Abstract
The automatic capture of attention by drug cues, or attentional bias, is associated with craving and predicts future drug use. Despite its clinical significance, the neural bases of attentional bias to drug cues is not well understood. To address this gap, we undertook a neuroimaging investigation of the neural correlates of attentional bias towards smoking cues. Twenty-nine adults, including 14 active smokers and 15 non-smokers, completed a spatial cuing task during fMRI. A multivariate pattern analysis (MVPA) decoded the neural responses to the brief presentation of smoking versus neutral images. These data were correlated with behavioral measures of attentional bias, which included analyses targeting the neural correlates of response facilitation and cue-related task interference. We detected a set of brain-behavioral correlates that were similar across both smokers and non-smokers, indicating a role for stimuli salience in the absence of nicotine conditioning in smoking cue attentional bias. However, multiple smoking-related modifications to the neural correlates of attentional bias and its components were also identified. For example, regions demonstrating smoking-related differences in the neural correlates of attentional bias included the rostral anterior cingulate cortex and inferior frontal gyrus. Response facilitation effects of smoking were observed in the right orbitofrontal gyrus and bilateral middle temporal gyrus. Smoking-cue related task interference was related to smoking-related effects in the frontal eye fields. Our findings suggest that multiple cognitive, affective, and visual object recognition processes contribute to attentional bias towards smoking cues, and suggest multiple circuit modifications that may contribute to perpetuation of addiction.
Keywords: attentional bias, nicotine, addiction, fMRI, orbitofrontal cortex
1. INTRODUCTION
Craving for addictive substances can be readily elicited by exposure to drug-related cues. Evidence from laboratory-based tasks suggests that cue-triggered urges may result in part from abnormal allocation of attention to these drug-related stimuli, a process known as attentional bias (Field and Cox, 2008). Attentional bias predicts relapse risk (Cox et al., 2002; Cox et al., 2007; Marissen et al., 2006; Streeter et al., 2008; Waters et al., 2003), time to next drug use (Waters and Feyerabend, 2000), and quantity of drug use (Marhe et al., 2013; Mitchell et al., 2005); however, its ability to predict clinical outcomes might more precisely reflect its association with motivational states (Christiansen et al., 2015), as highlighted by strong evidence that the degree of bias associates with the extent of drug craving (Attwood et al., 2008; Field et al., 2005; Field et al., 2009; Franken et al., 2000; Mogg et al., 2003). As reduction of craving is a major focus of medication development for addictive disorders (Waters et al., 2003), improving our understanding of its neural underpinnings is imperative.
In the laboratory, attentional bias towards drug cues is often indexed by variations in task performance associated with the presence of drug-related visual stimuli. Laboratory tasks designed to measure attentional bias to drug cues have included modified Stroop tasks utilizing drug-use words as stimuli (Ersche et al., 2010; Janes et al., 2010; Kilts et al., 2014), spatial cuing tasks (Chanon et al., 2010; Mogg et al., 2005; Wetherill et al., 2014), and change blindness paradigms (Jones et al., 2006; Jones et al., 2003). A spatial cuing task variant, the “dot-probe” task, consists of the brief presentation of image pairs, one drug use-related and one neutral, followed by a target (often a dot), appearing in the location previously occupied by either the drug cue or a neutral image. Studies indicate that when participants are instructed to respond quickly and accurately to the location of the target, individuals with substance use disorders generally respond more quickly to targets appearing in the same spatial location in which a drug-related image had appeared, compared with targets appearing in place of a neutral image.
Several distinct processes are hypothesized to contribute to attentional bias among individuals with substance use disorders (Field and Cox, 2008; Mogg and Bradley, 2002; Mogg et al., 2005). Due to the relationship between craving and attentional bias during laboratory tasks, bias towards drug-related cues may stem from internal motivational states (Franken et al., 2000). Attentional bias to smoking-related images during a spatial cuing task also correlates with approach bias (Mogg et al., 2005), indicating possible overlapping mechanisms between these behaviors. Attentional bias among nicotine-dependent active smokers during a smoking word Stroop task was associated with increased activity in the insula, amygdala, hippocampus, parahippocampal gyrus, proposed to represent enhanced processing of internal states reflecting conditioned responses to drug cues (Janes et al., 2010). Neural activation of the right dorsal anterior cingulate cortex during fMRI in response to cocaine-related stimuli, which predicted future cocaine use among individuals with cocaine dependence, has been attributed to the enhanced cognitive control required to manage the conflict between task response requirements and automated processes elicited by drug-related words (Marhe et al., 2013). Enhanced activity in visual cortex in that study also indicated a role for enhanced sensory processing in attentional bias. In another study of individuals with cocaine dependence, attentional bias to drug-related words was positively correlated with activation of a network of regions involved in salience attribution and a frontal-temporal network that also related to processing of negative emotional stimuli; activity within a sensorimotor network predicted less attentional bias toward cocaine words (Kilts et al., 2014). Individuals with stimulant dependence also demonstrate activation of the left inferior frontal gyrus and right cerebellar cortex related to attentional bias during a drug-word Stroop task (Ersche et al., 2010). Thus, although findings remain inconsistent across drug-addicted populations and task designs, emphasizing the need for additional work in this area, the above studies suggest that multiple processes likely contribute to attentional bias. In the current study, we utilized a task designed to differentiate some of these processes. We hypothesized that several distinct processes contributing to attentional bias could be resolved by parsing out components of the attentional bias behavioral measure.
In this study, 14 active smokers (AS) and 15 non-smokers (NS) participated in a functional magnetic resonance imaging (fMRI) investigation of attentional bias to visual smoking cues, which used an fMRI dot-probe spatial cuing task to measure automatic attentional capture. We opted to use a task that included naturalistic images rather than words in order to engage neural processes of attentional bias related to visual object processing that are particularly relevant for real-world attentional bias. Importantly, we included a set of double neutral image trials to support our goal of distinguishing between components of attentional bias (Chanon et al., 2010; Cisler and Koster, 2010). Two distinct processes that may underlie behavioral measures of attentional bias include the facilitation of attentional capture by smoking cues on congruent trials and/or conflict between automatic attention towards smoking cues and task goals in incongruent trials. Thus, in addition to investigating the neural correlates of smoking attentional bias as typically defined in this task - the difference in reaction time (RT) between incongruent and congruent trials, we further considered congruent versus neutral trials (response facilitation) and incongruent versus neutral trials (smoking cue interference) to identify the neural correlates of these previously unexplored processes. To sensitively classify patterns of voxels representing smoking cue-induced brain activation, we applied a multivariate pattern analysis (MVPA) to the fMRI data (Haxby, 2012; Norman et al., 2006). Our findings suggest that neural processes contributing to smoking cue-related attentional bias involve a constellation of brain regions implicated in cognitive, affective, motor, and visual processes.
2. METHODS
2.1. Participants
Active smokers (AS; n=14, 6 females) and non-smokers (NS; n=15, 6 females), were recruited from the University of North Carolina at Chapel Hill (UNC) campus and surrounding community via informational e-mails and posted flyers. Prior to recruitment, participants completed an online or telephone-based questionnaire regarding their smoking habits. For inclusion in the AS group, participants needed to smoke at least 5 cigarettes per day on average (group average: 12.0 cigarettes/day, standard deviation: 6.3 range: 5–25). There were no instructions to abstain from smoking prior to the study session; the number of minutes since participants smoked their last cigarette was recorded and is summarized in Table 1. Occasional and former smokers were excluded from the non-smoking group. All subjects were between 18–40 years of age, right-handed, and were screened and excluded for neurological and psychiatric disease, current use of other psychoactive drugs, including medications, excepting moderate caffeine or alcohol intake, and contraindications to fMRI. Sample characteristics are summarized in Table 1.
Table 1.
Sample characteristics
| Active Smokers (Mean ± SEM) |
Non-Smokers (Mean ± SEM) |
|
|---|---|---|
| N | 14 | 15 |
| Age (years) | 24.8 ± 1.7 | 23.4 ± 0.9 |
| Sex (Males/Females) | 8/6 | 9/6 |
| Race (white/black/other) | 10/2/2 | 7/3/5 |
| Cigarette Dependence Scale | 42.6 ± 2.7 | N/A |
| Time since last cigarette (min) | 182.5 ± 83.4 | N/A |
| Cigarettes/day | 12 ± 1.7 | N/A |
| Age smoking initiated (years) | 17.6 ± 0.6 | N/A |
| Years of cigarette use | 7.1 ± 1.7 | |
| Cigarette craving pre-scan | 4.7 ± 0.6 | N/A |
| Cigarette craving post-scan | 7.1 ± 0.7 | N/A |
| Barrett Impulsiveness Scale | 70.1 ± 1.7 | 69.8 ± 1.1 |
| State Anxiety (STAI) | 34.5 ± 2.6 | 31.5 ± 1.7 |
| Trait Anxiety (STAI) | 41.4 ± 3.1 | 35.5 ± 2.0 |
| Thought-Action Fusion Scale | 16.4 ± 2.7 | 19.7 ± 2.7 |
| Affect Misattribution Procedure: Smoking-Neutral ratings | −0.07 ± 0.05 | −0.10 ± 0.03 |
2.2. Experimental Task
After obtaining informed consent, participants complete a battery of self-administered, paper and pencil questionnaires including: The Cigarette Dependence Scale (CDS) (Etter et al., 2003), the Barratt Impulsiveness Scale (BIS) (Barratt and Stanford, 1995), the State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1970), the Thought-Action Fusion Scale (TAF-scale) (Shafran et al., 1996), and a questionnaire developed in-house to assess smoking history, habits, and motivations. Additionally, a measurement of breath carbon monoxide levels was taken before beginning the task practice. At that time, participants also rated current cigarette craving on a scale of 1 (no craving) −10 (most intense craving ever experienced). A second measure of craving w0as completed following the fMRI scan.
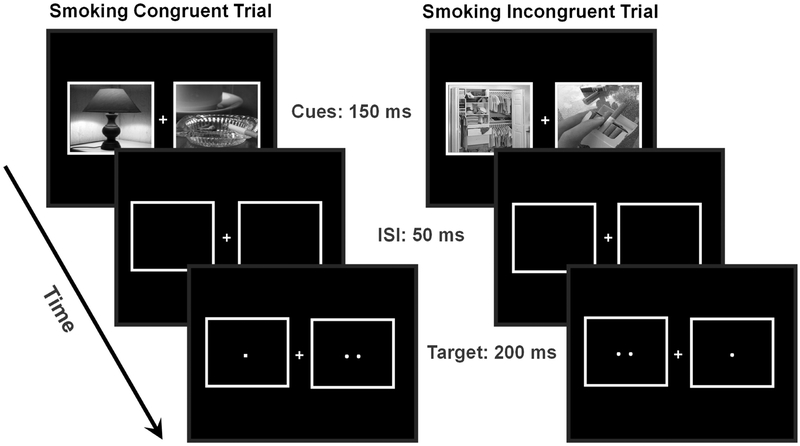
The spatial cuing task, implemented with E-Prime 1.2 (PST Inc., Pittsburgh, PA), was a variation of a dot-probe task, a type of behavioral task that has been used extensively to elicit attentional bias to drug-related stimuli (Chanon et al., 2010; Domaradzka and Bielecki, 2017; Ehrman et al., 2002; Wetherill et al., 2014), or to other types of salient stimuli (Lipp and Derakshan, 2005; MacLeod et al., 1986; Salemink et al., 2007). This particular version of the task has been previously described and validated in a behavioral study of attentional bias (Chanon et al., 2010). In that previous study, active smokers demonstrated significantly greater attentional bias toward smoking images compared with non-smokers, providing good evidence for construct validity for this task variant. An additional experiment further demonstrated that reaction time effects produced by smoking images in this task are not likely driven simply by smokers’ familiarity with the stimuli (Chanon et al., 2010). In each trial of the task, two grayscale images (11.1°×9.0°) appeared, one on each side of a fixation cross. Importantly, we selected a task that included images rather than words, as these more naturalistic stimuli were intended to engage neural processes of attentional bias related to visual object processing that are particularly relevant for real-world attentional bias. The pairs of pictures consisted of either two neutral images or one smoking and one neutral image. The task design included trials with two neutral images not only to provide a requisite neuroimaging contrast, but also to differentiate the distinct processes that contribute to behavioral indices of attentional bias (Chanon et al., 2010; Cisler and Koster, 2010). Each image pair was presented for 150 ms. Smoking images were randomly presented on the left or right side of the screen and appeared an equal number of times on each side. Following a 50 ms inter-stimulus interval, both images disappeared and were replaced by either one dot (non-target) or two dots (target) for 100 ms. One-third of all trials consisted of “catch trials,” in which the images were not followed by any targets, an fMRI task design strategy intended to enable statistical separation of the neural signals related to image presentation and target responding. The stimulus sets were matched in terms of the presence of people and hands and were also analyzed with respect to their spatial frequency content to ensure that they did not differ in terms of basic visual properties. On measures of both the spectral peak (Neutral: 0.020, Smoking: 0.021, t=−0.07, p=0.95), and spectral width (Neutral: 54.81, Smoking: 54.01, t=0.18, p=0.86), the two stimulus sets did not differ. Subjects practiced one block of the cuing task in a mock scanner prior to the actual scan.
Following the practice and prior to entering the fMRI scanner, participants completed a block of the Affect Misattribution Procedure, which measures implicit affective responses to visual cues (Payne et al., 2007). In this task, implemented using E-Prime, participants saw an image (either smoking or neutral) for 75ms, immediately followed by a blank screen for 125 ms, then a Chinese pictograph for 100ms, and finally a screen of white noise until the participant responded. Participants were instructed to ignore the image and respond with one button if they found the pictograph to be pleasant and another if they found it unpleasant. They were warned that the image appearing before the pictograph could influence their responses and were instructed to try to avoid that to the best of their ability.
Once in the MR scanner, participants completed 4 blocks of 120 trials each of the spatial cuing task (Figure 1). Subjects were instructed to fix their gaze on the cross and to respond with a button press corresponding to the side of the target (two dots) using an MRI-compatible button box.
Figure 1. Spatial cuing task to measure attentional bias to smoking cues.
Sample trial sequences are displayed for congruent trials (left), in which the target (i.e., two dots) appeared on the same side as the smoking-related image, and incongruent trials (right) in which the target appeared opposite of the smoking-related image. The task also included neutral trials in which both images were neutral images. ISI, inter-stimulus interval.
Subjects were compensated $20/hour. The entire experiment, including training, completion of questionnaires, and the scanning session lasted approximately 2 hours. The spatial-cuing task in the scanner lasted approximately 30 minutes, and an additional three blocks of an attentional blink task (not reported here) took an additional 30 minutes. All procedures were approved by the UNC Office of Human Research Ethics.
2.3. Analysis of Behavioral Data
Three trial types were defined: “neutral trials” included the presentation of side-by-side neutral images followed by a target appearing on either side; “congruent trials” included the side-by-side presentation of one smoking image and one neutral image followed by a target in the location previously occupied by the smoking image; “incongruent trials” included the side-by-side presentation of one smoking image and one neutral image followed by a target in the location previously occupied by the neutral image. The primary measure of attentional bias was determined by RT differences between incongruent and congruent trials. We further subdivided the attentional bias measure into distinct components. To isolate two potential mechanisms of attentional bias – facilitation of attentional capture by smoking cues in congruent trials or distraction from task goals by the smoking cues in incongruent trials – we calculated the RT differences between neutral and congruent trials (neutral minus congruent) and between incongruent and neutral trials (incongruent minus neutral). Only RTs from correct trials and trials with reaction times greater than 200 ms were included in calculations.
The effects of smoking status and trial type on accuracy and RT were tested in two-way repeated-measures analyses of variance (ANOVA) with group (active smokers and non-smokers) and trial type (neutral, congruent, and incongruent) as factors using the SAS (SAS 9.4. Cary, NC: SAS Institute Inc.) MIXED procedure. A follow-up analysis was conducted to explore whether a greater severity of smoking would associate with an enhanced attentional bias behavioral effect. For that analysis, the ANOVA models were altered to only include nonsmokers and the subset of smokers reporting smoking at least 10 cigarettes per day (n=8). A third set of ANOVAs included only smokers and tested factors of trial type, time since last cigarette, and their interaction on accuracy and RT.
2.4. Image Acquisition
Structural and functional images were acquired on a Siemens Magnetom Allegra 3T MR scanner. Echo-planar images were acquired during four runs of the spatial cuing task using the following parameters: TR=2200 ms, TE=30 ms, FA=80°, voxel size=3.5×3.5×3.5 mm3, FOV=224×224 mm2, 37 coronal slices. For registration and tissue segmentation purposes, a high resolution T1 structural image was acquired with an MPRAGE sequence using the following parameters: TR=1750 ms, TE=4.38 ms, FA=8°, voxel size=1.0×1.0×1.0 mm3, FOV=256×208 mm2, 160 axial slices. A low resolution structural image was acquired to aid in registration between the functional and high-resolution structural images: TR=500 ms, TE=9 ms, FA1=90°, FA2=150°, slice thickness= 3.5 mm, voxel size=1.8×1.8×3.5 mm3, FOV=224×224 mm2, 37 coronal slices.
2.5. Image Preprocessing
Prior to statistical analyses, functional images were slice timing corrected and realigned with Statistical Parametric Mapping 8 (SPM8). Time series were scaled to percent signal change using Analysis of Functional Neuroimages (AFNI (Cox, 1996), version 16.2.07). A gray matter mask was created for each subject using the FSL fast segmentation process applied to each subject’s high resolution T1 image. Images remained in subject space and were not spatially smoothed prior to MVPA in order to preserve subject-specific patterns of activations.
2.6. Calculation of Activation Maps
The task was modeled in SPM with events modeled as delta functions and convolved with a canonical hemodynamic response function. Since the task included cue-only catch trials, we were able to model image presentation and targets separately; therefore, we modeled five different event types: two neutral images (neutral trial), one smoking and one neutral image, targets following neutral trials, targets congruent with smoking images, and targets incongruent with smoking images. Each of the four fMRI task runs was further divided into 3, 52-frame data sets, yielding 12 total datasets per subject. A general linear model implemented in AFNI (3dDeconvolve and 3dREMLfit) tested the relationship between each of the five conditions and the fMRI time series across the 52 frames. Several nuisance variables representing likely sources of noise in the fMRI data were also included as covariates in the model: 6 directions of head motion, first 5 principal components of signals from masks encompassing cerebrospinal fluid and white matter voxels, and first and second order trends. Beta maps of neuroactivation estimates when two neutral images were presented versus when a smoking image was presented were subsequently entered into multivariate pattern analyses.
2.7. Multivariate Pattern Analysis
In this analysis, we sought to identify sets of brain voxels that accurately distinguished between the presence versus absence of a smoking-related image. The presentation of a smoking image, relative to the presentation of two neutral images, contributes to RT differences in the response to a subsequent target (Chanon et al., 2010). Therefore, differences in patterns of neural responses between smoking and neutral conditions should theoretically account for variance in RT to the target (or “probe”). Advantages of focusing our analysis on neural responses to the presentation of smoking and neutral images – rather than the statistically-independent probes that follow – include the ability to better isolate the neural correlates of attentional capture by smoking cues and an improvement in power for the multivariate pattern analysis (probe types were divided into three categories versus only two categories of image presentation trials).
MVPA has emerged as an alternative method of examining brain activation during task fMRI as opposed to univariate tests which are performed on each voxel independently (Haxby, 2012; Norman et al., 2006). Multivariate pattern analysis of fMRI data was performed with the Princeton MVPA toolbox for Matlab in MATLAB13a. A spherical “searchlight” (Kriegeskorte et al., 2006) consisting of a center voxel and all its immediate neighbors (1 voxel radius) was passed across every voxel within each subject’s gray matter mask. A Gaussian Naïve Bayes (GNB) binary classifier was created for sets of voxels within each searchlight location to classify patterns of voxel activation during trials containing smoking images versus those with two neutral images. A leave-one-out cross-validation procedure was applied across the 12 datasets to derive an unbiased measure of predictor accuracy. The center voxel of each searchlight was assigned the average cross-validation accuracy across the 12 datasets, resulting in a whole-brain map of accuracy values for each subject.
2.8. Group-level statistical analysis and correction for multiple comparisons
Subject-level datasets were warped to MNI standard space in AFNI for group analysis using a nearest neighbor interpolation. To improve correspondence between subjects, which is limited by variation in functional anatomy, and to improve the signal-to-noise ratio of the accuracy data, we spatially smoothed accuracy maps using a 10 mm full-width at half maximum (FWHM) Gaussian kernel. We then calculated a group gray matter mask and applied it to all images. We detected brain regions showing statistically significant discrimination between conditions with a two step process: 1) Heteroscedastic linear models employed in FVGWAS software for MATLAB (Huang et al., 2015) tested the relationship between attentional bias behavioral measures and accuracy maps; this is a nonparametric analysis which makes no assumptions regarding data distributions. 2) A correction for multiple comparisons was employed to control family-wise error rates. In FCGWAS, two wild bootstrapping methods (Huang et al., 2015; Zhu et al., 2007) were used to generate null distributions of the maximum test statistic and maximum cluster size statistic (given p<0.005), respectively. Voxels exceeding the 95th percentile maximum test statistic or clusters of voxels exceeding the 95th percentile maximum cluster size statistic were considered significant (α=0.05).
These group-level analyses tested two types of effects: 1) the effects of attentional bias on MVPA accuracy across all subjects and 2) the effects of the interaction of smoking group and attentional bias on MVPA accuracy. The first set of analyses examined the neural underpinnings of the visual detection of salient stimuli, a key process in the allocation of visual attention (Parkhurst et al., 2002) that has implications for attentional bias in addiction (Kilts et al., 2014; Marhe et al., 2013). The latter analysis was designed to isolate those elements of smoking-cue attentional bias specifically associated with nicotine conditioning among active smokers. All analyses controlled for age and sex.
3. RESULTS
Smokers and non-smokers did not differ on any sample characteristic measures (Table 1), aside from their use of cigarettes. Smokers’ scores on the Cigarette Dependence Scale (Table 1) were well within the norm of a random sample of adult smokers (Etter et al., 2003). Craving ratings among smokers were significantly higher after the fMRI scan than before, based on a paired samples t-test (t=3.59, p<0.003).
3.1. Behavioral
Participants completed the spatial cuing task with high levels of accuracy (see Table 2). An two-way repeated-measures analysis of variance indicated that average task accuracy did not differ between neutral, congruent, and incongruent trial types (F(2,27)=1.99, p=0.16; Table 2) or between active smoker and non-smoker groups (F(1,27)=0.14, p=0.91; Table 2), and there was no group-by-trial-type interaction (F(2,27)=0.79, p=0.46). However, there was a significant effect of trial type on RTs (F(2,27)=9.93, p<0.001; Table 2). RTs did not differ between the groups (F(1,27)=0.28, p=0.60; Table 2) and there was no group-by-trial-type interaction (F(2,27)=0.44, p=0.65).
Table 2.
Spatial-cuing attentional bias task accuracy and reaction times by trial type
| Overall (Mean ± SEM) |
Neutral (Mean ± SEM) |
Congruent (Mean ± SEM) |
Incongruent (Mean ± SEM) |
|
|---|---|---|---|---|
| Accuracy | ||||
| Active Smokers | 0.92 ± 0.02 | 0.92 ± 0.03 | 0.92 ± 0.02 | 0.93 ± 0.02 |
| Non-Smokers | 0.93 ± 0.02 | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.94 ± 0.02 |
| Average Reaction Time (ms) | ||||
| Active Smokers | 436.8 ± 6.8 | 437.6 ± 6.6 | 434.8 ± 7.3 | 438.1 ± 6.7 |
| Non-Smokers | 449.1 ± 14.4 | 450.5 ± 14.6 | 447.6 ± 14.2 | 449.3 ± 14.4 |
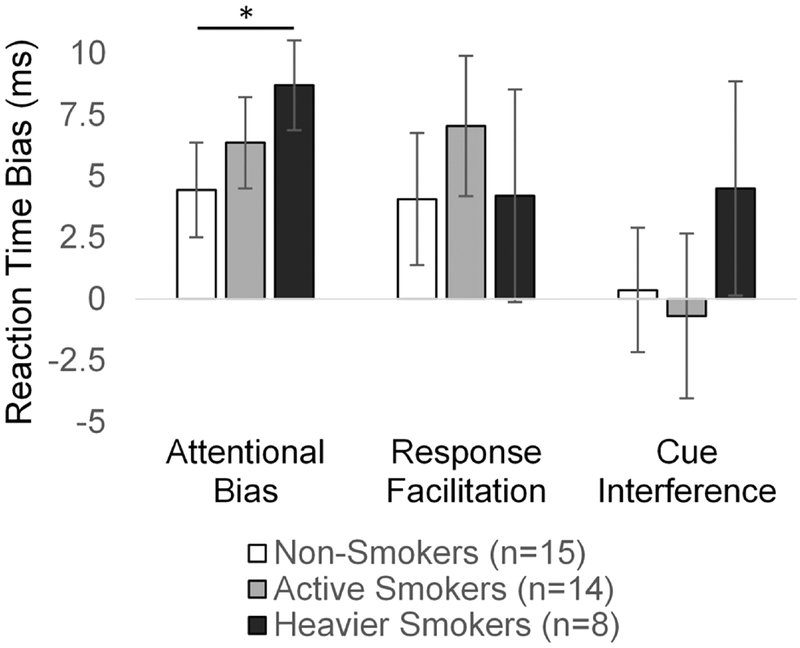
Post-hoc tests of RT effects indicated that congruent trial RTs were significantly faster than incongruent trial RTs, indicating a significant attentional bias effect (whole sample: t=−4.36, p<0.001), which was observed for both active smokers (t=2.36, p=0.004) and non-smokers (t=2.30, p=0.037). Congruent trial RTs were also significantly faster than neutral trial RTs (whole sample: t=−3.05, p=0.005), but the response facilitation effect of congruent trials was only significant for active smokers (smokers: t=2.47, p=0.028; non-smokers t=1.52, p=0.15). However, neutral and incongruent trial RTs did not differ (whole sample: t=0.07, p=0.94; smokers: t=−0.20, p=0.84; non-smokers t=0.15, p=0.89), suggesting that incongruent trials did not produce smoking cue interference. These effects are displayed in bar graphs for active smokers and non-smokers for each of the three RT contrasts in Figure 2.
Figure 2. Attentional bias to smoking cues: reaction time effects.
Bar plots represent group means and standard errors of the means for reaction times related to attentional bias towards smoking cues (incongruent minus congruent trials), response facilitation (neutral minus congruent trials), and smoking cue interference (incongruent minus neutral trials).
We also compared non-smokers to those smokers reporting smoking at least 10 cigarettes per day to explore whether the non-significant overall group differences in attentional bias may be related to the sample’s relatively low levels of cigarette consumption (Figure 2). Because this reduced the smoking sample to only 8 participants, we used a bootstrapping analysis (10,000 iterations) to statistically compare differences in group mean bias scores. The subsample of heavier smokers demonstrated significantly greater attentional bias (incongruent versus congruent RTs) compared with non-smokers (95% CI: 0.16–8.54; p=0.043). There were no differences detected for response facilitation (95% CI: −7.30–8.87; p=0.51) or cue interference (95% CI: −3.74–12.01; p=0.19).
The final set of ANOVAs did not detect a main effect of time since last cigarette on either RT (F(1,10)=0.44, p=0.52) or accuracy (F(1,10)=0.49, p=0.50), and there was no interaction of time since last cigarette with trial type on either RT (F(1,10)=0.31, p=0.74) or accuracy (F(2,10)=2.35, p=0.15).
Pearson’s correlation analyses of the entire sample (n=29) indicated that attentional bias towards smoking cues (incongruent RT minus congruent RT) was related to smoking cue interference (incongruent RT minus neutral RT) (r=0.40, p=0.03), but not to facilitation of responding to smoking-cue congruent targets (neutral RT minus congruent RT) (r=0.26, p=0.17). Interestingly, response facilitation effects and smoking cue interference effects were inversely correlated (r=−0.78, p<0.001), suggesting that attentional bias effects may be more strongly related to response facilitation in some individuals and to smoking cue interference in others. Behavioral data by smoking group and trial type are presented in Table 2.
Pleasantness ratings for smoking versus neutral images during the Affect Misattribution Procedure were also not significantly correlated with attentional bias score (r=−0.20, p=0.31), response facilitation (r=−0.14, p=0.48), or smoking cue interference (r=−0.00, p=0.99) based on Pearson’s correlations. Changes in craving were positively, but not significantly, related to attentional bias scores (ρ=0.32, p=0.29), response facilitation (ρ=0.32, p=0.28), and smoking cue interference (ρ=−0.31, p=0.29) based on Spearman’s correlations.
3.2. fMRI
The results of analyses of the MVPA neural correlates of attentional bias towards smoking cues are presented in Table 3.
Table 3.
Multivariate Pattern Analysis Correlates of Smoking Cue Attentional Bias
| A. Attentional bias towards smoking cues | ||||
|---|---|---|---|---|
| Region | # Voxels | x | y | z |
| negative | ||||
| L posterior insula/precentral gyrus | 6 | −4 6 | −4 | 9 |
| L middle frontal gyrus | 5 | −46 | 25 | 34 |
| 1 | −24 | 47 | 18 | |
| 1 | −38 | 34 | 18 | |
| R cerebellum | 4 | 14 | −51 | −44 |
| R anterior insula | 2 | 29 | 21 | 1 |
| 1 | 31 | −3 | 5 | |
| L fusiform gyrus | 2 | −32 | −50 | −15 |
| B. Attentional bias towards smoking cues interaction with group | ||||
| Region | # Voxels | x | y | z |
| positive | ||||
| R postcentral gyrus | 1 | 55 | −13 | 22 |
| negative | ||||
| L rostral anterior cingulate cortex | 13 | −8 | 45 | −4 |
| R rostral anterior cingulate cortex | 2 | 14 | 45 | 8 |
| L inferior frontal gyrus (pars triangularis) | 2 | −55 | −19 | 10 |
| L cerebellum | 2 | −16 | −46 | −43 |
| C. Bias attributed to response facilitation | ||||
| Region | # Voxels | x | y | z |
| positive | ||||
| L cerebellum | 10 | −1 4 | −56 | −23 |
| R superior parietal lobule | 3 | 21 | −45 | 64 |
| negative | ||||
| R insula | 3 | 35 | 8 | 15 |
| R inferior frontal gyrus (pars opercularis) | 1 | −34 | −41 | −17 |
| D. Bias attributed to response facilitation interaction with group | ||||
| Region | # Voxels | x | y | z |
| positive | ||||
| L middle temporal gyrus | 2 | −4 8 | −40 | 4 |
| R middle temporal gyrus | 1 | 58 | 9 | −13 |
| Mid-cingulate cortex | 1 | 8 | −26 | 43 |
| brainstem | 1 | 11 | −17 | −28 |
| negative | ||||
| R orbital frontal gyrus | 4 | 16 | 23 | −15 |
| E. Bias attributed to smoking cue interference | ||||
| Region | # Voxels | x | y | z |
| negative | ||||
| R postcentral gyrus | 5 | 17 | −35 | 67 |
| R temporal pole | 2 | 50 | 10 | −19 |
| L fusiform gyrus | 2 | −32 | −38 | −27 |
| L middle frontal gyrus | 1 | −50 | 22 | 23 |
| 1 | −27 | 63 | 9 | |
| L middle temporal gyru s | 1 | −64 | −43 | −2 |
| F. Bias attributed to smoking cue interference interaction with group | ||||
| Region | # Voxels | x | y | z |
| negative | ||||
| R frontal eye field | 4 | 35 | 3 | 44 |
| L frontal eye field | 2 | −41 | 4 | 52 |
3.2.1. Attentional bias towards smoking cues:
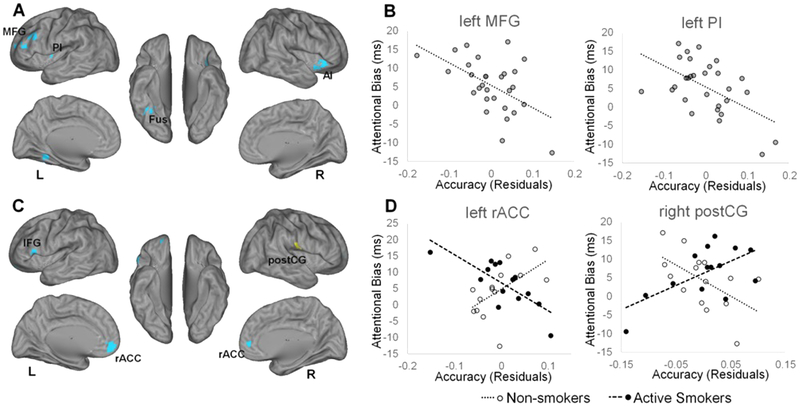
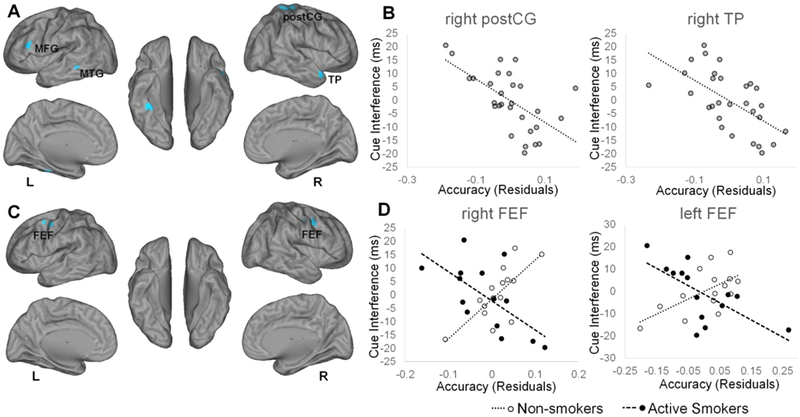
As our primary measure of bias towards smoking cues, we calculated the difference in RT between incongruent and congruent trials. We identified several brain regions in which accuracy was associated with decreased attentional bias across the full sample (Figure 3A–B; Table 3A). More critically, we also detected regions in which the relationship between accuracy and attentional bias significantly interacted with group. Specifically, we observed a more positive relationship in active smokers versus non-smokers between attentional bias and classification accuracy in the postcentral sulcus, whereas more negative relationships were detected in the rostral anterior cingulate cortex, left inferior frontal gyrus, and left cerebellum (Figure 3C–D; Table 3B).
Figure 3. Neural Correlates of attentional bias to smoking cues.
Regions for which multivariate pattern analysis accuracy was significantly associated with reaction time differences between incongruent and congruent trials, A) for all subjects, B) including scatter plots for visualization of representative relationships, and C) for the interaction with smoking group, D) including scatter plots for visualization of representative relationships, are displayed on left and right cerebral hemispheres. Accuracy scores represent residuals of cluster-average accuracy, adjusting for participant age and sex, and are therefore centered on zero. Yellow color represents positive relationships. L, left; R, right; AI, anterior insula; Fus, fusiform gyrus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PI, posterior insula; postCG, postcentral gyrus; rACC, rostral anterior cingulate cortex; postCG, postcentral gyrus.
3.2.2. Attentional bias attributed to response facilitation:
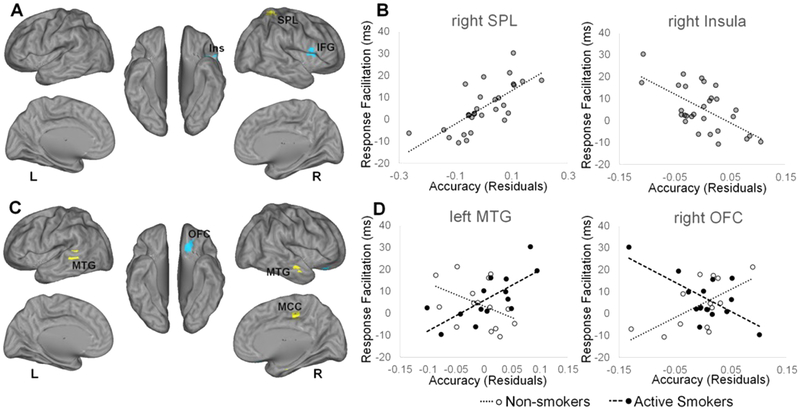
To measure facilitation of responding to targets appearing congruent with smoking images, we calculated the difference in RT between congruent and neutral trials. Across, the full sample, we identified regions in which classification accuracy was related to facilitation of responding to smoking congruent targets (Figure 4A–B; Table 3C). Of greater interest were differences in response facilitation-related accuracy detected between active smokers and non-smokers, which we found in the bilateral middle temporal gyrus, midcingulate cortex, brainstem, and right orbitofrontal gyrus (Figure 4C–D; Table 3D).
Figure 4. Neural correlates of facilitated responding to smoking cues.
Regions for which multivariate pattern analysis accuracy was significantly associated with reaction time differences between neutral and congruent trials, A) for all subjects, B) including scatter plots for visualization of representative relationships, and C) for the interaction with smoking group, D) including scatter plots for visualization of representative relationships, are displayed on left and right cerebral hemispheres. Accuracy scores represent residuals of cluster-average accuracy, adjusting for participant age and sex, and are therefore centered on zero. Yellow color represents positive relationships. Blue color represents negative relationships. L, left; R, right; IFG, inferior frontal gyrus; Ins, insula; MCC, mid-cingulate cortex; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; SPL, superior parietal lobule.
3.2.3. Attentional bias attributed to smoking cue interference:
As detailed above, attentional bias may also derive from difficulty in executing task goals in the presence of distracting smoking cues. Thus, we examined the smoking cue-related neural responses associated with incongruent versus neutral trial RTs. Among all subjects, smoking cue interference effects were directly of inversely related to activity in several regions (Figure 5A–B; Table 3E). However, group differences in the association between smoking cue interference and differential activation to smoking cues were only observed in the bilateral frontal eye fields, where a more positive relationship was observed for smokers versus non-smokers (Figure 5C–D; Table 3F).
Figure 5. Neural correlates of bias attributed to smoking cue interference.
Regions for which multivariate pattern analysis accuracy was significantly associated with reaction time differences between incongruent and neutral trials, A) for all subjects, B) including scatter plots for visualization of representative relationships, and C) for the interaction with smoking group, D) including scatter plots for visualization of representative relationships, are displayed on left and right cerebral hemispheres. Accuracy scores represent residuals of cluster-average accuracy, adjusting for participant age and sex, and are therefore centered on zero. Yellow color represents positive relationships. Blue color represents negative relationships. L, left; R, right; MFG, middle frontal gyrus; MTG, middle temporal gyrus; postCG, postcentral gyrus; TP, temporal pole; FEF, frontal eye field.
4. DISCUSSION
In this study, we identified the neural substrates of attentional bias to smoking-related cues using a multivariate analysis of fMRI data during a spatial cuing task. We explored several components of the attentional bias score, as well as the distinct neural correlates putatively related to nicotine conditioning among active smokers. The findings indicate that attentional bias in a laboratory spatial cuing task associates with differential patterns of activation in regions implicated in cognitive control, salience detection, reward processing, motor responding, and visual object recognition, which may contribute to response facilitation and cue interference components of attentional bias.
Among all subjects, regardless of nicotine use, greater attentional bias to smoking cues was associated with differential activation patterns in the left middle frontal gyrus, left posterior insula, right anterior insula, right cerebellum, and left fusiform gyrus. For each of the detected voxels and clusters, greater MVPA accuracy was associated with less attentional bias toward smoking cues. The left middle frontal gyrus and right anterior insula are regions involved in executive control (Vincent et al., 2008) and salience detection (Seeley et al., 2007), respectively, and may be involved in maintaining focus on relevant stimuli in the presence of distracting stimuli (Dosenbach et al., 2008), such as salient, but task-irrelevant, smoking cues. The posterior insula is implicated in the processing of visceral responses and representing internal motivational states (Gottfried et al., 2003). In the context of attentional bias, activation patterns in this region may relate to automatic visceral responses to smoking stimuli that function to facilitate or delay attentional shifts towards task-relevant stimuli. The left fusiform gyrus cluster was approximately at the location of the fusiform face area, which is selectively activated by faces compared with objects (Kanwisher et al., 1997). During the visual probe task, this process may contribute to the automatic diversion of subjects’ gaze during those trials that included images of people smoking.
Comparing active smokers to non-smokers in an attentional bias-by-group interaction analysis revealed a set of neural correlates of smoking cue attentional bias that may result from conditioned associations. The largest region of significant smoking group-specific MVPA accuracy consisted of two clusters spanning the left and right rostral anterior cingulate cortex (rACC)/ventromedial prefrontal cortex (vmPFC), a finding consistent with other drug cue-related attentional bias studies (Goldstein et al., 2009; Goldstein et al., 2007; Kilts et al., 2014). The rACC/vmPFC region is part of a dopamine circuit with the nucleus accumbens and ventral tegmental area (Der-Avakian and Markou, 2012; Haber and Knutson, 2010) and responds to subjectively rewarding stimuli (Haber and Knutson, 2010; Pujara et al., 2016; Vassena et al., 2014). This region has also been associated with cognitive control in the presence of conflicting emotional stimuli (Egner et al., 2008; Etkin et al., 2006; Kompus et al., 2009), suggesting that its role in drug cue attentional bias may involve regulating conditioned responses to emotionally-salient drug cues (Goldstein et al., 2007).
We also investigated the neural correlates of smoking cue facilitation of responding. Across all subjects, variation in MVPA accuracy within the cerebellum, posterior parietal lobe, right insula, and right inferior frontal gyrus explained variance in responses to congruent trials compared with neutral trials. The identified cerebellar cluster is highly correlated with the primary motor cortex and associated with motor execution (neurosynth.org, (Manto et al., 2012)), consistent with its association with the response facilitation component of attentional bias in the current study. The insula’s association with attentional bias to smoking cues has been previously attributed to its role in processing emotionally-salient stimuli (Janes et al., 2010). Although response facilitation by smoking cues did not significantly correlate with our primary measure of attentional bias, the fact that we detected significant response facilitation only in smokers (Figure 2), and that response facilitation was associated with a distinct set of neural correlates, suggests that variations in response facilitation processes may represent a functionally meaningful source of attentional bias for a subset of smokers. This assertion is further supported by our finding that response facilitation negatively correlated with smoking cue interference, indicating that these two mechanisms likely contribute independently to attentional bias to smoking cues. Differences between smokers and non-smokers in the neural correlates of response facilitation were observed in the bilateral middle temporal gyrus, mid-cingulate cortex, and brainstem (smokers > non-smokers), and in the right OFC (non-smokers > smokers). The lateral OFC is often engaged when responses for rewards require suppression of other reward-related cues (Elliott et al., 2000; Rogers et al., 1999), suggesting a potential role in facilitating responding among smokers, for whom smoking cues have acquired incentive salience. However, this region is also important for the fast recognition of visual stimuli (Bar et al., 2006; Kveraga et al., 2007), and may also integrate past affective experiences with current perceptions (Barrett and Bar, 2009); thus, the OFC may be differentially engaged among active smokers due to their more extensive familiarity and experience with smoking-related stimuli.
The neural correlates of attentional bias attributed to smoking cue interference across all subjects, quantified as RT differences between incongruent and neutral trials, included clusters in the postcentral gyrus, temporal pole, left fusiform gyrus, middle frontal gyrus, and middle temporal gyrus. Consistent with the positive correlations between the primary measure of attentional bias and smoking cue interference, there were some similarities in the fMRI analysis of these two behavioral measures, particularly in the middle frontal gyrus and fusiform gyrus. Thus, these regions, and perhaps their inferred roles in executive control and visual facial processing, respectively, may contribute to a mechanism of attentional bias related to engaging cognitive control over the automatic capture of attention by smoking cues. Group differences in smoking cue interference effects were present in the frontal eye fields, a region involved in saccadic eye movements and visual attention. In particular, the relationship was positive in non-smokers and negative in smokers, indicating a possible nicotine conditioning effect on visual attention systems.
The attentional bias effect based on reaction times was non-significantly larger in active smokers versus non-smokers. However, we observed faster responding to congruent versus incongruent trials in both the smokers and non-smokers. The finding of significant smoking-cue attentional bias even among non-smokers is consistent with several prior studies (e.g., (Chanon et al., 2010; Ehrman et al., 2002; Forestell et al., 2012)) and suggests that conditioned responses to smoking images can be acquired in the absence of personal nicotine use. In this sample, only heavier smokers (at least 10 cigarettes per day) demonstrated significantly greater attentional bias scores than non-smokers. It is possible that a more severe clinical sample or a larger sample size would have yielded more significant group differences in attentional bias. Nonetheless, the current study as well as many others that have reported small or absent group behavioral differences (i.e., (Goldstein et al., 2009; Goldstein et al., 2010; Luijten et al., 2012; Luijten et al., 2011; Nestor et al., 2011) detected neuroimaging group differences that indicate smoking-related effects on the neural correlates of attentional bias, possibly since similar behaviors may emerge from different neural mechanisms. Indeed, neuroimaging analyses enabled us to disentangle effects that are purely related to stimuli salience (whole group correlation), which are also observed in nonclinical samples, from conditioned drug effects or maladaptive neural processes among active smokers (interaction effects). Thus, the inclusion of a non-smoking sample represents a particular strength of the current study.
Several limitations of this study also deserve consideration. First, although we maximized our power by using correlational analyses, the study sample size is relatively small, raising concerns about undetected effects (Type II error), as well as the need for replication. Secondly, laboratory tasks likely provide imprecise correspondence to attentional bias in a real-world setting. For example, primary motor cortical activations may represent a task-specific finding relevant for manual button responses but not for real world drug-seeking behavior. Further efforts to include naturalistic imagery during fMRI sessions, as well as the evaluation of correspondence between attentional bias neuroactivation measures and drug use outcomes, are warranted. Additionally, although we attempted to interpret the roles of detected brain regions based on the known demands of the attentional bias task and the existing attentional bias literature, we cannot make conclusive inferences for these brain-behavior associations. Finally, we did not acquire eye tracking data during this study, which may have provided additional insight for interpreting the results.
4.1. Conclusions
We applied a multivariate approach to detecting the neural correlates of attentional bias to smoking cues. We report novel findings of the components of the neural response to smoking cues related to overall attentional bias, response facilitation, and smoking cue response interference. This study extends our knowledge of attentional processing in cigarette smokers by identifying a brain basis for behavioral responses to smoking-related images.
Highlights.
A multivariate analysis detected neural correlates of smoking cue attentional bias.
Analyses considered response facilitation and response interference by smoking cues.
We detected brain-behavioral correlates that were similar in smokers and non-smokers.
Smokers also recruited sets of brain regions that differed from non-smokers.
ACKNOWLEDGEMENTS:
The authors thank K. Cullen and R. Hay for technical assistance and M. Robertson for valuable comments on the manuscript draft.
FUNDING: This work was supported by Award Numbers UL1TR001111, KL2TR001109, and P60AA011605 (CAB), T32AA007573 (AE), and F32DA025442 (VC). The authors declare no competing financial interests related to the described work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafó MR, 2008. Attentional bias training and cue reactivity in cigarette smokers. Addiction (Abingdon, England) 103(11), 1875–1882. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E, 2006. Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America 103(2), 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, 1995. Impulsiveness, in: Costello CG (Ed.) Personality Characteristics of the Personality Disordered Client. Wiley, New York, pp. 91–118. [Google Scholar]
- Barrett L, Bar M, 2009. See it with feeling: affective predictions during object perception. Philos Trans R Soc Lond B Biol Sci 364(1521), 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanon VW, Sours CR, Boettiger CA, 2010. Attentional bias toward cigarette cues in active smokers. Psychopharmacology (Berl) 212(3), 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Schoenmakers TM, Field M, 2015. Less than meets the eye: reappraising the clinical relevance of attentional bias in addiction. Addict Behav 44, 43–50. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW, 2010. Mechanisms of Attentional Biases towards Threat in the Anxiety Disorders: An Integrative Review. Clin Psychol Rev 30(2), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH, 2002. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug and alcohol dependence 68(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG, 2007. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology 192(4), 499–510. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A, 2012. The Neurobiology of Anhedonia and Other Reward-Related Deficits. Trends Neurosci 35(1), 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domaradzka E, Bielecki M, 2017. Deadly Attraction – Attentional Bias toward Preferred Cigarette Brand in Smokers. Front Psychol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE, 2008. A dual-networks architecture of top-down control. Trends in Cognitive Sciences 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J, 2008. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex 18(6), 1475–1484. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP, 2002. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend 67(2), 185–191. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD, 2000. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex 10(3), 308–317. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW, 2010. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry 67(6), 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51(6), 871–882. [DOI] [PubMed] [Google Scholar]
- Etter JF, Le Houezec J, Perneger TV, 2003. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28(2), 359–370. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM, 2008. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 97(1–2), 1–20. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP, 2005. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol and alcoholism (Oxford, Oxfordshire) 40(6), 504–510. [DOI] [PubMed] [Google Scholar]
- Field M, Munafó MR, Franken IH, 2009. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological bulletin 135(4), 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestell CA, Dickter CL, Wright JD, Young CM, 2012. Clearing the smoke: parental influences on non-smokers’ attentional biases to smoking-related cues. Psychol Addict Behav 26(3), 638–643. [DOI] [PubMed] [Google Scholar]
- Franken IH, Kroon LY, Wiers RW, Jansen A, 2000. Selective cognitive processing of drug cues in heroin dependence. Journal of psychopharmacology (Oxford, England) 14(4), 395–400. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND, 2009. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A 106(23), 9453–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND, 2007. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 144(4), 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND, 2010. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A 107(38), 16667–16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ, 2003. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301(5636), 1104–1107. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, 2012. Multivariate pattern analysis of fMRI: The early beginnings. Neuroimage 62(2), 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Nichols T, Huang C, Yu Y, Lu Z, Knickmeyer RC, Feng Q, Zhu H, 2015. FVGWAS: Fast voxelwise genome wide association analysis of large-scale imaging genetic data. Neuroimage 118, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ, 2010. Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology 35(12), 2339–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BT, Bruce G, Livingstone S, Reed E, 2006. Alcohol-related attentional bias in problem drinkers with the flicker change blindness paradigm. Psychol Addict Behav 20(2), 171–177. [DOI] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Smith H, Copley N, 2003. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction (Abingdon, England) 98(2), 235–244. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM, 1997. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. The Journal of Neuroscience 17(11), 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, James GA, 2014. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology 39(5), 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L, 2009. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett 467(2), 76–80. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P, 2006. Information-based functional brain mapping. Proc Natl Acad Sci U S A 103(10), 3863–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M, 2007. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci 27(48), 13232–13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp OV, Derakshan N, 2005. Attentional bias to pictures of fear-relevant animals in a dot probe task. Emotion 5(3), 365–369. [DOI] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, Smits M, Pepplinkhuizen L, Franken IHA, 2012. Brain Activation Associated with Attentional Bias in Smokers is Modulated by a Dopamine Antagonist. Neuropsychopharmacology 37(13), 2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, Franken IH, 2011. Neurobiological substrate of smoking-related attentional bias. Neuroimage 54(3), 2374–2381. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P, 1986. Attentional bias in emotional disorders. J Abnorm Psychol 95(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, Habas C, Hagura N, Ivry RB, Mariën P, Molinari M, Naito E, Nowak DA, Ben Taib NO, Pelisson D, Tesche CD, Tilikete C, Timmann D, 2012. Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. Cerebellum 11(2), 457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJM, Smits M, Franken IHA, 2013. Individual Differences in Anterior Cingulate Activation Associated with Attentional Bias Predict Cocaine Use After Treatment. Neuropsychopharmacology 38(6), 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM, 2006. Attentional bias predicts heroin relapse following treatment. Addiction (Abingdon, England) 101(9), 1306–1312. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA, 2005. Impulsive responding in alcoholics. Alcoholism, clinical and experimental research 29(12), 2158–2169. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, 2002. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol 16(4), 385–392. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J, 2003. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction (Abingdon, England) 98(6), 825–836. [DOI] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP, 2005. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology 180(2), 333–341. [DOI] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H, 2011. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 56(4), 2258–2275. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV, 2006. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences 10(9), 424–430. [DOI] [PubMed] [Google Scholar]
- Parkhurst D, Law K, Niebur E, 2002. Modeling the role of salience in the allocation of overt visual attention. Vision Res 42(1), 107–123. [DOI] [PubMed] [Google Scholar]
- Payne BK, McClernon FJ, Dobbins IG, 2007. Automatic affective responses to smoking cues. Experimental and Clinical Psychopharmacology 15(4), 400–409. [DOI] [PubMed] [Google Scholar]
- Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M, 2016. Ventromedial Prefrontal Cortex Damage Is Associated with Decreased Ventral Striatum Volume and Response to Reward. J Neurosci 36(18), 5047–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW, 1999. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19(20), 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemink E, van den Hout MA, Kindt M, 2007. Selective attention and threat: quick orienting versus slow disengagement and two versions of the dot probe task. Behav Res Ther 45(3), 607–615. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran R, Thordarson DS, Rachman S, 1996. Thought-action fusion in obsessive compulsive disorder. Journal of Anxiety Disorders 10(5), 379–391. [Google Scholar]
- Spielberger CD, Gorsuch RR, Luchene RE, 1970. State-Trait Anxiety Inventory. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA, 2008. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology 33(4), 827–836. [DOI] [PubMed] [Google Scholar]
- Vassena E, Krebs RM, Silvetti M, Fias W, Verguts T, 2014. Dissociating contributions of ACC and vmPFC in reward prediction, outcome, and choice. Neuropsychologia 59, 112–123. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL, 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology 100(6), 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C, 2000. Determinants and effects of attentional bias in smokers. Psychol Addict Behav 14(2), 111–120. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH, 2003. Attentional Bias Predicts Outcome in Smoking Cessation. Health Psychol 22(4), 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Lohoff FW, Ehrman R, O’Brien CP, Childress AR, Franklin TR, 2014. Neural correlates of attentional bias for smoking cues: modulation by variance in the dopamine transporter gene. Addict Biol 19(2), 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Ibrahim JG, Tang N, Rowe DB, Hao X, Bansal R, Peterson BS, 2007. A Statistical Analysis of Brain Morphology Using Wild Bootstrapping. IEEE Trans Med Imaging 26(7), 954–966. [DOI] [PMC free article] [PubMed] [Google Scholar]