Abstract
Purpose.
Optimal meibomian gland (MG) function is critically important for the health and wellbeing of the ocular surface. We hypothesize that low oxygen (O2) conditions promote the function of human MG epithelial cells (HMGECs) and that human MGs exist in a relatively hypoxic environment. The purpose of this study was to test our hypotheses.
Methods.
We used human and mouse eyelid segments, and immortalized human MG epithelial cells (IHMGECs) in our studies. To evaluate oxygen (O2) levels in the mouse MG and vicinity, we injected pimonidazole (pimo), a hypoxia marker, before sacrifice. Human eyelid samples were stained with the hypoxia marker glucose transporter 1 (Glut-1). To determine the effect of low O2 levels on IHMGECs, we cultured cells under proliferating and differentiating conditions in both normoxic (21% O2) and hypoxic (3% O2) conditions for 5 to 15 days. IHMGECs were evaluated for cell number, neutral lipid content, lysosome accumulation, expression of biomarker proteins and DNase II activity.
Results.
Our results demonstrate that human and mouse MGs, but not the surrounding connective tissue, exist in a relatively hypoxic environment in vivo. In addition, our findings show that hypoxia does not influence IHMGEC numbers in basal or proliferating culture conditions, but does stimulate the expression of SREBP-1 in differentiating IHMGECs. Hypoxia also significantly increased DNase II activity, and apparently IHMGEC terminal differentiation.
Conclusions.
Our results support our hypotheses, and indicate that relative hypoxia promotes MG function.
Keywords: hypoxia, meibomian gland, pimonidazole, glucose transporter 1, DNase II
Introduction
Meibomian glands (MGs) are large sebaceous glands (SGs) located in the eyelids. Normally, MGs produce abundant lipids (e.g. cholesterol and phospholipids) that accumulate in lysosomes, are secreted in a holocrine fashion into lateral ducts, and are then released onto the ocular surface.[1, 2] This lipid secretion (i.e. meibum) serves to provide a clear optical surface for the cornea, interfere with bacterial colonization, and minimize tear overflow.[1, 2] Meibum also increases the stability and decreases the evaporation of the tear film, thereby playing an essential role in ocular surface health.[1, 3-5]
In contrast, MG dysfunction (MGD), and the consequent meibum deficiency, destabilizes the tear film and increases its osmolarity and evaporation.[1, 2, 6-8] In fact, MGD is the leading cause of DED,[2] which afflicts countless people throughout the world, and is one of the most frequent causes of patient visits to eye care practitioners. [9] A recent study found that over 85% of clinically-identified DED patients exhibited signs of MGD.[10]
The most common cause of human MGD is excretory duct obstruction, due to decreased meibum quality and hyperkeratinization of the terminal duct epithelium. [2] This obstruction, which often occurs during aging, androgen deficiency and 13-cis retinoic acid (RA) use,[11-14] may lead to cystic dilatation of MG ducts, atrophy and loss of MG epithelial cells (MGECs), and MG dropout.[2, 15] There is no global cure for MGD.[16]
We hypothesize that there is another major risk factor that promotes the development of human MGD. More specifically, we hypothesize that loss of the relative hypoxic status of the MG plays a major role in the pathogenesis of MGD, and that restoration of this hypoxic environment will serve a treatment for MGD. In support of our hypothesis, investigators have reported that the mouse MG environment, like that of human skin SGs,[17-20] is one of the most hypoxic areas in the body.[21, 22]
Accordingly, to begin to test our hypothesis, we sought to: first, determine whether human MGs, like those of mice, exist in a relatively hypoxic environment in vivo; and second, to examine whether low O2 conditions promote the function of human MG epithelial cells (HMGECs) in vitro.
Materials and Methods
Human and mouse tissues
Human eyelids were obtained after ectropion surgeries from patients (2 women, 1 man; age range, 70–82 years) without MGD, and immediately frozen in optimal cutting temperature compound (OCT, Tissue-Tek, Sakura USA, Torrance, CA). All tissues were deidentified, according to Health Insurance Portability and Accountability Act regulations. The use of human tissues was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary and Schepens Eye Research Institute (SERI) and adhered to the tenets of the Declaration of Helsinki.
C57BL/6J mice were bred in the SERI Animal Facility. The experiments with these mice were approved by the SERI Institutional Animal Care and Use Committee (IACUC) and adhere to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. For this study we administered 100 mg/kg Pimonidazole (pimo) HCl (Hypoxyprobe Inc., Burlington, MA, USA) intraperitoneally two hours before sacrifice. Eyelid tissues were fixed in 4% paraformaldehyde (PFA) for 24 hours, frozen and later sectioned (15 μm) with a cryostat.
Terminology
There are different definitions for hypoxia. According to various sources, the terms physiological, modest, moderate and severe hypoxia and anoxia are used to designate 10–14, 2.5, 0.5, 0.1 and 0% O2, respectively.[19]We use the terminology “relative hypoxia,” because although we currently do not have a device to measure the oxygen concentration in human or mouse tissues, pimo stains tissues that harbor oxygen levels below 1.3%. [23]).
Immunofluorescence staining
Human and mouse eyelid sections were fixed with cold methanol for 15 minutes at −20°C. Following three phosphate-buffered saline (PBS) rinses for 5 minutes each, samples were incubated with 2% bovine serum albumin (BSA, Sigma-Aldrich Corp., St. Louis, MO) in PBS for 60 minutes. The slides were then placed overnight at 4°C in a moist chamber with antibodies specific for pimo (1 : 50; Hypoxyprobe Inc.), Glucose transporter-1 (Glut-1, 1:500, ab652, Abcam, Cambridge, MA), human CD31 (1:500, ab24590, Abcam), Notch1 (1: 10, bTAN20, Developmental Studies Hybridoma Bank, University of Iowa), or cytokeratin 14 (CK14, 1:500, ab181595, Abcam). After 3 additional PBS rinses, donkey anti-rabbit (1:200, ab150075, Abcam,), donkey anti-mouse (1:200, 2492098, EMD Millipore, Temecula, CA), or goat anti-rat (1: 200, a10522, Thermo Fisher Scientific, Grand Island, NY) secondary antibodies were applied the sections for 1 hour at room temperature. The primary antibodies were replaced with mouse or rabbit IgG as negative controls.
Immunohistochemistry
Immunohistochemistry was performed using a mouse monoclonal IgG1 specific for pimo (1: 50, Hypoxyprobe Inc.) and an anti-FITC secondary antibody (1:100, Hypoxyprobe Inc.). For visualization, 3,3′-diaminobenzidine (DAB, Vector) was used, followed by counterstaining with hematoxylin, dehydration and mounting. As a negative control, the primary antibody was replaced with PBS, following the manufacturer’s protocol. For comparison, mouse lacrimal glands (LGs) were processed and stained the same way.
Cell Culture
All cell culture experiments (n = 3 wells/treatment) were repeated at least 3 times.
For cell proliferation studies immortalized (I) HMGECs[24], an authenticated cell line[25], were placed in basal keratinocyte serum-free medium (KSFM), or KSFM with 5 ng/mL epidermal growth factor (EGF) and 50 μg/mL bovine pituitary extract (BPE) (Thermo Fisher Scientific)[26] to stimulate cell proliferation (proliferation medium, (PM)) in 35mm polystyrene-coated dishes (Corning, Falcon). Cells were cultured for 5 days under 21% (normoxic) or 3% (hypoxic) O2 conditions.
The rationale for using 3% O2 in our experiments was to mimic the environment in vivo. In brief, the oxygen percentage in sebaceous glands is reported to be 0.1–1.3%.[20] Further, the O2 saturation in adult epidermis ranges from 0.5 to 5%.[19]Thus, we used 3% O2 to approximate the level in the skin and sebaceous gland area.
To achieve these low O2 levels, hypoxia chambers were prepared according to a published protocol.[27] In brief, cell culture dishes were inserted into 1,000-ml Nalgene PC Straight-Side Wide-Mouth Jars (Thermo Fisher Scientific, Waltham, MA), which were then flushed with a premade 3% O2, 5% CO2 and 92% N2 gas mixture (Linde) for 2 minutes.. Size 15D silicone rubber stoppers and silicone vacuum grease were used to prevent gas leakage. [27] Following cell media changes, the jars were reflushed with the gas mixture.
For cell differentiation experiments, IHMGECs were maintained in proliferating conditions until reaching 60% to 70% confluence, and then placed into differentiation medium (DM) composed of a 50:50 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 (DMEM/F12, Mediatech, Inc, a Corning Subsidiary, Manassas, VA), and supplemented with 10% fetal bovine serum[28] (FBS, Thermo Fisher Scientific).[29] Cells were cultured in 35mm polystyrene-coated dishes (Corning, Falcon ®) in the presence or absence of 10 μg/ml azithromycin (AZM, Santa Cruz Biotechnology, Dallas, TX) under normoxic or hypoxic conditions for 5 to 15 days.
After experimental termination, cells were counted with a hemocytometer.
Lipid and lysosome analyses
Cells were then stained for lysosome accumulation using LysoTracker® blue DND-22 and for neutral lipid with HCS LipidTOX™ Green neutral lipid stain.[30] Slides were viewed using an Eclipse E800 fluorescent microscope and images captured with NIS-Elements Basic Research software, version 4.2 (Nikon Instruments, Melville, NY,).[29, 31-34] Intensities were quantified using ImageJ (http://rsb.info.nih.gov/ij).
Western blot procedures
After 5 days of culture in various media, cells were lysed for Western Blot according to our published protocol.[33] Cell lysates were evaluated by primary antibodies specific to proliferating cell nuclear antigen (PCNA, D3H8P, 1: 1000, Cell Signaling Technology, Danvers, MA), sterol regulatory element-binding protein 1(SREBP-1, C-20, 1:500, Santa Cruz Biotechnology), lysosomal-associated membrane protein 1 (Lamp-1, H4A3, 1:500, Developmental Studies Hybridoma Bank), DNase II (1: 1000, Sigma-Aldrich) or β-actin (1:10,000, Cell Signaling Technology). Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat anti-rabbit or goat anti-mouse IgG (both 1:5000, Sigma-Aldrich Corp.). Densitometry was performed by using ImageJ (http://rsb.info.nih.gov/ii).
Single radial enzyme diffusion assay
The DNase II activity of cell lysates and cell culture media was determined by single radial enzyme diffusion assay, according to a published protocol.[35] Agarose gels were prepared by mixing together 1% (w/v) agarose (Boston Bioproducts, Ashland, MA), 0.05 mg/ml Type III salmon sperm DNA (Sigma-Aldrich), 5 μg/ml ethidium bromide (Boston Bioproducts), 0.5 M sodium acetate buffer (pH 4.7) and 10 mM EDTA (Boston Bioproducts). Then 5 ml of the mixture was poured into 60 mm polystyrene-coated dishes (Corning, Falcon) and the gel was allowed to solidify. Cylindrical wells (radius, 1.5 mm) were made with a disposable punch (Acuderm Inc., Ft. Lauderdale, FL).
To obtain cell lysates, IHMGECs were exposed to 70 μl M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific). For medium analysis, differentiating culture medium was replaced the day before collection with 10% FBS that was heat inactivated at 70°C for 30 minutes to remove the heat-stable nucleases in the serum.[36] Media samples were then obtained, centrifuged for 3 minutes at 2,500 RPM, and the resulting supernatants were assayed for activity.
Fresh cell lysates and cell culture supernatants were assayed by loading 1 μl aliquots in the cylindrical wells in the agarose gel. After incubation for 4-48 hours at 37°C, the area of the dark circle was measured under an UV transilluminator at 312 nm. The area was normalized to cell number.
Statistical analysis
The significance of the differences between groups was determined by using Student’s unpaired, two-tailed t-test (Prism 5, GraphPad Software, Inc., La Jolla, CA, USA). Values with p < 0.05 were considered statistically significant.
Results
Is the human MG environment relatively hypoxic in vivo?
To determine whether human MGs exist in a relatively hypoxic environment in vivo, we examined eyelid tissues for Glut-1 staining. Glut-1 is a widely utilized hypoxia biomarker in human tissues.[37]. As a positive control for the detection of a hypoxic environment, we analyzed mouse eyelids for Glut-1 and pimo presence. Pimo is a common marker used in vivo to stain hypoxic tissues.[38, 39]
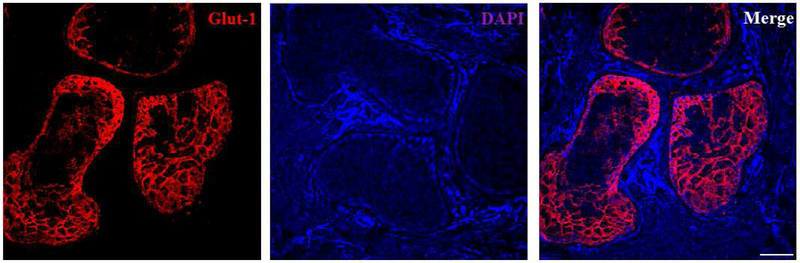
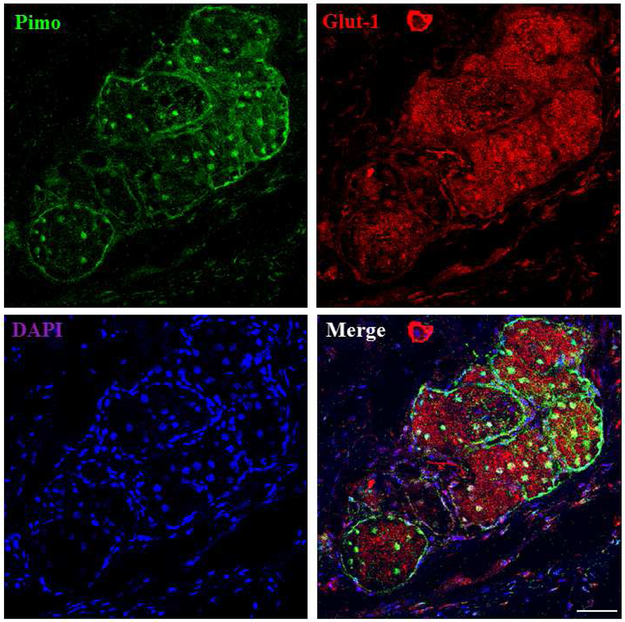
Our results demonstrate that human MGs, but not the surrounding connective tissues, express abundant Glut-1 (Figure 1). The pattern of Glut-1 staining appeared membranous in both human (Figure 1) and mouse (Figure 2) MGs. We tried different Glut-1 antibodies, and they all seemed to be more specific in human samples than in those of mice.
Figure 1.
Glut-1 staining inhuman MGs. High intensity Glut-1 staining is found in human MG acini, but not in the peripheral connective tissues. Data from one experiment are shown as a representative of three independent repeats. Scale bar = 50 μM
Figure 2.
The correlation of pimo and Glut-1 staining in mouse MGs. High intensity staining of pimo and Glut-1 is found in mouse MG acini. Pimo staining is found both in the nucleus and cytoplasm, and Glut-1 staining shows a membranous pattern. Data from one experiment are shown as a representative of three independent studies. Scale bar = 50 μM.
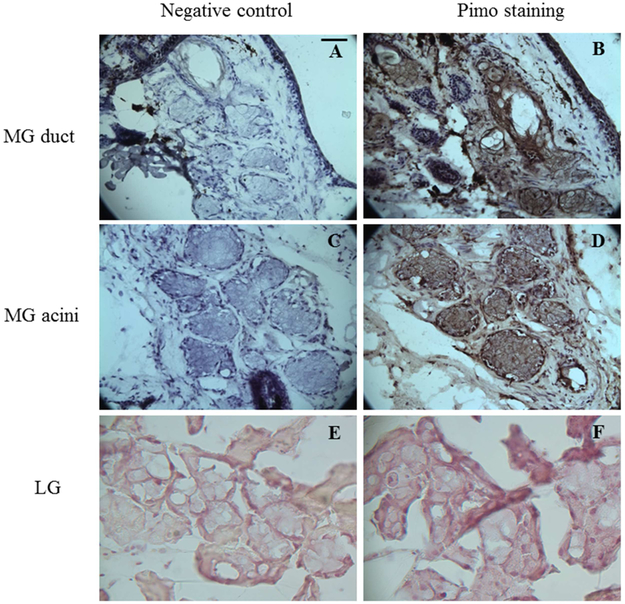
In the mouse eyelids, we observed intense pimo staining in the nucleus and cytoplasm of ductal and acinar epithelial cells (Figure 2, Figures 3B and 3D), We also found pimo staining in adjacent SGs within the hair follicle complex, as has previously been reported by others.[18, 40] The mouse LGs and connective tissue adjacent to the mouse MGs were negative for pimo staining (Figure 3F).
Figure 3.
Pimo staining of mouse MGs and LGs. Both the MG duct (arrows) and acini (notched arrows) show high intensity of Pimo, as do the mouse SGs (* in B). LG shows no staining of pimo. Data from one experiment are shown as a representative of three studies performed under the same conditions. Scale bar = 5 μM.
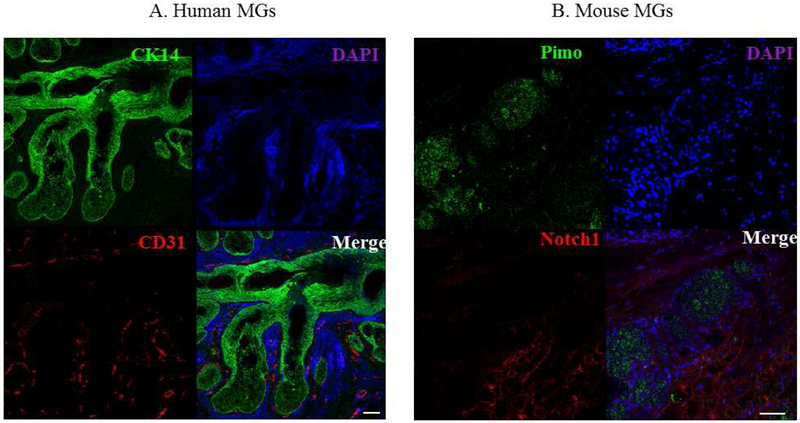
To examine the location of blood vessels, the O2 source, relative to human and mouse MGs, we used CD31 and Notch 1 as biomarkers for human and mouse vascular endothelial cells, respectively. CD31 is an endothelial marker commonly used for vessel staining[41], and Notch1 is a marker for arteries.[41] We also used CK14 staining to identify HMGECs [42, 43], and pimo staining to highlight the acini and ducts in the mouse MGs. Our results show that blood vessels are located around the acini and ducts of MGs in both human and mouse samples, but not inside the MGs (Figure 4).
Figure 4.
The vasculature of human and mouse MGs. (A) CK14 (green) and vessels (red) staining in human MGs. (B) Pimo (green) and vessels (red) staining in mouse MGs. The experiment was repeated three times. Scale bar = 50 μM.
Effect of low O2 levels on IHMGEC proliferation
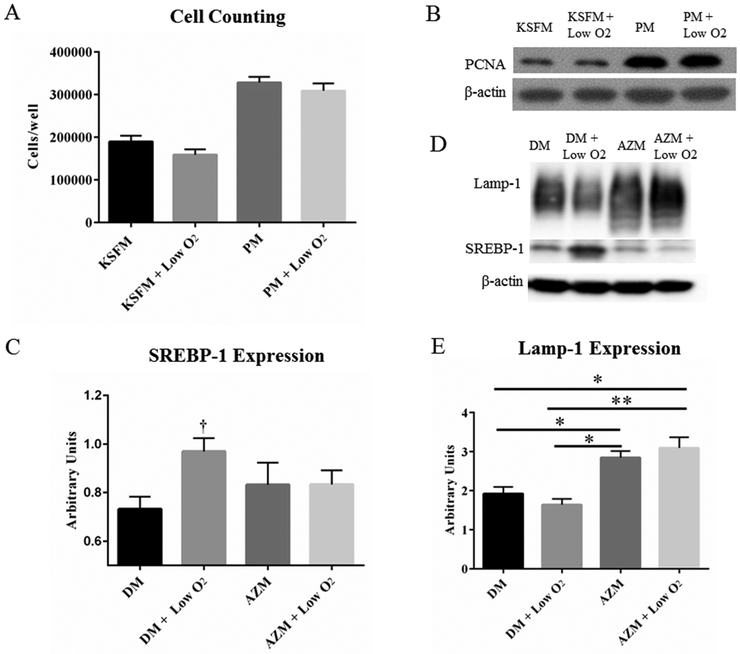
To determine whether a low O2 environment impacts the growth of IHMGECs, cells were cultured in either basal KSFM or EGF- and BPE-supplemented media for 5 days under normoxic and hypoxic conditions. Following experimental completion, cells were counted and then processed for PCNA analysis by Western blot. PCNA is an indicator of cell cycle entry. [44]
Our findings show that exposure to a 3% O2 environment had no impact on cell proliferation, as indicated by cell counts and PCNA expression (Figures 5A and B). This lack of effect was found in both cells cultured under basal and proliferating conditions (Figure 5A and B).
Figure 5.
Effects of low O2 on IHMGEC proliferation and differentiation. Low O2 treatment did not impact the cell growth (A) or the expression of PCNA (B). SREBP-1, but not Lamp-1, expression is significantly increased in a hypoxic environment (C and D, † p < 0.05, n = 3 experiments). AZM with or without low O2 significantly increased the levels of Lamp-1 protein. (E, * p < 0.05, ** p< 0.01, n=3 experiments). DM = Differentiation Medium (DMEM/F12 + 10% FBS). PM = Proliferation Medium (KSFM + EGF/BPE).
Influence of low O2 levels on IHMGEC differentiation
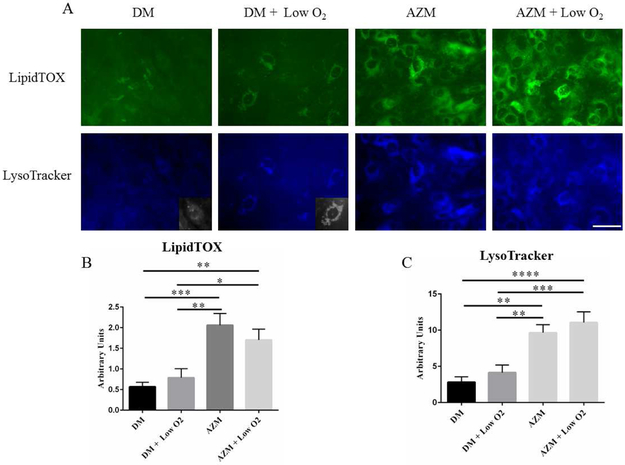
To test whether hypoxia influences IHMGEC differentiation, we cultured cells in differentiating medium in the presence or absence of AZM for 5 days. AZM, in turn, is known to facilitate IHMGEC differentiation and promote the accumulation of neutral and polar lipids and lysosomes; this cellular response appears to reflect a phospholipidosis effect.[32, 33]We then processed cells for the identification of neutral lipids with LipidTOX and lysosomes with LysoTracker. We also assess the expression of Lamp-1, a lysosome marker [45], and SREBP-1, a key regulator of lipid synthesis [46].
Our results indicate that hypoxia appears to increase the size of lysosomes (Figure 6A insets), but does not alter the overall lysosome or neutral lipid staining or the Lamp-1 levels (Figure 5D). However, 3% O2 exposure significantly increased SREBP-1 expression (Figure 5C). As a comparison, AZM significantly increased both lysosome and neutral lipid staining, and the combination of hypoxia with AZM elicited no further effect (Figure 6B and C).
Figure 6.
Influence of low O2 on lipid and lysosome accumulation in IHMGECs. IHMGECs were treated were cultured in normoxic (21% O2) and hypoxic (3% O2) conditions environments in the presence or absence of AZM and then stained for neutral lipids (LipidTox Neutral Green) and lysosomes (LysoTracker blue). (* p < 0.05, ** p < 0.01, *** p< 0.001, **** p < 0.0001, one-way ANOVA, n = 3 experiments). Inserts show a section of the LysoTracker staining in black and white. Scale bar = 50 μM.
Impact of low O2 levels on the terminal differentiation of IHMGECs
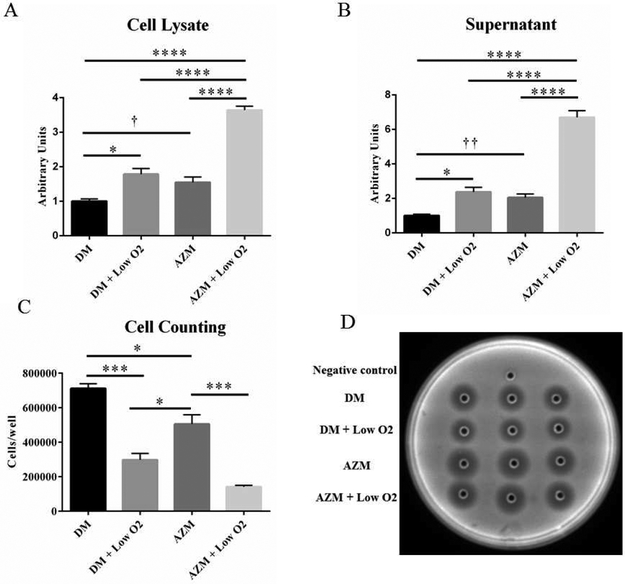
To investigate the effect of hypoxia on IHMGEC terminal differentiation (i.e. holocrine secretion), which is characterized by cell breakup and release of cellular content, we measured the activity of DNase II in cell lysates and medium after culturing cells in different conditions. DNase II activity is closely related to the terminal differentiation of SG epithelial cells and in holocrine secretion, [47, 48] We also examined DNase II protein expression in cell lysates by Western blot.
To obtain cell lysates, our experimental design involved culturing IHMGECs under proliferating conditions until reaching 60 to 70% confluence, then switching to differentiation media with or without 10μg/ml AZM[29] in normoxic or hypoxic chambers for 15 days. To collect cell culture media, we cultured the cells as described above for 14 days, then replaced the FBS with heat-inactivated FBS for 1 day, as described in the Methods.
Our results show that hypoxia significantly increased the activity of DNase II in both cell lysates and media supernatants (Figures 7A and B). The influence of low O2 appeared to be similar to that of AZM alone, while the combination of hypoxia and AZM had an additive effect on DNase II activity. These changes were paralleled a decrease in IHMGEC number after 15 days (Figure 7c). Neither hypoxia nor AZM impacted the expression of DNase II protein (data not shown).
Figure 7.
Impact of low O2 on DNase II activity and cell number in IHMGECs under differentiating conditions. DNase II activity is significantly increased by low O2 treatment in both cell lysate (A) and supernatant (B). Low O2 also significantly decreased the cell number under differentiating condition (C). DM = Differentiation Medium (DMEM/F12 + 10% FBS). (* p < 0.05, *** p< 0.001, **** p < 0.0001, one-way ANOVA. f p < 0.05, †† p < 0.01, two-tailed t-test. n = 3 experiments)
Discussion
Our results show that human and mouse MGs, but not the surrounding connective tissue, exist in a hypoxic environment in vivo. In addition, our findings demonstrate that low O2 does not alter IHMGEC numbers in basal or proliferating culture conditions, but does enhance lysosome size and stimulate SREBP-1 expression in differentiating IHMGECs. Hypoxia also significantly increases DNase II activity, and apparently the terminal differentiation of IHMGECs. These data support our hypothesis that a relatively hypoxic environment is beneficial for MG function.
Our hypothesis that relative hypoxia is physiologically beneficial for the MG may appear counterintuitive, because the lipid synthetic process would seem to require a considerable supply of O2.[2] Further, there are heat-related therapies designed to increase eyelid blood flow in an attempt to alleviate MGD.[49] Such an increase in circulation would deliver more O2 to the tissue. And the eyelid telangiectasia that often follows MGD could theoretically be a manifestation of the body’s attempt to increase the local blood flow and O2 supply to the MG and its environment.
However, in support of our hypothesis, investigators have reported that the environment of human skin SGs in general,[18-21] and mouse MGs in particular,[22,23] are some of the most hypoxic areas in the body. This observation is consistent with the location of MG-associated vasculature, which, as we show in this study, is situated beyond the basement membrane of MG acini. This distance, by Krogh’s law, would decrease the amount of O2 diffusing from blood vessels to the MG. This relative hypoxia makes sense, given that MG acinar epithelial cells accumulate lipids primarily in lysosomes,[32] rather than the cytoplasm as in adipocytes. Indeed, hypoxia can lead to an up-regulation of genes that function in lysosome and lipid metabolism.[50] Similarly, we discovered hypoxia promotes an increase in lysosome size and an upregulation of SREBP-1, and these low O2 effects are analogous to those found in other tissues.[50-53] Thus, it appears that MG epithelial cells do not require much O2 to produce and release lipids. As these cells mature, they move further away from the O2 source and lose mitochondria. This process is different than found with other lipid-producing cells (i.e. adipocytes).[2]
Another consideration is that acinar atrophy in human MGD is associated with a thickening of the basement membrane. [2] This anatomical development may represent a compensatory response to decrease O2 delivery from adjacent vessels and to restore the relative hypoxia needed for optimal MG function. Most importantly, low O2 concentrations have been found to allow stem cells to maintain their stemness, and may also be useful in maintaining and expanding a population of cells that is in limited supply.[54, 55] Such a process would be critical for MG regeneration after dropout in vivo. [56]
Of interest, it has been found that basal blood flow and transcutaneous partial pressure of O2 in human skin may increase with age. [57] This loss of hypoxic environment, if it occurs in human eyelids, may contribute to the heightened prevalence of MGD during aging. [2] Thus, strategies to create a hypoxic MG environment may serve as new and effective therapies for the treatment of human MGD. Such an approach has been shown to benefit other physiological systems in mice, with low O2 exposure inducing heart regeneration, reversing neurodegeneration, and ameliorating graft-versus-host disease.[56, 58, 59]
It is possible that hypoxia may also play a role in the reported efficacy of Intense Pulsed Light (IPL) for MGD treatment.[60] IPL is known to significantly reduce telangiectasia,[61] and the closure of these excessive vessels may help the MG to restore its hypoxic environment and normal function.
We found that hypoxia significantly increases the activity of DNase II in IHMGEC lysates and culture medium. DNase II is a lysosomal deoxyribonuclease [62], that mediates a unique mode of programmed cell death as part of holocrine secretion in SGs.[48] DNase II is usually activated by acidic conditions (pH 4.5-5.5),[63] where it translocates to the nucleus and initiates the DNase II-dependent cell death.[48, 64] The hypoxic state is known to modulate intracellular and intravesicular pH[65, 66], decrease lysosomal membrane stability and cause the intracellular release of enzymes.[67-69] Thus, it is possible that DNase II is stimulated and translocated because of hypoxia-induced intracellular acidosis and lysosomal disintegration. This finding could explain why MG epithelial cells undergo holocrine secretion in near the lateral duct, where they are furthest away from the O2 source at the acinar periphery. [2]
AZM also enhanced DNase II activity, but the mechanism(s) is likely different than that of hypoxia. AZM increases the lysosomal pH, impairs lysosomal function, and induces phospholipidosis.[32, 70] Theoretically, AZM should decrease the activity of lysosomal enzymes.[70] However, our finding is consistent with the effects of AZM on other lysosomal enzymes.[71] It is assumed that this function has no direct relation to the lysosomal phospholipidosis,[71] and its mechanism remains to be clarified.
Quite notable was our discovery that hypoxia and AZM stimulate the IHMGEC release of DNase II into the culture medium. During holocrine secretion, the entire cell content is ejected, including DNase II-loaded lysosomes. Therefore, the increased DNase II activity in cell culture medium likely represents an increased cell breakup, which is consistent with the decreased number of differentiated cells following hypoxia or AZM exposure. We conclude that either a hypoxic environment or AZM alone promotes the terminal differentiation of HMGECs, and that the combination of a hypoxia and AZM treatment has an additive effect.
In summary, we discovered that the MG is a hypoxic tissue, and hypoxia is beneficial for the function of IHMGECs. Hypoxia may be a breath of fresh air for the treatment of MGD in future.
Supplementary Material
Acknowledgments
We thank Dr. Petr Baranov, Donald Pottle and Bianai Fan (Boston, MA) for providing technical support.
Disclosure of Funding: This research was supported by NIH grants R21028653 and P30EY003790, the Margaret S. Sinon Scholar in Ocular Surface Research fund, the David A. Sullivan laboratory fund, the Yong Zhang Research Fund, and the One-Hundred-Talent Scholarship Program of Peking Union Medical College Hospital.
Footnotes
Disclosure
A provisional patent has been filed around this technology. The intellectual property for this application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investigative ophthalmology & visual science. 2011;52:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative ophthalmology & visual science. 2011;52:1938–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. The ocular surface. 2004;2:149–65. [DOI] [PubMed] [Google Scholar]
- [4].Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Experimental eye research. 2004;78:347–60. [DOI] [PubMed] [Google Scholar]
- [5].McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. The ocular surface. 2003;1:97–106. [DOI] [PubMed] [Google Scholar]
- [6].Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investigative ophthalmology & visual science. 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Investigative ophthalmology & visual science. 2011;52:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Investigative ophthalmology & visual science. 2011;52:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. The ocular surface. 2017;15:334–65. [DOI] [PubMed] [Google Scholar]
- [10].Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–8. [DOI] [PubMed] [Google Scholar]
- [11].Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. The Journal of clinical endocrinology and metabolism. 2000;85:4874–82. [DOI] [PubMed] [Google Scholar]
- [12].Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. The Journal of clinical endocrinology and metabolism. 2000;85:4866–73. [DOI] [PubMed] [Google Scholar]
- [13].Ding J, Kam WR, Dieckow J, Sullivan DA. The influence of 13-cis retinoic acid on human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2013;54:4341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Archives of ophthalmology. 2006;124:1286–92. [DOI] [PubMed] [Google Scholar]
- [15].Knop E, Knop N. [Meibomian glands : part IV. Functional interactions in the pathogenesis of meibomian gland dysfunction (MGD)]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2009;106:980–7. [DOI] [PubMed] [Google Scholar]
- [16].Sullivan DA, Hammitt KM, Schaumberg DA, Sullivan BD, Begley CG, Gjorstrup P, et al. Report of the TFOS/ARVO Symposium on global treatments for dry eye disease: an unmet need. The ocular surface. 2012;10:108–16. [DOI] [PubMed] [Google Scholar]
- [17].Haroon ZA, Raleigh JA, Greenberg CS, Dewhirst MW. Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann Surg. 2000;231:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenberger C, Solovan C, Rosenberger AD, Jinping L, Treudler R, Frei U, et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin. The Journal of investigative dermatology. 2007;127:2445–52. [DOI] [PubMed] [Google Scholar]
- [19].Evans SM, Schrlau AE, Chalian AA, Zhang P, Koch CJ. Oxygen levels in normal and previously irradiated human skin as assessed by EF5 binding. The Journal of investigative dermatology. 2006;126:2596–606. [DOI] [PubMed] [Google Scholar]
- [20].Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taieb A, et al. HIF-1alpha in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. The Journal of investigative dermatology. 2011;131:1793–805. [DOI] [PubMed] [Google Scholar]
- [21].Cobb LM, Hacker T, Nolan J. NAD(P)H nitroblue tetrazolium reductase levels in apparently normoxic tissues: a histochemical study correlating enzyme activity with binding of radiolabelled misonidazole. British journal of cancer. 1990;61:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cobb LM, Nolan J, O'Neill P. Microscopic distribution of misonidazole in mouse tissues. British journal of cancer. 1989;59:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gross MW, Karbach U, Groebe K, Franko AJ, Mueller-Klieser W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. International journal of cancer Journal international du cancer. 1995;61:567–73. [DOI] [PubMed] [Google Scholar]
- [24].Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2010;51:3993–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McDermott AM, Baidouri H, Woodward AM, Kam WR, Liu Y, Chen X, et al. Short Tandem Repeat (STR) profiles of commonly used human ocular surface cell lines. Current eye research. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2013;54:2541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wright WE, Shay JW. Inexpensive low-oxygen incubators. Nat Protoc. 2006;1:2088–90. [DOI] [PubMed] [Google Scholar]
- [28].Sullivan DA, Liu Y, Kam WR, Ding J, Green KM, Shaffer SA, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2014;55:3866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Y, Kam WR, Ding J, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA ophthalmology. 2014;132:226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kam WR, Liu Y, Ding J, Sullivan DA. Do Cyclosporine A, an IL-1 Receptor Antagonist, Uridine Triphosphate, Rebamipide, and/or Bimatoprost Regulate Human Meibomian Gland Epithelial Cells? Investigative ophthalmology & visual science. 2016;57:4287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Ding J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2014;55:5596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu Y, Kam WR, Ding J, Sullivan DA. One man's poison is another man's meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu Y, Kam WR, Ding J, Sullivan DA. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015;34:342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Y, Kam WR, Sullivan DA. Influence of Omega 3 and 6 Fatty Acids on Human Meibomian Gland Epithelial Cells. Cornea. 2016;35:1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].von Kockritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hoskin PJ, Sibtain A, Daley FM, Wilson GD. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. British journal of cancer. 2003;89:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Samoszuk MK, Walter J, Mechetner E. Improved immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2004;52:837–9. [DOI] [PubMed] [Google Scholar]
- [39].Olive PL, Banath JP, Aquino-Parsons C. Measuring hypoxia in solid tumours--is there a gold standard? Acta oncologica. 2001;40:917–23. [DOI] [PubMed] [Google Scholar]
- [40].Cobb LM, Nolan J, Butler SA. Distribution of pimonidazole and RSU 1069 in tumour and normal tissues. British journal of cancer. 1990;62:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang D, Stockard CR, Harkins L, Lott P, Salih C, Yuan K, et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem. 2008;83:179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Call M, Fischesser K, Lunn MO, Kao WW. A unique lineage gives rise to the meibomian gland. Mol Vis. 2016;22:168–76. [PMC free article] [PubMed] [Google Scholar]
- [43].Tektas OY, Yadav A, Garreis F, Schlotzer-Schrehardt U, Schicht M, Hampel U, et al. Characterization of the mucocutaneous junction of the human eyelid margin and meibomian glands with different biomarkers. Ann Anat. 2012;194:436–45. [DOI] [PubMed] [Google Scholar]
- [44].Morris GF, Mathews MB. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989;264:13856–64. [PubMed] [Google Scholar]
- [45].Meikle PJ, Brooks DA, Ravenscroft EM, Yan M, Williams RE, Jaunzems AE, et al. Diagnosis of lysosomal storage disorders: evaluation of lysosome-associated membrane protein LAMP-1 as a diagnostic marker. Clin Chem. 1997;43:1325–35. [PubMed] [Google Scholar]
- [46].Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109:1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zouboulis CC. Further Evidence of Sebaceous Differentiation Uniqueness: Holocrine Secretion of Sebocytes Is a Multistep, Cell-Specific Lysosomal DNase2-Mediated Mode of Programmed Cell Death. The Journal of investigative dermatology. 2017;137:537–9. [DOI] [PubMed] [Google Scholar]
- [48].Fischer H, Fumicz J, Rossiter H, Napirei M, Buchberger M, Tschachler E, et al. Holocrine Secretion of Sebum Is a Unique DNase2-Dependent Mode of Programmed Cell Death. The Journal of investigative dermatology. 2017;137:587–94. [DOI] [PubMed] [Google Scholar]
- [49].Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative ophthalmology & visual science. 2011;52:2050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lai MC, Chang CM, Sun HS. Hypoxia Induces Autophagy through Translational Up-Regulation of Lysosomal Proteins in Human Colon Cancer Cells. PLoS One. 2016;11:e0153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–11. [DOI] [PubMed] [Google Scholar]
- [52].Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. [DOI] [PubMed] [Google Scholar]
- [53].Choi H, Merceron C, Mangiavini L, Seifert EL, Schipani E, Shapiro IM, et al. Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling. Autophagy. 2016;12:1631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. [DOI] [PubMed] [Google Scholar]
- [55].Hawkins KE, Sharp TV, McKay TR. The role of hypoxia in stem cell potency and differentiation. Regen Med. 2013;8:771–82. [DOI] [PubMed] [Google Scholar]
- [56].Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, et al. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–7. [DOI] [PubMed] [Google Scholar]
- [57].Ogrin R, Darzins P, Khalil Z. Age-related changes in microvascular blood flow and transcutaneous oxygen tension under Basal and stimulated conditions. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60:200–6. [DOI] [PubMed] [Google Scholar]
- [58].Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Cheng KH, et al. Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E4241–E50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Investigative ophthalmology & visual science. 2015;56:1965–70. [DOI] [PubMed] [Google Scholar]
- [61].Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. The British journal of dermatology. 2008;159:628–32. [DOI] [PubMed] [Google Scholar]
- [62].Ohkouchi S, Shibata M, Sasaki M, Koike M, Safig P, Peters C, et al. Biogenesis and proteolytic processing of lysosomal DNase II. PloS one. 2013;8:e59148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322:1–15. [DOI] [PubMed] [Google Scholar]
- [64].Barry MA, Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993;300:440–50. [DOI] [PubMed] [Google Scholar]
- [65].Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lucien F, Pelletier PP, Lavoie RR, Lacroix JM, Roy S, Parent JL, et al. Hypoxia-induced mobilization of NHE6 to the plasma membrane triggers endosome hyperacidification and chemoresistance. Nat Commun. 2017;8:15884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Loegering DJ, Bonin ML, Smith JJ. Effect of exercise, hypoxia, and epinephrine on lysosomes and plasma enzymes. Experimental and molecular pathology. 1975;22:242–51. [DOI] [PubMed] [Google Scholar]
- [68].Smith RJ, Ignarro LJ, Heidger PM, Fisher JW. Lysosomal enzyme release in vivo: an evaluation of the mechanism of cobalt polycythemia. The Journal of pharmacology and experimental therapeutics. 1974;191:564–74. [PubMed] [Google Scholar]
- [69].Abraham R, Goldberg L, Grasso P. Hepatic response to lysosomal effects of hypoxia, neutral red and chloroquine. Nature. 1967;215:194–6. [DOI] [PubMed] [Google Scholar]
- [70].Nujic K, Banjanac M, Munic V, Polancec D, Erakovic Haber V. Impairment of lysosomal functions by azithromycin and chloroquine contributes to anti-inflammatory phenotype. Cellular immunology. 2012;279:78–86. [DOI] [PubMed] [Google Scholar]
- [71].Gerbaux C, Van Bambeke F, Montenez JP, Piret J, Morlighem G, Tulkens PM. Hyperactivity of cathepsin B and other lysosomal enzymes in fibroblasts exposed to azithromycin, a dicationic macrolide antibiotic with exceptional tissue accumulation. FEBS letters. 1996;394:307–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.