Abstract
Purpose
Primary fluid secretion in secretory epithelia relies on the unidirectional transport of ions and water across a single cell layer. This mechanism requires the asymmetric apico-basal distribution of ion transporters and intracellular Ca2+ signaling. The primary aim of the present study was to verify the localization and the identity of Ca2+-dependent ion channels in acinar cells of the mouse lacrimal gland.
Methods
Whole-cell patch-clamp-electrophysiology, spatially localized flash-photolysis of Ca2+ and temporally resolved digital Ca2+-imaging was combined. Immunostaining of enzymatically isolated mouse lacrimal acinar cells was performed.
Results
We show that the Ca2+-dependent K+-conductance is paxilline-sensitive, abundant in the luminal, but negligible in the basal membrane; and co-localizes with Cl−-conductance. These data suggest that both Cl− and K+ are secreted into the lumen and thus they account for the high luminal [Cl−] (~141 mM), but not for the relatively low [K+] (<17 mM) of the primary fluid. Accordingly, these results also imply that K+ must be reabsorbed from the primary tear fluid by the acinar cells. We hypothesized that apically-localized Na+-K+ pumps are responsible for K+-reabsorption. To test this possibility, immunostaining of lacrimal acinar cells was performed using anti-Na+-K+ ATP-ase antibody. We found positive fluorescence signal not only in the basal, but in the apical membrane of acinar cells too.
Conclusions
Based on these results we propose a new primary fluid-secretion model in the lacrimal gland, in which the paracellular pathway of Na+ secretion is supplemented by a transcellular pathway driven by apical Na+-K+ pumps.
Keywords: lacrimal gland, tear, fluid secretion, acinar cell, BK channel, maxiK, Na+-K+ ATP-ase
1. Introduction
Tear secretion is essential for maintaining the integrity and function of the corneal surface and conjunctiva. When the quantity or quality of tear secretion decreases, insufficient moisture of the ocular surface may lead to dry eye (keratoconjunctivitis sicca). The symptoms of dry eye include irritation, inflammation and in more severe cases ulceration of the cornea (1). However, dry eye can be managed with eye drops (artificial tears), which substitute the missing tear film and results in better eye comfort, unfortunately, there is no cure for the syndrome. As the acinar epithelium produces most of the volume of the fluid, designing better medications for dry eye requires the better understanding of the primary fluid secretion mechanism in the gland.
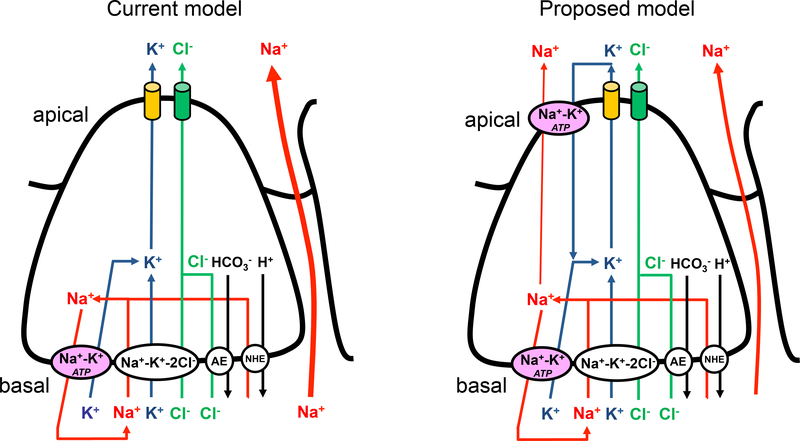
The primary fluid secretion in secretory epithelia is a result of unidirectional salt- and water transport across a single acinar cell layer. The current proposal for the mechanism of lacrimation is based on patch-clamp electrophysiology data, which described the ion transporter composition and polarized arrangement of K+ and Cl−-channels in lacrimal acinar cells earlier (2–8). The discovery of the uneven distribution of ion channels and other functionally coupled transporters in the plasma membrane led to the elaboration of the current model (see figure 1). According to this model, the secretory process is fueled by the electrochemical gradient of Na+ -established by the Na+-K+ ATP-ase-, which serves as a driving force for the ion transports mediated by the Na+-K+-2Cl− cotransporter, the Na+-H+ exchanger and after all, the Cl−-HCO3− exchanger (6–8). All of these carriers are believed to be located in the basolateral plasma membrane. As a result of their function, K+ and Cl− accumulate in the cytoplasm, while Na+ recirculates across the basolateral membrane. When the intracellular Ca2+ concentration ([Ca2+]i) increases, it activates Ca2+-dependent Cl−-channels in the luminal membrane and therefore, triggers Cl− efflux (9–18), which generates a transepithelial potential for paracellular Na+ transport. This mechanism is also referred to as Cl−-driven Na+-secretion. Ca2+-dependent K+-channels also play an important role in the process, because they maintain the driving force for Cl− efflux by holding the membrane potential close to the resting value (−45 mV). These K+-channels were shown to function in the luminal membrane of lacrimal acinar cells, implying that not only Cl− but K+ is also secreted to the lumen (5). Since the membrane potential does not depolarize significantly (only +12 mV) during stimulation (19), the anion and cation currents should be very similar in magnitude. However, contrary to our expectations, there is a huge difference between the intraluminal K+ and Cl− concentrations (17 vs. 152 mM). This discrepancy suggests either that K+-channels are not localized in the luminal membrane (thus, K+ may not be secreted) or K+ is secreted, but immediately reabsorbed from the lumen. To distinguish between these two possibilities, we measured the Ca2+-dependent currents selectively in the apical and basal membranes using the combination of whole-cell voltage-clamp electrophysiology, Ca2+-imaging and spatially limited flash photolysis (Ca2+ uncaging). We found that both Cl− and K+-channels are located in the apical plasma membrane, suggesting a functional K+-reabsorption from the lumen. In line with this finding, Na+-K+ pumps could be detected in the luminal membrane of the acinar cells. Based on these results we propose a new primary lacrimal fluid secretion model (Figure 1.), which verifies an old idea published by Mircheff in 1986 (25).
Figure 1. Cartoon representation of a new lacrimal fluid secretion model.
Please find description in Discussion. NHE-Na+-H+ exchanger, AE-anion (Cl−-HCO3+) exchanger.
2. Material and methods
2.1. Isolation of acinar cells from mouse lacrimal glands
All experiments complied with the 2010/63/EU guideline and were approved by the Animal Welfare Committee of the University of Rochester and the University of Debrecen. Lacrimal acinar cells were isolated by enzymatic dissociation of the lacrimal tissue. The enzymes were dissolved in SMEM (ThermoFisher Scientific Inc., Waltham, MA). 3–4 month old mice were sacrificed by cervical dislocation. Lacrimal glands were immediately removed, finely minced with scissors and digested with 28 μg/ml trypsin at 37°C in shaking waterbath for 8 min. The tissue was washed in SMEM medium, containing trypsin inhibitor and it was further digested in 2×10 ml of 0.18 Wünsch units/ml Liberase TL (an enzyme blend, containing collagenase from Roche Diagnostics GmbH, Mannheim, Germany) for 2×20 min. The tissue was gently pipetted through a cut 200 μl pipette tip applied on a 10 ml serological pipette. Finally, the cells were filtered through a 100 μm nylon mesh, washed, centrifuged (200xg, 3 min) and resuspended in BME medium (20, 21).
For the immunofluorescence study, larger cell clumps were isolated, using a slightly modified protocol, when trypsin digestion was omitted and collagenase was applied for only 20 minutes.
2.2. Spatially limited flash photolysis, Ca2+-imaging, and electrophysiological recording
UV-flash photolysis, Ca2+-imaging and electrophysiological recording was assembled and operated as described previously (20, 21, 22).
Whole-cell currents were recorded using an Axopatch 200A amplifier controlled by a Digidata 1322A interface (Axon Instruments Inc., Foster City, CA, USA). Acinar cells were voltage clamped at +40 mV (K+-current recordings) or −20 mV (Cl−-current measurements). Pipettes were filled with intracellular solution containing (in mM): 135 K-glutamate, 10 HEPES, 10 NP-EGTA, 2 CaCl2, and 0.25 Fluo-4-K, pH=7.2. External solutions contained (in mM) 135 Na-glutamate, 5 K-glutamate, 2 CaCl2, 2 MgCl2, and 10 HEPES, pH=7.2 or 140 TEA-Cl and 10 HEPES mM, pH=7.2).
Fluo-4 (Ca2+ indicator) fluorescence was imaged using a monochromator-based imaging system and a high-speed CCD camera (TILL Photonics GmbH, Munich, Germany). The dye was excited for 20 ms at 488 nm and the emitted fluorescence was collected through a 525 nm band-pass filter (Chroma Technology Corp, Bellows Falls, VT, USA) at 20 Hz. Data were displayed as ΔF/F0=(F−F0)/F0, where F was the recorded fluorescence and F0 was the average fluorescence of the first 10 frames of the image series (baseline). Images were scaled to 4096 gray levels and pseudocolored.
Ca2+ release was triggered in the intracellular space by flashing the photolabile Ca2+ chelator NP-EGTA (caged Ca2+) with UV light. Photolysis of NP-EGTA by UV illumination decreases its affinity for Ca2+ by >12-fold. This process is called Ca2+ uncaging, which was performed using a 375 nm laser and an appropriate condenser, coupled to an inverted microscope (TE200; Nikon Corp, Japan). The laser was brought to focus in the sample plane using a ×40 oil immersion objective (Nikon Corp, Japan). In this setup, the pixel size was 0.32 μm. The full width at half-maximum (FWHM) of the laser point was ∼0.7 μM in the x and y plane and ∼2.0 μm in the z plane (23). The laser power was controlled between 4 and 6 mW and the cells were flashed for one image frame (50 ms). The current recordings, fluorescence image acquisition, and triggering of the laser exposure were synchronized by the image controller and controlled by Vision software package (TILL Photonics GmbH, Munich, Germany).
2.3. Immunocytochemistry
Immediately after isolation, acinar cell clumps were fixed in ice-cold methanol in suspension for 15 min. Antisera raised against the Na+-K+ ATP-ase (Abcam, Cambridge, UK, ref. #ab76020) and occludin (Novus, NBP1–87402) were added to acinar cell suspension in dilutions of 1:250 and 1:100, respectively. Subsequently, the cells were probed with Dylight 488 horse anti rabbit secondary antibody (ThermoFisher Scientific Inc., Waltham, MA) in the dilution of 1:2000 or 1:500. Cells were incubated with the antibodies at room temperature for 1 h and washed three times for 5 min by resuspending the cells in 3% BSA-PBS solution. After each wash, cells were collected by centrifugation. Primary antibodies were omitted in negative control experiments. Finally, acinar cell clumps were seeded on coverslips and examined using a Zeiss 510 Meta (Carl Zeiss Microscopy GmbH, Jena, Germany) confocal microscope equipped with a suite of diode and gas lasers.
3. Results
3.1. Spatially limited flash photolysis, Ca2+-imaging and electrophysiological recording
In order to determine the localization of Ca2+-activated K+-channels in the plasma membrane, Ca2+ was transiently raised selectively in the apical or the basal membrane domain using flash-photolysis and the whole-cell current was measured simultaneously. The UV laser point (of ∼0.7 μM FWHM) was positioned near the intracellular side of the apical or basal plasma membrane in a cell loaded with caged-Ca2+ and Fluo-4 through a patch pipette and exposed with the UV laser for 50 ms. Intracellular Fluo-4 fluorescence reported that the flash-photolysis of caged Ca2+ resulted in a spatially limited Ca2+ signal as demonstrated in Figure 2.
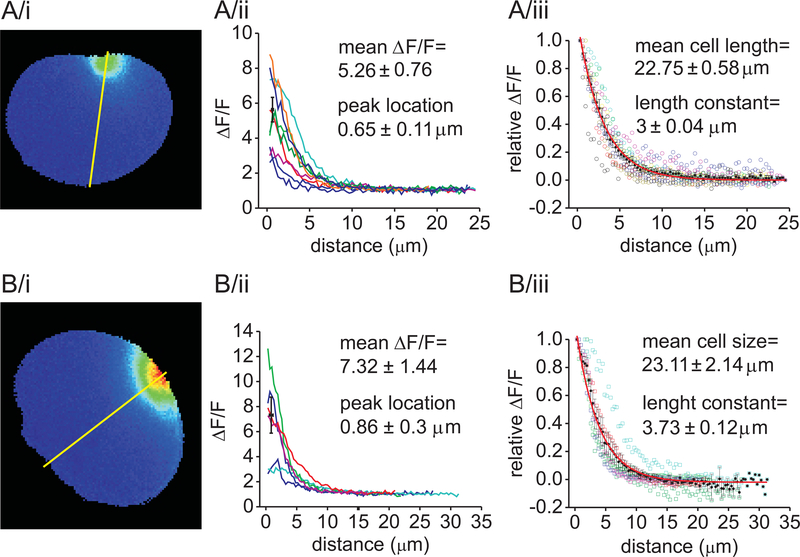
Figure 2. Fluo-4 fluorescence remained spatially localized after Ca2+ uncaging in lacrimal acinar cells.
Pseudocolored fluorescent images of acinar cells taken 50 ms after the UV laser exposure near the intracellular side of the apical (A/i) or basal membrane (B/i) of the cells. Spatial analysis of fluorescence intensities along the yellow line laid across the cells were plotted to demonstrate the spatial decay of [Ca2+i] in each cell (A/ii and B/ii). Average peak fluorescence intensities (±SEM) are shown by black squares. Similar analysis, in which the data of individual experiments were normalized to the peak-fluorescence: (A/iii and B/iii, empty spheres). Average fluorescence (±SEM) is shown by black symbols. The red lines represent the exponential fit of the data. The length constant was 3±0.04 μm whereas the average cell length was 22.75±0.58 μm in the apical- (A/iii) and 3.73±0.12 μm and 23.11±2.14 μm in the basal (B/iii) experiments, respectively.
Figure 2A shows a typical example of the image of peak fluorescence after uncaging Ca2+ in a cell dialyzed with the pipette solution containing 10 mM NP-EGTA and 2 mM Ca2+. The image clearly demonstrates that under these conditions, a sizeable Ca2+ transient could be generated without significant spread of the signal away from the photolysis site. In the experiments shown in Figure 2A, the laser was focused near the apical membrane domain of the cell. The apical membrane was identified by the granular region of acinar cells. Figure 2A/ii demonstrates that the average elevation of fluorescence was 5.26±0.76 times higher than the baseline. The spatial analysis of raw and normalized fluorescence intensities along a line running from the apical membrane across the cells was performed for each experiment (illustrated by Figure 2A/ii and 2A/iii). This analysis demonstrates that the increase in fluorescence peaks were 0.65±0.11 μm away from the apical plasma membrane and decayed exponentially from the photolysis site with a mean length constant of 3±0.04 μm, whereas the average cell diameter was 22.8±0.6 μm. Similar analysis was performed in cells whose basal membrane was targeted by the laser point, with similar results: the normalized peak-fluorescence was 7.32±1.44 compared to baseline, which located 0.86±0.3 μm away from the plasma membrane and decayed with a length constant of 3.73±0.12 μm. The average size of these cells was 23.1±2.1 μm. These data demonstrate that laser exposure under these buffering conditions resulted in a highly localized Ca2+-elevation near the selected aspect of the cell and that the [Ca2+i] in the opposite membrane domain remained unaltered. Consequently, these results verify that this method is suitable for the pole-selective elevation of [Ca2+i].
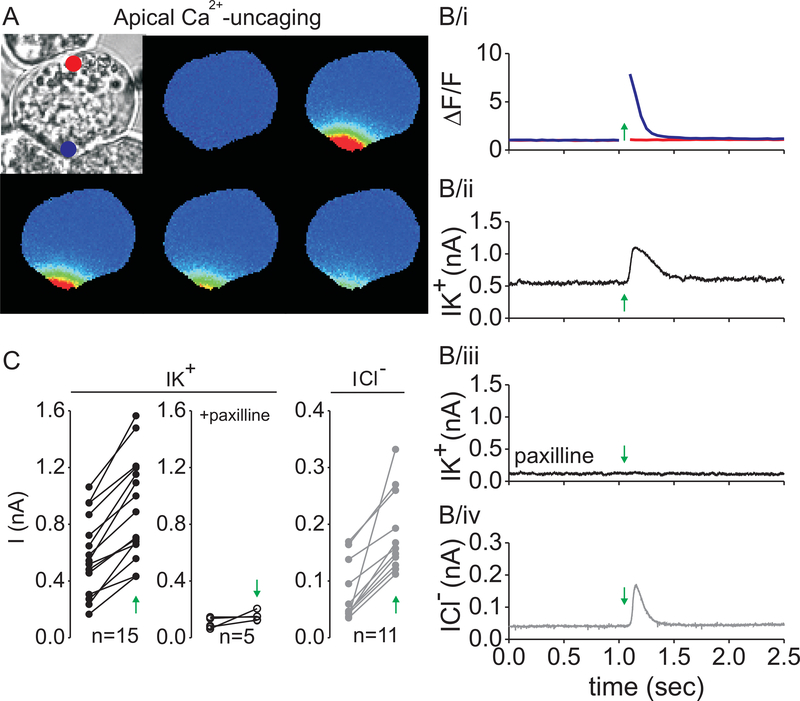
The aim of the first series of experiments was to investigate the function of Ca2+-dependent K+-channels in the apical membrane of lacrimal acinar cells. Local uncaging was combined with whole-cell current recording and Ca2+-activated K+-and Cl−-current was measured. The apical membrane region was identified by the granular region of the cells and was verified by the presence of the Ca2+-activated Cl− -current, which was previously shown to function exclusively in the apical membrane of acinar cells (5, 20). Data were collected from small group of cells (2–3 cell clusters). An example of this type of experiment is illustrated in Figure 3. A bright field image of a cell clump of 3 cells is shown in Figure 3A (top left). The cell in the center of the image was attached to the patch-pipette, which contained Fluo-4 Ca2+-indicator dye and caged-Ca2+. K+-current and Fluo-4 fluorescence was recorded simultaneously as described in Materials and methods. The UV laser beam was positioned near the apical plasma membrane (at the position indicated by the blue dot). The laser was flashed 1.05 s after the start of electrical recording (indicated by the green arrows in Figure 3B) for 50 ms time duration. The continuous recording of fluorescence in both the apical (Figure 3B/i, blue line) and basal membrane regions (red line) clearly shows that the flash evoked an instantaneous increase of [Ca2+i] in the apical region, which last for around 250 ms. Importantly, fluorescence did not change near the basal membrane. Figure 3B/ii shows the whole-cell current of the same cell before and after laser exposure (indicated by the green arrow). The graph clearly shows that the local increase of [Ca2+] resulted in a significant elevation of K+-current. In this particular experiment, the baseline of K+-current was 0.5 nA, because the membrane potential was clamped at +40 mV, which partially opens voltage- and Ca2+-dependent K+-channels (BK channel, MaxiK). The BK channel inhibitor paxilline decreased the current’s baseline and prevented the activation of the K+-current during Ca2+-uncaging in the same cell (Figure 3B/iii). After K+-current recording, the Na+- and K+-glutamate containing extracellular medium was replaced with 140 mM TEA-Cl, which inhibits K+-currents, but provides enough Cl− to record outward Cl− currents. In this solution, subsequent flash photolysis in the same spot resulted in a robust activation of the Cl−-current (Figure 3B/iv). These observations were consistent in all the 15 cells that we measured. Quantitative analysis of K+- and Cl−-current amplitudes of each cell before and after Ca2+ uncaging and in the presence of paxilline are shown in Figure 3C.
Figure 3. Apically localized increase in [Ca2+i] activates K+ and Cl− -channels.
The series of pseudocolored fluorescent images of a cell loaded with NP-EGTA and Fluo-4 before and after UV flash (A, blue dot). The laser was flashed 1.05 seconds after the start of recording (A and B, green arrow). In these images and kinetic plot, a marked increase in Ca2+ signal was evoked in the apical domain (B/i, blue line) and remained highly localized. The fluorescence near the opposite pole of the cell did not change (B/i, red line). The whole-cell K+ -current -change, evoked by the Ca2+ transient under control conditions (B/ii) and in the presence of 1 μM paxilline (B/iii). The increase in Cl− -current after a subsequent apical laser exposure in 140 mM TEA-Cl (B/iv). Whole-cell current data of paired experiments before and after flash (C, green arrow).
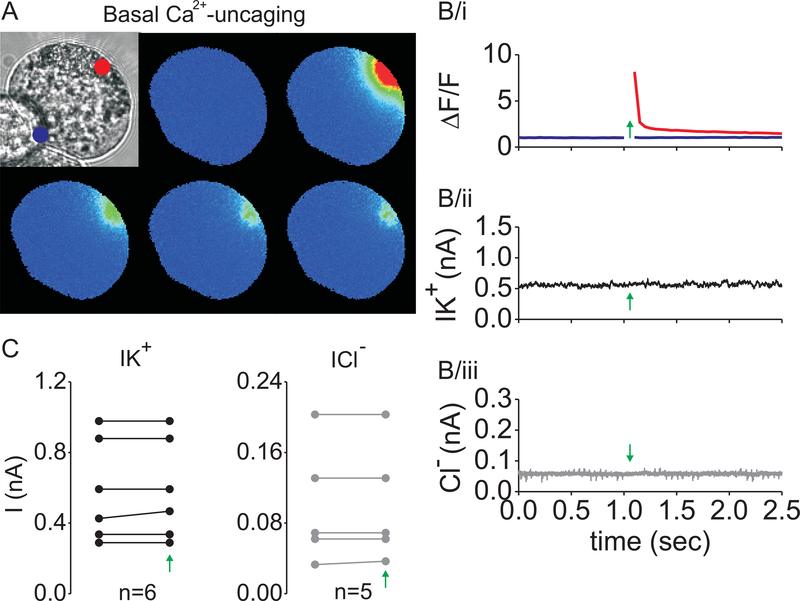
Next, the basal membrane was probed for K+- and Cl−-currents by using the same approach (Figure 4). Morphologically, this membrane domain was defined by the prominent reticular structures, whereas functionally, this membrane was defined by the absence of the Ca2+-activated Cl−-current. Although, in these experiments, the change in local [Ca2+i] was slightly higher (Figure 2B and Figure 4B/i), it failed to activate either K+- or the Cl−-currents (Figure 4B/ii and 4B/iii). Current amplitudes of 6 different cells are plotted in Figure 4C.
Figure 4. Localized increase in [Ca2+i] near the basal membrane does not activate K+ and Cl− -channels.
UV-photolysis of NP-EGTA was performed as in Figure 2, but near the basal membrane of lacrimal cells. The series of pseudocolored fluorescent images of a cell loaded with NP-EGTA and Fluo-4 before and after UV flash is shown in (A) (red dot). Kinetic plot of Fluo-4 fluorescence in the basal (B/i, red line) and apical area (blue line), after Ca2+-uncaging (labelled by green arrow). Whole-cell K+- and Cl− -current recordings of a lacrimal cell subject to Ca2+-uncaging (B/ii and B/iii). Whole-cell current data of paired experiments before and after flash (C, green arrow).
These data strongly suggest that the primary K+-channel in the mouse lacrimal acinar cell is the BK channel, which co-localizes with the Ca2+-activated Cl−-channels in the apical plasma membrane.
3.2. Immunocytochemistry
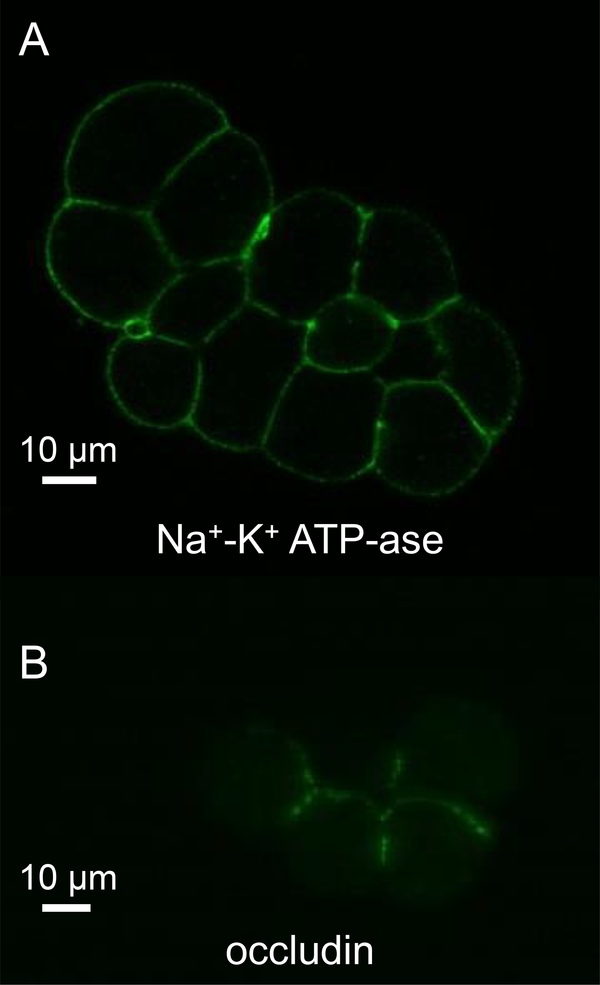
The apical localization of K+-channels implies that significant K+ secretion should take place during acinar cell stimulation, which raises the question that why [K+] of the tear is relatively low. We hypothesized that the reason is that K+ is reabsorbed from the acinar lumen. As the function of our hypothetical K+-transporter should be electronegative, in order to hold the membrane potential at hyperpolarized potentials during agonist-stimulation, the most plausible transporter to account for K+-reabsorption is the Na+-K+ pump. To test this hypothesis, Na+-K+ pumps were immunolabelled in freshly isolated acinar cell clumps, as described in Materials and Methods. Figure 5A shows a micrograph of a cell clump, where the localization of anti-Na+-K+ pump antibody was visualized with a Dylight488 secondary antibody. Notably, Na+-K+ pumps show clear membrane localization all over the plasma membrane, including the apical area, consistent with the pump being involved in K+-reabsorption across the apical membrane from the acinar lumen.
Figure 5. Localization of Na+-K+ ATP-ase in lacrimal acinar cell clumps by immunofluorescence.
Lacrimal acinar cells express Na+-K+ ATP-ase in both the basolateral and apical plasma membrane (A). The distribution of the apical plasma membrane marker occludin (B).
However, the functional significance of the apical pumps cannot be appreciated unless the proportion of the apical membrane surface area is known. To this end, the size of the apical membrane was estimated by evaluating the coverage of the plasma membrane by the apical membrane marker tight junction protein, occludin (24). Cells were probed with antibody raised against occludin. The immunostaining shown in Figure 5B demonstrates that the apical membrane area is relatively large (~30% of the apparent membrane circumference). This result presumably reflects the highly invaginated nature of the apical membrane and further suggests that the activity of apical Na+-K+ pumps may be functionally significant in determining the ionic composition of the primary tear fluid.
4. Discussion
Based on our experimental data we constructed a new model to represent primary tear-secretion, which is presented by a cartoon in Figure 1. In order to provide better understanding of our new concept, we compare the old and the new models in the same figure. Although, the basic idea of our new model is very similar to the hypothetic model proposed by Wood and Mircheff in 1986 (25), here we provide a thorough evidence to support this concept and we explain the ion movements across the epithelium more accurately and in deeper detail.
In the old model, Na+ is transported into the cytoplasm by the Na+-K+−2Cl− and Na+-H+ cotransporters and recirculates across the basal membrane through the Na+-K+ ATP-ase. K+ and Cl− also enter the cell through the basolateral membrane using the Na+-K+−2Cl− and Cl−-HCO3− cotransporters, but they exit on the apical membrane via Ca2+-activated K+ and Cl− channels (26). Conceptionally, a potential problem with this mechanism is that it suggests that the luminal osmotic gradient primarily relies on K+ secretion, which also implies that the role of Na+ secretion is small (because K+ flux would diminish the transepithelial driving force for Na+). Moreover, this concept is not consistent with the ionic concentrations in the tear.
In the current work, we confirmed Trautmann and Marty’s results that Ca2+-dependent K+- and Cl−-channels do colocalize in the apical plasma membrane of lacrimal acinar cells and concluded that a reabsorption mechanism must work in the apical membrane domain in order to maintain the intraluminal [K+] low. In accordance with this idea, our immunofluorescent images showed strong Na+-K+ pump expression in both the basolateral and luminal plasma membrane with equal density. These results are in accordance with some earlier data and suggest that the pump has significant role in K+-reabsorption (6, 25). However, the functional significance of the apical Na+-K+ pumps depends on the apical to basolateral ratio of the transporters. This ratio was estimated to be as high as 30:70, which surface is probably high enough to mediate significant K+-reabsorption from the lumen.
In our recent study, we constructed a mathematical model for fluid secretion in parotid acini. This model suggested that fluid secretion was optimal when 40% of the total K+-conductance and 30% of the pump activity was inserted into the apical membrane. Also, under these conditions it predicted that 27% of Na+ was secreted through the transcellular pathway into the lumen, while without apical pumps, Na+ used the paracellular pathway exclusively (21, 27). We propose a similar overall mechanism in lacrimal glands.
In summary, our new model proposes that, K+-efflux takes place through the apical membrane, but subsequently, K+ is immediately reabsorbed by apical Na+-K+ ATP-ases, which enhances Na+ secretion rate and redirects Na+ –at least partially- from the paracellular to the transcellular pathway. In addition, the pump’s electronegative function holds the membrane potential hyperpolarized during stimulation and maintains the electrochemical driving force for Cl−-efflux (Figure 1.).
Also, our model implies that K+-reabsorption is flow-rate dependent. During stimulation (reflex or emotional lacrimation), when the high fluid secretion rate allows shorter time for Na+-K+ exchange through the luminal membrane of the acinar cell, the [K+] of the primary tear would remain higher, while the [Na+] would remain lower, compared to low (basal) flow rates.
Acknowledgements
This work was supported by the Hungarian National Research Development and Innovation Office (PD112199 to JA and K115397 to PPN). JA is supported by the Lajos Szodoray Scholarship of the University of Debrecen and the János Bolyai scholarship of the Hungarian Academy of Sciences. The publication was supported also by the GINOP-2.3.2-15-2016-00040 and EFOP-3.6.2-16-2017-00006 projects (to PPN and JA), which are co-financed by the European Union and the European Regional Development Fund. The work was also partially supported by NIH grants R01DE014756 and DE019245 (DIY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Tóth-Molnár E, Venglovecz V, Ozsvári B, Rakonczay Z Jr, Varró A, Papp JG, Tóth A, Lonovics J, Takács T, Ignáth I, Iványi B, Hegyi P (2007) New experimental method to study acid/base transporters and their regulation in lacrimal gland ductal epithelia. Invest Ophthalmol Vis Sci. 48: 3746–3755. [DOI] [PubMed] [Google Scholar]
- (2).Marty A, Tan YP and Trautmann A (1984) Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 357: 293–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Trautmann A and Marty A. (1984) Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 81: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Evans MG, Marty A, Tan YP and Trautmann A. (1986) Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 406: 65–68. [DOI] [PubMed] [Google Scholar]
- (5).Tan YP, Marty A and Trautmann A (1992) High density of Ca2+-dependent K+ and Cl− channels on the luminal membrane of lacrimal acinar cells. Proc Natl Acad Sci U S A. 89: 11229–11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Selvam S, Thomas PB, Gukasyan HJ, Yu AS, Stevenson D, Trousdale MD, Mircheff AK, Schechter JE, Smith RE, Yiu SC (2007) Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. 293: C1412–1419. [DOI] [PubMed] [Google Scholar]
- (7).Mircheff AK. (1989) Lacrimal fluid and electrolyte secretion: a review. Curr Eye Res 8: 607–617. [DOI] [PubMed] [Google Scholar]
- (8).Dartt DA, Møller M, Poulsen JH (1981) Lacrimal gland electrolyte and water secretion in the rabbit: localization and role of (Na+-K+)-activated ATPase. J Physiol. 321: 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lechleiter JD, Dartt DA, Brehm P (1988) Vasoactive intestinal peptide activates Ca(2+)-dependent K+ channels through a cAMP pathway in mouse lacrimal cells. Neuron. 1: 227–235. [DOI] [PubMed] [Google Scholar]
- (10).Findlay I, Petersen OH (1985) Acetylcholine stimulates a Ca2+-dependent Cl- conductance in mouse lacrimal acinar cells. Pflugers Arch. 403: 328–330. [DOI] [PubMed] [Google Scholar]
- (11).Changya L, Gallacher DV, Irvine RF, Potter BV, Petersen OH. (1989) Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 109: 85–93. [DOI] [PubMed] [Google Scholar]
- (12).Toescu EC, Lawrie AM, Petersen OH, Gallacher DV (1992) Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 11: 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Smith PM, Harmer AR, Letcher AJ, Irvine RF (2000) The effect of inositol 1,3,4,5-tetrakisphosphate on inositol trisphosphate-induced Ca2+ mobilization in freshly isolated and cultured mouse lacrimal acinar cells. Biochem J. 347: 77–82. [PMC free article] [PubMed] [Google Scholar]
- (14).Putney JW Jr, Huang Y, Bird GS (1998) Calcium signalling in lacrimal acinar cells. Adv Exp Med Biol. 438: 123–128. [DOI] [PubMed] [Google Scholar]
- (15).Putney JW, Bird GS (2014) Calcium signaling in lacrimal glands. Cell Calcium. 55: 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Smith PM. (1992) Ins(1,3,4,5)P4 promotes sustained activation of the Ca(2+)-dependent Cl- current in isolated mouse lacrimal cells. Biochem J. 283: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bird GS, Rossier MF, Hughes AR, Shears SB, Armstrong DL, Putney JW Jr (1991) Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 352: 162–165. [DOI] [PubMed] [Google Scholar]
- (18).Kasai H and Augustine GJ (1990) Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 348: 735–738. [DOI] [PubMed] [Google Scholar]
- (19).Parod RJ, Dambach GE, Putney JW Jr. (1980) Membrane potential changes in lacrimal gland acinar cells elicited by carbachol and epinephrine. J Pharmacol Exp Ther. 213: 473–479. [PubMed] [Google Scholar]
- (20).Almassy J, Won JH, Begenisich TB and Yule DI (2012) Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol. 139: 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Almássy J, Siguenza E, Skaliczki M, Matesz K, Sneyd J, Yule DI and Nánási PP (2018) New saliva secretion model based on the expression of Na+-K+ pump and K+ channels in the apical membrane of parotid acinar cells. Pflugers Arch. 470: 613–621. [DOI] [PubMed] [Google Scholar]
- (22).Almassy J and Yule DI (2013) Investigating ion channel distribution using a combination of spatially limited photolysis, Ca(2+) imaging, and patch clamp recording. Cold Spring Harb Protoc. doi: 10.1101/pdb.prot072769. [DOI] [PubMed] [Google Scholar]
- (23).Won JH, Cottrell WJ, Foster TH, Yule DI (2007) Ca2+ release dynamics in parotid and pancreatic exocrine acinar cells evoked by spatially limited flash photolysis. Am J Physiol Gastrointest Liver Physiol. 293: G1166–1177. [DOI] [PubMed] [Google Scholar]
- (24).Larina O and Thorn P (2005) Ca2+ dynamics in salivary acinar cells: distinct morphology of the acinar lumen underlies near-synchronous global Ca2+ responses. J Cell Sci 118: 4131–4139. [DOI] [PubMed] [Google Scholar]
- (25).Wood RL, Mircheff AK (1986) Apical and basal-lateral Na/K-ATPase in rat lacrimal gland acinar cells. Invest Ophthalmol Vis Sci. 27: 1293–1296. [PubMed] [Google Scholar]
- (26).Walcott B (1998) The Lacrimal Gland and Its Veil of Tears. News Physiol Sci. 13: 97–103. [DOI] [PubMed] [Google Scholar]
- (27).Palk L, Sneyd J, Shuttleworth TJ, Yule DI and Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol. 266: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]