Abstract
We have previously identified associations of 2 circulating secondary bile acids (glycocholenate and glycolithocolate sulfate) with atrial fibrillation (AF) risk among 1,919 blacks in the ARIC cohort. We aimed to replicate these findings in an independent sample of 2,003 white and black ARIC participants, and performed a new metabolomic analysis in the combined sample of 3,922 participants, followed between 1987 and 2013. Metabolomic profiling was done in baseline serum samples using gas and liquid chromatography mass spectrometry. AF was ascertained from electrocardiograms, hospitalizations, and death certificates. We used multivariable Cox regression to estimate hazard ratios (HR) and 95% confidence intervals (95%CI) of AF by 1 standard deviation difference of metabolite levels. Over a mean follow-up of 20 years, 608 participants developed AF. Glycocholenate sulfate was associated with AF in the replication and combined samples (HR 1.10, 95%CI 1.00, 1.21 and HR 1.13, 95%CI 1.04, 1.22, respectively). Glycolithocolate sulfate was not related to AF risk in the replication sample (HR 1.02, 95%CI 0.92, 1.13). An analysis of 245 metabolites in the combined cohort identified 3 additional metabolites associated with AF after multiple-comparison correction: pseudouridine (HR 1.18, 95%CI 1.10, 1.28), uridine (HR 0.86, 95%CI 0.79, 0.93) and acisoga (HR 1.17, 95%CI 1.09, 1.26). In conclusion, we replicated a prospective association between a previously identified secondary bile acid, glycocholenate sulfate, and AF incidence, and identified new metabolites involved in nucleoside and polyamine metabolism as markers of AF risk.
Keywords: atrial fibrillation, metabolomics, epidemiology
INTRODUCTION
Atrial fibrillation (AF), a common cardiac arrhythmia, is a major risk factor for stroke and other cardiovascular diseases.1 Application of metabolomics, the systematic investigation of all small molecules in a biological system, to the study of AF risk could deepen our understanding of AF pathogenic pathways as well as contribute to the discovery of novel disease biomarkers.2 To date, however, metabolomic studies in this area have been few and limited in sample size. In an analysis of metabolomic data from 1,919 black participants in the community-based Atherosclerosis Risk in Communities (ARIC) study, including 183 who were newly diagnosed with AF, we reported an association of higher circulating levels of 2 secondary bile acids, glycolithocholate sulfate and glycocholenate sulfate, with incidence of AF, but no replication in independent cohorts was available.3 More recently, a report from the mostly European-American Framingham Heart Study including 2,458 participants with targeted metabolomic profiling, of which 156 developed AF, did not identify any molecule significantly associated with AF incidence after adjustment for multiple comparisons.4 Additional studies are required to replicate previous findings and increase statistical power for novel discoveries. In this manuscript, as a follow-up to our previous study in the ARIC cohort, we extend the metabolomic assessment to 2,003 additional ARIC participants. We aimed to replicate the findings from the prior ARIC analysis in the additional ARIC participants and to conduct a new hypothesis-generating analysis in the combined sample of 3,922 participants.
METHODS
In 1987–89, the ARIC study examined 15,792 men and women 45–64 years of age recruited from 4 communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; Washington County, MD).5 Participants were mostly white in the Minneapolis and Washington County sites, white and black in Forsyth County, while only black were recruited in Jackson. After their baseline exam, participants underwent follow-up visits in 1990–92, 1993–95, 1996–98, 2011–13, and 2016–17. Participants have been followed up via annual phone calls (semiannual since 2012). For the current analysis, we included 3,922 participants with available metabolomic data and without evidence of AF at baseline. The ARIC study has been approved by institutional review boards at all participating institutions. Participants provided written informed consent at baseline and follow-up visits.
As previously described, 1,977 randomly selected blacks in the Jackson field center had serum metabolomic profiling performed in 2010 in samples obtained at study baseline in 1987–89.6 The samples had been stored at −80°C and were assayed with an untargeted, gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry-based metabolomic protocol by Metabolon, Inc. (Durham, NC). Similarly in 2014, serum samples from an additional 2,055 randomly selected participants (76% white, 24% black) collected in 1987–89 and stored since then at −80°C were assayed by Metabolon, Inc. using the same protocol. Brief methodological details are provided in the online supplement.
We selected a set of 97 samples to measure their metabolome profiles using baseline serum samples at both 2010 and 2014. We calculated the Pearson correlation coefficients (r) between the 97 pairs for shared metabolites. For the present study, we limited the analysis to metabolites detected in both batches (n = 384) with: 1) no more than 25% missing values, and 2) Pearson correlation coefficients ≥0.3 between 2010 and 2014 measurements. After applying these criteria, 245 named metabolites were included (99 excluded due to >25% missing values, and 40 more excluded due to correlation <0.3). To evaluate the stability of samples in long-term storage, we compared metabolomic measures done at 2014 and 2016 with standard clinical laboratory measures done at ARIC baseline (1989) for urea, glucose, and cholesterol. All 3 metabolites showed Pearson correlation coefficients ≥0.65.
We have described elsewhere the details about AF ascertainment in the ARIC cohort.7 Briefly, we identified AF cases through the end of 2013 from 3 sources: electrocardiograms (ECG) done at scheduled study visits, discharge diagnosis codes from hospitalizations, and death certificates. At all study visits, participants underwent a standard 12-lead 10-second ECG, which was transmitted electronically to the ARIC ECG reading center at EPICARE (Wake Forest School of Medicine, Winston-Salem, NC) for review and analysis using the GE Marquette 12-SL program (GE Marquette, Milwaukee, WI). A computer algorithm identified the presence of AF in the ECG, with a cardiologist confirming the diagnosis.
Participants’ hospitalizations during follow-up were identified through phone calls and surveillance of local hospitals (response rate >90%). Trained abstractors collected information from these hospitalizations, including all discharge codes. We considered AF present if ICD-9-CM codes 427.31 or 427.32 were listed as discharge diagnoses in any given hospitalization. We excluded AF cases associated with open cardiac surgery. We and others have demonstrated adequate validity of this approach for the ascertainment of AF.7,8 Finally, we also defined AF from death certificates if ICD-9 427.3 or ICD-10 I48 were listed as any cause of death. We provide details about covariate assessment in the online supplement.
We conducted 2 separate sets of analyses. In the first one, we aimed to replicate the findings from our prior ARIC publication, estimating the association of glycolithocholate sulfate and glycocholenate sulfate with AF incidence in 2,003 participants without AF at baseline not included in our published analysis. A 2-tailed p-value of 0.05 was used as threshold for statistical significance in the replication analysis. A second analysis combined participants from the 2 metabolomic assessment batches (n = 3,922). We used a modified Bonferroni correction to determine statistical significance.9 Using this approach, p-values <3.538 × 10−4 were considered statistically significant for 245 tested metabolites.
For all analyses, the association of individual metabolites with the incidence of AF was estimated with Cox proportional hazards regression. Time of follow-up was defined as the time in days from the baseline visit to incidence of AF, death, loss to follow-up or December 31, 2013, whichever occurred earlier. Metabolites were mean centered and modeled as continuous variables in standard deviation units. Missing values were imputed with the lowest detected value in each batch. We ran 3 separate models with increasing number of covariates. A first model adjusted for age, sex, race, center, and batch (when applicable). A second model additionally adjusted for smoking, body mass index, systolic blood pressure, hypertension medications, diabetes mellitus, history of heart failure, and history of coronary heart disease. A final model additionally adjusted for eGFR. We selected model covariates based on prior knowledge of risk factors for AF.10 We assessed effect measure modification by race and sex using stratified analysis. The dose-response shape of the association between metabolite concentration and AF incidence was evaluated modeling metabolites using a restricted cubic spline with 5 knots. To test the robustness of the observed significant associations, we conducted a series of sensitivity analyses, adjusting for blood lipids and lipid-lowering medications and excluding participants with a prior history of prevalent coronary heart disease or heart failure, as well as adjusting for aspartate aminotransferase (AST) and alanine aminotransferase (ALT), measured in visit 2 samples, in the analyses of bile acids.
We conducted several additional analyses to explore potential mechanisms of the association between metabolites and AF incidence. First, we evaluated the association of statistically significant metabolites with electrocardiographic endophenotypes of AF risk using linear regression (PR duration, in ms) or logistic regression (abnormal P wave axis and elevated P wave terminal force in V1). Second, we evaluated the association of statistically significant metabolites with 23 single nucleotide polymorphisms (SNPs) associated with AF in a prior genome-wide association study (GWAS) from the AFGen consortium, and a genetic score calculated by adding the number of risk alleles weighted by the beta coefficient from the published genome-wide study.11 Finally, we explored whether variation in rs2272996 in gene VNN1, a SNP previously related to circulating concentrations of acisoga (one of the metabolites associated with AF incidence in this analysis),12 was associated with AF incidence in the latest GWAS of AF.
RESULTS
Of 15,792 participants in the ARIC cohort, the present analysis included 3,922 with available metabolomic data and free of AF at baseline, 1,919 of them included in our previous publication and 2,003 with newly available data. Participants were followed up for a mean (standard deviation) of 20.4 (7.0) years, during which 608 AF events were identified (incidence rate, 7.6 cases per 1,000 person-years). Table 1 reports participants’ characteristics overall and by AF incidence status during follow-up. As expected, participants who developed AF during follow-up were older, had higher systolic blood pressure and worse kidney function at baseline. They were also more likely to be white, male and have a baseline diagnosis of diabetes, heart failure or coronary heart disease.
Table 1.
Selected baseline characteristics by atrial fibrillation (AF) status during follow-up in 3,922 participants with available metabolomic data and free of AF at baseline, ARIC study, 1987–89
| (n = 3,922) | No (n = 3,314) | Yes (n = 608) | |
|---|---|---|---|
| Age (years) | 54±6 | 53±6 | 56±6 |
| Women | 60% | 62% | 50% |
| Black | 61% | 64% | 47% |
| White | 39% | 36% | 53% |
| Body mass index (kg/m2) | 29±6 | 29±6 | 30±6 |
| Current smoker | 28% | 27% | 29% |
| Systolic blood pressure (mmHg) | 125±21 | 124±21 | 129±22 |
| Anti-hypertensive medication | 32% | 31% | 39% |
| Diabetes mellitus | 14% | 13% | 19% |
| eGFR (mL/min/1.73 m2) | 99±18 | 100±18 | 94±19 |
| Prevalent heart failure | 5.1% | 4.5% | 8.4% |
| Prevalent coronary heart disease | 4.8% | 4.0% | 9.2% |
Values correspond to mean (standard deviation) or percentages. eGFR: estimated glomerular filtration rate
In an initial analysis, we aimed to replicate the findings from our previous publication showing that higher levels of glycolithocholate sulfate and glycocholenate sulfate were associated with increased risk of AF. In an age and sex-adjusted analysis including 2,003 participants and 386 incident AF events, higher levels of glycocholenate sulfate but not of glycolithocholate sulfate were associated with AF incidence in the replication analysis (Table 2, Model 1). The association of glycocholenate sulfate with incidence of AF became weaker after multivariable adjustment (Table 2, Model 2). Given the strong attenuation after multivariable adjustment, we explored if any individual covariate was responsible for this change. Adding each covariate to Model 1 individually did not point to any particular variable as responsible for the attenuation (Supplementary Figure 1). The hazard ratio (HR) and 95% confidence interval (CI) of AF per 1-standard deviation (SD) difference in glycocholenate sulfate in the combined derivation and replication samples was 1.23 (95%CI 1.14–1.32, p = 9.5 × 10−8) in minimally adjusted models and 1.13 (95%CI 1.04, 1.22, p = 0.003) after additional adjustment for cardiovascular risk factors. Additional adjustment for concentrations of ALT and AST in 3,401 participants with available information on liver enzymes did not modify the associations (HR 1.15, 95%CI 1.07, 1.23, p = 2.5 × 10−5). Analysis stratified by race and sex showed a weaker association between glycolithocholate sulfate and AF in whites compared to blacks (HR 1.04, 95%CI 0.94, 1.16 versus HR 1.19, 95%CI 1.10, 1.28, p for interaction = 0.05). No other interactions were identified (Supplementary Figures 2 and 3).
Table 2.
Association of two secondary bile acids (glycocholenate sulfate and glycolithocholate sulfate) with incidence of AF, by analytical batch. Hazard ratios per 1-standard deviation difference. ARIC study, 1987–2013
| HR(95%CI) | p-value | HR (95%CI) | p-value | HR(95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Glycocholenate sulfate | ||||||
| Model 1 | 1.27 (1.16, 1.39) | 1.9 × 10−7 | 1.21 (1.10, 1.33) | 0.0001 | 1.23 (1.14, 1.32) | 9.5 × 10−8 |
| Model 2 | 1.20 (1.08, 1.33) | 0.0006 | 1.10 (1.00, 1.21) | 0.05 | 1.13 (1.04, 1.22) | 0.003 |
| Model 3 | 1.20 (1.08, 1.33) | 0.0006 | 1.09 (0.99, 1.20) | 0.09 | 1.12 (1.04, 1.21) | 0.004 |
| Glycolithocholate sulfate | ||||||
| Model 1 | 1.22 (1.13, 1.31) | 1.4 × 10−7 | 1.02 (0.93, 1.13) | 0.69 | 1.09 (1.01, 1.17) | 0.03 |
| Model 2 | 1.21 (1.11, 1.31) | 5.5 × 10−6 | 1.02 (0.92, 1.13) | 0.67 | 1.07 (0.99, 1.15) | 0.11 |
| Model 3 | 1.21 (1.12, 1.31) | 4.0 × 10−6 | 1.02 (0.92, 1.13) | 0.72 | 1.07 (0.99, 1.15) | 0.10 |
Model 1 adjusted for age, sex and race, center and batch where applicable. Model 2 additionally adjusted for smoking, body mass index, systolic blood pressure, use of antihypertensive medication, diabetes, prevalent heart failure, and prevalent coronary heart disease. Model 3 additionally adjusted for estimated glomerular filtration rate
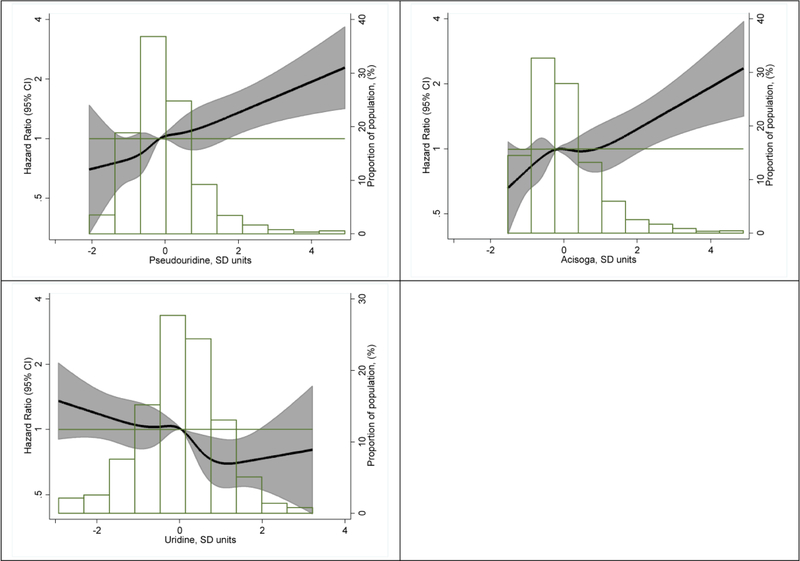
Subsequently, we performed a metabolome-wide, hypothesis-free analysis combining the 2 study samples. Of the 245 studied metabolites, 9 were associated with the incidence of AF with p-values <0.001 after multivariable adjustment (Table 3, Model 2). These metabolites included molecules involved in the metabolism of pyrimidines (pseudouridine and uridine), polyamines (acisoga), amino acids (N-acetylalanine and N-acetylthreonine), and bile acids (glycoursodeoxycholate and glycochenodeoxycholate), as well as one lysolipid (1-docosahexaenoylglycerophosphocholine), and a xenobiotic (O-sulfo-L-tyrosine). Pearson correlation coefficients for these metabolites between repeated measures in 97 samples as well as percentage of observations with missing values are presented in Supplementary Table 1. Three of these molecules, pseudouridine, acisoga, and uridine, were significantly associated with AF with p-values <3.538 × 10−4. Specifically, higher levels of pseudouridine and acisoga were associated with higher rates of AF while higher uridine levels were associated with reduced AF rates. Complete results for the 245 metabolites are available as a supplementary file. The correlation matrix of the 9 metabolites is shown in Supplementary Table 2. Uridine was not correlated with pseudouridine (r = −0.02) or acisoga (r = −0.03), though there was a modest association between pseudouridine and acisoga (r = 0.42). Associations for pseudouridine and acisoga weakened, but were still present, in a model including the 3 metabolites simultaneously (HR 1.16, 95%CI 1.06, 1.26 for pseudouridine, HR 1.11, 95%CI 1.02, 1.20 for acisoga). The inverse association between uridine and AF risk did not change after adjustment for pseudouridine and acisoga (HR 0.85, 95%CI 0.79, 0.92). The association remained essentially unchanged after adjustment for blood lipids and in those without CVD (Supplementary Table 3). Figure 1 presents the dose-response associations of pseudouridine, acisoga, and uridine with AF risk, which were approximately linear for the 3 molecules. Multivariable adjustment led to meaningful attenuation in the association of pseudouridine with AF. None of the individual covariates in the multivariable model seemed particularly responsible for this attenuation, as evaluated by adding each covariate individually to the minimally adjusted model (Supplementary Figure 1). Associations were similar across race and sex groups (Supplementary Figures 2 and 3).
Table 3.
Association of individual metabolites with incidence of atrial fibrillation, ARIC study, 1987–2013. Hazard ratios per 1-standard deviation difference. Only metabolites with an FDR-adjusted p-value <0.05 in the multivariable model 2 are shown.
| HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Pseudouridine | 1.31 (1.22, 1.41) | 4.5×10−13 | 1.18 (1.10, 1.28) | 1.7×10−5 | 1.16 (1.06, 1.27) | 9.6×10−4 |
| Acisoga | 1.20 (1.12, 1.30) | 1.3×10−6 | 1.17 (1.09, 1.26) | 4.0×10−5 | 1.15 (1.06, 1.24) | 3.7×10−4 |
| Uridine | 0.82 (0.75, 0.88) | 5.4×10−7 | 0.86 (0.79, 0.93) | 1.3×10−4 | 0.86 (0.79, 0.93) | 1.7×10−4 |
| 1-docosahexaenoylglycerophosphocholine | 0.82 (0.75, 0.90) | 2.2×10−5 | 0.85 (0.77, 0.93) | 3.6×10−4 | 0.85 (0.77, 0.93) | 4.0×10−4 |
| O-sulfo-L-tyrosine | 1.18 (1.09, 1.28) | 5.4×10−5 | 1.16 (1.07, 1.25) | 4.0×10−4 | 1.12 (1.03, 1.23) | 0.01 |
| Glycoursodeoxycholate | 1.15 (1.08, 1.23) | 3.0×10−5 | 1.13 (1.05, 1.20) | 5.2×10−4 | 1.13 (1.05, 1.20) | 5.4×10−4 |
| Glycochenodeoxycholate | 1.16 (1.08, 1.24) | 1.8×10−5 | 1.13 (1.05, 1.21) | 5.8×10−4 | 1.13 (1.06, 1.21) | 4.8×10−4 |
| N-acetylalanine | 1.22 (1.14, 1.32) | 5.6×10−8 | 1.14 (1.06, 1.23) | 6.0×10−4 | 1.11 (1.02, 1.21) | 0.02 |
| N-acetylthreonine | 1.21 (1.12, 1.31) | 7.3×10−7 | 1.14 (1.05, 1.23) | 9.2×10−4 | 1.11 (1.02, 1.21) | 0.02 |
FDR p: False Discovery Rate-adjusted p-values. Model 1: Proportional hazards model adjusted for age, sex, race, study site, and batch. Model 2: As Model 1, additionally adjusted for smoking, body mass index, systolic blood pressure, use of antihypertensive medication, diabetes mellitus, prevalent heart failure and prevalent coronary heart disease. Model 3: As Model 2, additionally adjusted for eGFR.
Figure 1.
Association of concentrations of pseudouridine (top left panel), acisoga (top right panel) and uridine (bottom right panel) with incidence of atrial fibrillation presented as hazard ratio (HR; solid line) and 95% confidence intervals (CI; shaded area). Results from Cox proportional hazards model with metabolites modeled using restricted cubic splines (knots at 5th, 27.5th,50th, 72.5th, and 95th percentiles), adjusted for age, sex, race, batch, study site, body mass index, smoking, diabetes, systolic blood pressure, use of antihypertensive medication, prevalent coronary heart disease, and prevalent heart failure. Median value of the metabolite was considered the reference (HR = 1). The histograms represent the frequency distribution of metabolites levels. ARIC study, 1987–2013
To characterize in more detail the association of glychocholenate sulfate, pseudouridine, uridine and acisoga with AF, we explored their cross-sectional association with selected intermediate phenotypes of AF (PR interval, elevated P wave terminal force in V1, abnormal P wave axis) (Table 4). None of the 3 metabolites were associated with the odds of abnormal P wave axis or elevated P wave terminal force in V1. The results were suggestive of a possible association of higher glycocholenate sulfate, pseudouridine and acisoga with shorter PR interval and higher uridine with longer PR interval.
Table 4.
Association of glycocholenate sulfate, pseudouridine, acisoga and uridine with selected ECG measures, ARIC study, 1987–1989
| PR duration, msa | Abnormal P wave axis | Elevated P wave terminal force in V1 | |||||
|---|---|---|---|---|---|---|---|
| Diff (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | ||
| Model 1 | −0.82 (−1.63, −0.01) | 0.05 | 0.98 (0.86, 1.11) | 0.72 | 1.05 (0.97, 1.14) | 0.19 | |
| Model 2 | −0.69 (−1.52, 0.13) | 0.10 | 0.95 (0.83, 1.09) | 0.45 | 1.01 (0.93, 1.10) | 0.81 | |
| Model 3 | −0.69 (−1.52, 0.14) | 0.10 | 0.95 (0.83, 1.09) | 0.45 | 1.01 (0.93, 1.10) | 0.81 | |
| Model 1 | 0.15 (−0.66, 0.97) | 0.71 | 0.86 (0.74, 1.00) | 0.04 | 1.11(1.03, 1.20) | 0.01 | |
| Model 2 | −0.56 (−1.39, 0.27) | 0.18 | 0.92 (0.79, 1.06) | 0.25 | 1.03 (0.94, 1.12) | 0.55 | |
| Model 3 | −0.90 (−1.71, −0.09) | 0.03 | 1.02 (0.89, 1.16) | 0.79 | 1.02 (0.94, 1.11) | 0.68 | |
| Model 1 | −0.52 (−1.31, 0.27) | 0.19 | 1.04 (0.92, 1.18) | 0.54 | 1.07 (0.99, 1.16) | 0.09 | |
| Model 2 | −0.81 (−1.60, −0.02) | 0.05 | 1.01 (0.89, 1.15) | 0.86 | 1.03 (0.95, 1.11) | 0.51 | |
| Model 3 | −0.90 (−1.71, −0.09) | 0.03 | 1.02 (0.89, 1.16) | 0.79 | 1.02 (0.94, 1.11) | 0.68 | |
| Model 1 | 0.79 (0.01, 1.57) | 0.05 | 0.92 (0.81, 1.04) | 0.17 | 0.96 (0.88, 1.04) | 0.30 | |
| Model 2 | 0.58 (−0.21, 1.37) | 0.15 | 1.00 (0.88, 1.15) | 0.95 | 1.00 (0.92, 1.08) | 0.93 | |
| Model 3 | 0.59 (−0.20, 1.38) | 0.15 | 1.00 (0.88, 1.15) | 0.96 | 1.00 (0.92, 1.09) | 0.98 | |
Model 1: Adjusted for age, sex, race, study site, and batch. Model 2: As Model 1, additionally adjusted for smoking, body mass index, systolic blood pressure, use of antihypertensive medication, diabetes mellitus, prevalent heart failure and prevalent coronary heart disease. Model 3: As Model 2, additionally adjusted for eGFR
Models additionally adjusted for resting heart rate
We assessed whether any of the AF-related genetic variants identified in a previously published GWAS of AF among individuals of European ancestry were associated with levels of glycocholenate sulfate, pseudouridine, acisoga or uridine among white participants with genomic data (N = 1421). In this analysis, neither the individual genetic variants nor the AFGen genetic risk score predicted serum levels of these 3 metabolites (Supplementary Table 4).
Finally, variation in rs2272996 in gene VNN1, previously associated with circulating levels of acisoga, was not predictive of AF risk (p = 0.88 in the most recent GWAS from the AFGen consortium).
DISCUSSION
In this metabolomic study of 3,922 men and women from a diverse prospective cohort we replicated a previously described association of glycocholenate sulfate, a secondary bile acid, with the incidence of AF. Also, we identified 3 additional metabolites (2 related to pyrimidine metabolism, pseudouridine and uridine, and 1 related to polyamine metabolism, acisoga) associated with incidence of AF using a stringent Bonferroni correction. Several additional analyses showing lack of association of these metabolites with AF electrical endophenotypes and gene variants associated with AF in a previously published GWAS suggest that these metabolites may affect AF pathogenesis through alternative mechanisms.
Consistent with our prior analysis of the ARIC cohort,3 we found an association of circulating glychocholenate sulfate with increased incidence of AF. The previously described association of another secondary bile acid, glycholithocholate sulfate, with AF was not replicated in this new analysis. Glychocholenate sulfate is possibly derived from 3-beta-hydroxy-5-cholenoic acid (cholenate). Prior literature has described elevations of cholenate in patients with liver disease.13 Thus, liver injury, which has been associated with AF previously, could explain the association of bile acids with incident AF. Alternative mechanisms, including the cardiometabolic implications of systemic activation of farnesoid X receptor by circulating bile acids14 or changes in the gut microbiota,15 instrumental in bile acid metabolism, could underlie the described associations. Our results, together with a prior study describing potential arrhythmogenic effects of bile acids,16 provide the rationale for future work exploring the impact of bile acids on the development of AF.
Pseudouridine and uridine are nucleosides involved in RNA synthesis and metabolism. Pseudouridine results from enzymatic posttranscriptional modification of uridine in RNA, with stress conditions influencing the occurrence of this process.17 In turn, RNA pseudouridylation can affect gene expression regulation through mRNA stability and proteome diversity.18 Because of its physiological roles, circulating or urinary pseudouridine is considered a marker of RNA degradation and cell turnover.19 Prior studies have reported higher concentrations of circulating pseudouridine in patients with some cardiovascular diseases and impaired kidney function.20,21 The relationships between circulating pseudouridine and posttranscriptional pseudouridylation of RNA and what role, if any, pseudouridine has in processes contributing to AF risk, requires further investigation.
Uridine is a ribonucleoside potentially involved in modulation of the metabolism of multiple systems and critical for cellular function and survival, though its specific targets have not been identified.22 Recent studies indicate that plasma uridine plays a key role in energy homeostasis and thermoregulation, modulating leptin signaling and potentially affecting glucose and insulin metabolism.23 Given the involvement of obesity and diabetes in the development of AF, deeper understanding of the physiological role of uridine in cardiometabolic disorders is needed. Also, in the Framingham Heart Study, higher concentrations of uridine were associated with a nonsignificant lower risk of AF (HR 0.84, 95%CI 0.70, 1.00, p = 0.05, per 1-standard deviation higher concentrations).4
Acisoga (N-(3-acetamidopropyl)pyrrolidin-2-one) is a catabolic product of spermidine formed from N1-acetylspermidine, and involved in the metabolism of polyamines.24 Its precise role is unknown, but 2 prior studies have found associations of elevated acisoga concentrations with higher body mass index,25,26 and a potential association with the incidence of diabetes mellitus in the ARIC study.27 Polyamines are key players in a range of processes, including cell-cell interactions, cellular signaling, and ion channel regulation.28 Acisoga, as an end product of polyamine metabolism, may be a marker of dysregulation in this pathway.
Our study has important strengths, including the inclusion of a large and diverse cohort with excellent follow-up, an adequate number of AF cases to identify associations, and the availability of extensive covariates to reduce confounding. Moreover, we have considered only metabolites that passed rigorous quality control criteria. However, the method of AF ascertainment—relying predominantly on hospital discharge diagnoses—has probably led to missed events, including asymptomatic AF and AF managed exclusively in outpatient settings. Other limitations include the risk of false negatives, due to the limited number of events, the absence of an independent sample for replication, which may result in false positive results, and the extended time between sample collection and metabolomic measurements, which could have influenced the concentrations of some metabolites.
In conclusion, this study replicated the association of one bile acid with AF reported in a previous study and identified 3 additional metabolites from 2 metabolic pathways associated with AF. Our findings suggest that metabolomic approaches in large epidemiologic studies can be valuable in biomarker discovery and advancing our understanding of the pathogenesis of complex diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
SOURCES OF FUNDING
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The metabolomics research was sponsored by the National Human Genome Research Institute (3U01HG004402-02S1). This work was additionally supported by American Heart Association grant 16EIA26410001 (Alonso). Dr. Yu is supported in part by American Heart Association (17SDG33661228) and the National Heart, Lung, and Blood Institute (HL141824 and HL142003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no competing interests.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ussher JR, Elmariah S, Gerszten RE, Dyck RF. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol 2016;68:2850–2870. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Yu B, Qureshi WT, Grams ME, Selvin E, Soliman EZ, Loehr LR, Chen LY, Agarwal SK, Alexander D, Boerwinkle E. Metabolomics and incidence of atrial fibrillation in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. PLoS One 2015;10:e0142610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko D, Riles EM, Marcos EG, Magnani JW, Lubitz SA, Lin H, Long MT, Schnabel RB, McManus DD, Ellinor PT, Ramachandran VS, Wang TJ, Gerszten RE, Benjamin EJ, Yin X, Rienstra M. Metabolomic profiling in relation to new-onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol 2016;118:1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 6.Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol 2013;178:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 1997;16:2529–2542. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JoD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Solisman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF Consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, Lin H, Arking DE, Smith AV, Albert CM, Chaffin M, Tucker NR, Li M, Klarin D, Bihlmeyer NA, Low S-K, Weeke PE, Müller-Nurasyid M, Smith JG, Brody JA, Niemeijer MN, Dörr M, Trompet S, Huffman J, Gustafsson S, Schurmann C, Kleber ME, Lyytikäinen L-P, AFGen Consortium, METASTROKE Consortium of the ISGC, Neurology Working Group of the CHARGE Consortium. Large-scale analysis of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet 2017;49:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu B, de Vries PS, Metcalf GA, Wang Z, Feofanova EV, Liu X, Muzny DM, Wagenknecht LE, Gibbs RA, Morrison AC, Boerwinkle E. Whole genome sequence analysis of serum amino acid levels. Genome Biol 2016;17:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minder EI, Karlaganis G, Paumgartner G. Radioimmunological determination of serum 3beta-hydroxy-5-cholenoic acid in normal subjects and patients with liver disease. J Lipid Res 1979;20:986–993. [PubMed] [Google Scholar]
- 14.Schaap FG, Trauner M, Jansen PLM. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 2014;11:55–67. [DOI] [PubMed] [Google Scholar]
- 15.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker J-D, Raes J, Hansen T, Meta H. I. T. consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–546. [DOI] [PubMed] [Google Scholar]
- 16.Rainer PP, Primessnig U, Harenkamp S, Doleschal B, Wallner M, Fauler G, Stojakovic T, Wachter R, Yates A, Groschner K, Trauner M, Pieske BM, von Lewinski D. Bile acids induce arrhythmias in human atrial myocardium--implications for altered serum bile acid composition in patients with atrial fibrillation. Heart 2013;99:1685–1692. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 2015;11:592–597. [DOI] [PubMed] [Google Scholar]
- 18.Karijolich J, Yi C, Yu YT. Transcriptome-wide dynamics of RNA pseudouridylation. Nat Rev Mol Cell Biol 2015;16:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander G, Topp H, Wieland J, Heller-Schöch G, Schöch G. Possible use of urinary modified RNA metabolites in the measurement of RNA turnover in the human body. Hum Nutr Clin Nutr 1986;40:103–118. [PubMed] [Google Scholar]
- 20.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, Pepke-Zaba J, Toshner MR, Wort SJ, Gibbs JSR, Lawrie A, Gräf S, Morrell NW, Wilkins MR. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017;135:460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Römisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, Dettmer K, Krumsiek J, Oefner PJ, Valdes AM, Meisinger C, Coresh J, Spector TD, Mohney RP, Suhre K, Kastenmüller G, Köttgen A. A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol 2016;27:1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly GP, Duley JA. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci 1999;20:218–225. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Wang ZV, Gordillo R, An Y, Zhang C, Liang Q, Yoshino J, Cautivo KM, De Brabander J, Elmquist JK, Horton JD, Hill JA, Klein S, Scherer PE. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 2017;355:eaaf5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg GA, Kingma AW, Elzinga H, Muskiet FA. Determination of N-(3-acetamidopropyl)pyrrolidin-2-one, a metabolite of spermidine, in urine by isotope dilution mass fragmentography. J Chromatogr 1986;383:251–258. [DOI] [PubMed] [Google Scholar]
- 25.Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, Göring H, Cole SA, Comuzzie AG. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr 2015;102:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore SC, Playdon MC, Sampson JN, Hoover RN, Trabert B, Matthews CE, Ziegler RG. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. J Natl Cancer Inst 2018;110:558–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebholz CM, Yu B, Zheng Z, Chang P, Tin A, Köttgen A, Wagenknecht LE, Coresh J, Boerwinkle E, Selvin E. Serum metabolomic profile of incident diabetes. Diabetologia 2018;61:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life 2009;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.