Abstract
The ability of the extracellular matrix (ECM) to direct cell fate has generated the potential for developing a materials-only strategy for tissue regeneration. Previously we described a nanoparticulate mineralized collagen glycosaminoglycan (MC-GAG) material that efficiently induced osteogenic differentiation of human mesenchymal stem cells (hMSCs) and calvarial bone healing without exogenous growth factors or progenitor cell expansion. In this work, we evaluated the interactions between MC-GAG and primary human osteoclasts (hOCs). In the absence of hMSCs, mineralized Col-GAG materials directly inhibited hOC viability, proliferation, and resorption in contrast to non-mineralized Col-GAG which demonstrated a modest inhibition of resorptive activity only. Co-cultures containing differentiating hMSCs with hOCs demonstrated increased hOC-mediated resorption only on Col-GAG while MC-GAG co-cultures continued to inhibit resorption. Unlike Col-GAG, hMSCs on MC-GAG expressed increased amounts of osteoprotegerin (OPG) protein, the major endogenous osteoclast inhibitor. Interestingly, OPG expression was found to be antagonized by small mothers against decapentaplegic1/5 (Smad1/5) phosphorylation, an obligate pathway for osteogenic differentiation of hMSCs on MC-GAG, and potentiated by extracellular signal-regulated kinase (ERK1/2) phosphorylation. Collectively, these results suggested that the MC-GAG material both directly inhibited the osteoclast viability, proliferation, and resorptive activity as well as induced hMSCs to secrete osteoprotegerin, an anti-osteoclastogenic factor, via a signaling pathway distinct from osteogenic differentiation.
Keywords: bone regeneration, bone resorption, collagen glycosaminoglycan, nanoparticle, osteoclast, osteoprotegerin
Introduction
The major axis controlling bone homeostasis via osteoclast regulation is mediated by the receptor activator of nuclear factor-κB (RANK), receptor activator of nuclear factor-κB ligand (RANKL), and osteoprotegerin (OPG) (Lee et al., 2011; Maxhimer, Bradley, & Lee, 2015). RANK, a tumor necrosis factor superfamily receptor, is essential to osteoclast differentiation and activation upon binding to its cognate ligand, RANKL (Anderson et al., 1997; Kong et al., 1999). Both in vitro and in vivo genetic studies have demonstrated that RANK or RANKL deficiencies result in uncontrolled bone formation (Lee et al., 2011; J. Li et al., 2000; Yun et al., 2001). As a decoy receptor, OPG is the endogenous negative regulator of RANK/RANKL interactions (Maxhimer et al., 2015). In the absence of OPG, uncontrolled bone resorption and osteoporosis occurs (Simonet et al., 1997; Yun et al., 2001). Thus, the relative quantities of RANKL and OPG have been hypothesized to be important to both the locoregional or organismal balance towards bone formation or resorption (Kadriu et al., 2017). Targeted therapies to this axis are now being investigated for treatment of osteoporosis and other pathologic conditions to yield a net osteogenic state (Faienza et al., 2018; Flick et al., 2003). Similarly, temporary alterations of the RANKL/OPG relative quantities may also be utilized for augmentation of bone regenerative therapies.
In addition to the major regulatory axis, components of the extracellular matrix (ECM) are known to direct changes in osteoclastogenesis and osteoclast activity (Barrow et al., 2011; Kram, Kilts, Bhattacharyya, Li, & Young, 2017; M. Li et al., 2018). Collagen I, the major species in bone ECM and the basis for the majority of bone ECM-based regenerative materials, is the ligand for the osteoclast-associated receptor (OSCAR), the co-receptor necessary for osteoclast activation (Barrow et al., 2011). Fibronectin has been described to both positively and negatively regulate osteoclast activation by several groups (Gramoun et al., 2010; M. Li et al., 2018). Glycosaminoglycans (GAGs) such as hyaluronan have also been known to serve anti-osteoclastogenic effects via Toll-like receptor 4 (TLR4) and inhibition of macrophage-colony stimulating factor (M-CSF) activated signaling pathways (Chang et al., 2007). Additionally, collagen-based coatings that contain sulfated GAGs have been differentially reported to inhibit osteoclast activation (Salbach, Kliemt, et al., 2012; Salbach, Rachner, et al., 2012; Salbach-Hirsch et al., 2013; Salbach-Hirsch et al., 2014). Kram and colleagues demonstrated that expression of the small leucine rich proteoglycans (SLRP) biglycan and fibromodulin by osteoprogenitors attenuated the abilities for osteoclast precursor cells to differentiate through sequestration of RANKL in mouse genetic models (Kram et al., 2017). Lastly, mineral content has also been found to have an anti-osteoclastogenic effect indirectly through OPG secretion by differentiating osteoprogenitors (Jiao et al., 2015). Thus, ECM components not only have direct effects on osteoclast regulation but also indirectly through differentiation of osteoprogenitors.
Due to the abilities of components of the ECM to differentially direct cell fate, understanding the net effects of individual bone regenerative materials require characterization of interactions with both osteoprogenitors and osteoclast progenitors. We have previously reported that a nanoparticulate mineralized collagen glycosaminoglycan (MC-GAG) material induces efficient osteogenic differentiation of primary human mesenchymal stem cells (hMSCs) and in vivo calvarial healing in a rabbit model in an exogenous growth factor-independent manner (X. Ren et al., 2015; Ren, Tu, et al., 2016a). We also detailed that an autogenous activation of the canonical bone morphogenetic protein receptor (BMPR) signaling pathway was necessary for osteogenesis of hMSCs on MC-GAG, whereas the mitogen activated protein kinase (MAPK) pathways were not required (Zhou et al., 2017). Our current work aims to characterize the effects of MC-GAG on osteoclastogenesis and osteoclast activity.
Materials and Methods
Fabrication and chemical crosslinking of non-mineralized and mineralized collagen scaffolds
Col-GAG and MC-GAG scaffolds were prepared using lyophilization as described previously (Harley, Leung, Silva, & Gibson, 2007; Harley et al., 2010; Weisgerber, Kelkhoff, Caliari, & Harley, 2013). Briefly, microfibrillar, type I collagen (Collagen Matrix, Oakland, NJ) and chondroitin-6-sulfate (Sigma-Aldrich, St. Louis, MO) were combined in suspension in the absence and presence of calcium salts (calcium nitrate hydrate: Ca(NO3)2·4H2O; calcium hydroxide: Ca(OH)2, Sigma-Aldrich, St. Louis, MO) in an acetic acid (Col-GAG) or phosphoric acid (MC-GAG) solution. Using a constant cooling rate technique at a rate of 1 °C/min, the solution was frozen from room temperature to −10 °C using a freeze dryer (Genesis, VirTis). Following sublimation of the ice phase, scaffolds were sterilized via ethylene oxide and cut into 8 mm disks for culture.
Crosslinking of scaffolds was performed after rehydration in phosphate buffered saline (PBS) for 4 hours using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC, Sigma-Aldrich) and N-hydroxysuccinimide (NHS, Sigma Aldrich) at a molar ratio of 5:2:1 EDC:NHS:COOH where COOH represents the amount of collagen in the scaffold as we previously described (Olde Damink et al., 1996). Scaffolds were washed with PBS to remove any of the residual chemical.
Cell culture
Primary human mesenchymal stem cells (hMSCs; Lonza, Inc., Allendale, NJ) were expanded in proliferation media composed of Dulbecco’s Modified Eagle Medium (DMEM; Corning Cellgro, Manassas, VT) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA), 2 mM L-glutamine (Life Technologies, Carlsbad, CA), 100 IU/mL penicillin/100 μg/mL streptomycin (Life Technologies).
Osteogenic differentiation of hMSCs on Col-GAG and MC-GAG:
3 × 105 hMSCs were seeded onto 8 mm discs of CG-GAG and MC-GAG scaffolds in proliferation media. 24 h after seeding, media was switched to osteogenic differentiation media consisting of 10 mM β-glycerophosphate, 50 μg/mL ascorbic acid, and 0.1 μM dexamethasone. For inhibitor studies, scaffolds were treated or untreated with dorsomorphin homologue 1 (DMH1; Sigma-Aldrich) or PD98059 (Cell Signaling Technologies, Beverly, MA) separately, all at a concentration of 50 μM. Fresh DMH1 and PD98059 were added to each media change every 3 days.
Indirect hMSC and hOC co-cultures:
2 × 105 hMSCs were seeded onto 6 mm Col-GAG and MC-GAG scaffolds in proliferation media. 24 h after seeding hMSCs, 6 × 104 primary human osteoclast precursors (hOCs; Lonza, Inc., Allendale, NJ) were separately cultured in Osteoclast Precursor Basal Medium (Lonza, Allendale NJ) supplemented with 33 ng/mL macrophage-colony stimulating factor (M-CSF), 66 ng/mL of RANKL, 10 mM β-glycerophosphate, 50 μg/mL ascorbic acid, 0.1 μM dexamethasone on 24 well Corning Osteo Assay Surface Microplates (Corning, NY), as the lower chamber of the co-culture. After 2 h, Col-GAG and MC-GAG scaffolds were transferred to 8 μm Transwell inserts (Corning, NY), the upper chamber of the co-culture. Media were changed every 3 days for 3 weeks.
Direct hMSC and hOC co-cultures:
3.5 × 105 hMSCs were seeded onto 8 mm Col-GAG and MC-GAG scaffolds in proliferation media for 24 h. 6 × 104 hOCs were cultured in Osteoclast Precursor Basal Medium (Lonza, Allendale NJ) supplemented with 33 ng/mL M-CSF, 66 ng/mL RANKL, 10 mM β-glycerophosphate, 50 μg/mL ascorbic acid, 0.1 μM dexamethasone on 24 well Osteo Assay Microplates. 2 h after hOCs were seeded, Col-GAG and MC-GAG scaffolds were transferred to the Osteo Assay Plates and directly co-cultured with hOCs. Media were changed every 3 days for 2 weeks.
OPG Enzyme Linked Immunosorbent Assay (ELISA)
Supernatants were collected from hMSC only, osteoclast only, or hMSC and hOC co-cultures. OPG protein concentrations were determined using the Human OPG DuoSet ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Briefly, a 96 well microplate was coated with the capture antibody and incubated overnight at room temperature. After blocking, samples were incubated for 2 h at room temperature with the detection antibody followed by incubation with streptavidin- horseradish peroxidase (HRP) for 20 min. The reaction was quenched by adding 100 μL of 2N H2SO4. Plates were read at 450 and 540 nm wavelengths on the Epoch microplate reader (BioTex, Winooski, VT).
Microcomputed tomographic (micro-CT) imaging
Scaffolds were fixed using 10% formalin and mineralization was quantified by micro-computed tomographic imaging (micro-CT) using Scanco 35 (Scanco Medical AG, Bruttisellen, Switzerland) in triplicate for each timepoint. Scans were performed at medium resolution with a source voltage of 70 E (kVp) and I (μA) of 114. The images had a final element size of 12.5 μm. Images were analyzed using software supplied from Scanco (Image Processing Language version 5.6) and reconstructed into three-dimensional (3D) volumes of interest. Optimum arbitrary threshold values of 20 (containing scaffold and mineralization) and 80 (containing mineralization alone) were used uniformly for all specimens to quantify mineralized areas from surrounding unmineralized scaffold. Analysis of 3D reconstructions was performed using Scanco Evaluation script #2 (3D segmentation of two volumes of interest: solid dense in transparent low-density object) and script #6 (bone volume/density only bone evaluation) for volume determinations.
Western blot
Lysates were prepared from scaffolds at 0, 4, 14, and 24 days of culture using SDS sample buffer and equal amounts were subjected to 4–20% SDS-PAGE (Bio-Rad, Hercules, CA). Western blot analysis was carried out with antibodies against OPG, RANKL, phosphorylated small mothers against decapentaplegic 1/5 (p-Smad1/5), total Smad5, phosphorylated extracellular regulated kinase 1/2 (p-ERK1/2), total ERK1/2, and β-actin followed by 1:4000 dilutions of HRP-conjugated IgG antibodies (Bio-Rad, Hercules, CA) and an enhanced chemiluminescent substrate (Thermo Scientific, Rockford, IL). For detection of p-Smad1/5 and total Smad5, 10 μg of lysate was loaded per lane. For detection of OPG, RANKL, p-ERK1/2, total ERK1/2, and β-actin, 20 μg of lysate was loaded per lane. All antibodies were obtained from Cell Signaling Technologies (Beverly, MA) with the exception of RANKL, OPG and β-actin antibodies which were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Imaging analysis was carried out using ImageJ (NIH, Bethesda, MD).
Water Soluble Tetrazolium-1 (WST-1) Assay
Culture media was supplemented with cell proliferation reagent WST-1 (Roche, Basel, Switzerland) at a 1:10 concentration. Scaffolds were incubated for 3–4 h at 37 °C in a humidified atmosphere with 5% CO2. Absorbance of the incubation medium was measured at 450 and 690 nm (Epoch spectrophotometer, BioTek, Winooski, VT).
Tartrate-Resistant Acid Phosphatase (TRAP) Staining
hOCs were detected using Leukocyte TRAP Kit 387-A (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, cultured cells were fixed with formaldehyde for 5 min at room temperature, washed, and air-dried. After staining, TRAP-positive multinucleated cells were observed under a phase-contrast microscope at 20x magnification and digitally photographed.
Resorption Pit Assay
Activity of hOCs in single culture or co-cultured with scaffolds with and without hMSCs was evaluated for resorption pit formation on Osteo Assay microplates. At the completion of the culture period, culture media was aspirated and 500 μL of 10% bleach solution was added for 5 minutes at room temperature. The wells were washed with distilled water and allowed to dry at room temperature for 3–5 h. Pits were observed using a standard microscope and digitally photographed. Percentage of resorption for the whole well of the culture at magnification 2x was calculated by ImageJ.
Statistical analysis
All statistical analyses were performed using SPSS Version 24 (Chicago, IL). Data points were composed of duplicates of at least three independent experiments, unless otherwise indicated. Mean measurements of mRNA expression were analyzed for statistical significance by analyses of variance (ANOVA) followed by post hoc tests using the Tukey criterion. A value of p<0.05 was considered significant.
Results
hMSCs undergoing osteogenic differentiation induce expression of osteoprotegerin in a differential manner on non-mineralized versus mineralized collagen glycosaminoglycan materials
We have previously demonstrated the capabilities of MC-GAG to induce in vitro hMSC osteogenic differentiation and mineralization as well as in vivo bone healing beyond that of a non-mineralized Col-GAG control material (Lee et al., 2015; X. Ren et al., 2015; Ren, Tu, et al., 2016b; Ren, Weisgerber, et al., 2016; Weisgerber, Caliari, & Harley, 2015; Zhou et al., 2017). To evaluate the role of MC-GAG in the regulation of osteoclast activation during osteoprogenitor differentiation, bone marrow-derived primary hMSCs (CD105+CD166+CD29+CD44+CD14−CD34−CD45−) were cultured in osteogenic differentiation medium and expression of OPG protein was evaluated (Figure 1A and B). Over the course of osteogenic differentiation, OPG protein expression was significantly increased in MC-GAG at day 3, 14, and 24 when compared to hMSCs differentiated on Col-GAG as assessed by western blot analysis (Figure 1A).
Figure 1. OPG is expressed and secreted at higher levels by hMSCs on MC-GAG compared to Col-GAG in the absence and presence of differentiating hOCs.
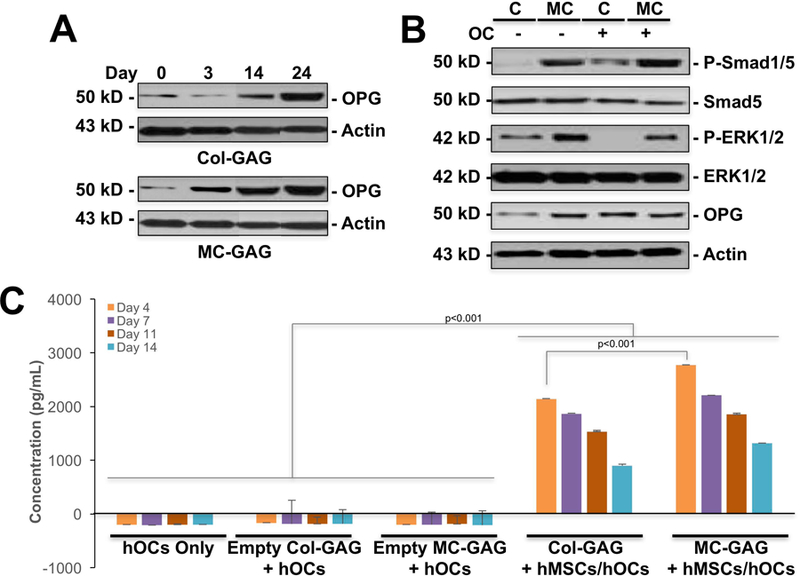
A) Western blot of primary hMSCs cultured on Col-GAG or MC-GAG materials for 0, 3, 14, and 24 days in osteogenic differentiation medium for OPG and β-actin. B) Western blot for p-Smad1/5, total Smad5, p-ERK1/2, ERK1/2, OPG, and β-actin of hMSCs differentiated on Col-GAG or MC-GAG for 3 weeks in the absence and presence of differentiating primary hOCs. C) OPG ELISA of hOCs only, hOCs co-cultured with empty Col-GAG (Empty Col-GAG + hOCs), empty MC-GAG (Empty MC-GAG + hOCs), hMSCs differentiated on Col-GAG (Col-GAG + hMSCs/hOCs), or hMSCs differentiated on MC-GAG (MC-GAG + hMSCs/hOCs) for 4, 7, 11, and 14 days. Bars represent mean concentrations in pg/mL, errors bars represent standard deviation. Significant posthoc comparisons following ANOVA indicated with p values.
To understand the coordination of osteoclast differentiation and hMSCs undergoing osteogenic differentiation on Col-GAG and MC-GAG, we devised an indirect in vitro co-culture assay. hMSCs seeded on Col-GAG and MC-GAG were cultured in the upper chamber of Transwell inserts in the absence or presence of primary hOCs plated in the lower chamber. Co-cultures were induced to simultaneously undergo osteogenic and osteoclastogenic differentiation with RANKL (66 ng/mL), M-CSF (33 ng/mL), β-glycerophosphate, and dexamethasone for three weeks and western blot analysis of the cultures were performed. In the presence of osteoclasts, the expression of phosphorylated Smad1/5 (p-Smad1/5) increased significantly in both Col-GAG and MC-GAG scaffolds (Figure 1B). Additionally, the expression of OPG also increased for hMSCs on Col-GAG in the presence of hOCs but not on MC-GAG in the presence of hOCs while ERK1/2 phosphorylation was decreased in hOC co-cultures.
To determine the effect of hOCs on OPG secretion, OPG ELISAs were performed (Figure 1C). Significant differences in secreted OPG were found between cultures on ANOVA [F(15,48)=172.56, p<0.001]. OPG was undetected in the absence of hMSCs (hOC Only, Empty Col-GAG/hOCs, or Empty MC-GAG/hOCs) when compared to co-cultures with hMSCs at every timepoint on posthoc comparisons (p<0.001). Between co-cultures of hOCs with differentiating hMSCs on Col-GAG versus MC-GAG, OPG was elevated in MC-GAG co-cultures particularly at day 4 (p<0.001). At later timepoints, OPG trended higher in MC-GAG co-cultures, but was no longer found to be significant.
hOCs augment hMSC mineralization on MC-GAG
To understand the effects of primary human osteoclast differentiation on hMSCs on Col-GAG and MC-GAG, we evaluated mineralization in hMSCs undergoing osteogenic differentiation in the absence and presence of hOCs (Figure 2A and 2B). Empty scaffolds, scaffolds seeded with hMSCs, or co-cultures with hMSCs on scaffolds in Transwell inserts and hOCs in the lower chamber were evaluated with micro-CT scanning after 3 weeks of culture in concurrent osteogenic and osteoclastogenic differentiation medium. Overall, differences in mineralization were found to be present [F(5,8)=22.44, p<0.001]. Posthoc comparisons between groups did not demonstrate statistically significant differences between mineralization on empty scaffolds or scaffolds cultured with hMSCs as single cultures on either material, an expected result at 3 weeks of culture. However, in the presence of hOCs, a significant increase in new mineral formation was seen in MC-GAG when compared to empty scaffolds (p=0.02) or scaffolds cultured with hMSCs in single culture (p=0.004). While co-cultures of hOC and hMSCs cultured on Col-GAG demonstrated a qualitative increase in mineralization, this difference did not reach statistical significance. These data suggest that hOCs increase hMSC osteogenic differentiation on MC-GAG scaffolds via soluble factors in a paracrine fashion.
Figure 2. hMSC mineralization on Col-GAG and MC-GAG is increased in the presence of differentiating hOCs.
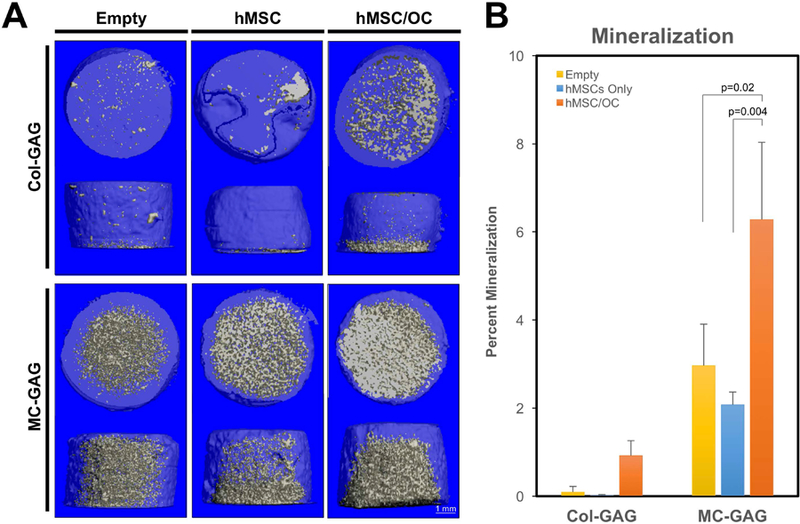
A) Representative micro-CT images and B) quantitative analysis of empty scaffolds, scaffolds cultured with hMSCs only, or scaffolds cultured with hMSCs in co-culture with hOC (hMSC/OC) for 3 weeks. Significant posthoc comparisons following ANOVA indicated with p values.
MC-GAG diminishes hOC activation and resorption directly and indirectly
Next, osteoclasts were evaluated in a direct co-culture system in order to account for both direct and indirect effects on osteoclast activity (Figure 3A-C). hMSCs were cultured on Col-GAG or MC-GAG materials that were then co-cultured directly with primary hOCs 24 hours after seeding. The co-cultures were differentiated simultaneously in osteogenic differentiation medium supplemented with M-CSF (33 ng/mL) and RANKL (33 ng/mL).
Figure 3. Empty MC-GAG and MC-GAG with differentiating hMSCs diminish the viability, proliferation, and resorption of hOCs.
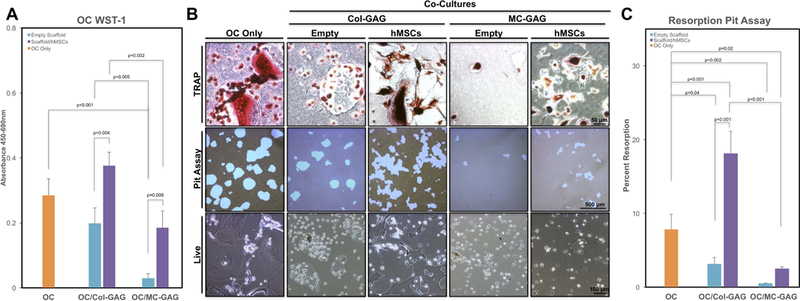
A) WST-1 proliferation and viability assays of primary hOCs in single culture (OC Only), co-cultured with empty Col-GAG or MC-GAG (Empty Scaffold), or co-cultured with Col-GAG or MC-GAG loaded with hMSCs (Scaffold/hMSCs) after 14 days. B) TRAP staining (upper row), resorption pits (middle row), and live images (lower row) of hOCs in single culture (OC Only) or co-cultured with empty or hMSCs-loaded Col-GAG or MC-GAG. C) Quantitative analysis of pit assays as percentage of total area of well in differentiated osteoclasts without hMSCs (OC Only) and osteoclasts co-cultured with Col-GAG and MC-GAG as empty scaffolds (Empty Scaffold) or scaffolds loaded with differentiating hMSCs (Scaffold/hMSCs). Significant posthoc comparisons following ANOVA indicated with p values.
At 14 days of culture, hOC proliferation and viability on the plate were assessed with WST-1 assays and found to demonstrate significant differences between the conditions by ANOVA [F(4,10)=26.38, p<0.001] (Figure 3A). Post hoc comparisons between groups demonstrated that hOC viability and proliferation were significantly diminished in the presence of empty MC-GAG (p<0.001) but not Col-GAG scaffolds when compared to hOCs alone. When comparing the differences between empty versus hMSC-seeded materials, osteogenic differentiation of hMSCs clearly demonstrated an increase in hOC viability and/or proliferation on both Col-GAG (p=0.004) and MC-GAG (p=0.009) materials. However, the increase in hOC viability and proliferation in the presence of hMSCs on MC-GAG was significantly lower compared to hMSCs on Col-GAG (p=0.002).
To evaluate hOC differentiation and activity, TRAP staining and resorption pit assays were performed for each co-culture condition and corresponding controls (Figure 3B). Both TRAP staining and resorption were diminished in co-culture with either empty Col-GAG or MC-GAG. Additionally, live images demonstrated qualitatively small rounded cells as opposed to large, differentiated multi-nucleated osteoclasts. When co-cultured with differentiating hMSCs on Col-GAG, TRAP staining and resorption pits increased. Simultaneously, an increase in larger, multi-nucleated cells was clearly evident in live cell imaging. When co-cultured with differentiating hMSCs on MC-GAG, TRAP staining and resorption pits increased, however this was qualitatively less compared to hOC single culture or hOCs co-cultured with hMSCs on Col-GAG.
To objectively evaluate the differences in hOC activity, percentages of the area of resorption were quantified for the different conditions (Figure 3C). Significant differences were found between the conditions on ANOVA [F(4,10)=53.98, p<0.001]. On posthoc comparisons, both co-culture conditions of hOC with empty Col-GAG or MC-GAG materials demonstrated decreased resorptive activity (p=0.04 and p=0.002, respectively). Similar to the WST-1 results, addition of hMSCs to the materials resulted in augmentation of resorptive activity. In the presence of hMSCs differentiated on Col-GAG, hOC-mediated resorption was significantly higher than in the presence of empty Col-GAG (p<0.001) as well as hOC single cultures (p<0.001). In the presence of hMSCs differentiated on MC-GAG, the recovery of hOC-mediated resorption was significantly less compared to that mediated by hMSCs differentiated on Col-GAG (p<0.001) or hOC single cultures (p=0.02).
OPG expression on MC-GAG is upregulated by ERK1/2 activation and antagonized by canonical BMPR signaling
We previously reported differential regulation of osteogenic differentiation and mineralization of primary hMSCs on Col-GAG and MC-GAG (X. Ren et al., 2015; Ren, Weisgerber, et al., 2016; Zhou et al., 2017). MC-GAG demonstrated an autogenous activation of the BMPR signaling in hMSCs that greatly surpasses Col-GAG. In both materials, BMPR signaling was essential for mineralization, however, Col-GAG also requires MEK1/ERK1/2-mediated signaling for mineralization whereas MC-GAG-mediated mineralization was completely independent of ERK1/2 phosphorylation.
To characterize the mechanism responsible for differential OPG expression by osteogenically differentiated hMSCs cultured on MC-GAG versus Col-GAG, we utilized DMH1 and PD98059 small molecule inhibitors for the canonical BMP receptor and MEK1/ERK1/2 signaling pathways, respectively (Figure 4). As we previously reported, in the presence of DMH1, phosphorylation of Smad1/5 was partially decreased at all timepoints in either material on western blot analyses (Figure 4A). Due to the massive upregulation of Smad1/5 phosphorylation in MC-GAG, a smaller inhibition was noted compared to Col-GAG. No differences in total Smad5, total ERK1/2, RANKL, or actin were detected. In contrast, OPG expression was increased in the presence of DMH1 on both Col-GAG and MC-GAG, particularly at the day 14 timepoint. A small elevation in phosphorylated ERK1/2 could also be seen in the presence of DMH1 specifically in MC-GAG at day 4.
Figure 4. Western blot of intracellular signaling molecules expressed by hMSCs cultured on Col-GAG and MC-GAG in the absence and presence of DMH1 or PD98059.
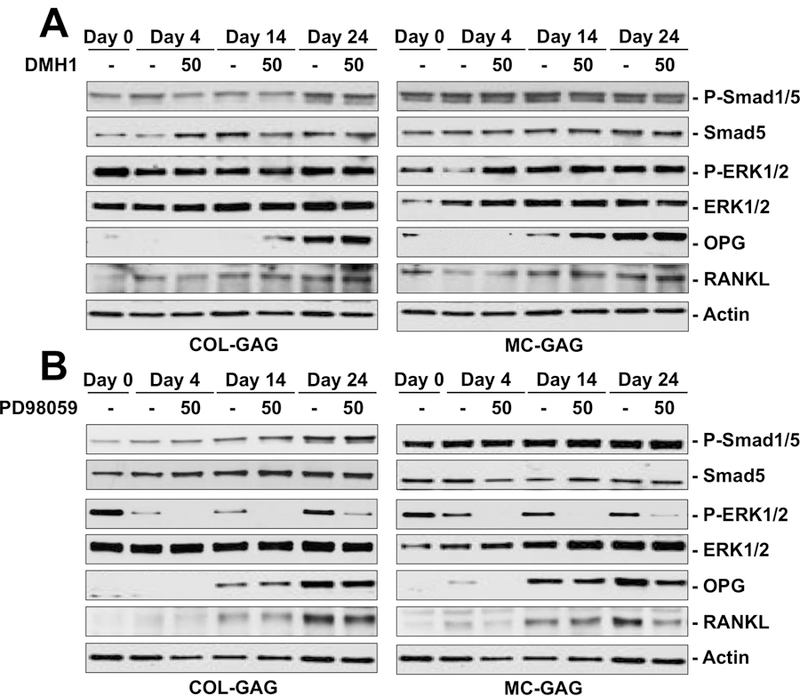
Western blot of phosphorylated Smad1/5 (P-Smad1/5) and total Smad (Smad5), phosphorylated ERK1/2 (P-ERK1/2) and total ERK1/2 (ERK1/2), OPG, RANKL, and β-actin in hMSCs cultured on Col-GAG and MC-GAG at day 0, 4, and 24 days of culture with and without A) 50 μM DMH1 or B) 50 μM PD98059.
To determine the contribution of the ERK1/2-mediated pathways, primary hMSCs osteogenically differentiated on Col-GAG or MC-GAG were treated with PD98059 (Figure 4B). As expected PD98059 decreased phosphorylated ERK1/2 at all timepoints in either material, whereas no effects were noted for phosphorylated Smad1/5, total Smad5, total ERK1/2, or actin. Unlike DMH1, OPG protein expression was decreased in MC-GAG in the presence of PD98059 at all timepoints, while no differences were detected on Col-GAG. Similarly, RANKL expression was also downregulated, particularly at day 24, in the presence of PD98059 on MC-GAG whereas no differences were detected on Col-GAG. In combination, these data suggest that the necessary signaling mechanisms for osteogenic differentiation are both distinct and antagonistic to the mechanisms responsible for osteoclast regulation in hMSCs differentiated on MC-GAG.
Discussion
In this work, we investigated the effect of nanoparticulate mineralized collagen glycosaminoglycan on osteoclastogenesis and osteoclast activity. Through activation of osteogenic differentiation, MC-GAG and Col-GAG, its non-mineralized counterpart, induced an elevation of OPG protein expression by osteoprogenitors (Figure 1A). Early in differentiation, MC-GAG surpassed Col-GAG in the amount of total OPG protein and the amount of secreted OPG (Figure 1C). The quantity of OPG protein expression was potentiated by the addition of differentiating osteoclasts in co-culture (Figure 1B). With respect to differentiating hMSCs, the presence of hOCs on either material augmented the canonical BMPR signaling pathway as seen by an increase in phosphorylated Smad1/5 (Figure 1) as well as mineralization particularly on MC-GAG (Figure 2). When the interactions between materials and hOCs were investigated, hOCs cultured in the presence of either Col-GAG or MC-GAG demonstrated a significant decrease in osteoclast-mediated resorption in the absence of hMSCs (Figure 3C). While Col-GAG only affected resorption but not viability or proliferation, MC-GAG appeared to diminish both osteoclast activity and viability/proliferation (Figure 3A and 3C). The addition of differentiating hMSCs on either material improved hOC viability/proliferation significantly (Figure 3A). However, while osteogenic differentiation of hMSCs on Col-GAG augmented osteoclast resorption (Figure 3C), hOCs in the presence of MC-GAG continued to demonstrate diminished resorptive activity suggesting an indirect inhibitory effect of differentiating osteoprogenitors on MC-GAG. To mechanistically understand the indirect effects induced by MC-GAG on osteoclasts through osteogenic differentiation of hMSCs, the canonical BMPR and ERK1/2 pathways were inhibited with respective small molecule inhibitors (Figure 4). Inhibition of the canonical BMPR pathway resulted in an increase in OPG protein expression by hMSCs cultured on both Col-GAG and MC-GAG materials. In contrast, inhibition of ERK1/2 phosphorylation downregulated both OPG and RANKL expression on MC-GAG whereas Col-GAG was not affected. These results suggest several conclusions regarding the direct and indirect influences of MC-GAG on hOC viability, proliferation, and activity: 1. Col-GAG and MC-GAG have direct inhibitory effects on osteoclast resorptive activity; 2. MC-GAG possesses an additional intrinsic ability to directly diminish osteoclast viability and proliferation that is not present in Col-GAG; 3. Indirectly, the addition of hMSCs undergoing osteogenic differentiation improves osteoclast viability or proliferation on either Col-GAG or MC-GAG; 4. Differentiating hMSCs on MC-GAG continue to inhibit the resorptive activity of hOCs whereas Col-GAG augments hOC-mediated resorption, correlating to the increased protein expression and secretion of OPG from hMSCs on MC-GAG. 5. While the canonical BMPR signaling pathway is essential for osteogenic differentiation of hMSCs cultured on either Col-GAG or MC-GAG, the MEK1/ERK1/2 pathway regulates OPG and RANKL expression on MC-GAG but not Col-GAG.
The combination of these conclusions suggests differing models in the interactions between Col-GAG and MC-GAG with osteoprogenitors and osteoclast progenitors (Figures 5 and 6). With respect to osteoprogenitors, we previously reported that the necessary mechanism for osteogenic differentiation was an autogenous activation of the canonical BMP receptor signaling pathway through elevated Smad1/5 phosphorylation for both Col-GAG and MC-GAG (Figure 5, Mechanism 1; Figure 6, Mechanism 1) (D. Ren et al., 2015; Ren, Tu, et al., 2016a; Zhou et al., 2017). Whereas Col-GAG also required activation of the ERK1/2 pathway, MC-GAG was independent of ERK1/2 for mineralization. With respect to osteoclast progenitors, both Col-GAG and MC-GAG had the ability to directly diminish osteoclast activation and resorptive activity (Figure 5, Mechanism 2A; Figure 6, Mechanism 2), thus suggesting that the activation of osteoclasts is diminished in the presence of collagen and glycosaminoglycan in the form of chondroitin-6-sulfate. Unlike Col-GAG, the direct inhibition of osteoclasts by MC-GAG materials is also accompanied with a diminished viability and proliferation of osteoclast precursors, suggesting a role for nanoparticulate mineral content in decreasing osteoclast activity and proliferation. In the presence of differentiating osteoprogenitors, differing net effects on osteoclast activation are observed between Col-GAG and MC-GAG (Figure 5, Mechanism 2B; Figure 6, Mechanism 3). Although both materials induce OPG expression by osteoprogenitors, the quantity of OPG expression is higher in hMSCs differentiated on MC-GAG which is correlated to a net effect of continued inhibition of osteoclast-mediated resorption (Figure 5, Mechanism 2B). While differentiating osteoprogenitors on either material improves the viability and/or proliferation of osteoclasts, a net effect of increased resorptive activity is only observed in the presence of Col-GAG (Figure 6, Mechanism 3).
Figure 5. Mechanisms induced by MC-GAG on osteoprogenitors and osteoclast progenitors.

MC-GAG induces osteogenic differentiation of primary hMSCs via an autogenous activation of the canonical BMPR signaling pathway with phosphorylation of Smad1/5/8 (Mechanism 1, green arrow). MC-GAG directly inhibits viability, proliferation, and resorptive activity of osteoclasts even in the absence of differentiating hMSCs (Mechanism 2A, red lines). MC-GAG also upregulates OPG expression through an ERK1/2 dependent pathway, correlating to an indirect inhibition of resorptive activity but not viability or proliferation in co-culture with differentiating hMSCs (Mechanism 2B, red arrows and lines).
Figure 6. Mechanisms induced by Col-GAG on osteoprogenitors and osteoclast progenitors.
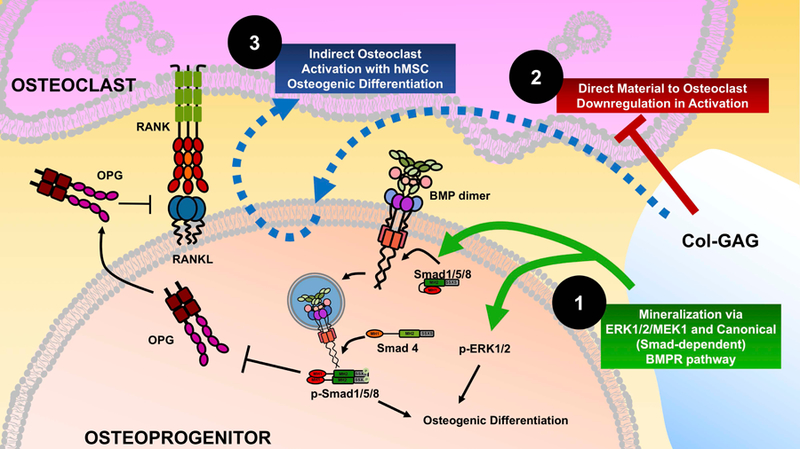
Col-GAG induces osteogenic differentiation of primary hMSCs via both an autogenous activation of the canonical BMPR signaling pathway with phosphorylation of Smad1/5/8 and phosphorylation of ERK1/2 (Mechanism 1, green arrows). Col-GAG directly inhibits resorptive activity of osteoclasts even in the absence of differentiating hMSCs (Mechanism 2, red line). Although OPG expression is present in hMSCs differentiated on Col-GAG, a net activation of osteoclast mediated resorption occurs through unclear mechanisms (Mechanism 3, blue dashed line).
Direct inhibitory effects of Col-GAG and MC-GAG on hOC-mediated resorption are likely to be related to glycosaminoglycan content. Similar to our results, chondroitin sulfate has been previously reported to inhibit resorptive abilities of osteoclasts, but not the viability, proliferation, or maturation in two-dimensional cultures (Cantley, Rainsford, & Haynes, 2013; Salbach, Kliemt, et al., 2012). The mechanism of this effect has been suggested to be related to the sulfation status of GAGs such that a dose dependent suppression of osteoclast activation occurs in highly sulfated GAGs (sGAGs) (Salbach, Kliemt, et al., 2012). Two reasons for sGAG-mediated osteoclast suppression have been reported in the literature. Similar to reports on growth factor sequestration by sGAGs in tendon regeneration (Hortensius & Harley, 2013), sGAGs have also been reported to prevent RANKL from interacting with its cognate receptor (Ling, Murali, Stein, van Wijnen, & Cool, 2010). Secondly, separate from RANKL sequestration, a direct inhibitory effect of sGAGs on osteoclast adherence and spreading has also been reported (Baud’huin et al., 2011). Both effects are likely to be important in our current system. In terms of RANKL sequestration, we have found that the inhibitory effects on osteoclasts can be overcome with increases in RANKL concentration. We have also found that both Col-GAG and MC-GAG-mediated osteoclast inhibition require direct contact of the material with cells such that indirect cultures (via Transwell inserts) demonstrate no statistically significant effects on resorption (unpublished observations, JCL).
MC-GAG also demonstrated an additional direct effect of reducing hOC viability and proliferation. As the major difference in composition between Col-GAG and MC-GAG is the presence of nanoparticulate calcium phosphate in the latter, one potential explanation would be negative regulation by calcium or phosphate ion-induced signaling pathways. High levels of extracellular calcium ion have been identified to induce osteoclast apoptosis dependent on L-type voltage gated calcium channels but not the calcium sensing receptor (Lorget et al., 2000). Similarly high extracellular phosphate concentrations have also been identified to inhibit osteoclastogenesis as well as induce osteoprogenitors to upregulate osteoprotegerin, thereby acting as both a direct and indirect inhibitor of osteoclastogenesis and osteoclast activation (Kanatani, Sugimoto, Kano, Kanzawa, & Chihara, 2003). Conversely, both calcium and phosphate ions are known to be activators of osteogenic differentiation (Barradas et al., 2012; Dvorak et al., 2004; Shih et al., 2014).Thus, bone regenerative materials that include mineral content are likely to be able to utilize the dichotomy of osteogenic activation and osteoclast inhibition imparted by calcium and phosphate ion signaling.
A second inhibitory effect on osteoclast activity was also produced indirectly by osteoprogenitors differentiated on MC-GAG. The correlation of increased OPG expression by osteoprogenitors on MC-GAG suggests that the anti-resorptive effect may be due to an alteration in the relative equilibrium between RANKL and OPG within this system. The balance of RANKL and OPG has been suggested to be responsible for the net effect towards bone formation or resorption on the locoregional or organismal environment (Gori et al., 2000; Kadriu et al., 2017). Tanaka and colleagues have demonstrated that the RANKL/OPG ratio changes over time following injury with an low ratio in the early stages, favoring bone formation, followed by a higher ratio, favoring bone resorption and remodeling, later after injury (Tanaka, Mine, Ogasa, Taguchi, & Liang, 2011). The increase OPG secretion in MC-GAG was higher than Col-GAG only early in differentiation (day 4). Thus, further in vivo studies evaluating the effects of MC-GAG on local osteoclast differentiation may be necessary to understand the ultimate net effects of MC-GAG on OPG over time.
Although OPG is generally considered a marker of osteogenic differentiation, mechanisms governing OPG expression in osteoprogenitors on Col-GAG and MC-GAG are clearly distinct from the necessary pathways for mineralization. Whereas both Col-GAG and MC-GAG requires Smad1/5 phosphorylation for hMSC mineralization, the canonical BMPR pathway antagonized OPG expression such that inhibition by DMH1 resulted in higher levels of OPG. Both OPG and RANKL expression in hMSCs depended upon ERK1/2 phosphorylation for MC-GAG but not Col-GAG. These data suggested that osteogenic differentiation and expression of osteoclast regulatory proteins may be separately modulated by specific extracellular matrix compositions in regenerative materials. Future materials-based regenerative strategies for controlled augmentation of osteogenesis with concurrent, temporary downregulation of osteoclastogenesis are underway.
Acknowledgements:
This work was supported by the US Department of Veterans Affairs under award number IK2 BX002442 (JCL), the Aramont Foundation (TAM), the Jean Perkins Foundation (JCL), and the Plastic Surgery Foundation (JCL) under award number 234813. Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R21 AR063331 (BACH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement:
There are no conflicts of interest to declare among any of the authors.
References
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, … Galibert L (1997). A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature, 390(6656), 175–179. 10.1038/36593 [DOI] [PubMed] [Google Scholar]
- Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, … de Boer J (2012). A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials, 33(11), 3205–3215. 10.1016/j.biomaterials.2012.01.020 [DOI] [PubMed] [Google Scholar]
- Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, … Trowsdale J (2011). OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest, 121(9), 3505–3516. 10.1172/JCI45913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud’huin M, Ruiz-Velasco C, Jego G, Charrier C, Gasiunas N, Gallagher J, … Heymann D (2011). Glycosaminoglycans inhibit the adherence and the spreading of osteoclasts and their precursors: role in osteoclastogenesis and bone resorption. Eur J Cell Biol, 90(1), 49–57. 10.1016/j.ejcb.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Cantley MD, Rainsford KD, & Haynes DR (2013). Comparison of the ability of chondroitin sulfate derived from bovine, fish and pigs to suppress human osteoclast activity in vitro. Inflammopharmacology, 21(6), 407–412. 10.1007/s10787-013-0171-y [DOI] [PubMed] [Google Scholar]
- Chang EJ, Kim HJ, Ha J, Ryu J, Park KH, Kim UH, … Kim HH (2007). Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci, 120(Pt 1), 166–176. 10.1242/jcs.03310 [DOI] [PubMed] [Google Scholar]
- Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, & Riccardi D (2004). Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci U S A, 101(14), 5140–5145. 10.1073/pnas.0306141101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faienza MF, Chiarito M, D’amato G, Colaianni G, Colucci S, Grano M, & Brunetti G (2018). Monoclonal antibodies for treating osteoporosis. Expert Opin Biol Ther, 18(2), 149–157. 10.1080/14712598.2018.1401607 [DOI] [PubMed] [Google Scholar]
- Flick LM, Weaver JM, Ulrich-Vinther M, Abuzzahab F, Zhang X, Dougall WC, … Schwarz EM (2003). Effects of receptor activator of NFkappaB (RANK) signaling blockade on fracture healing. J Orthop Res, 21(4), 676–684. 10.1016/S0736-0266(03)00011-1 [DOI] [PubMed] [Google Scholar]
- Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, & Riggs BL (2000). The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology, 141(12), 4768–4776. 10.1210/endo.141.12.7840 [DOI] [PubMed] [Google Scholar]
- Gramoun A, Goto T, Nordström T, Rotstein OD, Grinstein S, Heersche JN, & Manolson MF (2010). Bone matrix proteins and extracellular acidification: potential co-regulators of osteoclast morphology. J Cell Biochem, 111(2), 350–361. 10.1002/jcb.22705 [DOI] [PubMed] [Google Scholar]
- Harley BA, Leung JH, Silva EC, & Gibson LJ (2007). Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater, 3(4), 463–474. 10.1016/j.actbio.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, & Gibson LJ (2010). Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res A, 92(3), 1066–1077. 10.1002/jbm.a.32361 [DOI] [PubMed] [Google Scholar]
- Hortensius RA, & Harley BA (2013). The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials, 34(31), 7645–7652. 10.1016/j.biomaterials.2013.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Niu LN, Li QH, Chen FM, Zhao W, Li JJ, … Tay FR (2015). Biphasic silica/apatite co-mineralized collagen scaffolds stimulate osteogenesis and inhibit RANKL-mediated osteoclastogenesis. Acta Biomater, 19, 23–32. 10.1016/j.actbio.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ, … Zarate CA (2017). Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry 10.1038/mp.2017.109 [DOI] [PMC free article] [PubMed]
- Kanatani M, Sugimoto T, Kano J, Kanzawa M, & Chihara K (2003). Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J Cell Physiol, 196(1), 180–189. 10.1002/jcp.10270 [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, … Penninger JM (1999). OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature, 397(6717), 315–323. 10.1038/16852 [DOI] [PubMed] [Google Scholar]
- Kram V, Kilts TM, Bhattacharyya N, Li L, & Young MF (2017). Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci Rep, 7(1), 12627 10.1038/s41598-017-12651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Pereira CT, Ren X, Huang W, Bischoff D, Weisgerber DW, … Miller TA (2015). Optimizing Collagen Scaffolds for Bone Engineering: Effects of Cross-linking and Mineral Content on Structural Contraction and Osteogenesis. J Craniofac Surg 10.1097/SCS.0000000000001918 [DOI] [PMC free article] [PubMed]
- Lee JC, Spiguel L, Shenaq DS, Zhong M, Wietholt C, He TC, & Reid RR (2011). Role of RANK-RANKL-OPG axis in cranial suture homeostasis. J Craniofac Surg, 22(2), 699–705. 10.1097/SCS.0b013e3182077fbd [DOI] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, … Boyle WJ (2000). RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A, 97(4), 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen X, Yan J, Zhou L, Wang Y, He F, … Liu T (2018). Inhibition of osteoclastogenesis by stem cell-derived extracellular matrix through modulation of intracellular reactive oxygen species. Acta Biomater, 71, 118–131. 10.1016/j.actbio.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Murali S, Stein GS, van Wijnen AJ, & Cool SM (2010). Glycosaminoglycans modulate RANKL-induced osteoclastogenesis. J Cell Biochem, 109(6), 1222–1231. 10.1002/jcb.22506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorget F, Kamel S, Mentaverri R, Wattel A, Naassila M, Maamer M, & Brazier M (2000). High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Commun, 268(3), 899–903. 10.1006/bbrc.2000.2229 [DOI] [PubMed] [Google Scholar]
- Maxhimer JB, Bradley JP, & Lee JC (2015). Signaling pathways in osteogenesis and osteoclastogenesis: Lessons from cranial sutures and applications to regenerative medicine. Genes Dis, 2(1), 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, & Feijen J (1996). Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials, 17(8), 765–773. [DOI] [PubMed] [Google Scholar]
- Ren D, Wei F, Hu L, Yang S, Wang C, & Yuan X (2015). Phosphorylation of Runx2, induced by cyclic mechanical tension via ERK1/2 pathway, contributes to osteodifferentiation of human periodontal ligament fibroblasts. J Cell Physiol, 230(10), 2426–2436. 10.1002/jcp.24972 [DOI] [PubMed] [Google Scholar]
- Ren X, Bischoff D, Weisgerber DW, Lewis MS, Tu V, Yamaguchi DT, … Lee JC (2015). Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomaterials, 50, 107–114. 10.1016/j.biomaterials.2015.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, … Lee JC (2016a). Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials, 89, 67–78. 10.1016/j.biomaterials.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, … Lee JC (2016b). Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials, 89, 67–78. 10.1016/j.biomaterials.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Weisgerber DW, Bischoff D, Lewis MS, Reid RR, He TC, … Lee JC (2016). Nanoparticulate Mineralized Collagen Scaffolds and BMP-9 Induce a Long-Term Bone Cartilage Construct in Human Mesenchymal Stem Cells. Adv Healthc Mater, 5(14), 1821–1830. 10.1002/adhm.201600187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbach J, Kliemt S, Rauner M, Rachner TD, Goettsch C, Kalkhof S, … Hofbauer LC (2012). The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials, 33(33), 8418–8429. 10.1016/j.biomaterials.2012.08.028 [DOI] [PubMed] [Google Scholar]
- Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, … Hofbauer LC (2012). Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med (Berl), 90(6), 625–635. 10.1007/s00109-011-0843-2 [DOI] [PubMed] [Google Scholar]
- Salbach-Hirsch J, Kraemer J, Rauner M, Samsonov SA, Pisabarro MT, Moeller S, … Hintze V (2013). The promotion of osteoclastogenesis by sulfated hyaluronan through interference with osteoprotegerin and receptor activator of NF-κB ligand/osteoprotegerin complex formation. Biomaterials, 34(31), 7653–7661. 10.1016/j.biomaterials.2013.06.053 [DOI] [PubMed] [Google Scholar]
- Salbach-Hirsch J, Ziegler N, Thiele S, Moeller S, Schnabelrauch M, Hintze V, … Hofbauer LC (2014). Sulfated glycosaminoglycans support osteoblast functions and concurrently suppress osteoclasts. J Cell Biochem, 115(6), 1101–1111. 10.1002/jcb.24750 [DOI] [PubMed] [Google Scholar]
- Shih YR, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, … Varghese S (2014). Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci U S A, 111(3), 990–995. 10.1073/pnas.1321717111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, … Boyle WJ (1997). Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell, 89(2), 309–319. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Mine T, Ogasa H, Taguchi T, & Liang CT (2011). Expression of RANKL/OPG during bone remodeling in vivo. Biochem Biophys Res Commun, 411(4), 690–694. 10.1016/j.bbrc.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Weisgerber DW, Caliari SR, & Harley BA (2015). Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater Sci, 3(3), 533–542. 10.1039/C4BM00397G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber DW, Kelkhoff DO, Caliari SR, & Harley BA (2013). The impact of discrete compartments of a multi-compartment collagen-GAG scaffold on overall construct biophysical properties. J Mech Behav Biomed Mater, 28, 26–36. 10.1016/j.jmbbm.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun TJ, Tallquist MD, Aicher A, Rafferty KL, Marshall AJ, Moon JJ, … Clark EA (2001). Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol, 166(3), 1482–1491. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ren X, Bischoff D, Weisgerber DW, Yamaguchi DT, Miller TA, … Lee JC (2017). Nonmineralized and Mineralized Collagen Scaffolds Induce Differential Osteogenic Signaling Pathways in Human Mesenchymal Stem Cells. Adv Healthc Mater, 6(23). 10.1002/adhm.201700641 [DOI] [PMC free article] [PubMed] [Google Scholar]


