilized for in vivo studies.
Herein, we evaluate the second generation RSV M-null as a potential RSV vaccine candidate in vivo by assessing its pathogenicity, immunogenicity, and protective capacity in BALB/c mice, which have been widely used to study RSV-induced pathogenesis and immunity. In comparison to its recombinant WT control virus (RSV recWT), RSV M-null-infected mice exhibited significantly reduced lung viral titers, weight loss, and pulmonary dysfunction. Remarkably, RSV M-null infection elicited robust acute and late memory immune responses that were similar in magnitude to RSV recWT infection. Importantly, RSV M-null immunization provided protection against a secondary viral challenge as efficiently as immunization with RSV recWT. Therefore, RSV M-null combines for the first time an effective and highly stable attenuation phenotype without a loss of immunogenicity resulting in fully protective immunity. The stable attenuation due to the absence of the entire M ORF may overcome the past difficulties experienced with attempting to balance the level of attenuation by constraining genome replication while maintaining a high level of efficacy. Together, our results indicate that RSV M-null is a promising prototype from which to develop a safe live-attenuated RSV vaccine candidate.
Materials and Methods
Mice
Female BALB/cAnNCr and C57BL/6NCr mice between 6–8 weeks old were purchased from the National Cancer Institute (Frederick, MD). First generation C57BL/6 x BALB/c (CB6F1) mice were bred at the University of Iowa Animal Facility and utilized between 6–8 weeks old. All experimental procedures utilizing mice were approved by the University of Iowa Animal Care and Use Committee under Animal Protocols #4101196 and #7041999. The experiments were performed under strict accordance to the Office of Laboratory Animal Welfare guidelines and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Viruses and infection
RSV recWT in which the small hydrophobic (SH) ORF was replaced with that of enhanced GFP (EGFP) was generated as previously described (25). A second generation RSV M-null in which the SH ORF was replaced with that of EGFP and the M ORF was replaced with that of a Tet transactivator sequence was propagated in HEp-2 cells containing a plasmid in which the M ORF was expressed behind a Tet-inducible promoter (H2-M helper cells) (25). The second generation RSV M-null and the H2-M helper cell line will be described in detail in a future manuscript. The second generation RSV M-null behaves identically to the previously published first generation RSV M-null virus in vitro, as the only difference between them is the genetic method for M ablation (25). Both RSV recWT and RSV M-null were generated from the A2 strain of RSV (RSV-A2). Mice were infected with 1.0 × 106 PFU of either RSV recWT or RSV M-null intranasally in 100 μl volume. RSV-A2 was a gift from Dr. Barney Graham (National Institutes of Health, Bethesda, MD) and was purified as previously described (26). Recombinant IAV-M282 was provided by Dr. Ryan Langlois (University of Minnesota, Minneapolis, MN) and was generated as previously described (26, 27). Naive mice or mice that were infected 6 or 12 weeks prior with either RSV recWT or RSV M-null were challenged with either 1.5 × 106 PFU of purified RSV-A2 or a 5 LD50 dose of IAV-M282 intranasally.
Real-time PCR
Lungs were harvested from either RSV recWT- or RSV M-null-infected mice and RNA was isolated as previously described (28). cDNA was prepared using SuperScript VILO Master Mix (Invitrogen, Carlsbad, CA) and used as a template for real-time PCR. Real-time PCR to detect the RSV nucleoprotein (N) or M genes was performed with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) on an ABI 7900 Real-Time PCR System (Applied Biosystems) using universal thermocycling parameters. RSV N and M gene primers and probe sequences have been described previously (29, 30). Probes were synthesized with a 5’−6FAM reporter dye and a 3’-TAMRA quencher dye (Integrated DNA Technologies, Coralville, IA). Results were analyzed using Sequence Detection System analysis software (Applied Biosystems). Samples were compared with known standard dilutions of a plasmid containing either the RSV N or M gene. The number of RSV N and M gene copies per lung were calculated based on the number of RSV gene copies in the sample and the total RNA isolated from the lung.
Plaque assay for RSV and IAV
Whole lungs were harvested from mice, weighed, mechanically homogenized, and supernatants were flash-frozen in liquid nitrogen prior to storage at −80°C. Plaque assays were performed using either H2-M helper cells or VERO cells (American Type Culture Collection, Manassas, VA) as indicated in the figure legends to determine RSV titers as previously described (26). Plaque assays on MDCK cells (American Type Culture Collection) to determine IAV titers were performed as previously described (26).
Assessment of weight loss and pulmonary function
Mice were evaluated daily following infection for weight loss and pulmonary function using an unrestrained whole-body plethysmograph (Buxco Electronics, Wilmington, NC). Whole-body plethysmography parameters were measured as changes in respiration from baseline values prior to infection and did not utilize methacholine administration. Pressure and volume changes in the chamber caused by respiration were averaged over a five minute period and used to calculate enhanced pause (Penh), mid-tidal expiratory flow (EF50), and respiratory rate.
Flow cytometry analysis
Lungs and mediastinal lymph nodes (mLN) were harvested from mice and single-cell suspensions were generated as previously described (31, 32). Cells were stained extracellularly with CD8 tetramers (all tetramers made in house) and monoclonal antibodies specific to CD90.2 (BioLegend, San Diego, CA; clone 53–2.1), CD4 (clone RM4–5), CD8 (clone 53–6.7), CD11a (clone M17/4), CD49d (clone R1–2), CD45 (BioLegend; clone 30-F11), CD3 (clone 145–2C11), CD19 (clone 6D5), Fas (clone 15A7), GL-7 (BioLegend; clone GL7), CD69 (BioLegend; clone H1.2F3), and CD103 (clone 2E7) in FACS buffer (PBS, 2% FCS, 0.02% sodium azide) for 30 minutes at 4°C and fixed using 1-step Fix/Lyse solution for 10 minutes at room temperature (eBioscience, San Diego, CA). For CXCR5 staining, cells were incubated with the monoclonal antibody specific to CXCR5 (biotin; BD Biosciences, San Jose, CA) at room temperature for 30 minutes followed by streptavidin-PE in FACS buffer. For transcription factor staining, cells were fixed with Foxp3 staining buffer set (eBioscience) and stained intracellularly for Bcl6 (clone BCL-DWN) according to manufacturer’s instructions. For intracellular cytokine staining, cells were stimulated with 1 μM of either M282–90 or G183–195 peptides (Bio-Synthesis Inc, Lewisville, TX) in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) for 5 hours at 37°C in 10% FCS-supplemented RPMI 1640. Stimulated cells were stained for surface markers as indicated above and subsequently stained intracellularly for IFN-γ (clone XMG1.2) in FACS buffer containing 0.5% saponin (Sigma-Aldrich) for 30 minutes at 4°C. All antibodies were purchased from eBioscience unless otherwise indicated. Samples were run on a BD LSRFortessa and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Cell types were phenotyped as follows: CD4 T cells (CD90.2+CD4+), CD8 T cells (CD90.2+CD8+), and B cells (CD45+CD3−CD19+).
RSV Antibody ELISA
Mouse serum was collected on days 14, 28, and 84 following infection with either RSV recWT or RSV M-null. RSV-specific serum antibody was measured by ELISA as previously described (33). Briefly, serum was serially diluted 1:2 starting at 1:32 over 8 total dilutions, and incubated overnight at 4°C on plates that were previously coated with 1 × 105 PFU/well of RSV-A2 and blocked with 5% non-fat dry milk in PBS. RSV-specific antibody was detected using biotinylated goat anti-mouse antibody specific for total Ig, IgG1, or IgG2a (Southern Biotech, Birmingham, AL). Plates were incubated with streptavidin-horse radish peroxidase conjugate (Sigma-Aldrich), developed in 3,3’,5,5’-tetramethylbenzidine solution (Sigma-Aldrich), and the reaction was stopped with 2M sulfuric acid. Absorbance values (450 nm) were measured and assessed using Gen5 software (BioTek, Winooski, VT).
Intravascular staining
Mice were injected intravascularly with 1 μg CD45-PE antibody (anti-CD45 labeled with phycoerythrin) (clone 30-F11) 3 minutes prior to euthanasia. Cells from the lung were processed as previously described (34).
Statistical analysis
All statistical analyses are described in each figure legend and were performed using Prism software (GraphPad Software, San Diego, CA). Data were evaluated using either unpaired, two-tailed Student’s t tests between two groups or one-way ANOVA with Tukey-Kramer’s post-test for more than two groups to determine a statistical significance of at least α = 0.05.
Results
RSV M-null infection does not produce infectious progeny in vivo and induces lower magnitude clinical signs of disease
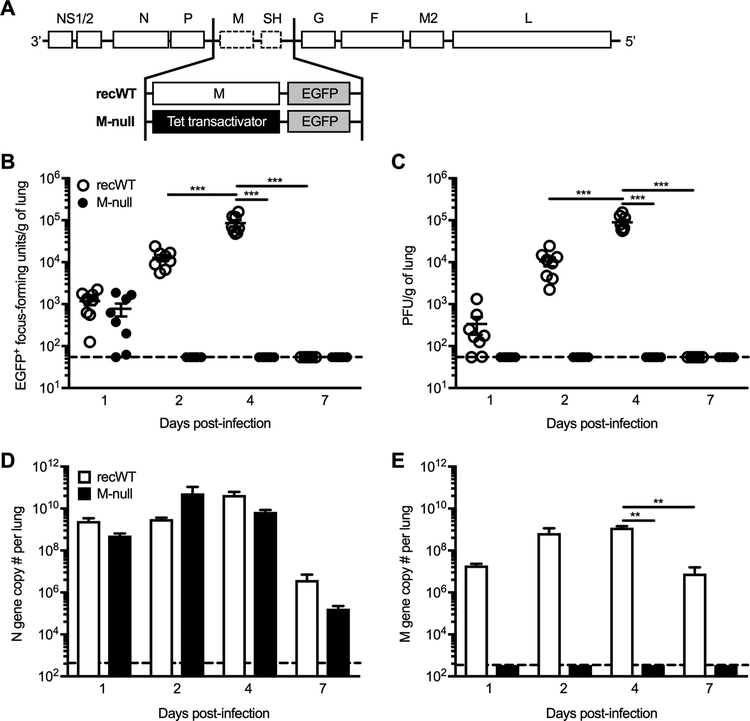
The single-cycle virus RSV M-null was generated such that the M protein ORF was replaced with a Tet transactivator sequence to facilitate its growth in vitro in the H2-M helper cell line, which drives expression of the M gene by a Tet-responsive promoter (Fig. 1A). Additionally, EGFP was inserted in place of the SH ORF, which is dispensable for virus replication both in vitro and in vivo, to allow for visualization of the virus via microscopy (Fig. 1A) (35). In all experiments, RSV M-null was compared to RSV recWT, a recombinant WT RSV-A2 strain that encodes the RSV M gene but also contains EGFP in place of the SH ORF (Fig. 1A). To confirm that RSV M-null behaves as a single-cycle virus in vivo, plaque assays using H2-M helper cells were performed to quantify both EGFP+ focus-forming units (Fig. 1B) and infectious PFU (Fig. 1C) in the lungs of RSV recWT- and RSV M-null-infected mice. RSV recWT was detected by both EGFP+ foci and PFU on day 1 p.i., increased approximately two additional logs to peak viral titers on day 4 p.i., and was completely cleared by day 7 p.i. (Fig. 1B, 1C). In contrast, RSV M-null EGFP+ foci were visualized on day 1 p.i. and were no longer detected by day 2 p.i. (Fig. 1B). Although RSV M-null EGFP+ foci were seen on day 1 p.i., no infectious PFU were present on day 1 p.i. (Fig. 1B, 1C). However, the number of EGFP+ cells were not significantly different between RSV recWT- and RSV M-null-infected mice at day 1 p.i., confirming that the number of initially infected cells within the lung were similar between infections (Fig. 1B). Importantly, infectious PFU were undetectable in the lungs of RSV M-null-infected mice at all time-points evaluated (Fig. 1C). These results confirm that RSV M-null does not produce infectious progeny following infection and behaves as a single-cycle virus in vivo.
Figure 1. No infectious progeny is detected following RSV M-null infection in vivo.
(A) Diagram depicting the genome content of RSV recWT and RSV M-null. (B-E) BALB/c mice were infected with either RSV recWT or RSV M-null, and lungs were harvested on days 1, 2, 4, and 7 p.i. (B) EGFP+ focus-forming units and (C) infectious PFU were quantified in the lung by plaque assay using H2-M helper cells. Open (recWT) and closed (M-null) circles represent values for individual mice and lines indicate mean ± SEM of 2 independent experiments (n=8). (D) RSV N gene and (E) RSV M gene copy numbers per lung were determined by real-time PCR. Data are presented as mean ± SEM of representative results from 1 of 2 independent experiments (n=4). Dashed lines denote the limit of detection for each assay. Groups were compared using one-way ANOVA, ** p<0.01, *** p<0.001.
Given its lack of infectious progeny production in vivo, we performed real-time PCR to evaluate RSV M-null’s ability to express viral genes. Interestingly, RSV N gene copy numbers within the lung were similar following infection with RSV recWT and RSV M-null at all time-points evaluated (Fig. 1D). Importantly, RSV M gene copies were only detected in the lungs of RSV recWT-infected mice and not in RSV M-null-infected mice, confirming that production of the M gene is absent following RSV M-null infection (Fig. 1E). Therefore, RSV M-null exhibits robust viral gene expression of the N gene, despite its inability to generate infectious particles and spread beyond the initially infected cell.
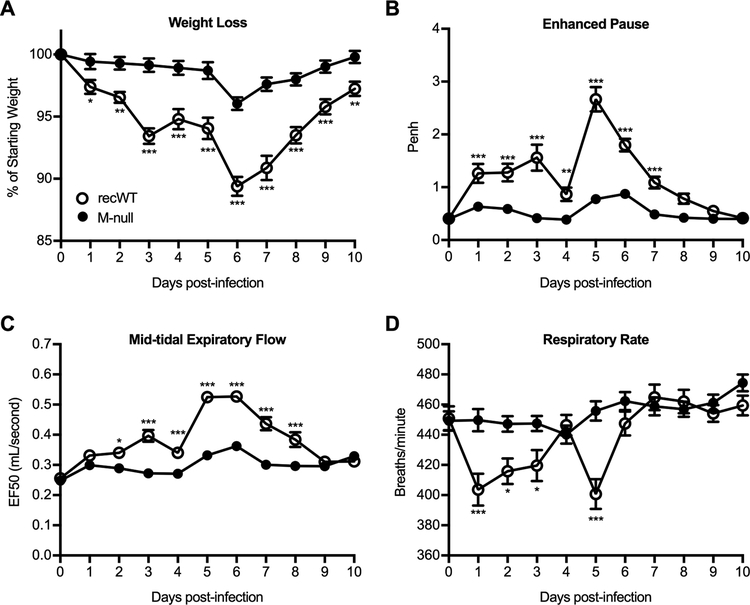
Due its demonstrated attenuation of in vivo, we next evaluated whether RSV M-null induced lower magnitude clinical signs of disease following infection compared to RSV recWT infection. RSV recWT infection induced weight loss up to 10 percent of the animal’s total body weight prior to recovery, as is typical of an acute RSV infection of BALB/c mice (Fig. 2A) (32, 36, 37). In contrast, RSV M-null-infected mice exhibited significantly reduced weight loss at all time points examined (Fig. 2A). Additionally, whole-body plethysmography was utilized to measure several parameters of pulmonary function including Penh as an indicator of airway obstruction, EF50 as a measure of bronchoconstriction, and respiratory rate (38–40). Infection with RSV recWT induced pulmonary dysfunction as defined by an elevation in Penh and EF50 and a decline in respiratory rate from baseline (Fig. 2B–D). In contrast, RSV M-null-infected mice exhibited little change in Penh, EF50, and respiratory rate values from baseline, indicating a significant reduction in pulmonary dysfunction compared to RSV recWT infection (Fig. 2B–D). Overall, these results indicate that RSV M-null infection significantly reduces clinical signs of disease, including weight loss and pulmonary dysfunction, compared to RSV recWT.
Figure 2. RSV M-null infection reduces weight loss and pulmonary dysfunction.
BALB/c mice were infected with either RSV recWT or RSV M-null and monitored daily for (A) weight loss and respiratory parameters including (B) Penh, (C) EF50, and (D) respiratory rate by whole body plethysmography. Data are presented as mean ± SEM of 6 independent experiments (n=35 for recWT and n=36 for M-null). Groups were compared using Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
RSV M-null infection induces robust immune responses of similar magnitude to RSV recWT
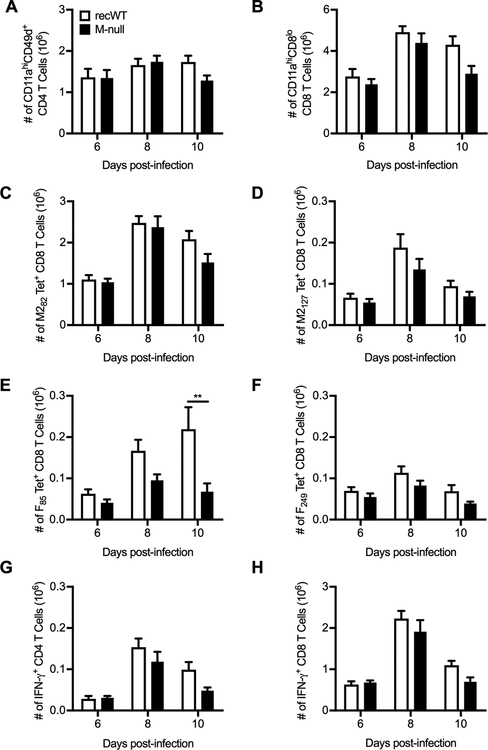
Due to the strong attenuation of RSV M-null’s production of infectious viral progeny and pathogenesis, we evaluated the immunogenicity of RSV M-null vaccination. First, we determined the ability of RSV M-null to induce a T cell response, as both CD4 and CD8 T cells are critical for viral clearance following an acute RSV infection (37, 41, 42). Remarkably, the total numbers of activated CD4 (Fig. 3A) and CD8 T cells (Fig. 3B) in the lung, as determined by surrogate activation marker staining, were similar between RSV recWT- and RSV M-null-infected BALB/c mice at days 6, 8, and 10 p.i. (43, 44). RSV M-null infection also induced CD8 T cells specific to the immunodominant M282 CD8 T cell epitope in the lung to a similar magnitude as observed following RSV recWT infection (Fig. 3C) (45–47). Similar results were observed for CD8 T cell responses in the lung against the subdominant CD8 T cell epitopes M2127, F85, and F249 (Fig. 3D–F) (48–50). The only significant difference observed between T cell responses induced by RSV recWT and RSV M-null infection was the number of F85-specific CD8 T cells in the lung at day 10 p.i. (Fig. 3E). Lung CD4 and CD8 T cells induced by RSV M-null infection were also capable of producing effector cytokines, as RSV recWT- and RSV M-null-infected mice exhibited similar numbers of IFN-γ+ CD4 (Fig. 3G) and CD8 T cells (Fig. 3H) in the lung following ex vivo stimulation with G183 and M282 peptides, respectively. Overall, these results indicate that despite its inability to produce infectious progeny following infection, RSV M-null vaccination induces robust T cell responses of similar magnitude to that of RSV recWT.
Figure 3. RSV M-null infection induces T cell responses of similar magnitude to recWT infection.
BALB/c mice were infected with either RSV recWT or RSV M-null, and lungs were harvested on days 6, 8, and 10 p.i. Total numbers of (A) CD11ahiCD49d+ CD4 T cells, (B) CD11ahiCD8lo CD8 T cells, and (C) M282-, (D) M2127-, (E) F85-, and (F) F249-tetramer+ CD8 T cells were quantified. Total numbers of (G) CD4 T cells and (H) CD8 T cells producing IFN-γ after re-stimulation with G183 and M282 peptides, respectively, were determined by intracellular cytokine staining. Data are presented as mean ± SEM of 2–3 independent experiments (n=8 for days 6 and 10 and n=12 for day 8). Groups were compared using Student’s t test, ** p<0.01.
We next utilized C57BL/6 mice to evaluate the magnitude of T cell responses directed against the RSV M protein following RSV M-null vaccination, as BALB/c mice do not contain any defined CD4 or CD8 T cell epitopes within the RSV M protein (50). Similar to what was observed in BALB/c mice, C57BL/6 mice exhibited a similar number of total activated CD4 (Fig. S1A) and CD8 T cells (Fig. S1B) in the lung following infection with either RSV recWT or RSV M-null. In contrast, the evaluation of M protein-specific CD8 T cell responses by staining with a tetramer specific to the immunodominant M187 CD8 T cell epitope in C57BL/6 mice revealed a dramatically reduced frequency and total number of M-specific CD8 T cells in the lung following RSV M-null infection compared to RSV recWT infection (Fig. S1C, S1D) (51). Despite RSV M-null’s inability to produce the M protein, a small frequency of M187-specific CD8 T cells were detected in the lungs following RSV M-null infection, indicating that the initial amount of M protein within the virus inoculum is sufficient to prime the activation of a fraction of the normal M187-specific CD8 T cell response (Fig. S1C).
Additionally, we evaluated the ability of RSV M-null to induce a T cell response in CB6F1 mice, a cross between C57BL/6 and BALB/c mice, which are frequently used as a murine model for RSV infection (52–56). Similar to what was observed in BALB/c and C57BL/6 mice, CB6F1 mice infected with RSV M-null exhibited similar numbers of total activated CD4 (Fig. S2A) and CD8 T cells (Fig. S2B) as those infected with RSV recWT at day 8 p.i. M282-specific CD8 T cells in the lung were also similar in both frequency (Fig. S2C) and total number (Fig. S2D) following infection with either RSV recWT or RSV M-null. In contrast, M187-specific CD8 T cell responses were significantly reduced in RSV M-null-infected CB6F1 mice compared to mice infected with RSV recWT (Fig. S2C, S2D). Therefore, M-specific CD8 T cell responses are significantly reduced following infection with RSV M-null, but the magnitude of non-M protein-specific CD8 T cell responses are largely unaffected by RSV M-null’s inability to produce infectious viral progeny.
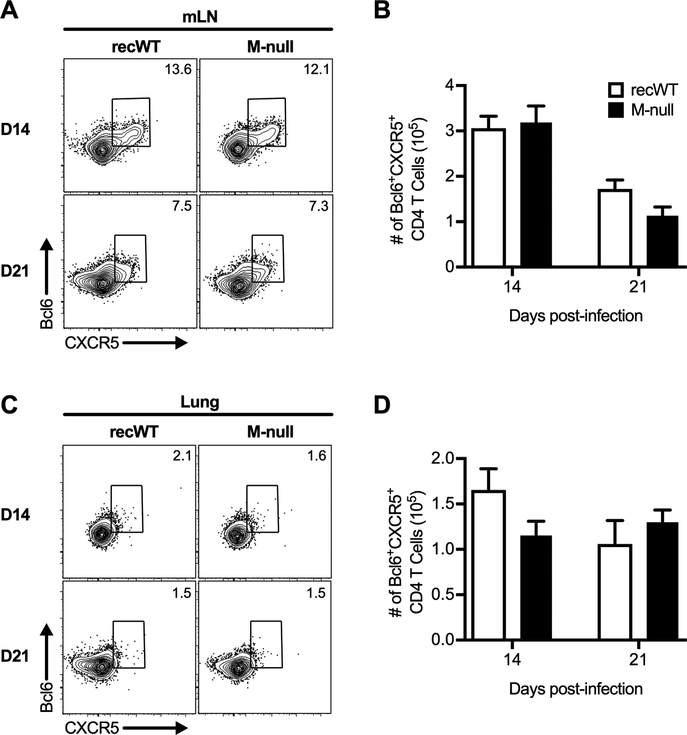
Given that overall CD4 T cell activation following RSV M-null infection was similar to that of RSV recWT infection, we next evaluated whether T follicular helper (Tfh) cells were generated by RSV M-null infection in BALB/c mice. Tfh cells are a subset of CD4 T cells that interact with germinal center (GC) B cells and are critical for the generation of long-lived plasma cells and memory B cells (57–59). We evaluated Tfh generation in the mLN by staining for the Tfh marker CXCR5 and the Tfh lineage-defining transcription factor Bcl6 (Fig. 4A) (60–64). Tfh cells were present in the mLN 14 days following RSV M-null infection at a similar magnitude to that observed following RSV recWT infection (Fig. 4A, 4B). The Tfh populations in the mLN for both RSV recWT and RSV M-null declined in both frequency (Fig. 4A) and total number (Fig. 4B) at day 21 but remained comparable between the two infections. In addition, a small but detectable population of Tfh cells in the lung was also induced by RSV M-null infection that was similar in magnitude to that of RSV recWT infection (Fig. 4C, 4D). Overall, RSV M-null infection generates Tfh cells in the mLN and lung of BALB/c mice comparable to RSV recWT infection.
Figure 4. T follicular helper cells are generated by RSV M-null infection.
BALB/c mice were infected with either RSV recWT or RSV M-null, and mLN and lungs were harvested on days 14 and 21 p.i. Cells were gated on CD4 T cells, and gates were placed using fluorescence minus one controls. Representative staining panels of Bcl6+CXCR5+ Tfh cells in the (A) mLN and (C) lung. Total numbers of Bcl6+CXCR5+ Tfh cells in the (B) mLN and (D) lung. Data are presented as mean ± SEM of 2 independent experiments (n=8). Groups were compared using Student’s t test.
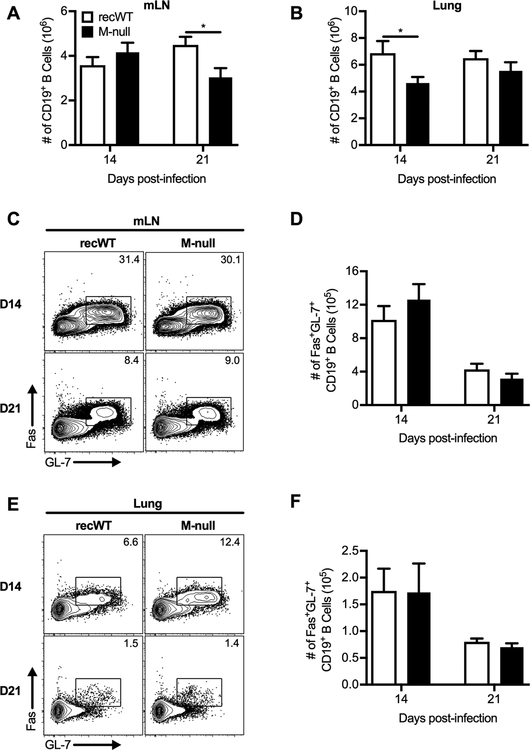
In addition to T cell responses, we also determined the ability of RSV M-null infection to generate B cells in the mLN and lung. The number of B cells in the mLN were similar in magnitude between RSV recWT and RSV M-null infection at day 14 p.i., but RSV M-null-infected mice exhibited a decreased number of B cells at day 21 p.i. compared to RSV recWT-infected mice (Fig. 5A). In contrast, the total number of B cells in the lung of RSV M-null-infected mice was decreased at day 14 p.i. compared to RSV recWT infection but similar in magnitude at day 21 p.i. (Fig. 5B). We also evaluated whether GC B cells, which differentiate into antibody-secreting B cells, were generated following infection with RSV M-null (58, 59). Using the GC B cell markers GL-7 and Fas, we detected a sizeable GC B cell population in the mLN of both RSV recWT- and RSV M-null-infected mice at day 14 p.i. (Fig. 5C, 5D). The GC B cell population in the mLN declined by day 21 p.i. but remained similar in magnitude between RSV recWT and RSV M-null infections at both day 14 and 21 p.i. (Fig. 5C, 5D). Similar results were also observed for GC B cells in the lung of RSV recWT- and RSV M-null-infected mice (Fig. 5E, 5F). Therefore, RSV M-null infection generates B cell responses, including GC B cells, at a similar magnitude to that of RSV recWT infection. Overall, immunization with the single-cycle virus RSV M-null induces robust immune responses, including CD4 T cells, CD8 T cells, Tfh cells, and GC B cells, that are of similar magnitude to infection with the replication-competent RSV recWT virus.
Figure 5. RSV M-null infection induces similar numbers of germinal center B cells as recWT infection.
BALB/c mice were infected with either RSV recWT or RSV M-null, and mLN and lungs were harvested on days 14 and 21 p.i. Total numbers of CD19+ B cells in the (A) mLN and (B) lung. Representative staining panels of Fas+GL-7+ GC B cells in the (C) mLN and (E) lung. Total numbers of Fas+GL-7+ GC B cells in the (D) mLN and (F) lung. Data are presented as mean ± SEM of 2 independent experiments (n=8). Groups were compared using Student’s t test, * p<0.05.
RSV M-null infection generates long-lasting antibodies and memory T cell responses
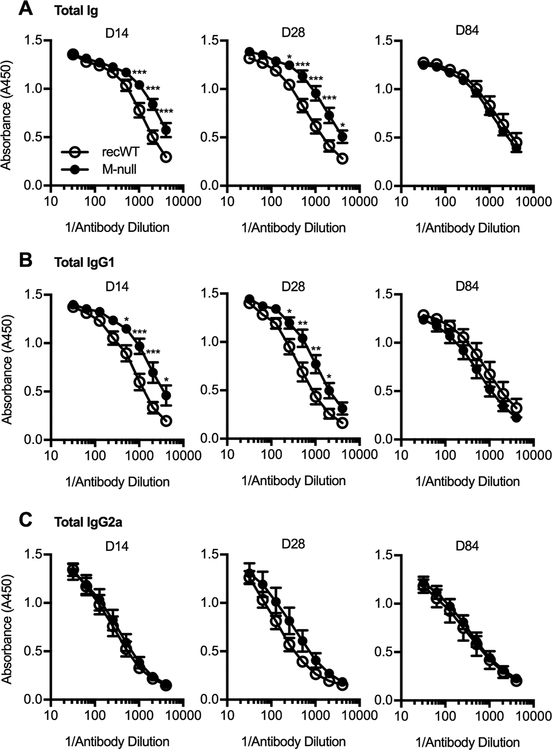
The induction of RSV-specific antibodies in the serum has been correlated with a reduced risk of secondary RSV infection and amelioration of RSV-associated disease in humans (65–68). Therefore, we determined the degree to which RSV M-null infection induces RSV-specific antibodies in the serum compared to RSV recWT infection of BALB/c mice. Early after infection at days 14 and 28 p.i., RSV M-null-infected mice exhibited a robust induction of RSV-specific serum antibodies, including total Ig-, IgG1-, and IgG2a-specific antibodies (Fig. 6A–C). The serum of RSV M-null-infected mice exhibited an elevated level of both total Ig- and IgG1-specific antibodies at the lower antibody dilutions compared to RSV recWT-infected mice (Fig. 6A, 6B). Importantly, the RSV-specific serum antibodies generated by RSV M-null infection were long-lasting, with antibody levels similar to that of RSV recWT-infected mice at the late memory timepoint of day 84 p.i. (Fig. 6A–C). Overall, RSV M-null infection induces serum antibody responses that are long-lasting and similar in magnitude to RSV recWT infection.
Figure 6. RSV M-null infection generates long-lasting RSV-specific serum antibody responses.
BALB/c mice were infected with either RSV recWT or RSV M-null, and serum was collected on days 14, 28, and 84 p.i. Serum was assessed for levels of RSV-specific (A) total Ig, (B) IgG1, and (C) IgG2a by ELISA. Data are presented as mean ± SEM of representative results from 1 of 2 independent experiments (n=5). Groups were compared using Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
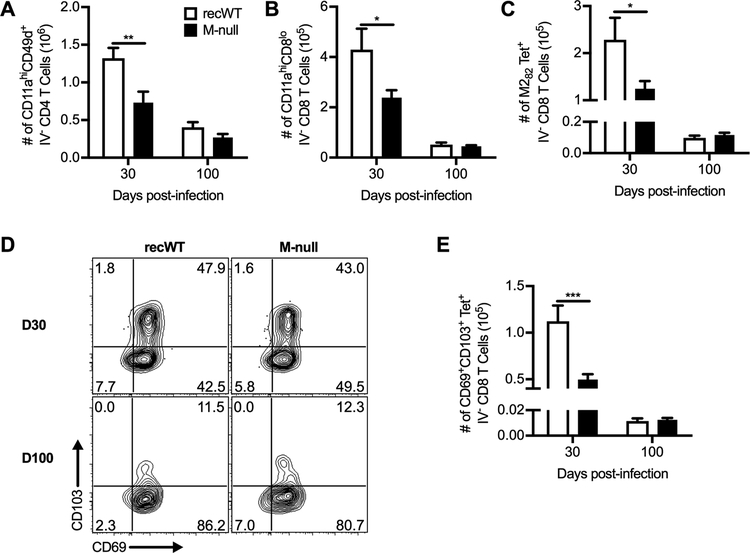
In addition to evaluating serum antibodies, we also determined the ability of RSV M-null to generate memory T cell responses, which are important for providing protection against secondary infection (26, 69). We utilized an intravascular staining technique to ensure that the memory T cells were located within the lung parenchyma rather than the pulmonary vasculature (34, 70). The number of total activated CD4 T cells (Fig. 7A) and CD8 T cells (Fig. 7B) within the lung parenchyma were decreased in RSV M-null-infected mice at the early memory timepoint of 30 days p.i. compared to RSV recWT-infected mice. However, the number of activated CD4 (Fig. 7A) and CD8 T cells (Fig. 7B) in RSV M-null-infected mice contracted down to a similar magnitude as RSV recWT-infected mice at the late memory timepoint of 100 days p.i. These results indicate that while the T cell contraction kinetics may be accelerated in RSV M-null-infected mice, memory T cells contract to form a stable memory T cell population of similar magnitude to RSV recWT infection. Similar results were observed for M282-specific memory CD8 T cells in the lung parenchyma (Fig. 7C). Additionally, we evaluated the ability of RSV M-null immunization to generate RSV-specific tissue-resident memory CD8 T cells (TRM), which have been shown to be elicited following RSV infection (69, 70). As expected, RSV recWT infection induced a large proportion of M282-specific memory CD8 T cells within the lung parenchyma expressing both CD69 and CD103, the canonical markers of TRM, at day 30 p.i. (Fig. 7D) (71, 72). The TRM population generated by RSV recWT infection contracted to a lower number by 100 days p.i. (Fig. 7D). RSV M-null infection also induced a population of M282-specific TRM at day 30 p.i. (Fig. 7D). Similar to both total activated and M282-specific memory CD8 T cells, the number of M282-specific TRM was decreased in magnitude as compared to RSV recWT infection (Fig. 7E). Importantly, both the frequency (Fig. 7D) and total number (Fig. 7E) of RSV-specific TRM generated by RSV M-null infection were similar to RSV recWT infection at day 100 p.i., indicating that RSV M-null vaccination generates long-lived RSV-specific TRM. Overall, RSV M-null infection induces memory CD4 and CD8 T cell responses that are similar in magnitude to RSV recWT infection at a late memory timepoint.
Figure 7. RSV M-null infection induces RSV-specific tissue-resident memory CD8 T cells.
BALB/c mice were infected with either RSV recWT or RSV M-null and administered anti-CD45 antibody intravascularly 3 minutes prior to euthanasia on days 30 and 100 p.i. Cells negative for staining with the CD45 intravascular antibody (IV−) were gated, and total numbers of (A) CD11ahiCD49d+ CD4 T cells, (B) CD11ahiCD8lo CD8 T cells, and (C) M282-tetramer+ CD8 T cells within the lung parenchyma are shown. (D) Representative staining panels and (E) total numbers of CD69+CD103+ IV− M282-tetramer+ CD8 T cells. Data are presented as mean ± SEM of 2 independent experiments (n=10 for day 30 and n=9 for day 100). Groups were compared using Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
RSV M-null immunization provides protection against secondary viral challenge
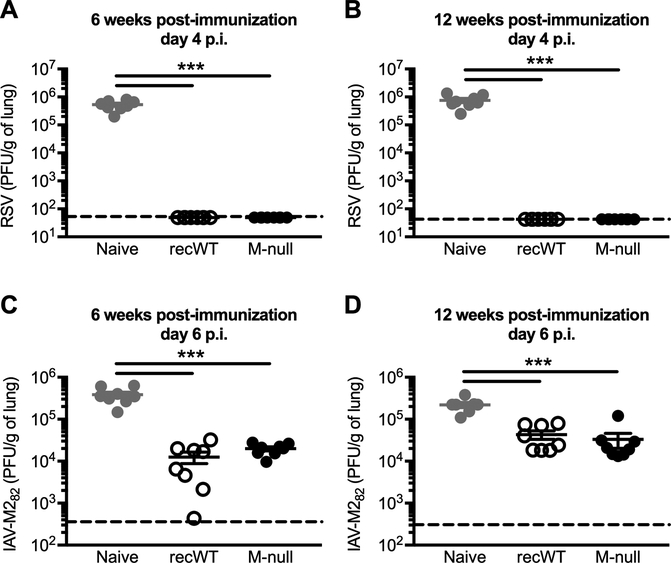
We next evaluated the ability of RSV M-null vaccination to provide protection against a secondary RSV infection. BALB/c mice were immunized with either RSV recWT or RSV M-null and challenged with RSV 6 weeks later (Fig. 8A). While naive mice receiving a primary RSV infection exhibited high levels of virus in their lungs at day 4 post-challenge, immunization with either RSV recWT or RSV M-null resulted in complete viral clearance in the lungs (Fig. 8A). Importantly, the protection afforded by RSV M-null vaccination is long-lasting, as mice challenged with RSV at 12 weeks post-vaccination also completely cleared the virus from the lungs (Fig. 8B). Therefore, RSV M-null vaccination protects against a secondary RSV infection by reducing lung viral titers after RSV challenge.
Figure 8. RSV M-null infection provides protection against secondary viral challenge.
(A/B) Naive BALB/c mice were infected with either RSV recWT or RSV M-null and challenged with RSV either (A) 6 or (B) 12 weeks p.i. Lung RSV titers on day 4 p.i. were determined by plaque assay using VERO cells. (C/D) Naive BALB/c mice were infected with either RSV recWT or RSV M-null and challenged with IAV-M282 either (C) 6 or (D) 12 weeks p.i. Lung IAV titers on day 6 p.i. were determined by plaque assay using MDCK cells. Data are presented as mean ± SEM of 2 independent experiments (n=8). Groups were compared using one-way ANOVA, *** p<0.001.
RSV-specific memory CD8 T cells have previously been shown to significantly reduce lung viral titers following a secondary RSV challenge independently of both RSV-specific antibodies and memory CD4 T cells (26). Given the protective potential of RSV-specific memory CD8 T cells, we wanted to determine the specific ability of RSV-specific memory CD8 T cells induced by RSV M-null vaccination to provide protection against a secondary viral challenge. Therefore, BALB/c mice that were previously immunized with either RSV recWT or RSV M-null 6 weeks prior were challenged with IAV-M282, a recombinant IAV strain expressing the RSV M282 CD8 T cell epitope. Using this approach, we can evaluate the isolated ability of M282-specific CD8 T cells to reduce viral titers, as the only RSV-specific cells that will specifically respond to IAV-M282 challenge are the M282-specific CD8 T cells generated by vaccination. M282-specific memory CD8 T cells generated by immunization with either RSV recWT or RSV M-null significantly reduced lung viral titers at day 6 post-challenge compared to naive mice receiving a primary IAV-M282 infection (Fig. 8C). However, there was no difference in IAV-M282 lung viral titers between mice vaccinated with either RSV recWT or RSV M-null, indicating that memory CD8 T cells generated by RSV M-null vaccination provided protection as efficiently as those induced by RSV recWT infection (Fig. 8C). Importantly, the protection provided by RSV-specific memory CD8 T cells was long-term, as mice challenged with IAV-M282 at 12 weeks post-immunization also exhibited a significant reduction in lung viral titers compared to naive controls (Fig. 8D). Overall, RSV M-null vaccination provides long-lasting protection against both secondary RSV and IAV-M282 challenges by significantly reducing lung viral titers.
Discussion
The RSV M protein is critical for the production of infectious viral progeny and the formation of viral filaments in vitro (25). Thus, infection with the single-cycle virus RSV M-null, which does not contain the RSV M gene, cannot generate infectious viral particles in WT cell lines (25). However, in the H2-M helper cell line, which provides expression of the M protein via a trans-complementing plasmid, RSV M-null is capable of spreading to neighboring uninfected cells (25). These results correspond to what we observed following in vivo infection with RSV M-null in BALB/c mice, which also did not produce infectious viral progeny in the lung at any of the time-points evaluated following infection. While RSV M-null infection in vivo did not result in mature virion formation, viral gene expression of RSV N was robust and similar in magnitude to RSV recWT infection. Given that the peak of infectious virus replication is day 4 p.i., it is particularly interesting that the magnitude of RSV N gene expression remains similar between RSV recWT- and RSV M-null-infected mice at day 4 p.i. It is not clear why RSV N gene copy levels are similar between RSV recWT- and RSV M-null-infected mice. In vitro, the first generation RSV M-null replicated and transcribed genomic RNA at higher levels than RSV recWT due to the absence of replication-inhibition by the RSV M protein (25, 73). Therefore, the lack of replication-inhibition by RSV M may also contribute to the unexpectedly high copy levels of the RSV N gene following in vivo infection with the second generation RSV M-null, which may be masking potential differences. Alternatively, the large viral inoculum required for RSV infection in mice, which are semi-permissive hosts, may be the primary driver of viral gene expression (74, 75). The amount of input virus for RSV recWT and RSV M-null (1 × 106 PFU) was substantially larger than the amount of virus detected in the lungs of RSV recWT-infected mice (1 × 105 PFU per gram of lung, approximately 2.5 × 104 per whole lung). Thus, it is likely that viral gene expression is primarily a result of genome replication by the initial viral inoculum, and the relatively small amount of infectious progeny produced following infection with a replication-competent virus has minimal impact on gene expression levels. If this is the case, the lack of difference in RSV N gene expression observed following RSV recWT and RSV M-null infection would be expected given that the initial infection dose was identical. We are currently examining this further using the RSV M-null virus as a tool to evaluate the mechanisms involved. Regardless of the underlying mechanism, the data clearly demonstrate that a single-cycle live RSV is sufficient to induce a comprehensive, protective, and long-lasting immune response.
The attenuation of live viral vaccine candidates to reduce pathogenesis following immunization is a critical component of vaccine development. Importantly, RSV M-null-infected mice exhibited significantly reduced weight loss and pulmonary dysfunction, as measured by the respiratory parameters Penh, EF50, and respiratory rate, compared to RSV recWT infection. These results are similar to what was previously observed following vaccination of mice with single-cycle virus vaccines against IAV, herpes simplex virus, and vesicular stomatitis virus (16, 19, 20, 22). Clinical signs of disease including early weight loss and pulmonary dysfunction were almost completely abrogated following RSV M-null infection with little change from pre-immunization baseline levels. While RSV M-null infection induced a small amount of weight loss and pulmonary dysfunction at the later phase of infection between days 5–7 p.i., it was dramatically reduced compared to infection with RSV recWT. The clinical signs of disease that occurs following RSV infection of BALB/c mice between day 5 and day 7 p.i. is largely attributed to the induction of the RSV-specific T cell response (37, 41, 42). However, the acute T cell response induced by RSV M-null vaccination was similar in magnitude to that of an RSV recWT infection. Thus, it is likely that inflammatory cytokines, such as IFN-γ and TNF, induced by continued RSV production of infectious progeny contribute to the T cell-mediated pathology observed following RSV recWT infection (76–79). Overall, RSV M-null is sufficiently attenuated to prevent pathogenesis, as the induction of clinical signs of disease following vaccination is minimal.
While attenuation of pathogenesis following vaccination is critical, it often corresponds with a marked reduction in immunogenicity. Indeed, the immune responses generated following immunization with single-cycle virus vaccine candidates against IAV and cytomegalovirus were substantially reduced compared to their WT counterparts (16, 80). In contrast, single-cycle adenovirus, rabies virus, and vesicular stomatitis virus vectors induced robust immune responses that were comparable to WT controls (15, 81, 82). Therefore, the immunogenicity of single-cycle virus vaccine candidates varies widely. Remarkably, RSV M-null immunization induced robust acute immune responses, including CD4 T cells, CD8 T cells, B cells, and serum antibodies, that were similar in magnitude to those generated by RSV recWT infection. These results correlated with the robust expression of the RSV N gene in the lung following RSV M-null infection, despite its lack of infectious progeny production. These results suggest that sustained viral gene expression, rather than the production and spread of infectious viral progeny, is responsible for the majority of the immune activation that occurs following RSV infection in mice. Interestingly, although significantly reduced compared to RSV recWT infection, a small population of M187-specific CD8 T cells were detectable in the lungs of RSV M-null-infected C57BL/6 and CB6F1 mice. This observation suggests that a portion of the CD8 T cell response is primed solely by the RSV M protein that is contained within the initial viral inoculum. It is possible that antigen-presenting cells in the lung and the airway that were directly infected with the initial RSV M-null inoculum primed the small M187-specific CD8 T cell response observed following infection of C57BL/6 and CB6F1 mice. RSV M-null provides an interesting tool to further examine the degree to which immune responses are primed by the original inoculum prior to the production of viral progeny. Overall, these results indicate that despite its significant attenuation, RSV M-null infection is strongly immunogenic in mice.
In addition to robust acute immune responses, RSV M-null vaccination also induced serum antibody and memory T cell responses that were similar in magnitude to RSV recWT infection at a late memory timepoint. A portion of the memory CD8 T cells induced by RSV M-null vaccination were RSV-specific TRM. TRM persist in the lung and rapidly respond to pathogen exposure by producing inflammatory cytokines, such as IFN-γ (71, 83, 84). Importantly, TRM in the lung have been demonstrated to provide protection against secondary infection with IAV (83–85). Therefore, vaccination to induce RSV-specific TRM may provide a critical component of the protective immune response. Interestingly, the magnitude of memory T cell responses induced by RSV M-null vaccination was reduced at day 30 p.i. but was similar by day 100 p.i. compared to RSV recWT vaccination. These results indicate that the T cell contraction kinetics may be accelerated following infection with RSV M-null compared to RSV recWT. Importantly, the serum antibodies and memory T cells induced by RSV M-null vaccination provided protection against secondary viral challenge with both RSV-A2 and IAV-M282. These results indicate that both antibody and T cell responses contribute to the protection afforded by RSV M-null vaccination. The ability of RSV M-null to provide protection is particularly striking given that despite its inability to produce infectious progeny, a single immunization was sufficient to provide protection as efficiently as was observed with immunization with the replication-competent RSV recWT virus. The current RSV M-null vaccine does not improve on cross-protection against RSV B strains compared to other RSV-A2-based live-attenuated vaccines. Improvement of cross-protection remains an important consideration for the future development of any live-attenuated vaccines against RSV. Overall, RSV M-null vaccination induced serum antibody and memory T cell responses that were able to provide protection against secondary viral challenges.
In summary, we have evaluated the novel single-cycle virus strain RSV M-null as a potential live-attenuated vaccine candidate in mice. RSV M-null behaved as a single-cycle virus in vivo, as it did not produce infectious viral particles following infection. RSV M-null is sufficiently attenuated, as RSV M-null-infected mice exhibited significantly reduced weight loss and pulmonary dysfunction compared to infection with RSV recWT. Surprisingly, despite its inability to produce infectious progeny and spread to neighboring cells, RSV M-null vaccination induced robust immune responses, including long-lasting serum antibodies and memory T cell responses, that were similar in magnitude to that of an RSV recWT infection. Importantly, RSV M-null vaccination provided protection against secondary viral challenges by reducing lung viral titers as effectively as immunization with RSV recWT. Therefore, a single immunization with RSV M-null is strongly immunogenic and protective in mice. Humans are more susceptible to RSV infection than mice. Furthermore, intranasal vaccination of humans results in virus deposition primarily in the upper respiratory tract, while infection of mice results in direct virus deposition in the lungs. Thus, whether RSV M-null would be similarly efficacious following intranasal vaccination in humans will need to be determined in future studies. Our data indicate that RSV M-null may provide a safety advantage over other line-attenuated vaccine candidates for use in humans. As the entire M gene sequence is absent from the viral genome, the RSV M-null vaccine cannot regain virulence by restoring M function. This in contrast to live vaccines attenuated by point mutations, which have the potential to revert and regain virulence. Overall, our results suggest that the single-cycle virus RSV M-null has potential to overcome the tremendous hurdle of balancing vaccine efficacy with safety and therefore represents a promising novel live-attenuated RSV vaccinate candidate.
Supplementary Material
Key Points.
The live-attenuated single-cycle RSV M-null vaccine is significantly attenuated.
RSV M-null vaccination in mice induces robust acute and memory immune responses.
Vaccination with RSV M-null provides protection against secondary viral challenge.
Acknowledgments
We thank Stacey Hartwig for excellent technical assistance and Garry Hauser for assistance with real-time PCR.
Footnotes
Address correspondence and reprint requests to: Dr. Steven M. Varga, 51 Newton Road, 3–532 Bowen Science Building, University of Iowa, Iowa City, IA 52242. steven-varga@uiowa.edu
This work was supported by the Department of Microbiology and Immunology at the University of Iowa (to SMV), the Oklahoma Center for the Advancement of Science and Technology under award number HR08–1395 (to AGPO), and the National Institute of Allergy and Infectious Diseases and the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award numbers R01AI124093 (to SMV), T32AI007485 (to MES), and P20GM103648 (to AGPO). Data presented herein were obtained at the Genomics Division of the Iowa Institute of Human Genetics which is supported, in part, by the University of Iowa Carver College of Medicine, the Holden Comprehensive Cancer Center, and the National Cancer Institute of the NIH under award number P30CA086862. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used in this article: EF50, mid-tidal expiratory flow; EGFP, enhanced GFP; FI-RSV, formalin-inactivated respiratory syncytial virus; GC, germinal center; IAV, influenza A virus; M, matrix protein; mLN, mediastinal lymph node; N, nucleoprotein; ORF, open-reading frame; Penh, enhanced pause; p.i., post-infection; RSV, respiratory syncytial virus; RSV-A2, A2 strain of RSV; Tfh, T follicular helper; TRM, tissue-resident memory CD8 T cells; SH, small hydrophobic protein; WT, wild-type
References
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, and Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lazaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccala G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, Nair H, and R. S. V. G. E. Network. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, and Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 4.Chin J, Magoffin RL, Shearer LA, Schieble JH, and Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89: 449–463. [DOI] [PubMed] [Google Scholar]
- 5.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, and Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89: 435–448. [DOI] [PubMed] [Google Scholar]
- 6.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, and Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89: 405–421. [DOI] [PubMed] [Google Scholar]
- 7.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, and Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89: 422–434. [DOI] [PubMed] [Google Scholar]
- 8.Ambrose CS, Wu X, and Belshe RB. 2010. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J 29: 806–811. [DOI] [PubMed] [Google Scholar]
- 9.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, and Belshe RB. 2011. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 204: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karron RA, Buchholz UJ, and Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 372: 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O’Shea AF, Gruber WC, and Murphy BR. 2007. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 25: 7372–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KY, Root M, and McGregor A. 2016. A novel non-replication-competent cytomegalovirus capsid mutant vaccine strategy is effective in reducing congenital infection. J Virol 90: 7902–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosby CM, and Barry MA. 2014. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 462–463: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DT, Bricker JE, and Desrosiers RC. 2004. A novel approach for producing lentiviruses that are limited to a single cycle of infection. J Virol 78: 11715–11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomme EA, Faul EJ, Flomenberg P, McGettigan JP, and Schnell MJ. 2010. Characterization of a single-cycle rabies virus-based vaccine vector. J Virol 84: 2820–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Baker SF, Martinez-Sobrido L, and Topham DJ. 2014. Induction of CD8 T cell heterologous protection by a single dose of single-cycle infectious influenza virus. J Virol 88: 12006–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa T, Widman DG, Bourne N, Konishi E, and Mason PW. 2008. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine 26: 2772–2781. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Qin Y, He Y, Tao W, Zhang N, Tsai C, Zhou P, and Zhong J. 2011. Production of hepatitis C virus lacking the envelope-encoding genes for single-cycle infection by providing homologous envelope proteins or vesicular stomatitis virus glycoproteins in trans. J Virol 85: 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, and Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts A, Buonocore L, Price R, Forman J, and Rose JK. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 73: 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan C, Xie X, Zou J, Zust R, Zhang B, Ambrose R, Mackenzie J, Fink K, and Shi PY. 2018. Using a virion assembly-defective dengue virus as a vaccine approach. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si L, Xu H, Zhou X, Zhang Z, Tian Z, Wang Y, Wu Y, Zhang B, Niu Z, Zhang C, Fu G, Xiao S, Xia Q, Zhang L, and Zhou D. 2016. Generation of influenza A viruses as live but replication-incompetent virus vaccines. Science 354: 1170–1173. [DOI] [PubMed] [Google Scholar]
- 23.Snyder CM, Allan JE, Bonnett EL, Doom CM, and Hill AB. 2010. Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One 5: e9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widman DG, Ishikawa T, Fayzulin R, Bourne N, and Mason PW. 2008. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine 26: 2762–2771. [DOI] [PubMed] [Google Scholar]
- 25.Mitra R, Baviskar P, Duncan-Decocq RR, Patel D, and Oomens AG. 2012. The human respiratory syncytial virus matrix protein is required for maturation of viral filaments. J Virol 86: 4432–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, and Varga SM. 2018. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog 14: e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, and Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97: 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castilow EM, Meyerholz DK, and Varga SM. 2008. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol 180: 2376–2384. [DOI] [PubMed] [Google Scholar]
- 29.Hu A, Colella M, Tam JS, Rappaport R, and Cheng SM. 2003. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J Clin Microbiol 41: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuypers J, Wright N, and Morrow R. 2004. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 31: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulton RB, Olson MR, and Varga SM. 2008. Regulation of cytokine production by virus-specific CD8 T cells in the lungs. J Virol 82: 7799–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, and Varga SM. 2011. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J Immunol 187: 3145–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knudson CJ, Hartwig SM, Meyerholz DK, and Varga SM. 2015. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog 11: e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, and Masopust D. 2012. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189: 2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukreyev A, Whitehead SS, Murphy BR, and Collins PL. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol 71: 8973–8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulton RB, Meyerholz DK, and Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol 185: 2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham BS, Bunton LA, Wright PF, and Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest 88: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaab T, Ziegert M, Baelder R, Korolewitz R, Braun A, Hohlfeld JM, Mitzner W, Krug N, and Hoymann HG. 2005. Invasive versus noninvasive measurement of allergic and cholinergic airway responsiveness in mice. Respir Res 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huck B, Neumann-Haefelin D, Schmitt-Graeff A, Weckmann M, Mattes J, Ehl S, and Falcone V. 2007. Human metapneumovirus induces more severe disease and stronger innate immune response in BALB/c mice as compared with respiratory syncytial virus. Respir Res 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Schaik SM, Enhorning G, Vargas I, and Welliver RC. 1998. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J Infect Dis 177: 269–276. [DOI] [PubMed] [Google Scholar]
- 41.Alwan WH, Record FM, and Openshaw PJ. 1992. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol 88: 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alwan WH, Kozlowska WJ, and Openshaw PJ. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med 179: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott DS, and Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J Immunol 187: 5568–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai D, Pham NL, Harty JT, and Badovinac VP. 2009. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol 183: 7672–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni AB, Morse HC 3rd, Bennink JR, Yewdell JW, and Murphy BR. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol 67: 4086–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni AB, Collins PL, Bacik I, Yewdell JW, Bennink JR, Crowe JE Jr., and Murphy BR. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol 69: 1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Openshaw PJ, Anderson K, Wertz GW, and Askonas BA. 1990. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol 64: 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang J, Srikiatkhachorn A, and Braciale TJ. 2001. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol 167: 4254–4260. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Miller SA, Wright DW, Rock MT, and Crowe JE Jr. 2007. Tissue-specific regulation of CD8+ T-lymphocyte immunodominance in respiratory syncytial virus infection. J Virol 81: 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDermott DS, Knudson CJ, and Varga SM. 2014. Determining the breadth of the respiratory syncytial virus-specific T cell response. J Virol 88: 3135–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rutigliano JA, Rock MT, Johnson AK, Crowe JE Jr., and Graham BS. 2005. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology 337: 335–343. [DOI] [PubMed] [Google Scholar]
- 52.Malloy AM, Ruckwardt TJ, Morabito KM, Lau-Kilby AW, and Graham BS. 2017. Pulmonary dendritic cell subsets shape the respiratory syncytial virus-specific CD8+ T cell immunodominance hierarchy in neonates. J Immunol 198: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruckwardt TJ, Bonaparte KL, Nason MC, and Graham BS. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol 83: 3019–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruckwardt TJ, Luongo C, Malloy AM, Liu J, Chen M, Collins PL, and Graham BS. 2010. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J Immunol 185: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, Thomas PG, and Graham BS. 2011. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog 7: e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruckwardt TJ, Malloy AM, Morabito KM, and Graham BS. 2014. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog 10: e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crotty S 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shlomchik MJ, and Weisel F. 2012. Germinal center selection and the development of memory B and plasma cells. Immunol Rev 247: 52–63. [DOI] [PubMed] [Google Scholar]
- 59.Victora GD, and Nussenzweig MC. 2012. Germinal centers. Annu Rev Immunol 30: 429–457. [DOI] [PubMed] [Google Scholar]
- 60.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, and Cyster JG. 1999. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med 190: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, and Cyster JG. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol 179: 5099–5108. [DOI] [PubMed] [Google Scholar]
- 62.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, and Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, and Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, and Vinuesa CG. 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31: 457–468. [DOI] [PubMed] [Google Scholar]
- 65.Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, Johnson SM, Moore-Clingenpeel M, Ramilo O, Mejias A, and Peeples ME. 2017. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis 216: 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falsey AR, and Walsh EE. 1998. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 177: 463–466. [DOI] [PubMed] [Google Scholar]
- 67.Lee FE, Walsh EE, Falsey AR, Betts RF, and Treanor JJ. 2004. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res 63: 191–196. [DOI] [PubMed] [Google Scholar]
- 68.Piedra PA, Jewell AM, Cron SG, Atmar RL, and Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21: 3479–3482. [DOI] [PubMed] [Google Scholar]
- 69.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, and Tregoning JS. 2018. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol 11: 249–256. [DOI] [PubMed] [Google Scholar]
- 70.Knudson CJ, Weiss KA, Hartwig SM, and Varga SM. 2014. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol 88: 9010–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. [DOI] [PubMed] [Google Scholar]
- 72.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, and Farber DL. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghildyal R, Mills J, Murray M, Vardaxis N, and Meanger J. 2002. Respiratory syncytial virus matrix protein associates with nucleocapsids in infected cells. J Gen Virol 83: 753–757. [DOI] [PubMed] [Google Scholar]
- 74.Prince GA, Horswood RL, Berndt J, Suffin SC, and Chanock RM. 1979. Respiratory syncytial virus infection in inbred mice. Infect Immun 26: 764–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stark JM, McDowell SA, Koenigsknecht V, Prows DR, Leikauf JE, Le Vine AM, and Leikauf GD. 2002. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J Med Virol 67: 92–100. [DOI] [PubMed] [Google Scholar]
- 76.Ostler T, Davidson W, and Ehl S. 2002. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol 32: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 77.Rutigliano JA, and Graham BS. 2004. Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection. J Immunol 173: 3408–3417. [DOI] [PubMed] [Google Scholar]
- 78.van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, and Welliver RC. 2000. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol 62: 257–266. [DOI] [PubMed] [Google Scholar]
- 79.Tregoning JS, Pribul PK, Pennycook AM, Hussell T, Wang B, Lukacs N, Schwarze J, Culley FJ, and Openshaw PJ. 2010. The chemokine MIP1alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PLoS One 5: e9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snyder CM, Cho KS, Bonnett EL, Allan JE, and Hill AB. 2011. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog 7: e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crosby CM, Nehete P, Sastry KJ, and Barry MA. 2015. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J Virol 89: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Publicover J, Ramsburg E, and Rose JK. 2005. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol 79: 13231–13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McMaster SR, Wilson JJ, Wang H, and Kohlmeier JE. 2015. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-gamma production. J Immunol 195: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, and Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, and Harty JT. 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.