Abstract
Background:
Dietary intake of choline has been linked to systemic inflammation through the microbial production of two metabolites trimethylamine (TMA) and trimethylamine-N-oxide (TMAO). Herein we explore the association between choline metabolites and inflammation in psoriatic arthritis (PsA) patients.
Material and Methods:
Thirty-eight patients with PsA, all of whom satisfied the CASPAR classification criteria for PsA, were studied. Outcomes reflecting the activity of peripheral arthritis as well as skin psoriasis, Disease Activity Score (DAS)28, Clinical Disease Index (CDAI) and Body Surface Area (BSA) were assessed. Serum concentration of choline metabolites (choline, TMA, TMAO, betaine and carnitine) were determined by LC-MS, and metabolite levels associated with disease scores.
Results:
Among the 38 PsA patients included the mean of DAS28PCR was 2.74 ± 1.29. Twenty-seven patients had active skin disease, with an average BSA of 7.2 ± 16.22. TMAO, but not TMA or choline, significantly correlated with measures of disease activity for both skin and peripheral joints.
Conclusion:
In our cohort, only TMAO, but not TMA, choline, betaine or carnitine, was associated with inflammation in PsA patients, establishing a mechanistic link between TMAO and PsA phenotypes. Future studies will explore modulation of TMAO and disease severity in PsA.
Keywords: psoriatic arthritis, psoriasis, metabolomics, choline, trimethylamine-N-oxide, disease activity
Introduction
Choline metabolism has been recently strongly related to inflammation in relation to the pathogenesis of atherosclerosis (1). Choline is a semi-essential nutrient found in a variety of foods, but it is particularly abundant in egg yolk, meats, liver, fish, dairy products, nuts, and soybean (2). Choline is a component of phosphatidylcholine, and following oxidation, precursor to betaine (3). Choline and other trimethylamine (TMA) containing species like carnitine are metabolized to TMA by the gut microbiota (4). TMA is subsequently oxidized by at least one member of the flavin-containing monooxygenases, FMO3, forming trimethylamine-N-oxide (TMAO), which is then released into circulation (4). Several studies have shown that an elevated level of choline, betaine and TMAO increases the risk of atherosclerosis and subsequently of cardiovascular disease (CVD) (5). Also, a recent study revealed a link between the TMAO-producing enzyme FMO3 and obesity (6, 7).
Psoriatic arthritis (PsA) is an inflammatory disease affecting the joints and connective tissue and is associated with psoriasis of the skin and nails. Among the risk factors for psoriasis, evidence is accumulating that nutrition plays a major role in psoriasis pathogenesis. In particular, body weight, nutrition, and diet may exacerbate the clinical manifestations (8). Obesity is a known risk factor for developing PsA in patients with psoriasis and several studies have described that a successful weight loss might be associated with a higher rate of achievement of minimal disease activity in overweight/obese patients with PsA who started treatment with TNFα blockers (9). Metabolic syndrome and high CVD risk are also more frequent in PsA than other rheumatic diseases such as rheumatoid arthritis (10). As choline metabolism is associated to all these comorbidities, we examined the relationship between circulating levels of choline, betaine, carnitine, TMA and TMAO, and skin and joint clinical scores in a cohort of PsA patients.
Methods
Patients
A cross-sectional cohort of 38 adult patients with PsA fulfilling the classification for PsA (CASPAR) criteria was recruited from the University of California San Diego (UCSD) Arthritis Clinics. The study was approved by the UCSD Institutional Review Board (#150272) and obtained the patient’s written informed consent to publish the material. Clinical assessment included: evaluation of the number of tender (TJC) and swollen joints (SJC) (out of 28), the number of tender entheseal sites, the percentage of the body surface affected by psoriasis, functional status as assessed by Health Assessment Questionnaire (HAQ), and assessments of pain, fatigue, global disease severity by patients, and a global assessment of disease by physicians, using a Visual Analogue Scale ranging from 0 to 10. Composite measures of peripheral arthritis were calculated using the above measures: Disease Assessment Score using a 28 joint count and C reactive protein (DAS28-CRP), Clinical Disease Activity Index (CDAI) and Simple Disease Activity Index (SDAI). Blood samples were processed immediately, and sera aliquots were stored at −80oC until analysis.
Metabolite Measure
Choline, TMA, TMAO, betaine and carnitine were assayed in serum samples using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as detailed in supplementary methods.
Data Analysis
The data, consisting of 38 patient samples measured across 5 choline metabolites and 14 clinical outcomes were processed using R, Version 3.4.1. (www.r-project.org) as detailed in supplementary methods.
Results
Cohort demographics and disease characteristics
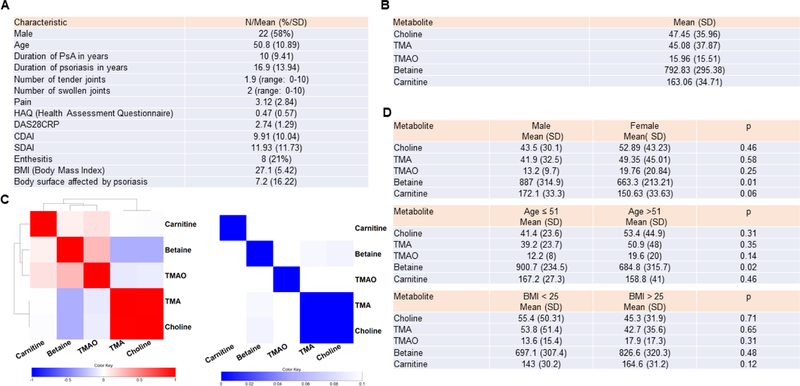
Patient characteristics are summarized in Figure 1A. Of the 38 PsA patients included in this study, 58% were male and the mean age was 50.8 years ± 10.9 (range 23 to 75 years). The mean number of tender joints and swollen joints were 1.9 ± 3 (range 0 to 10) and 2 ± 3.2 (range 0 to 10) respectively. The average DAS28-CRP score was 2.74 ± 1.29 (range 1–5.18). Twenty-seven patients (71%) had active skin disease, with an average BSA of 4.73 ± 14.5. Eight patients had enthesitis. 65% were receiving biological therapy (46% of them in association with a synthetic disease modifying drug (DMARD), mostly methotrexate. 14% received sDMARD as monotherapy, and 21% received no systemic treatment.
Figure 1. Cohort demographics and metabolites clustering.
A) Demographic and disease characteristics of the patients (N=38). B) The average value of the metabolites in our cohort of 38 patients is displayed in Figure 1B. C) Linear regression was performed between each metabolite – metabolite pair, controlling for age and gender. Left: The regression coefficients for each pair were used to form a clustered heatmap. Abbreviations: TMA: trimethylamine, TMAO: trimethylamine N-oxide. Right: Metabolite regression p-values are displayed, where the row and column order are preserved from 1C left. D) The average value of metabolites per age, gender and body max index (BMI) are displayed. T-test p-values are also displayed. Concentrations are provided as ng/ml.
Choline metabolite profiling and clustering
Concentration of circulating betaine, carnitine and choline, TMA and TMO are shown in Figure 1B. We also analyzed choline metabolite clustering (Figure 1C). Of interest, choline strongly correlated with its metabolite TMA, but did not correlate with TMAO. Betaine and carnitine did not correlate with any of the other metabolites. Concentration of betaine and carnitine were higher in males than in females (p = 0.01 for betaine and p = 0.06 for carnitine). Betaine was lower in patients older than 51 years old (p = 0.02). There were no significant differences in the concentrations of choline and its metabolites between patient with normal BMI versus overweight and obese patients adjusted by age and gender. (Figure 1D)
Linear and logistic regression analysis between grouped serum choline metabolites and skin and joint clinical parameters..
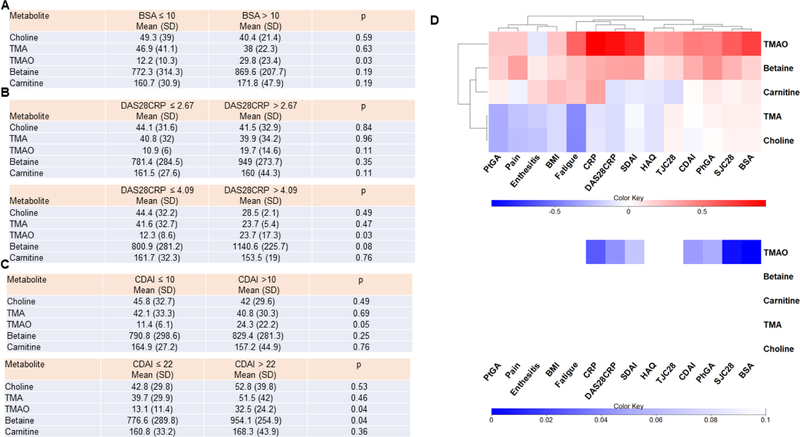
We then analyzed whether choline metabolites differentiate between high and low skin or joint disease activity scores. Figure 2A shows logistic regression analysis adjusted by age and gender by including these factors as covariates in the model, of each choline metabolite of patients classified between mild plus moderate (BSA ≤ 10) versus severe psoriasis (BSA > 10). We also conduct this analysis of each choline metabolite of patients classified as low or high joint disease activity (Figure 2B and C). TMAO was the only metabolite that significantly increased in patients with higher skin and joint scores. We also conducted linear regression that was performed between each choline metabolite-clinical parameter pair, controlling for age and gender. The regression coefficients for each choline metabolite-clinical parameter pair were used to form a clustered heatmap to lend insight into which groups of choline metabolites were correlated with which clinical parameters (Figure 2D-left panel). Metabolite regression p-values are displayed in Figure 2D (right panel), where the row and column order are preserved from left panel. Interestingly, TMAO correlated with parameters of both skin (BSA) and joint disease activity (TJC, SJC, CDAI, SDAI, DAS28CRP). TMAO also correlated with HAQ and fatigue. Of note, two metabolites, choline and TMA, negatively correlated with fatigue.
Figure 2. Relation of choline metabolites with skin and joint disease activity.
A) Logistic regression was performed for each metabolite in patients with BSA ≤10 compared with patient with BSA > 10, adjusting for age and gender. B and C) Logistic regression was performed for each metabolite in patients with low disease activity (measured by either DAS28CRP ≤ 2.67 or CDAI ≤10) and patients with moderate and high disease activity (DAS28CRP > 2.67 or CDAI > 10), adjusting for age and gender. Logistic regression was also performed for each metabolite in patients with mild or moderate disease activity (measured by either DAS28CRP ≤ 4.09 or CDAI ≤ 22) and patients with high disease activity (DAS28CRP > 4.09 or CDAI > 22) adjusting for age and gender. D) Linear regression was performed between each clinical score – metabolite pair, controlling for age and gender. In the left panel, the regression coefficients for each pair were used to form a clustered heatmap, to lend insight into which clinical scores were correlated with which metabolite. Row clusters have been identified by cophenetic cutting of the row dendrogram. In the right panel, metabolite regression p-values are displayed, where the row and column order are preserved from left panel. TJC: tender joint count; SJC: swollen joint count; CDAI: clinical Disease Activity Index; SDAI: Simple Disease Activity Index; HAQ: Health Assessment Questionnaire; PhGA: physician global assessment; PtGA: patient global assessment; BMI: body mass index; BSA: body surface area; DAS28CRP: disease assessment score 28. Concentrations are provided as ng/ml.
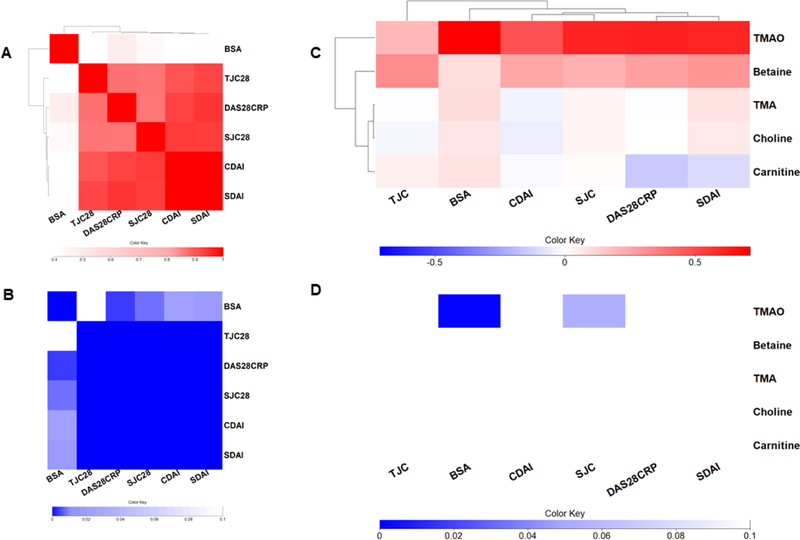
Although some other studies have reported a relatively low correlation between items assessing joint symptoms with items assessing skin symptoms (11), in our series, these parameters showed high correlation between them (Figure 3A and B). We then conducted linear regression between each choline metabolite-joint disease scores (TJC, SJC, DAS28, CDAI and SDAI) adjusted for BSA. We also conducted linear regression between each choline metabolite and BSA adjusted for CDAI. Figure 3C shows regression coefficients and Figure 3D metabolite regression p-values. Of note, TMAO still significantly correlates with both skin and joint clinical scores.
Figure 3. Choline metabolite regression after BSA and CDAI.
A) The Pearson correlation coefficients for each pair of clinical scores were used to form a clustered heatmap, to evaluate the relationship between joint and skin disease scores. B) The p values are displayed in Figure 3B where the row and columns are preserved from Figure 3A. C) Linear regression was performed between each clinical score – metabolite pair, controlling for age and gender. TJC, SJC, DAS28CRP, CDAI and SDAI were also adjusted by BSA, and BSA was also adjusted by CDAI. The regression coefficients for each pair were used to form a clustered heatmap. D) Metabolite regression p-values are displayed in Figure 3D, where the row and column order are preserved from Figure 3C. TJC: tender joint count; SJC: swollen joint count; CDAI: clinical Disease Activity Index; SDAI: Simple Disease Activity Index; BSA: body surface area; DAS28CRP: disease assessment score 28.
Discussion
In the present study, we show that circulating levels of TMAO correlates with skin and joint severity in a cohort of PsA patients. Previous studies showed that choline, betaine and TMAO were all associated with atherosclerosis and higher risk of CVD risk (5). Also, TMAO concentration was dependent on the dietary intake of choline (1), hence it was suggested that changes in diet could be a therapeutic approach for decreasing CVD risk. In our patients, however, there is no correlation between the concentration of choline and TMAO, and, furthermore, only TMAO and not choline and betaine correlated with skin and joint disease activity. This suggests that TMAO concentration does not depend on choline concentration in PsA patients, but more likely on the activity of FMO3, which might be elevated in patients with inflammation and obesity.
A recent study showed that expression of FMO3 in adipose tissue in overweight patients positively correlated with BMI and waist-to-hip ratio, and negatively correlated with insulin sensitivity (6, 7), suggesting a link between TMAO-producing enzyme FMO3 and obesity. In our cohort, although TMAO levels did not reach significance in overweight patients, the ratio TMAO/TMA was higher in the obese group (0.3 ± 0.2 in BMI ≤ 30 vs 0.7 ± 0.5 in BMI > 30, p=0.08) suggesting a higher activity of FMO3 in our cohort as well. Given that there is solid epidemiologic evidence linking psoriasis and PsA to metabolic syndrome (12, 13) an increase of FMO3 activity could explain the elevated TMAO levels in PsA patients.
Very few studies have attempted to study whether or not elevated levels of choline metabolites have an impact on cell function in vitro or in vivo. Although previous studies have suggested that TMAO promotes vascular inflammation by activating several inflammatory pathways including the NLRP3 inflammasome (14), mitogen-activated protein kinase and nuclear factor-κB pathwaya (15), further studies are needed to explore the mechanistic links between TMAO and inflammation in PsA.
In conclusion, in our cohort, only TMAO, but not TMA, choline, betaine or carnitine, was associated with inflammation in PsA patients. Future studies are needed to explore the mechanistic links between TMAO and skin and joint inflammation.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grants from the National Institutes of Health (K08AR064834 and R03AR068094 to MG; and R01ES027595 and S10OD020025 to MJ), UC San Diego Frontiers of Innovation Scholars Program (KL), and the Spanish Society of Rheumatology to RC.
Footnotes
Authors do not have any conflict of interest.
REFERENCES
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7. [DOI] [PubMed] [Google Scholar]
- 3.Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis 2011;34:3–15. [DOI] [PubMed] [Google Scholar]
- 4.Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, et al. Isoform specificity of trimethylamine n-oxygenation by human flavin-containing monooxygenase (fmo) and p450 enzymes: Selective catalysis by fmo3. Biochem Pharmacol 1998;56:1005–12. [DOI] [PubMed] [Google Scholar]
- 5.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, et al. The tmao-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep 2017;19:2451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrea L, Nappi F, Di Somma C, Savanelli MC, Falco A, Balato A, et al. Environmental risk factors in psoriasis: The point of view of the nutritionist. Int J Environ Res Public Health 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis 2014;73:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feld J, Nissan S, Eder L, Rahat MA, Elias M, Rimar D, et al. Increased prevalence of metabolic syndrome and adipocytokine levels in a psoriatic arthritis cohort. J Clin Rheumatol 2018;24:302–7. [DOI] [PubMed] [Google Scholar]
- 11.Wittkowski KM, Leonardi C, Gottlieb A, Menter A, Krueger GG, Tebbey PW, et al. Clinical symptoms of skin, nails, and joints manifest independently in patients with concomitant psoriasis and psoriatic arthritis. PLoS One 2011;6:e20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelfand JM, Yeung H. Metabolic syndrome in patients with psoriatic disease. J Rheumatol Suppl 2012;89:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol 2014;41:1357–65. [DOI] [PubMed] [Google Scholar]
- 14.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-n-oxide induces vascular inflammation by activating the nlrp3 inflammasome through the sirt3-sod2-mtros signaling pathway. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine n-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappab. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.