Abstract
The application of human amniotic membrane (AM) has a wide spectrum of indications in the treatment of ocular surface disorders. Transplantation of AM has been incorporated routinely as a component of ocular surface reconstruction in a variety of ocular pathologies. The application of human AM can be combined with nearly all types of limbal transplantation in treating limbal stem cell deficiency (LSCD). AM provides support and possible protection to the transplanted limbal tissues and limbal stem cells owing to its mechanical and biological properties, and these properties are thought to enhance the success rate of LSC transplantation. This paper reviews the current literatures on the applications of AM in the surgical management of LSCD and summarizes the outcome of different surgical approaches. The current literature contains mostly low-level evidences in supporting the role of AM. The efficacy of AM in LSC transplantation needs to be confirmed by randomized controlled clinical trials.
I. Introduction
Limbal stem cells (LSCs) are responsible for the regeneration of corneal epithelial cells and the maintenance of the integrity and transparency of the corneal epithelium.1 The destruction to LSCs and/or the stem cell niche leads to dysfunction or deficiency of LSCs. Limbal stem cell deficiency (LSCD) is characterized with impaired epithelial wound healing, recurrent epithelial erosions, and scarring and opacity of corneal stroma. It is one of the causes of corneal blindness. The common etiologies of LSCD include chemical/thermal burn, contact lens wear, congenital abnormalities, iatrogenic trauma, severe microbial infection, and chronic cicatricial inflammation such as Stevens-Johnson syndrome and mucous membrane pemphigoid.2
The treatment of LSCD is challenging because corneal transplant cannot treat LSCD and will fail after the surgery. Medical treatment has limited success. Only a few mild LSCD cases are reversible by medical treatments.3 Surgical management is usually performed in cases of moderate to severe LSCD. The surgical treatment of LSCD to restore a stable ocular surface can be divided into three groups: direct transplantation of limbal tissues, transplantation of ex vivo/in vivo expanded LSCs, and transplantation of cultivated oral mucosal epithelium. Transplantation of AM has been incorporated into the LSC transplantation in nearly all surgical approaches. AM can be used alone, or as a substrate and cell carrier of LSCs. Therefore, we performed a systematic review to investigate the application of AM in the surgical management of LSCD.
II. Search Method
We performed a systematic literature search on PubMed and Medline for the papers published before December 31, 2017. The following combined search terms were used: “limbal stem cell deficiency”, “amniotic membrane”, “surgical treatment”, “limbal transplantation”, “cultivated limbal epithelial transplantation”, “simple epithelial transplantation”, “cultivated oral mucosal epithelial transplantation”, “conjunctival limbal autograft”, “conjunctival limbal allograft” and “keratolimbal allograft”. Only human studies with 15 or more cases are included in the outcome evaluation. Literature reviews, correspondence, notes, editorials and conference abstracts were excluded in the outcome evaluation. Neither language filter nor limitation of publication time was applied during the literature search. The non-English articles were translated to English to obtain the needed information. We also reviewed the references from retrieved studies manually to identify relevant articles. The data on the preparation and preservation of AM, indications of the surgery, surgical techniques, and clinical outcomes were collected.
III. Results
A. Property and preparation of human amniotic membrane
1. Structure
Amniotic membrane, which is semi-transparent, is the innermost layer of the placenta. It is composed of three layers: a monolayer of epithelium, a thick basement membrane and the avascular stroma. The basement membrane of AM, one of the thickest membranes found in human, is similar to the basement membrane of human corneal and conjunctival epithelium in composition.4 The structural integrity of this layer does not alter after current cryopreservation techniques.5
2. Properties
Human AM has multiple functions in the reconstruction of ocular surface. Mechanically, its toughness and elasticity provides mechanical support and protection to the epithelial cells. Biologically, it could promote the adhesion and migration of limbal epithelial cells and retain their in vivo properties.6,7 Moreover, it has the properties of anti-fibrosis, anti-inflammation, anti-angiogenesis, and anti-bacteria.8 Several recent studies show that a novel matrix component termed heavy chain-hyaluronan/pentraxin 3 (HC-HA/PTX3) purified from cryopreserved AM is the active component responsible for the aforementioned AM's biological properties.9,10 HC-HA/PTX3 complex also uniquely maintains limbal niche cells to support the quiescence of LSCs.10 In addition, AM has low immunogenicity because there is a lacking expression of human leukocyte antigen-A, B, or DR antigens.
3. Preparation, sterilization and preservation
a. Preparation
The method of AM preparation was first described by Tseng.11 In brief, the donors of placenta are selected by serological tests to exclude hepatitis B virus, hepatitis C virus, human immunodeficiency virus (HIV), and syphilis. The placenta is washed by a sterile antibiotic solution, which contains 50μg/ml of penicillin, 50μg /ml of streptomycin, 100μg g/ml of neomycin, and 2.5μg /ml of amphotericin B. Then the AM is separated from the rest of the chorion by blunt dissection, and placed on the nitrocellulose filter paper (pore size: 0.45μm) with the stromal side facing down. The filter paper and the adherent AM was then cut into pieces with the approximate size of 3 cm ✕ 4 cm. This method are used by many study centers with some minor modifications in different studies.12-15
b. Sterilization
The cryopreserved AM is usually treated with antibiotics and antimycotics as mentioned above to prevent microbial infection from contamination during processing. Alternatively, the freeze-dried or air-dried AM is usually sterilized either by 25 kGy gamma irradiation,16-18 or by peracetic acid/ethanol mixture.19 It is also reported that supercritical carbon dioxide can be used to sterilize AM tissue grafts with good preservation of their biological features.20
c. Preservation
Although non-preserved AM was used in some studies,21,22 it is generally recommended that AM is preserved for at least 4-6 months before the confirmation of the HIV negative status of the donor by repeated serology. 18,23,24 The most common method of preservation is cryopreservation. The AM is mounted on a nitrocellulose filter paper is stored at −80 °C in a sterile vial containing Dulbecco modified Eagle medium and glycerol at the ratio of 1:1 (v/v). The cryopreservation of a suspension containing homogenized amniotic membrane was also reported in a small clinical trial.25
AM can also be preserved under a freeze-dried (lyophilized) 26 or air-dried 16 condition. Only one small study compared fresh and dried AM in the treatment of partial LSCD. The outcomes at 24 weeks were similar between these two preservation methods.27
d. Removal of epithelium
According to different clinical purposes, AM (cryopreserved, lyophilized or dry) might be used as intact (with intact epithelium) or denuded (epithelium is removed). Denuded AM has been shown to have less immunogenicity, support the proliferation of LSCs better and preserve a higher clonogenicity.12,28 Removal of the epithelium could be accomplished by NaOH, urea (5M) treatment or mechanically scraping by using a cell scraper with or without the combination of trypsin, EDTA, dispase, or thermolysin.13,14,29-32
e. Effect of AM preparation, sterilization and preservation on its biological properties
A laboratory study 12 compared the effect of different methods of epithelial removal (intact, partial denuded, fully denuded), sterilization (peracetic acid sterilized, nonperacetic acid sterilized) and cryopreservation (DMEM/glycerol, glycerol only, no glycerol) on AM and its impact on the cultured LSCs. The findings showed that complete removal of epithelium facilitated the migration and confluence of LSCs and did not affect the biological properties of LSCs. However, the use of glycerol as a cryoprotectant seemed to impair the function of AM to support the growth of LSCs, leading to a poorer morphology of LSCs and a lower percentage of cells expressing LSC biomarkers. Moreover, sterilization by gamma irradiation has been shown to cause a significant decrease of growth factors and the structural alteration of basement membrane.33,34 The optimal method to prepare, sterilize and preserve AM still needs to be investigated to optimize the function of AM for different applications in ophthalmology.
B. Application of AM in surgical treatment of LSCD
1. Transplantation of AM alone
a. Indications
AM transplantation (AMT) is widely used in the treatment of acute phase of chemical burn, thermal injury or Stevens-Johnson Syndrome to promote epithelium healing, and alleviate ocular surface inflammation which might rescue the residual LSCs. 21,22,35-37 In these cases, AM is serving as a temporary overlay patch to mechanically protect the ocular surface, promote normal epithelial wound healing and prevent intermediate-term ocular cicatricial sequelae.37 However, prospective, randomized, controlled clinical trials showed that no definite long-term advantage of AMT alone over medical therapy in terms of final visual outcome, appearance of symblepharon and corneal vascularization.38-40
AM transplantation also have been used to treat partial LSCD.15,24,27,41-44 Although AM is believed to serve as the permanent graft in these cases and to provide a surrogate basement membrane for the regenerated epithelium, the histological study confirmed the complete integration of AM with corneal stromal tissue,45 which suggests the effect of AM was more through its biological properties than mechanical properties.
b. Surgical technique
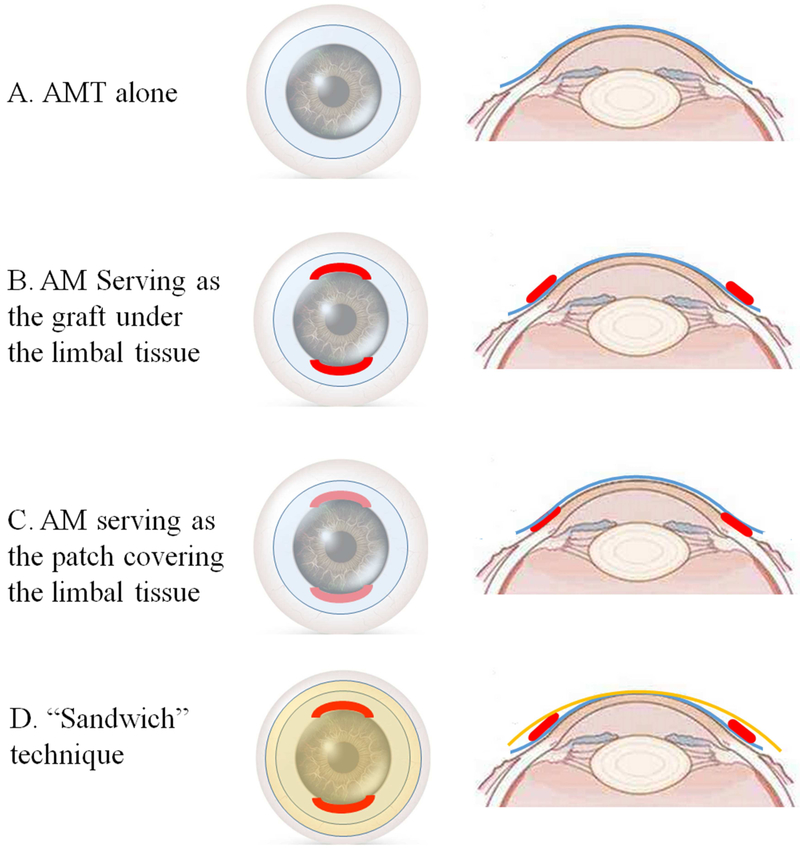
After debridement of fibrovascular pannus and removal of scarring and inflamed tissue, AM is removed from the storage medium, and placed over the denuded cornea, limbus and conjunctiva (Figure 1A). In a majority of studies, AM was placed with the epithelium/basement membrane side facing up.24,27,35-37,41,43,45-48 The placement of AM with the stromal side facing up was only used in only two studies.35,41 However, some studies did not specify the orientation of the basement membrane.15,49-51 AM was then secured to the cornea with 10-0 or 11-0 nylon sutures 24,48 or/and to the surrounding conjunctiva with 9-0 or 10-0 Vicryl sutures.35,49 Recent studies showed that fibrin glue could be used to avoid suture-related disadvantages and complications.37,42
Figure 1.
Schematic diagram of amniotic membrane transplantation (AMT) alone (A) and combination of direct limbal transplantation with AMT (B to D). In AMT alone procedure, AM depicted in blue is placed over the denuded cornea, limbus and conjunctiva (A). When combined with limbal stem cell transplantation, AM is either serving as a graft under the limbal tissues depicted in red (B), or as a patch covering the limbal tissues (C). In “Sandwich” technique, AM is placed both beneath and on top of the limbal grafts. The AMs beneath and on top of the limbal tissues are labeled as blue and orange, respectively (D).
Occasionally sectorial sequential conjunctival epitheliectomy (SSCE) combined with AMT is used in the treatment of partial LSCD.44,50 It is a surgical procedure in which the abnormal conjunctival epithelium on the cornea is removed by mechanical superficial debridement. The denuded corneal and limbal surface could be re-epithelialized by corneal epithelial cells that migrate from the unaffected area of cornea and limbus.52 The limitation of SSCE is that it could cause persistent epithelial defect and pain from the epithelial debridement. Multiple treatments are often required to achieve satisfactory outcome in successful cases. The combined AMT might reduce bleeding, pain and promote epithelialization.
c. Outcome
As shown in Table 1, a total of 8 studies reported the outcome of AMT alone in the treatment of partial LSCD. After AMT for the treatment of partial LSCD, the mean time of complete corneal and conjunctival re-epithelialization is usually 2-3 weeks.15,24,43 The mean time of the maintenance of a stable corneal epithelial surface is 14-25 months after surgery, along with less stromal opacity and vascularization.24,46,47 Visual improvement is found in 25%-81% eyes. 15,24,27,42,43,46,49 However, the long term success rate of AMT following superficial keratectomy in cases with partial LSCD is only 40%–54% at an average follow-up period of 52 months.43
Table 1.
Demographic characteristics of studies with AMT involved in the treatment of limbal stem cell deficiency
| Author (Year) | Gender | Etiology | Range of LSCD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Type |
No. of Eyes |
Male | Female | Mean Age |
Chemical/ Thermal Injury |
chronic cicatricial inflammation (SJS/OCP) |
Others | Total | Partial | Follow-up in Months |
|
| AMT alone | |||||||||||
| Chung JP. 2015 | P | 30 | 15 | 15 | 48.9±16.3 | 3 | 7 | 30 | 0 | 30 | 6 |
| Konomi K. 2013 | R | 16 | 9 | 7 | 57.4±16.4 | 2 | 2 | 12 | 0 | 16 | 52.3±26.3 |
| Kheirkhah A. 2008 | R | 11 | 5 | 6 | 32.4±18.4 | 3 | 2 | 6 | 0 | 11 | 14.2±7.7 |
| Lopez-Garcia JS. 2005 | P | 14 | ND | ND | 37 | 0 | 0 | 14 | ND | ND | 24 |
| Ivekovic R. 2005 | ND | 5 | 3 | 2 | 31.6±12.3 | 5 | 0 | 0 | ND | ND | 18±4.3 |
| Gomes JA. 2003 | P | 4 | 4 | 0 | 34.5±26.3 | 4 | 0 | 0 | 0 | 4 | 17.5±5.1 |
| Anderson DF. 2001 | R | 17 | 9 | 8 | 42.3±4.6 | 8 | 0 | 9 | 0 | 17 | 25.8±2.5 |
| Tseng SC. 1998 | R | 10 | 4 | 6 | 36.8±8.4 | 4 | 0 | 6 | ND | ND | 12.3±9.3 |
| CLAU/CLAL+AMT | |||||||||||
| Arora R. 2017 | P | 10 | ND | ND | 18±8 | 10 | 0 | 0 | 3 | 7 | 6 |
| Moreira PB. 2015 | R | 28 | 19 | 9 | 40.3 | 20 | 3 | 5 | ND | ND | 24.8 |
| Barreiro TP. 2014 | R | 15 | 13 | 2 | 36.3 | 15 | 0 | 0 | 15 | 0 | 19.7±5.6 |
| Baradaran-Rafii. 2012 | P | 34 | 32 | 2 | 27.3±9.4 | 34 | 0 | 0 | ND | ND | 17.2±6.3 |
| Miri A. 2010 | R | 27 | 19 | 8 | ND | 14 | 0 | 13 | 27 | 0 | 38±35.9 |
| Scocco C. 2008 | R | 39 | ND | ND | 33.6±18.9 | 12 | 15 | 12 | ND | ND | 48.7±30.6 |
| Santos MS. 2005 | P | 33 | 26 | 5 | 35±16 | 22 | 11 | 0 | 33 | 0 | 33±12 |
| Lopez-Garcia JS. 2005 | P | 14 | ND | ND | 47 | 7 | 2 | 5 | 14 | 0 | 24 |
| Ivekovic R. 2005 | ND | 4 | 4 | 0 | 27.8±7.8 | 4 | 0 | 0 | ND | ND | 12.8±1.7 |
| Shimazaki J. 2004 | R | 11 | 11 | 1 | 40.2±14.3 | 11 | 0 | 0 | 11 | 0 | 15 |
| Gomes JA. 2003 | P | 16 | 15 | 1 | 42.3±11.2 | 16 | 0 | 0 | 16 | 0 | 18.3±6.1 |
| KLAL+AMT | |||||||||||
| Baradaran-Rafii. 2013 | R | 45 | 41 | 4 | 26.7±8.7 | 41 | 4 | 0 | ND | ND | 26.1±11.8 |
| Eberwein P. 2012 | R | 20 | 13 | 7 | 44 | 8 | 6 | 6 | 20 | 0 | 22.4 |
| Han ES. 2011 | R | 24 | 17 | 5 | 39.4±17.4 | 8 | 8 | 8 | ND | ND | 47.3±22 |
| Shi W. 2008 | R | 39 | 33 | 5 | ND | 39 | 0 | 0 | 28 | 11 | 32 |
| Maruyama-Hosoi F. 2005 | R | 85 | 38 | 40 | 52.5±19.5 | 17 | 43 | 25 | 85 | 0 | 46.6 |
| Shimazaki J. 2004 | R | 21 | 18 | 3 | 43.2±19.1 | 21 | 0 | 0 | 21 | 0 | 15 |
| Solomon A. 2002 | R | 39 | 21 | 10 | 40.1±14.6 | 16 | 11 | 12 | 39 | 0 | 34±21.5 |
| Ilari L. 2002 | R | 23 | 12 | 8 | 45 | 8 | 9 | 6 | ND | ND | 60 |
| Tsubota K. 1999 | ND | 43 | 26 | 13 | 49±23 | 29 | 14 | 0 | ND | ND | 38.7 |
| Tseng SC. 1998 | R | 7 | 4 | 3 | 54.3±17.6 | 2 | 3 | 2 | ND | ND | 11.3±4.6 |
| CLET (AM as the substrate) | |||||||||||
| Parihar JK. 2017 | P | 25 | 14 | 11 | 46±6 | 15 | 6 | 4 | 20 | 5 | 12 |
| Cheng J. 2017 | R | 80 | 73 | 7 | 42.4±13.7 | 80 | 0 | 0 | 57 | 23 | 26.4±13.6 |
| Scholz SL. 2016 | R | 61 | 46 | 11 | 48.9±17.5 | 34 | 0 | 27 | ND | ND | 50.8±32.7 |
| Ramirez BE. 2015 | P | 20 | 12 | 8 | 51.6±14.2 | 7 | 4 | 9 | 12 | 8 | 36 |
| Ganger A. 2015 | R | 62 | 41 | 13 | 14.7±10 | 60 | 1 | 1 | ND | ND | 21.4±17.8 |
| Zakaria N. 2014 | P | 18 | 11 | 7 | 40.7±19.4 | 7 | 0 | 11 | 15 | 3 | 23.7±13.3 |
| Vazirani J. 2014 | R | 70 | 56 | 14 | 24±12.5 | 64 | 1 | 5 | ND | ND | 17.5±7 |
| Subramaniam SV. 2013 | R | 40 | 3 | 9 | 16.8±9.3 | 36 | 0 | 4 | ND | ND | 33.4±29.2 |
| Sejpal K. 2013 | R | 107 | ND | ND | 7.5±3.72 | 107 | 0 | 0 | 92 | 15 | 41.2±26 |
| Qi X.2013 | R | 42 | ND | ND | 38±14.7 | 42 | 0 | 0 | 42 | 0 | 17.8±3.8 |
| Prabhasawat P. 2012 | P | 19 | 12 | 7 | 44.7±15.2 | 13 | 1 | 5 | 11 | 8 | 26.1±13.5 |
| Basu S. 2012 | R | 50 | 35 | 15 | 20.7±11.4 | 50 | 0 | 0 | 50 | 0 | 27.6±16.8 |
| Basu S. 2012 | R | 28 | 17 | 3 | 27.9±17.4 | 18 | 3 | 7 | 28 | 0 | 58±33.6 |
| Sharma S, 2011 | P | 50 | 40 | 10 | 14.5±10 | 47 | 2 | 1 | ND | ND | 13.8±2.9 |
| Sangwan VS. 2011 | R | 200 | 159 | 41 | 24.1±9.9 | 200 | 0 | 0 | 200 | 0 | 36±19.2 |
| Pauklin M. 2010 | P | 44 | 27 | 11 | 47.4±20.1 | 22 | 0 | 22 | 32 | 12 | 28.5±14.9 |
| Meller D. 2010 | R | 30 | 22 | 6 | 47.4±20.1 | 16 | 0 | 14 | 18 | 12 | 28.9±15.5 |
| Shimazaki J. 2007 | R | 27 | 18 | 9 | 50.2±20.7 | 9 | 17 | 1 | 27 | 0 | 31.8 |
| Sangwan VS. 2006 | R | 88 | 74 | 12 | 21.1±12.5 | 78 | 0 | 10 | 61 | 27 | 18.3±11.2 |
| Schwab IR. 2000 | P | 14 | 11 | 3 | 49.4±14 | 6 | 1 | 7 | ND | ND | 11.5±6.6 |
| COMET (AM as the substrate) | |||||||||||
| Prabhasawat P. 2016 | P | 20 | 7 | 11 | 48.2±15.5 | 7 | 10 | 3 | 15 | 5 | 31.9±12.1 |
| Dobrowolski D. 2015 | P | 17 | 3 | 10 | 31.1±11.5 | 0 | 0 | 17 | 14 | 3 | 16±2.2 |
| Hirayama M. 2012 | R | 16 | 11 | 5 | 58.4±17.7 | 6 | 10 | 0 | 16 | 0 | 35±17.6 |
| Satake Y. 2011 | R | 40 | 22 | 14 | 58.5 | 11 | 21 | 8 | 40 | 0 | 25.5 |
| Nakamura T. 2011 | R | 19 | 7 | 10 | 54±21 | 1 | 15 | 3 | 19 | 0 | 55±17 |
| Inatomi T. 2006 | R | 15 | 9 | 6 | 48.4±22.3 | 6 | 8 | 1 | 15 | 0 | 20±11 |
| SLET | |||||||||||
| IyerG. 2017 | R | 18 | 8 | 9 | ND | 18 | 0 | 0 | ND | ND | 10.3±6.7 |
| Arora R. 2017 | P | 10 | ND | ND | 15.2±10.8 | 10 | 0 | 0 | 7 | 3 | 6 |
| Vazirani J. 2016 | R | 68 | 51 | 17 | 22 | 62 | 0 | 6 | 46 | 22 | 12 |
| Basu S. 2016 | P | 125 | 82 | 43 | ND | 125 | 0 | 0 | 107 | 18 | 18 |
AM: amniotic membrane; AMT: amniotic membrane transplantation; CLAL: conjunctival limbal allograft transplantation; CLAU: conjunctival limbal autograft transplantation; CLET: cultivated limbal epithelial transplantation; COMET: cultivated oral mucosal epithelial transplantation; KLAL: keratolimbal allograft transplantation; ND: not documented; OCP: ocular cicatricial pemphigoid; P: prospective; R: retrospective; SLET: simple limbal epithelial transplantation; SJS: Stevens-Johnson Syndrome
2. Direct transplantation of limbal tissues with AM
a. Indications
Direct LSCs transplantation includes conjunctival limbal autograft transplantation (CLAU), conjunctival limbal allograft transplantation (CLAL) and keratolimbal allograft transplantation (KLAL). Keratolimbal autograft transplantation (KLAU) has only been published by two case reports53,54 because of the requirement of large graft size (around 180 degree) on the donor eye and the need to reconstruct the conjunctiva in LSCD eyes with abnormal conjunctiva.
CLAU is usually performed in unilateral total LSCD cases, while CLAL and KLAL are considered in bilateral LSCD cases. All of these procedures can be performed with the combination of AMT.
b. Surgical technique
(1). Under the limbal tissue in the recipient eye (inlay)
After the removal of conjunctival and dermal-like epithelium covering the cornea, the dissection of fibrous tissues and the releasement of existing symblephon, AM was placed on the denuded ocular surface and secured with suture or fibrin glue. Then the limbal graft is sutured to the original limbal area (Figure 1B).15,49,50,55-72 In these cases, AM is thought to reduce postoperative inflammation and scarring in the underlying stroma. Moreover, many researchers thought that a combination of AMT might secure an environment favorable for the regeneration of LSCs,58,64-66 thus reducing the requirement of graft size and decreasing the risk of iatrogenic of LSCD in the donor eye.
(2). Covering the limbal tissue in the recipient eye (overlay)
After the fixation of limbal grafts, AM was used as a temporary patch to cover the limbal grafts and the entire ocular surface at the end of the surgery (Figure 1C). 63,68,73-77 In some studies, AM are placed both under and over the limbal grafts, which is called “Sandwich” technique (Figure 1D).58,61,63-65,67,78 The role of AM in this condition is similar to the contact lens, which provides mechanical protection to the limbal grafts and regenerated epithelium from external insults, and relieves ocular symptoms such as pain, photophobia and discomfort after surgery.
(3). Serving as the patch in the donor eye
The efficacy of the transplantation of 2 clock hours (60°) of donor limbus for a permanent and stable epithelialization of the cornea has been reported.58,69 However, it is generally presumed that at least three to four clock hours (90°–120°) of a conjunctival–limbal graft is usually required to obtain enough amount of LSCs in the graft, either from the healthy contralateral eye (CLAU) or from an eye of a living relative (lr-CLAL).15,46,66,79 Therefore, there is a risk of developing LSCD in the donor eye. In these cases, AM used as a temporary patch in the donor eye 58,64 may be helpful to reduce the risk of iatrogenic LSCD after graft removal because AM is thought to provide support for restoring the remaining functional LSCs.36,64,68 However, all these reports are retrospective uncontrolled studies. There is no high-level data demonstrating the advantage of AMT in the donor eye.
(4). CLAU combined with AM-assisted SSCE
It is recently reported that a modified AM-assisted SSCE, named as amnion-assisted conjunctival epithelial redirection, could be combined with CLAU.80,81 It was advocated that AM might play a role in redirecting conjunctival epithelium and preventing admixtures of conjunctival epithelial cells and limbal explant-derived corneal epithelial cells on to the corneal surface.
c. Outcome
(1). Conjunctival limbal autograft/conjunctival limbal allograft
A total of 17 studies reported the outcome of CLAU/CLAL with or without combined AMT after the follow-up of ≥12 months. Among them, only two studies directly compared the outcome with or without the use of AM in CLAU/CLAL. Ivekovic et al46 compared the time required to re-epithelialize after AMT, CLAU, and CLAU combined with AMT. The mean re-epithelialization time was 24.6 days, 14 days and 15.3 days in each group, respectively. There was no difference between CLAU and CLAU+AMT, both of which were shorter than AMT only. However, Barreiro et al59 reported that although the final graft survival rate was similar between groups with or without the use of AMT, re-epithelialization time was significantly longer in the group using AMT.
The other studies are non-comparative studies. They only focused either on CLAU/CLAL with AMT,15,62,65-67,70,75,78,82 or CLAU/CLAL without AMT. 83-88 Although a higher or similar successful rate with AMT (Table 2) was reported in the majority of studies , the study designs and patient populations were quite different (Table 1). Therefore, there is insufficient evidence to support the advantages of combined use of AMT in CLAU/CLAL either to promote epithelial healing or to increase the graft survival, even though AMT is used as a routine procedure in many cases of CLAU/CLAL.
Table 2.
Comparisons on the outcome among CLAU/CLAL/KLAL with or without combined use of AMT
| AMT not used/mentioned | with AMT | |
|---|---|---|
| Reepithelization time (Days) | ||
| CLAU/CLAL | 6.4-35.6 46,59,83,88 | 5.6-23.8 15,46,59 |
| KLAL | 8.4-12.7 88,94 | |
| Successful rate | ||
| CLAU | ||
| 1Y | 75% 83 | 43%-91% 65,67 |
| 1.5Y | 77%-81% 59,84,85 | 67%-92% 15,59,78 |
| 2Y | 67% 82 | |
| 3Y | 76% 86 | 33% 67 |
| CLAL | ||
| 1Y | 53%-70% 87,88 | 38%-85% 15,66,70 |
| 1.5Y | 7.1%-40%59,83,84 | 67% 59 |
| 2Y | 33%-71% 15,62,66 | |
| 3Y | 39%-59% 86,87 | 23%-67% 67,70 |
| KLAL | ||
| 1Y | 40%-83% 87,90,93,94 | 33%-83% 49,65,72,76 |
| 2Y | 59%-86% 90,91 | 33%-73% 72,77,89 |
| 3Y | 74% 92 | 27%-54% 55,56,72,76 |
| 4Y | 58% 90 | 33%-66% 63,71 |
| 5Y | 51% 90 | 21%-47% 56,72 |
AMT: amniotic membrane transplantation; CLAL: conjunctival limbal allograft transplantation; CLAU: conjunctival limbal autograft transplantation; KLAL: keratolimbal allograft transplantation; Y: year
(2). Keratolimbal allograft
A total of 16 studies reported the outcome of KLAL after the follow-up of ≥12 months, 10 studies with AMT, 49,55,56,63,65,71,72,76,77,89 and 6 without AMT. 88,90-94 No comparative studies have been performed yet. The successful rate of KLAL, no matter AMT is used or not, has a similar decreasing tendency with the prolongation of follow-up. Table 2 showed that AM played a minor role in the graft survival after KLAL. Although the authors suggested that the application of AM could reduce the postoperative inflammation and complications in these cases, the function of AM in KLAL needs to be investigated by further comparative studies.
3. Transplantation of ex vivo cultured cells on AM
a. Indications and presumed function of AM
For patients who have bilateral total limbus damage without residual LSCs, or those who do not have enough healthy limbal tissue in the other eye to harvest sufficient amount of LSCs, transplantation of ex vivo cultured and expanded cells is one of main approaches for the treatment of LSCD to restore the structural and functional integrity of corneal surface. The most commonly used cell sources for transplantation are human limbal epithelium95 and oral mucosal epithelium.30 The procedure is called “cultivated limbal epithelial transplantation (CLET)” and “cultivated oral mucosal epithelial transplantation (COMET)” respectively. The applications of human bone marrow mesenchymal stem cells,96 human conjunctival epithelial cells,97 and human nasal mucosal epithelial cells 98 have also been reported. The cell source can be taken either from the patient (autologous), or from an eye of a living relative or cadaveric tissue (allogenic). The biggest advantages of this technique is the minimal need of donor tissue (less than 1mm2)99,100 and the lowest risk for the donor eye.
Many materials such as AM,95,100,101 fibrin sheet, 99,102,103 contact lenses,104 and nylon sheet105 have been reported to serve as the substrate and carriers of cultured LSCs or oral mucosal epithelial cells. Among them, AM is still most commonly used. AM usually serves as a surrogate basement membrane for cultured cells and the substrate as a cell carrier in CLET or COMET. Although both de-epithelialized (denuded) and intact AM can be used, de-epithelialized AM is better than intact AM because it preserves the properties of LSCs better and facilitates the migration and confluence of LSCs.12 Moreover, it has been reported that some limbal epithelial stem cells underwent epithelial-mesenchymal transition and invaded the limbal stroma when cultured on intact AM.28
It has been shown that AM preferentially preserves and expands limbal epithelial cells that retain their in vivo properties of slow cycling, putative marker expression, and an undifferentiated state.6,106-114 The maintenance of a limbal epithelial phenotype indicates that AM provides a unique stromal microenvironment beneficial to the preservation and expansion of LSCs. AM also prevent cultured LSCs from undergoing apoptosis through interleukin-1 receptor antagonist.115
b. Methods of cultivation on AM
After the biopsy of limbus or oral mucosa, careful removal of excessive tissue, and rinsing with culture medium containing antibiotics, there are two methods to culture cells on AM. One is chopping the tissue into small pieces and then placing the explant on the epithelium/basement membrane side of AM.6,32,95,100,108-113,116-120 The orientation of limbal explant on AM, either epithelial side or stromal side facing up, does not influence tissue adhesion and cell expansion.100 The other method is incubating the biopsy tissue with trypsin, EDTA and dispase to obtain single cell suspension first. Then these single cells are seeded on AM with or without the presence of irradiation- or mitomycin C-treated 3T3 feeder cells.30,96,107,110,121-131 These two methods do not have differences regarding the cell growth and phenotype.110
A minimum size of 0.3mm2 live limbal tissue or 0.5mm2 cadaveric limbal explant is required to achieve sufficient cells for expansion and transplantation.100 Limbal explant takes more time to reach a linear growth phase if it is retrieved from corneo-limbal rings or discs with a longer duration of organ culture.132 As for oral mucosal biopsy, at least a specimen with the size of 2 ∼3 mm2 is needed.122 The successful rate of ex vivo cultured and expanded cells on AM is reported to be 96.2%-98.5%.6,112
c. Surgical technique
Corneal fibrovascular tissue and perilimbal subconjunctival scarring tissue are dissected and removed to the bare sclera at least 2 to 3 mm behind the limbus. Symblepharon are released if necessary. Then cultured epithelial cell sheet, together with the amniotic membrane substrate, is placed on the cornea with the epithelial side up. The graft is secured with either suture or fibrin glue.
d. Outcome
(1). CLET
Owing to the small size of tissue needed for ex vivo culture and the fact that antigen presenting cells do not survive during culture,133 the rejection rate of CLET is relatively low even in allogenic cases. The overall successful rate of CLET is stable after one year postoperatively.29,32,93,110,113,114,117-120,134-142,143,144 Nevertheless, the successful rate is influenced by many factors including age, donor source, and cell quality. 99,117,120,134,136,137 it should be noted that the clinical outcomes of the transplantation of LSCs cultured on AM and fibrin are similar, as shown in Table 3. Fibrin is easier to be standardized, but AM has a wider accessibility, especially in the developing countries.
Table 3.
Comparisons on the outcome between AM and fibrin as the substrate in CLET and COMET
| substrate | AM | Fibrin |
|---|---|---|
| Reepithelization time (Day) | ||
| CLET | 5-13.7 138,144 | |
| COMET | 5.2 127 | |
| Successful rate | ||
| CLET | ||
| 1Y | 60%-91% 29,93,113,118-120,136,138,142-144 | 62%-80% 102,103,158 |
| 2Y | 56%-81% 32,110,114,117-120,135-138,140,141 | |
| 3Y | 47%-75% 119,120,134,136,138 | 77% 99 |
| 4Y | 45% 118 | |
| 5Y | 64%-75% 136,139 | |
| 8Y | 66% 159 | |
| COMET | ||
| 1Y | 63%-88% (substrate free)146,147 | |
| 44%-65% 123,127,129,147 | ||
| 2Y | 59%-79% 127,129 | 64% (fibrin) 160 |
| 3Y | 53%-71% 126,129,145 | |
AM: amniotic membrane; CLET: cultivated limbal epithelial transplantation; COMET: cultivated oral mucosal epithelial transplantation;
(2). COMET
The overall successful rate of COMET is stable after two years postoperatively. 123,126,127,129,145 Although Kim146 and Hirayama147 reported that the transplantation of substrate-free oral mucosal cell sheet achieved better clinical outcomes (87.5% and 62.5%, respectively) than AM group (44%), the mean follow-up was only one year after surgery, as shown in Table 3. Its midterm and long-term outcome needs to be evaluated by further studies.
The result of immunostaining and RT-PCR showed that the oral mucosal epithelial cells cultured on AM expressed putative markers of progenitor stem cells, namely p63 and ABCG2, and markers of epithelial differentiation such as CK3 and connexin 43. 123-125,127,128,148,149 However, neither CK12, the corneal epithelium-specific marker, nor Pax6, an eye-specific transcription factor, was expressed in these transplanted oral mucosal cells.150 These results suggest that although oral mucosal epithelial cells cultured on AM achieved a similar phenotype of limbal and corneal epithelium, they do not undergo a true transdifferentiation.
4. Transplantation of in vivo expanded LSCs on AM
a. Indications
A novel surgical technique named as “simple limbal epithelial transplantation (SLET)” was firstly described by Sangwan.151 It allows the in vivo expansion of small pieces of limbal biopsy on AM, combining the advantages of CLAU (low cost, single staged, no requirement of clinical-grade laboratory) and CLET (using minimal donor tissue). Both fresh AM and cryopreserved AM are applicable.151,152 This technique is mainly used in the treatment of unilateral LSCD.
b. Surgical technique
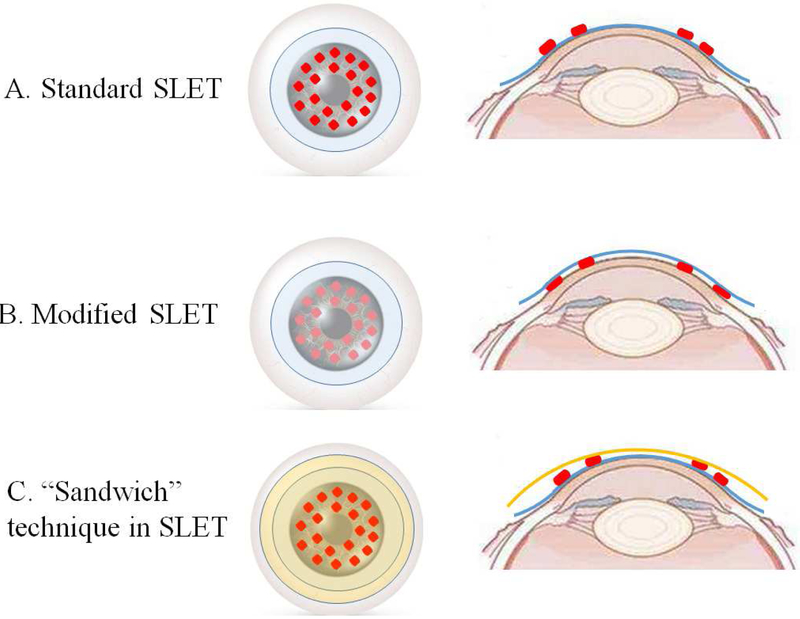
AM is considered to provide a suitable substrate and create a nourishing ocular surface microenvironment, allowing in-vivo expansion of LSCs from the donor tissue explants. In most SLET cases, AM is placed over the bare ocular surface and donor limbal lenticule is secured on AM with the epithelial side up (Figure 2A).75,151,153-155 Instead, Vasquez-Perez 156 and Vazirani 157 described a modified SLET. Donor tissue explants were placed on the bared cornea surface and AM is used to cover the grafts and entire corneal surface (Figure 2B). The authors believed that placing the AM either above or below the donor tissue explants is equally effective and safe. Amescua et al 152 reported another modified SLET named as “sandwich technique” in which the limbal biopsy explants were placed between the two layers of AM with the intention of replicating a fetal environment for the stem cells (Figure 2C). This technique provides protection to the graft and stem cell niche without negative effect on the clinical outcome.
Figure 2.
Schematic diagram of different surgical techniques of SLET
In a standard SLET (A), AM depicted in blue is placed on top of the ocular surface and donor limbal biopsy explants depicted in red are secured on AM. In a modified SLET (B), donor limbal biopsy explants are placed on the bared cornea surface and AM covers the limbal grafts and the entire corneal surface. The technique in which AMs are both used beneath (blue) and on top of (orange) the limbal tissues is called “Sandwich” technique (C).
c. Outcome
SLET has an excellent outcome in the treatment of partial and total LSCD. The longest follow-up has been only 18 months (Table 1). Complete epithelialization is usually achieved within four weeks after surgery.155 A stable and avascular corneal surface is found in 100% eyes at 6 months and 9 months, in 80% eyes at 12 months, and in 76% eyes at 18 months.75,153,154 AMT is used in all reported cases of SLET and the actual function of AMT in SLET is unknown.
IV. Conclusions
The surgical approaches to treat LSCD vary depending on the severity of LSCD. The transplantation of AM alone seems to have limited long term effect. AMT combined with various types of LSC transplantation is commonly performed based on the presumption that AM provides biologically and mechanically support, and protection to the transplanted tissues and cells. High level studies are lacking to support the efficacy of AMT in LSC transplantation. Future randomized controlled clinical trials are needed to demonstrate the efficacy of AMT in the treatment of LSCD.
Acknowledgement
The study is supported in part by an unrestricted grant from Research to Prevent Blindness to Stein Eye Institute. SXD received grant support from National Eye Institute (5P30EY000331 and R01EY021797), California Institute for Regenerative Medicine (TR2-01768, CLIN1-08686).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product described in this article.
References:
- 1.Ramos T, Scott D, Ahmad S. An Update on Ocular Surface Epithelial Stem Cells: Cornea and Conjunctiva. Stem cells international. 2015;2015:601731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. The ocular surface. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of sub-chains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea and conunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 5.Rodriguez-Ares MT, Lopez-Valladares MJ, Tourino R, et al. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009;87(4):396–403. [DOI] [PubMed] [Google Scholar]
- 6.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh HJ, Yao CL, Chen HI, Cheng HC, Hwang SM. Cryopreservation of human limbal stem cells ex vivo expanded on amniotic membrane. Cornea. 2008;27(3):327–333. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World Journal of Transplantation. 2014;4(2):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng SC. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFh1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SY, Han B, Zhu YT, et al. HC-HA/PTX3 Purified From Amniotic Membrane Promotes BMP Signaling in Limbal Niche Cells to Maintain Quiescence of Limbal Epithelial Progenitor/Stem Cells. Stem Cells. 2015;33(11):3341–3355. [DOI] [PubMed] [Google Scholar]
- 11.Lee S-H, Tseng SCG. Amniotic Membrane Transplantation for Persistent Epithelial Defects With Ulceration. American Journal of Ophthalmology. 1997;123(3):303–312. [DOI] [PubMed] [Google Scholar]
- 12.Shortt AJ, Secker GA, Lomas RJ, et al. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009;30(6):1056–1065. [DOI] [PubMed] [Google Scholar]
- 13.Saghizadeh M, Winkler MA, Kramerov AA, et al. A simple alkaline method for decellularizing human amniotic membrane for cell culture. PLoS One. 2013;8(11):e79632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5(8):1470–1479. [DOI] [PubMed] [Google Scholar]
- 15.Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros Jde N, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110(3):466–473. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Gupta P, Kumar P, Kumar A, Chacharkar MP. Properties of Air Dried Radiation Processed Amniotic Membranes under Different Storage Conditions. Cell Tissue Bank. 2003;4(2–4):95–100. [DOI] [PubMed] [Google Scholar]
- 17.Gajiwala K, Gajiwala AL. Evaluation of lyophilised, gamma-irradiated amnion as a biological dressing. Cell Tissue Bank. 2004;5(2):73–80. [DOI] [PubMed] [Google Scholar]
- 18.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. [DOI] [PubMed] [Google Scholar]
- 19.von Versen-Hoynck F, Syring C, Bachmann S, Moller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts--light and scanning electron microscopic studies. Cell Tissue Bank. 2004;5(1):45–56. [DOI] [PubMed] [Google Scholar]
- 20.Wehmeyer JL, Natesan S, Christy RJ. Development of a Sterile Amniotic Membrane Tissue Graft Using Supercritical Carbon Dioxide. Tissue Eng Part C Methods. 2015;21(7):649–659. [DOI] [PubMed] [Google Scholar]
- 21.Ucakhan OO, Koklu G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002;21(2):169–172. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Zhou S, Huang T, Liu Z, Chen L, Lin Y. A clinical study on fresh amniotic membrane transplantation for treatment of severe ocular surface disorders at acute inflammatory and cicatricial stage. Zhonghua Yan Ke Za Zhi. 2000;36(1):13–17. [PubMed] [Google Scholar]
- 23.Qureshi IZ, Fareeha A, Khan WA. Technique for Processing and Preservation of Human Amniotic Membrane for Ocular Surface Reconstruction Int J Biotechnol Bioeng. 2010;4(9):710–713. [Google Scholar]
- 24.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85(5):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonci P, Bonci P, Lia A. Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol. 2005;15(4):441–445. [PubMed] [Google Scholar]
- 26.Nakamura T, Yoshitani M, Rigby H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–99. [DOI] [PubMed] [Google Scholar]
- 27.Chugh JP, Jain P, Sen R. Comparative analysis of fresh and dry preserved amniotic membrane transplantation in partial limbal stem cell deficiency. Int Ophthalmol. 2015;35(3):347–355. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48(2):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000; 19(4):421–426. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopkinson A, Shanmuganathan V, Gray T, et al. Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods. 2008;14(4):371–381. [DOI] [PubMed] [Google Scholar]
- 32.Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54(1):29–34. [DOI] [PubMed] [Google Scholar]
- 33.Paolin A, Trojan D, Leonardi A, et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and gamma-irradiated human amniotic membranes. Cell Tissue Bank. 2016;17(3):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrazova H, Koller J, Kubisova K, Fujerikova G, Klincova E, Babal P. Comparison of structural changes in skin and amnion tissue grafts for transplantation induced by gamma and electron beam irradiation for sterilization. Cell Tissue Bank. 2016;17(2):255–260. [DOI] [PubMed] [Google Scholar]
- 35.Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107(5):980–989; discussion 990. [DOI] [PubMed] [Google Scholar]
- 36.Tejwani S, Kolari RS, Sangwan VS, Rao GN. Role of amniotic membrane graft for ocular chemical and thermal injuries. Cornea. 2007;26(1):21–26. [DOI] [PubMed] [Google Scholar]
- 37.Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant Role of Amniotic Membrane Transplantation in Acute Ocular Stevens-Johnson Syndrome: A Randomized Control Trial. Ophthalmology. 2016;123(3):484–491. [DOI] [PubMed] [Google Scholar]
- 38.Tamhane A, Vajpayee RB, Biswas NR, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005; 112(11):1963–1969. [DOI] [PubMed] [Google Scholar]
- 39.Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95(2):199–204. [DOI] [PubMed] [Google Scholar]
- 40.Sharma N, Singh D, Maharana PK, et al. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: A Randomized Controlled Trial. Am J Ophthalmol. 2016;168:157–163. [DOI] [PubMed] [Google Scholar]
- 41.Saw VP, Minassian D, Dart JK, et al. Amniotic membrane transplantation for ocular disease: a review of the first 233 cases from the UK user group. Br J Ophthalmol. 2007;91 (8):1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145(5):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konomi K, Satake Y, Shimmura S, Tsubota K, Shimazaki J. Long-term results of amniotic membrane transplantation for partial limbal deficiency. Cornea. 2013;32(8):1110–1115. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Valle D, Santos-Bueso E, Benitez-Del-Castillo JM, et al. [Sectorial conjunctival epitheliectomy and amniotic membrane transplantation for partial limbal stem cells deficiency]. Arch Soc Esp Oftalmol. 2007;82(12):769–772. [DOI] [PubMed] [Google Scholar]
- 45.Tosi GM, Traversi C, Schuerfeld K, et al. Amniotic membrane graft: histopathological findings in five cases. J Cell Physiol. 2005;202(3):852–857. [DOI] [PubMed] [Google Scholar]
- 46.Ivekovic R, Tedeschi-Reiner E, Novak-Laus K, Andrijevic-Derk B, Cima I, Mandic Z. Limbal graft and/or amniotic membrane transplantation in the treatment of ocular burns. Ophthalmologica. 2005;219(5):297–302. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Garcia JS, Rivas L, Garcia-Lozano I. [Moderate limbal deficiency in patients with congenital aniridia treated with amniotic membrane transplantation]. Arch Soc Esp Oftalmol. 2005;80(9):517–523. [DOI] [PubMed] [Google Scholar]
- 48.Westekemper H, Figueiredo FC, Siah WF, Wagner N, Steuhl KP, Meller D. Clinical outcomes of amniotic membrane transplantation in the management of acute ocular chemical injury. Br J Ophthalmol. 2017;101(2):103–107. [DOI] [PubMed] [Google Scholar]
- 49.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116(4):431–441. [DOI] [PubMed] [Google Scholar]
- 50.Burcu A, Yalniz-Akkaya Z, Ozdemir MF, Erdem E, Onat MM, Ornek F. Surgical rehabilitation following ocular chemical injury. Cutan Ocul Toxicol. 2014;33(1):42–48. [DOI] [PubMed] [Google Scholar]
- 51.Seitz B, Kasmann-Kellner B, Viestenz A. [Stage-related therapy of congenital aniridia]. Ophthalmologe. 2014; 111(12):1164–1171. [DOI] [PubMed] [Google Scholar]
- 52.Dua HS, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990;110(6):646–656. [DOI] [PubMed] [Google Scholar]
- 53.Jarade E, Amro M, Haydar AA, Hemade A. Simultaneous Keratolimbal Autograft and Penetrating Autokeratoplasty: A Single-Stage Procedure to Restore Monocular Vision of a Blind Patient With Limbal Stem Cell Deficiency. Cornea. 2017;36(6):749–751. [DOI] [PubMed] [Google Scholar]
- 54.Celis Sanchez J, Mesa Varona DV, Avendano Cantos E, Lopez-Romero Moraleda S, Cebrian Rosado E, Gonzalez Del Valle F. Keratolimbal autograft transplantation as a possible new treatment of Lisch epithelial corneal dystrophy. Arch Soc Esp Oftalmol. 2016;91(7):333–336. [DOI] [PubMed] [Google Scholar]
- 55.Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697–1703. [DOI] [PubMed] [Google Scholar]
- 56.Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109(6):1159–1166. [DOI] [PubMed] [Google Scholar]
- 57.Stoiber J, Muss WH, Pohla-Gubo G, Ruckhofer J, Grabner G. Histopathology of human corneas after amniotic membrane and limbal stem cell transplantation for severe chemical burn. Cornea. 2002;21(5):482–489. [DOI] [PubMed] [Google Scholar]
- 58.Kheirkhah A, Raju VK, Tseng SC. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea. 2008;27(6):730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barreiro TP, Santos MS, Vieira AC, de Nadai Barros J, Hazarbassanov RM, Gomes JA. Comparative study of conjunctival limbal transplantation not associated with the use of amniotic membrane transplantation for treatment of total limbal deficiency secondary to chemical injury. Cornea. 2014;33(7):716–720. [DOI] [PubMed] [Google Scholar]
- 60.Capozzi P, Petroni S, Buzzonetti L. Combined HLA matched limbal stem cells allograft with amniotic membrane transplantation as a prophylactic surgical procedure to prevent corneal graft rejection after penetrating keratoplasty: case report. Ann Ist Super Sanita. 2014;50(3):298–300. [DOI] [PubMed] [Google Scholar]
- 61.Liang L, Sheha H, Tseng SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol. 2009;127(11):1428–1434. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Garcia JS, Rivas L, Garcia-Lozano I. [Severe limbal deficiency treated by combined limbal allograft and amniotic membrane transplantation]. Arch Soc Esp Oftalmol. 2005;80(7):405–412. [DOI] [PubMed] [Google Scholar]
- 63.Maruyama-Hosoi F, Shimazaki J, Shimmura S, Tsubota K. Changes observed in keratolimbal allograft. Cornea. 2006;25(4):377–382. [DOI] [PubMed] [Google Scholar]
- 64.Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110(8):1585–1592. [DOI] [PubMed] [Google Scholar]
- 65.Shimazaki J, Shimmura S, Tsubota K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology. 2004;111(1):38–44. [DOI] [PubMed] [Google Scholar]
- 66.Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr., Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223–230. [DOI] [PubMed] [Google Scholar]
- 67.Moreira PB, Magalhaes RS, Pereira NC, Oliveira LA, Sousa LB. Limbal transplantation at a tertiary hospital in Brazil: a retrospective study. Arq Bras Oftalmol. 2015;78(4):207–211. [DOI] [PubMed] [Google Scholar]
- 68.Park G, Je J, Kim J. Stepwise surgical approach for in vivo expansion of epithelial stem cells to treating severe acute chemical burns with total limbal deficiency. Korean J Ophthalmol. 2003;17(2):75–82. [DOI] [PubMed] [Google Scholar]
- 69.Baradaran-Rafii A, Akbari M, Shirzadeh E, Shams M. Single block conjunctival limbal autograft for unilateral total limbal stem cell deficiency. J Ophthalmic Vis Res. 2015;10(1):90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scocco C, Kwitko S, Rymer S, Marinho D, Bocaccio F, Lindenmeyer R. HLA-matched living-related conjunctival limbal allograft for bilateral ocular surface disorders: long-term results. Arq Bras Oftalmol. 2008;71(6):781–787. [DOI] [PubMed] [Google Scholar]
- 71.Han ES, Wee WR, Lee JH, Kim MK. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1697–1704. [DOI] [PubMed] [Google Scholar]
- 72.Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278–1284. [DOI] [PubMed] [Google Scholar]
- 73.Espana EM, Grueterich M, Ti SE, Tseng SC. Phenotypic study of a case receiving a keratolimbal allograft and amniotic membrane for total limbal stem cell deficiency. Ophthalmology. 2003;110(3):481–486. [DOI] [PubMed] [Google Scholar]
- 74.Kafle PA, Singh SK, Sarkar I, Surin L. Amniotic membrane transplantation with and without limbal stem cell transplantation in chemical eye injury. Nepal J Ophthalmol. 2015;7(1):52–55. [DOI] [PubMed] [Google Scholar]
- 75.Arora R, Dokania P, Manudhane A, Goyal JL. Preliminary results from the comparison of simple limbal epithelial transplantation with conjunctival limbal autologous transplantation in severe unilateral chronic ocular burns. Indian J Ophthalmol. 2017;65(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi W, Gao H, Wang T, Xie L. Combined penetrating keratoplasty and keratolimbal allograft transplantation in comparison with corneoscleral transplantation in the treatment of severe eye burns. Clin Exp Ophthalmol. 2008;36(6):501–507. [DOI] [PubMed] [Google Scholar]
- 77.Eberwein P, Bohringer D, Schwartzkopff J, Birnbaum F, Reinhard T. Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin C, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology. 2012;119(5):930–937. [DOI] [PubMed] [Google Scholar]
- 78.Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31(8):893–899. [DOI] [PubMed] [Google Scholar]
- 79.Cheung AY, Sarnicola E, Holland EJ. Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes. Cornea. 2017;36(9):1031–1035. [DOI] [PubMed] [Google Scholar]
- 80.Dua HS, Miri A, Elalfy MS, Lencova A, Said DG. Amnion-assisted conjunctival epithelial redirection in limbal stem cell grafting. Br J Ophthalmol. 2017;101(7):913–919. [DOI] [PubMed] [Google Scholar]
- 81.Mataix B, Alcantara A, Caro M, Montero J, Ponte B, Rodriguez de la Rua E. Variations in the technique for autologous limbal transplantation. Arch Soc Esp Oftalmol. 2016;91(10):501–504. [DOI] [PubMed] [Google Scholar]
- 82.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010; 117(6):1207–1213. [DOI] [PubMed] [Google Scholar]
- 83.Ozdemir O, Tekeli O, Ornek K, Arslanpence A, Yalcindag NF. Limbal autograft and allograft transplantations in patients with corneal burns. Eye (Lond). 2004;18(3):241–248. [DOI] [PubMed] [Google Scholar]
- 84.Torres J, Fernandez I, Quadrado MJ, et al. [Limbal transplantation: multicenter retrospective case series analysis]. Arch Soc Esp Oftalmol. 2008;83(7):417–422. [DOI] [PubMed] [Google Scholar]
- 85.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709–722; discussion 722–703. [DOI] [PubMed] [Google Scholar]
- 86.Wylegala E, Dobrowolski D, Tarnawska D, et al. Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol. 2008; 18(6):886–890. [DOI] [PubMed] [Google Scholar]
- 87.Javadi MA, Jafarinasab MR, Feizi S, Karimian F, Negahban K. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118(7):1272–1281. [DOI] [PubMed] [Google Scholar]
- 88.Titiyal JS, Sharma N, Agarwal AK, Prakash G, Tandon R, Vajpayee R. Live Related versus Cadaveric Limbal Allograft in Limbal Stem Cell Deficiency. Ocul Immunol Inflamm. 2015;23(3):232–239. [DOI] [PubMed] [Google Scholar]
- 89.Baradaran-Rafii A, Eslani M, Djalillian AR. Complications of keratolimbal allograft surgery. Cornea. 2013;32(5):561–566. [DOI] [PubMed] [Google Scholar]
- 90.Qi X, Xie L, Cheng J, Zhao J. Clinical results and influential factors of modified large-diameter lamellar keratoplasty in the treatment of total limbal stem cell deficiency. Cornea. 2013;32(5):555–560. [DOI] [PubMed] [Google Scholar]
- 91.Shen C, Chan CC, Holland EJ. Limbal Stem Cell Transplantation for Soft Contact Lens Wear-Related Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015; 160(6):1142–1149 e1141. [DOI] [PubMed] [Google Scholar]
- 92.Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003; 110(1):125–130. [DOI] [PubMed] [Google Scholar]
- 93.Parihar JKS, Parihar AS, Jain VK, Kaushik J, Nath P. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2017;37(6):1323–1331. [DOI] [PubMed] [Google Scholar]
- 94.Nassiri N, Pandya HK, Djalilian AR. Limbal allograft transplantation using fibrin glue. Arch Ophthalmol. 2011;129(2):218–222. [DOI] [PubMed] [Google Scholar]
- 95.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343(2):86–93. [DOI] [PubMed] [Google Scholar]
- 96.Rohaina CM, Then KY, Ng AM, et al. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl Res. 2014;163(3):200–210. [DOI] [PubMed] [Google Scholar]
- 97.Silber PC, Ricardo JR, Cristovam PC, Hazarbassanov RM, Dreyfuss JL, Gomes JA. Conjunctival epithelial cells cultivated ex vivo from patients with total limbal stem cell deficiency. Eur J Ophthalmol. 2014:0. [DOI] [PubMed] [Google Scholar]
- 98.Kim JH, Chun YS, Lee SH, et al. Ocular surface reconstruction with autologous nasal mucosa in cicatricial ocular surface disease. Am J Ophthalmol. 2010;149(1):45–53. [DOI] [PubMed] [Google Scholar]
- 99.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. [DOI] [PubMed] [Google Scholar]
- 100.Kethiri AR, Basu S, Shukla S, Sangwan VS, Singh V. Optimizing the role of limbal explant size and source in determining the outcomes of limbal transplantation: An in vitro study. PLoS One. 2017;12(9):e0185623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shortt AJ, Secker GA, Rajan MS, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008; 115(11):1989–1997. [DOI] [PubMed] [Google Scholar]
- 102.Fasolo A, Pedrotti E, Passilongo M, et al. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol. 2017;101(5):640–649. [DOI] [PubMed] [Google Scholar]
- 103.Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72(9):1478–1485. [DOI] [PubMed] [Google Scholar]
- 104.Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, Watson SL. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87(10):1571–1578. [DOI] [PubMed] [Google Scholar]
- 105.Daya SM, Watson A, Sharpe JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112(3):470–477. [DOI] [PubMed] [Google Scholar]
- 106.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120(6):783–790. [DOI] [PubMed] [Google Scholar]
- 107.Harkin DG, Barnard Z, Gillies P, Ainscough SL, Apel AJ. Analysis of p63 and cytokeratin expression in a cultivated limbal autograft used in the treatment of limbal stem cell deficiency. Br J Ophthalmol. 2004;88(9):1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim MN, Umapathy T, Baharuddin PJ, Zubaidah Z. Characterization and safety assessment of bioengineered limbal epithelium. Med J Malaysia. 2011;66(4):335–341. [PubMed] [Google Scholar]
- 109.Pathak M, Olstad OK, Drolsum L, et al. The effect of culture medium and carrier on explant culture of human limbal epithelium: A comparison of ultrastructure, keratin profile and gene expression. Exp Eye Res. 2016;153:122–132. [DOI] [PubMed] [Google Scholar]
- 110.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143(6):945–953. [DOI] [PubMed] [Google Scholar]
- 111.Dhamodaran K, Subramani M, Matalia H, Jayadev C, Shetty R, Das D. One for all: A standardized protocol for ex vivo culture of limbal, conjunctival and oral mucosal epithelial cells into corneal lineage. Cytotherapy. 2016;18(4):546–561. [DOI] [PubMed] [Google Scholar]
- 112.Fatima A, Sangwan VS, Iftekhar G, et al. Technique of cultivating limbal derived corneal epithelium on human amniotic membrane for clinical transplantation. J Postgrad Med. 2006;52(4):257–261. [PubMed] [Google Scholar]
- 113.Sharma S, Tandon R, Mohanty S, et al. Culture of corneal limbal epithelial stem cells: experience from benchtop to bedside in a tertiary care hospital in India. Cornea. 2011;30(11):1223–1232. [DOI] [PubMed] [Google Scholar]
- 114.Zakaria N, Possemiers T, Dhubhghaill SN, et al. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun CC, Su Pang JH, Cheng CY, et al. Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells. 2006;24(9):2130–2139. [DOI] [PubMed] [Google Scholar]
- 116.Pauklin M, Kakkassery V, Steuhl KP, Meller D. Expression of membrane-associated mucins in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Curr Eye Res. 2009;34(3):221–230. [DOI] [PubMed] [Google Scholar]
- 117.Ganger A, Vanathi M, Mohanty S, Tandon R. Long-Term Outcomes of Cultivated Limbal Epithelial Transplantation: Evaluation and Comparison of Results in Children and Adults. Biomed Res Int. 2015;2015:480983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subramaniam SV, Sejpal K, Fatima A, Gaddipati S, Vemuganti GK, Sangwan VS. Coculture of autologous limbal and conjunctival epithelial cells to treat severe ocular surface disorders: long-term survival analysis. Indian J Ophthalmol. 2013;61(5):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ramirez BE, Sanchez A, Herreras JM, et al. Stem Cell Therapy for Corneal Epithelium Regeneration following Good Manufacturing and Clinical Procedures. Biomed Res Int. 2015;2015:408495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525–1529. [DOI] [PubMed] [Google Scholar]
- 121.Balasubramanian S, Jasty S, Sitalakshmi G, Madhavan HN, Krishnakumar S. Influence of feeder layer on the expression of stem cell markers in cultured limbal corneal epithelial cells. Indian J Med Res. 2008;128(5):616–622. [PubMed] [Google Scholar]
- 122.Ang LP, Nakamura T, Inatomi T, et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006; 124(11):1543–1551. [DOI] [PubMed] [Google Scholar]
- 123.Dobrowolski D, Orzechowska-Wylegala B, Wowra B, et al. Cultivated Oral Mucosa Epithelium in Ocular Surface Reconstruction in Aniridia Patients. Biomed Res Int. 2015;2015:281870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako M, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells. 2014;32(8):2135–2146. [DOI] [PubMed] [Google Scholar]
- 125.Ma DH, Kuo MT, Tsai YJ, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye (Lond). 2009;23(6):1442–1450. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95(7):942–946. [DOI] [PubMed] [Google Scholar]
- 127.Prabhasawat P, Ekpo P, Uiprasertkul M, et al. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016;17(3):491–503. [DOI] [PubMed] [Google Scholar]
- 128.Priya CG, Arpitha P, Vaishali S, et al. Adult human buccal epithelial stem cells: identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye (Lond). 2011;25(12):1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118(8):1524–1530. [DOI] [PubMed] [Google Scholar]
- 130.Sotozono C, Inatomi T, Nakamura T, et al. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014;92(6):e447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takeda K, Nakamura T, Inatomi T, Sotozono C, Watanabe A, Kinoshita S. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011;152(2):195–201 e191. [DOI] [PubMed] [Google Scholar]
- 132.Baylis O, Rooney P, Figueiredo F, Lako M, Ahmad S. An investigation of donor and culture parameters which influence epithelial outgrowths from cultured human cadaveric limbal explants. J Cell Physiol. 2013;228(5):1025–1030. [DOI] [PubMed] [Google Scholar]
- 133.Shortt AJ, Tuft SJ, Daniels JT. Corneal stem cells in the eye clinic. British medical bulletin. 2011;100:209–225. [DOI] [PubMed] [Google Scholar]
- 134.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013; 131(6):731–736. [DOI] [PubMed] [Google Scholar]
- 135.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153(4):643–650, 650 e641–642. [DOI] [PubMed] [Google Scholar]
- 136.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(12):1504–1509. [DOI] [PubMed] [Google Scholar]
- 137.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. [DOI] [PubMed] [Google Scholar]
- 138.Prabhasawat P, Ekpo P, Uiprasertkul M, Chotikavanich S, Tesavibul N. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol. 2012;6:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scholz SL, Thomasen H, Hestermann K, Dekowski D, Steuhl KP, Meller D. [Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency]. Ophthalmologe. 2016;113(4):321–329. [DOI] [PubMed] [Google Scholar]
- 140.Cheng J, Zhai H, Wang J, Duan H, Zhou Q. Long-term outcome of allogeneic cultivated limbal epithelial transplantation for symblepharon caused by severe ocular burns. BMC Ophthalmol. 2017; 17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meller D, Pauklin M, Westekemper H, Steuhl KP. [Autologous transplantation of cultivated limbal epithelium]. Ophthalmologe. 2010; 107(12):1133–1138. [DOI] [PubMed] [Google Scholar]
- 142.Vazirani J, Basu S, Kenia H, et al. Unilateral partial limbal stem cell deficiency: contralateral versus ipsilateral autologous cultivated limbal epithelial transplantation. Am J Ophthalmol. 2014;157(3):584–590 e581-582. [DOI] [PubMed] [Google Scholar]
- 143.Qi X, Xie L, Cheng J, Zhai H, Zhou Q. Characteristics of immune rejection after allogeneic cultivated limbal epithelial transplantation. Ophthalmology. 2013;120(5):931–936. [DOI] [PubMed] [Google Scholar]
- 144.Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108(9):1569–1574. [DOI] [PubMed] [Google Scholar]
- 145.Inatomi T, Nakamura T, Koizumi N, Sotozono C, Yokoi N, Kinoshita S. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141(2):267–275. [DOI] [PubMed] [Google Scholar]
- 146.Kim YJ, Lee HJ, Ryu JS, et al. Prospective Clinical Trial of Corneal Reconstruction With Biomaterial-Free Cultured Oral Mucosal Epithelial Cell Sheets. Cornea. 2018;37(1):76–83. [DOI] [PubMed] [Google Scholar]
- 147.Hirayama M, Satake Y, Higa K, Yamaguchi T, Shimazaki J. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci. 2012;53(3):1602–1609. [DOI] [PubMed] [Google Scholar]
- 148.Inatomi T, Nakamura T, Kojyo M, Koizumi N, Sotozono C, Kinoshita S. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006;142(5):757–764. [DOI] [PubMed] [Google Scholar]
- 149.Sen S, Sharma S, Gupta A, et al. Molecular characterization of explant cultured human oral mucosal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52(13):9548–9554. [DOI] [PubMed] [Google Scholar]
- 150.Madhira SL, Vemuganti G, Bhaduri A, Gaddipati S, Sangwan VS, Ghanekar Y. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis. 2008;14:189–196. [PMC free article] [PubMed] [Google Scholar]
- 151.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(7):931–934. [DOI] [PubMed] [Google Scholar]
- 152.Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158(3):469–475 e462. [DOI] [PubMed] [Google Scholar]
- 153.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple Limbal Epithelial Transplantation: Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns. Ophthalmology. 2016;123(5):1000–1010. [DOI] [PubMed] [Google Scholar]
- 154.Vazirani J, Ali MH, Sharma N, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. Br J Ophthalmol. 2016;100(10):1416–1420. [DOI] [PubMed] [Google Scholar]
- 155.Iyer G, Srinivasan B, Agarwal S, Tarigopula A. Outcome of allo simple limbal epithelial transplantation (alloSLET) in the early stage of ocular chemical injury. Br J Ophthalmol. 2017;101(6):828–833. [DOI] [PubMed] [Google Scholar]
- 156.Vasquez-Perez A, Nanavaty MA. Modified Allogenic Simple Limbal Epithelial Transplantation Followed by Keratoplasty as Treatment for Total Limbal Stem Cell Deficiency. Ocul Immunol Inflamm. 2017:1–3. [DOI] [PubMed] [Google Scholar]
- 157.Vazirani J, Lal I, Sangwan V Customised simple limbal epithelial transplantation for recurrent limbal stem cell deficiency. BMJ Case Rep. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marchini G, Pedrotti E, Pedrotti M, et al. Long-term effectiveness of autologous cultured limbal stem cell grafts in patients with limbal stem cell deficiency due to chemical burns. Clin Exp Ophthalmol. 2012;40(3):255–267. [DOI] [PubMed] [Google Scholar]
- 159.Pellegrini G, Rama P, Matuska S, et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med. 2013;8(5):553–567. [DOI] [PubMed] [Google Scholar]
- 160.Burillon C, Huot L, Justin V, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53(3):1325–1331. [DOI] [PubMed] [Google Scholar]