Abstract
BACKGROUND/OBJECTIVES:
Maternal obesity impacts fetal growth as early as 2nd trimester of pregnancy, yet little is known about the molecular mechanisms involved. We aimed to examine associations between maternal adipokines throughout pregnancy and neonatal size by pre-pregnancy obesity status.
METHODS:
In a prospective cohort of 2,802 U.S. pregnant women from the NICHD Fetal Growth Studies-Singleton Cohort (2009–2013) biospecimens were analyzed in a matched case-control subset of 321 women. Blood was collected at 10–14, 15–26 (fasting), 23–31, and 33–39 gestational weeks. Plasma leptin and soluble leptin receptor (sOB-R) and total and high-molecular-weight (HMW)-adiponectin were measured. Free leptin was calculated as leptin/sOB-R. Birthweight was abstracted from medical records. Neonatal length and skinfolds were measured.
RESULTS:
Leptin and sOB-R in late pregnancy tended to be positively and negatively associated with neonatal length, respectively, while free leptin throughout pregnancy tended to be positively associated with length. Free leptin associations with neonatal length were differential by obesity (i.e., inversely among women without obesity and positively among women with obesity). A per unit increase in free leptin at 33–39 weeks was associated with a shorter neonatal length by −0.55cm (95%CI −0.83, −0.28) in women without obesity and longer length by 0.49cm (95%CI 0.34, 0.65) in women with obesity. HMW-adiponectin at 33–39 weeks was inversely associated with neonatal length (β=−1.29cm; 95%CI, −1.74, −0.85) and skinfold thickness (β=−1.46mm; 95%CI, −1.58, −0.56) among women with obesity. Free leptin across pregnancy tended to be negatively associated with neonatal skinfold thickness among women without obesity, while free leptin in early pregnancy was positively associated with skinfold thickness.
CONCLUSIONS:
Maternal adipokines were associated with multiple pathways that influence neonatal size including length and adiposity, which differed in timing across pregnancy and by pre-pregnancy obesity. These findings provide new potential insights into mechanisms and timing by which maternal obesity may impact fetal growth.
INTRODUCTION
It is widely recognized that maternal obesity is strongly associated with offspring birthweight. Compared to offspring of women with normal weight, offspring of women with pre-pregnancy obesity are over three times more likely to be macrosomic at birth and this excess weight persists as overweight/obesity in adolescence.(1) Specifically, maternal obesity impacts fetal growth as early as the second trimester of pregnancy.(2) Pregnancy is a state of increased energy expenditure with substantial metabolic changes,(3) yet it is not fully understood how these metabolic alterations are affected by maternal obesity or their role in fetal growth.
Two main cytokines released by white adipose tissue which participate in energy homeostasis are leptin and adiponectin. In short, leptin acts on the hypothalamus in conjunction with other adipokines and hormones to inhibit food intake and increase energy expenditure.(4) Women with obesity tend to have higher levels, however, this signaling and feedback mechanism is disrupted by leptin resistance. Soluble leptin receptor (sOB-R), the major circulating binding protein of leptin, is a potential marker of leptin sensitivity.(5) Leptin tends to increase in pregnancy, albeit at a lower rate in women with pre-pregnancy overweight or obesity.(6) It is hypothesized that leptin influences maternal energy expenditure through increased catabolism of maternal adipose tissue via increases in lipid oxidation enzymes.(7) Acting in an opposing manner, adiponectin regulates insulin sensitivity and glucose uptake.(8) Adiponectin rises in early pregnancy, and then decreases corresponding to decreased maternal insulin sensitivity.(9)
Studies related to maternal leptin and birthweight have yielded inconsistent findings with either inverse(6, 10, 11) or null associations,(12) while adiponectin has been inversely associated with birthweight.(13–15) Most studies have examined these biomarkers only at a single time in gestation and given the dynamic changes of these adipokines in pregnancy, capturing full relations with fetal growth is difficult with only one measurement in pregnancy. In addition, other studies have focused on adipokines at delivery via cord blood and thus are addressing a different physiologic question than the impact of maternal levels. Furthermore, birthweight remains a crude measure of neonatal size and does not necessarily capture differences in neonatal length or adiposity. For example, birth length is correlated with health(16) and later height(17) and neonatal body fat is a better predictor of child weight than birthweight.(18, 19) The state of the literature with adiponectin and leptin remains limited and inconsistent regarding the latter outcomes. Lastly, there is little research incorporating leptin in the context of sOB-R, as an indicator of leptin resistance, and its associations with neonatal size.
The objectives of this analysis were to examine longitudinal associations between maternal adipokine levels including leptin, sOB-R, and adiponectin measured four-times across pregnancy with neonatal size including birthweight, length, and adiposity and assess if the associations differed by pre-pregnancy obesity.
SUBJECTS AND METHODS
Study sample
We used data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton Cohort (n=2,802), which enrolled at 12 U.S. clinical centers (2009–2013) between gestational weeks 8–13 2,334 low-risk pregnant women without pre-pregnancy obesity and 468 pregnant women with pre-pregnancy obesity.(20) Primary aims of the NICHD Fetal Growth Studies were to develop fetal growth standards, thus enrollment was restricted to women without preexisting chronic diseases, medical conditions, or obesity (n=2,334).(21) Secondary aims were to examine etiology of gestational diabetes (GDM) and associations of obesity and fetal growth and thus a supplemental cohort of women with obesity was recruited (n=468).(2) This analysis leveraged existing longitudinal biospecimen data measured in 321 women at four visits across pregnancy for a prior nested case-control biospecimen analysis on GDM.(22) Specifically, biospecimens were assayed on 107 women with GDM and 214 women without GDM matched 1:2 to each GDM case. Non-GDM controls were selected to identify a control for each GDM case that was of same race-ethnicity, ±2 years of age, and all of biospecimens across pregnancy were collected within ±2 weeks of the case. Since GDM was not part of the main research question of the current manuscript, the sample was re-weighted to represent the full original cohort of pregnant women without and with obesity (re-weighting described in the Statistical Methods section below).
Questionnaire data, research exams, and biospecimens were collected throughout pregnancy and at delivery. Medical record abstraction of routine prenatal exams and delivery discharge diagnoses was completed after delivery. Institutional review board approval was obtained for all the participating clinical sites (Christiana Care Health System, Columbia University, Fountain Valley, Long Beach Memorial Medical Center, Medical University of South Carolina, New York Hospital Queens, Northwestern University, St. Peter’s University Hospital, Tufts University, University of Alabama at Birmingham, University of California, Irvine, Women and Infants Hospital of Rhode Island), data coordinating centers (Clinical Trials & Surveys Corporation and the Emmes Corporation), and NICHD. All participants provided written, informed consent.
Maternal Biospecimen Collection and Analysis
Biospecimens were collected following a standardized protocol at enrollment (targeted 8–13; actual 10–14 weeks), visit 1 (fasting; targeted 16–22 weeks; actual 15–26 weeks), visit 2 (targeted 24–29; actual 23–31 weeks), and visit 4 (targeted 34–37; actual 33–39 weeks). Research visits were scheduled according to a pre-determined schedule based on randomization groups designed to provide data equally distributed across all gestational weeks. Biospecimens were assayed at all four-time points for women with GDM and one of the two non-GDM controls, while only one of the two controls was randomly selected to be assayed for visits 2 and 4 to minimize biospecimen analysis cost and maximize efficiency.
Blood samples were immediately processed into EDTA plasma and stored at <−70ᵒC until they were thawed immediately before analysis. Assays were performed by a certified clinical laboratory at the University of Minnesota (Minneapolis, MN). Plasma leptin (ng/ml) was measured using a quantitative direct sandwich enzyme immunoassay (Mercodia, Uppsala, Sweden). Plasma sOB-R (ng/ml) was measured using a quantitative sandwich enzyme immunoassay (R&D Systems, Minneapolis, MN). Plasma total and high molecular weight (HMW)-adiponectin (ng/ml) was measured using a quantitative sandwich enzyme immunoassay (R&D Systems, Minneapolis, MN) and converted to μg/ml. Intraassay CVs were <17% for all assays. The free leptin index was calculated as the ratio of leptin to sOB-R.
Neonatal Outcomes
Birthweight and gestational age at delivery were abstracted from neonatal medical records. Neonatal length and skinfolds were measured by trained, certified study personnel. Certification included review of study protocol and manual operations, review of training procedures,(23) and received individual training by a certified examiner. Measurements were obtained prior to discharge, 12–24 hours after delivery; if not possible to obtain measurements at birth because of NICU admission or neonatal complications, infants born very preterm (≤32 weeks) were measured at 32 completed weeks of gestation-corrected age and infants born moderately preterm (33–36 weeks) were measured once stabilized. Measurements were obtained in duplicate; a third measurement was obtained if the difference between first two measurements exceeded prespecified tolerances based on expected technical errors of measurement,(24–26) and the two closest measurements were averaged. Neonatal length, distance from soles of infant’s feet to top of head, was measured with infant supine using an Infantometer (seca 416 Infantometer). Skinfold measurements were taken on the right side of the infant’s body at abdominal flank, anterior thigh, subscapular, and triceps using Lange Skinfold Caliper (Beta Technology, Inc., Santa Cruz, CA) and summed for total adiposity.(27, 28) One of the study sites used incorrect calipers; skinfold measurements at this site (n=12) were excluded.
Additional Variables
Ultrasound at enrollment confirmed accurate pregnancy dating by last menstrual period, which was used to calculate gestational weeks at each visit. At enrollment, women completed questionnaires regarding medical history and behavioral and socio-demographic characteristics. Maternal race-ethnicity was self-reported (non-Hispanic white, non-Hispanic Black, Hispanic, Asian/Pacific Islander). Maternal height and pre-pregnancy weight were self-reported. Pre-pregnancy body mass index (BMI; kg/m2) was calculated and categorized as normal (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). Gestational weight gain at each visit was calculated as measured weight minus pre-pregnancy weight. As part of the inclusion criteria for the main study (primary aim was to define a fetal growth standard), non-obese women who smoked were not eligible. At enrollment, obese women reported their smoking habits in the 6 months prior to pregnancy (yes/no). Women reported attained education (high school or less, some college/associate degree, 4-year college degree or higher). GDM and preeclampsia cases were identified by medical record review.(29)
Statistical Methods
This study leveraged biomarker data measured as part of a nested GDM case-control study in which the primary aim was to assess relations between biomarkers and GDM. The current analysis aim was to assess relations between biomarkers and birth outcomes irrespective of GDM. Because women with GDM were overrepresented in the analytic sample with biomarkers, the sample was re-weighted to represent the full cohort (e.g., in the re-weighted sample 4% of women had GDM opposed to 33% in the non-weighted sample). We confirmed effective reweighting by confirming that 95% confidence intervals around estimates of distributions of the reweighted sample contained the original estimate based on the full cohort (Supplemental Table 1). Weights were created following the idea of pseudolikelihood in Samuelsen (1997),(30) weighting each subject by the inverse of her sampling probability. Sampling probability of each non-GDM subject was calculated from a logistic regression in the full cohort, excluding GDM cases. Predictors included matching factors for selecting controls: age, race/ethnicity, and gestational week at blood collection. GDM subjects had a sampling probability of 1. We used bootstrapping with 200 replicates to confirm the variance of our weighted models and observed similar findings.
Univariate sample characteristics were presented as weighted medians with interquartile ranges or percentages overall and by pre-pregnancy obesity status. Differences in sample characteristics by pre-pregnancy obesity were estimated using a weighted non-parametric model with robust error variance. Weighted linear regressions with robust standard errors were used to estimate associations between continuous biomarkers at each visit and continuous neonatal outcomes. A major aim of this study was to understand mechanisms by which pre-pregnancy obesity impacts fetal growth, thus we stratified all models by pre-pregnancy obesity. Stratification isolated women with pre-pregnancy obesity from women with normal weight and overweight. Stratified models adjusted for pre-pregnancy BMI as a continuous variable to remove residual confounding of adiposity within each strata and the following covariates: age, race-ethnicity, education, parity, gestational weight gain up to the respective visit, and gestational week at blood collection. Models of neonatal length and skinfold thickness were adjusted for number of days post-delivery when measurements were made. Maternal height was not adjusted for as it was not associated with adipokine levels.
Multiple sensitivity analyses were performed to ensure robustness of findings. First, missing data was usually due to inadequate aliquot biospecimen volume or missing neonatal anthropometric measurements. Supplemental Figure 1 displays the sample size for each of the models. We ran sensitivity analyses using multiple imputation with 50 replicates to address missing data among the biomarker sample (n=321) and included women’s baseline characteristics and relevant longitudinal data for time-varying variables.(31) Second, to assess if the difference in adiposity was due to differences in overall size, models of skinfold thickness were additionally adjusted for neonatal weight at the exam. Third, to ensure that the less restrictive enrollment criteria among obese women did not bias the results, we excluded women with pre-pregnancy obesity who smoked prior to pregnancy (n=5). Fourth, to ensure that preterm deliveries were not driving the findings, we excluded women with a preterm delivery <37 weeks (n=22). Fifth, we repeated analyses using a race/ethnic specific birthweight for gestational age z-score created based on a U.S. natality reference.(32) Sixth, the main analysis models were not adjusted for pregnancy complications (e.g., GDM, preeclampsia). Pathologic changes associated with GDM and preeclampsia may occur earlier than the diagnosis date and thus it is unclear at what timepoint these conditions may be on the causal pathway and adjustment for mediators can induce bias.(33) However, given that diagnosis of GDM is typically between 24–28 weeks we performed sensitivity analyses where for the associations at weeks 33–39, after the typical diagnosis date for GDM, we adjusted for GDM and preeclampsia.
We also completed a latent-class trajectory approach to identify specific trajectories of how maternal adipokines change over gestation and their association with neonatal size. A flexible data-driven semiparametric approach was used to identify trajectories.(34) We compared model fit for each biomarker based on 2–4 trajectory groups and fit linear, quadratic, and cubic models. Final models were selected based on maximum Bayesian information criterion value (i.e., least negative). (34) We required that final selected models had ≥5% of the data in each identified group. Each trajectory group was then associated with each neonatal outcome, stratified by pre-pregnancy obesity status, adjusting for age, pre-pregnancy BMI, race-ethnicity, education, parity, gestational weight gain up to the last visit (33–39 weeks), and number of days post-delivery when measurements were made (length and skinfold thickness only). The reference for each model was group 1 (i.e., group with lowest first trimester biomarker concentrations ).
R version 3.0.2 (Vienna, Austria) was used to calculate sampling weights. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all other analyses. Two-sided P-values <0.05 were considered significant. Requests for code or data should be directed to the corresponding author.
Results
The study sample was diverse with respect to race-ethnicity and socioeconomic factors (Table 1). Women with obesity were more likely to be non-Hispanic Black or Hispanic. Furthermore, women with obesity were less likely to have a 4-year college degree or higher and gain more weight later in pregnancy. Throughout gestation median adipokine levels differed significantly between women with and without pre-pregnancy obesity (Table 2). Furthermore, adipokine levels at each visit were highly correlated with levels at the prior visit (r=0.86–0.93) (Supplemental Table 2).
Table 1.
Sample characteristics overall and according to maternal pre-pregnancy obesity status.
| Pre-Pregnancy BMI | ||||
|---|---|---|---|---|
| Overall (n=321) | < 30.0 kg/m2 (n=255) | ≥ 30.0 kg/m2 (n=66) | ||
| n (%) or median (IQR) | n (%) or median (IQR) | n (%) or median (IQR) | Pa | |
| Age, y | 27.6 (23.2, 31.9) | 27.7 (23.2, 31.7) | 27.3 (23.0, 32.6) | 0.84 |
| Race-ethnicity | 0.004 | |||
| Non-Hispanic White | 75 (30.9) | 60 (32.9) | 15 (20.0) | |
| Non-Hispanic Black | 45 (23.3) | 31 (21.5) | 14 (33.3) | |
| Hispanic | 123 (27.2) | 87 (23.8) | 36 (46.6) | |
| Asian & Pacific Islander | 78 (18.5) | 77 (21.8) | 1 (0.2) | |
| Education | 0.01 | |||
| High school or less | 81 (25.1) | 60 (22.7) | 21 (38.3) | |
| Some college/associate degree | 117 (35.2) | 85 (33.2) | 32 (46.5) | |
| 4-year college degree or higher | 123 (39.8) | 110 (44.2) | 13 (15.2) | |
| Nulliparous | 144 (51.1) | 117 (53.2) | 27 (39.5) | 0.17 |
| Smokedb | 5 (0.7) | 0 (0.0) | 5 (4.6) | -- |
| Pre-pregnancy BMI, kg/m2 | 24.6 (22, 27.4) | 23.9 (21.6, 25.7) | 35.0 (32.4, 38.0) | <0.001 |
| 19.0–24.9 | 156 (51.7) | 156 (61.0) | 0 (0.0) | |
| 25.0–29.9 | 99 (33.1) | 99 (39.0) | 0 (0.0) | |
| 30.0–45.0 | 66 (15.2) | 0 (0.0) | 66 (100.0) | |
| Maternal weight gainc, kg | ||||
| 10–14 weeks | 1.9 (0.4, 3.2) | 2.0 (0.4, 3.2) | 1.7 (−1.4, 4.0) | 0.36 |
| 15–26 weeks | 4.5 (1.8, 6.8) | 4.6 (2.2, 6.9) | 2.3 (−0.3, 5.4) | 0.20 |
| 23–31 weeks | 8.7 (5.9, 11.4) | 9.3 (6.6, 11.4) | 5.5 (2.5, 8.2) | <0.001 |
| 33–39 weeks | 13.6 (10.9, 17.2) | 14 (11.3, 17.6) | 11.5 (6.9, 13.5) | 0.01 |
| Preterm delivery, <37 weeks | 22 (9.1) | 17 (8.4) | 5 (12.6) | 0.54 |
| Gestational diabetes | 107 (3.9) | 75 (3.2) | 32 (7.6) | 0.002 |
| Preeclampsia | 10 (2.2) | 6 (2.0) | 4 (3.2) | 0.65 |
Abbreviations: BMI, body mass index; IQR, interquartile range.
P-value represents the difference between the median level in women with a pre-pregnancy BMI <30.0 kg/m2 and ≥ 30.0 kg/m2
Women with a pre-pregnancy BMI < 30.0 kg/m2 who smoked were not eligible for the study
Maternal weight gain calculated as the difference between the maternal weight measured at the research visit and pre-pregnancy weight self-reported at enrollment.
Table 2.
Adipokine distributions across pregnancy overall and according to maternal pre-pregnancy obesity status.
| Pre-Pregnancy BMI | ||||
|---|---|---|---|---|
| Overall (n=321) | < 30.0 kg/m2 (n=255) | ≥ 30.0 kg/m2 (n=66) | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Pa | |
| Leptin, ng/ml | ||||
| 10–14 weeks | 30.7 (20.3, 48.6) | 27.6 (19.6, 43.6) | 65.6 (46.1, 79.1) | <0.001 |
| 15–26 weeks | 38.8 (26.9, 53.5) | 34.6 (25.4, 46.8) | 71.8 (57.3, 86.3) | <0.001 |
| 23–31 weeks | 40.6 (27.5, 59.1) | 38.6 (26.1, 52.5) | 78.2 (59.1, 93.7) | <0.001 |
| 33–39 weeks | 40.4 (26.2, 67.5) | 35.4 (24.3, 57.9) | 79.7 (53.1, 93.2) | <0.001 |
| sOB-R, ng/ml | ||||
| 10–14 weeks | 26.6 (22.4, 33.8) | 28.5 (23.0, 34.4) | 19.7 (14.9, 23.0) | <0.001 |
| 15–26 weeks | 31.7 (23.9, 39.6) | 33.9 (27.1, 41.4) | 20.4 (17.7, 24.2) | <0.001 |
| 23–31 weeks | 29.9 (23.4, 38.3) | 31.0 (25.4, 39.6) | 19.1 (13.7, 23.9) | <0.001 |
| 33–39 weeks | 29.7 (23.1, 37.6) | 31.8 (24.3, 38.3) | 20.5 (13.0, 28.2) | <0.001 |
| Free leptin indexb | ||||
| 10–14 weeks | 1.2 (0.7, 2.2) | 1.0 (0.6, 1.8) | 3.5 (2.4, 4.6) | <0.001 |
| 15–26 weeks | 1.2 (0.7, 2.1) | 1.0 (0.7, 1.6) | 3.8 (2.9, 4.4) | <0.001 |
| 23–31 weeks | 1.3 (0.8, 2.3) | 1.2 (0.7, 2.1) | 4.1 (2.6, 6.7) | <0.001 |
| 33–39 weeks | 1.4 (0.7, 2.4) | 1.1 (0.6, 2.3) | 3.4 (2.2, 5.8) | <0.001 |
| HMW-adiponectin, μg/ml | ||||
| 10–14 weeks | 4.1 (2.9, 6.5) | 4.7 (3.2, 7.2) | 2.5 (1.8, 3.5) | <0.001 |
| 15–26 weeks | 3.8 (2.7, 5.6) | 4.1 (3.0, 6.2) | 2.4 (1.8, 3.5) | <0.001 |
| 23–31 weeks | 3.2 (2.3, 4.9) | 3.5 (2.3, 5.2) | 2.3 (1.8, 3) | <0.001 |
| 33–39 weeks | 2.9 (1.9, 4.5) | 3.0 (2.1, 4.8) | 2.2 (1.0, 3.8) | <0.001 |
| Total Adiponectin, μg/ml | ||||
| 10–14 weeks | 7.1 (4.8, 10.6) | 7.9 (5.1, 11.3) | 4.5 (3.8, 6.5) | <0.001 |
| 15–26 weeks | 6.7 (4.7, 9.4) | 7.2 (5, 9.8) | 4.6 (3.5, 6.3) | <0.001 |
| 23–31 weeks | 5.8 (4.1, 7.9) | 5.8 (4.2, 8.5) | 5.1 (3.3, 5.8) | 0.003 |
| 33–39 weeks | 5.1 (3.5, 7.5) | 5.3 (3.6, 7.9) | 4.1 (2.5, 6.2) | 0.03 |
Abbreviations: BMI, body mass index; HMW, high molecular weight; IQR, interquartile range; sOB-R, soluble leptin receptor.
P-value represents the difference between the median level in women with a pre-pregnancy BMI <30.0 kg/m2 and ≥ 30.0 kg/m2
Free leptin index calculated as Leptin (ng/ml)/sOB-R (ng/ml).
Table 3 displays adjusted longitudinal associations of leptin, sOB-R, free leptin, HMW-adiponectin, and total adiponectin with neonatal birthweight, length, and skinfold thickness. Higher leptin and free leptin later in pregnancy, in weeks 33–39, was associated with lower birthweight independent of maternal pre-pregnancy BMI and other characteristics including maternal weight gain. Higher sOB-R in weeks 33–39 was positively associated with neonatal length and sOB-R early and late in pregnancy was positively associated with increased skinfold thickness. Lastly, total and HMW-adiponectin later in pregnancy were inversely associated with birthweight; associations were similar, yet slightly stronger with HMW-adiponectin than total adiponectin.
Table 3.
Longitudinal maternal plasma leptin, sOB-R, and HMW-adiponectin concentrations and the association with neonatal birthweight, length, and sum of skinfolds.
| Neonatal Outcome |
|||
|---|---|---|---|
| Maternal Biomarker | Birthweight, g Adjusteda β (95% CI) | Length, cm Adjusteda β (95% CI) | Sum of Skinfolds, mm Adjusteda β (95% CI) |
| Leptin, ng/ml | |||
| 10–14 weeks | −2.70 (−8.27, 2.88) | −0.01 (−0.04, 0.02) | 0.01 (−0.05, 0.06) |
| 15–26 weeks | −1.97 (−7.30, 3.37) | −0.02 (−0.04, 0.01) | −0.01 (−0.05, 0.04) |
| 23–31 weeks | −3.69 (−7.81, 0.44) | −0.01 (−0.04, 0.01) | −0.01 (−0.06, 0.04) |
| 33–39 weeks | −4.13 (−7.68, −0.59) | 0.00 (−0.03, 0.02) | 0.00 (−0.04, 0.04) |
| sOB-R, ng/ml | |||
| 10–14 weeks | −1.73 (−9.31, 5.86) | 0.02 (−0.04, 0.08) | 0.11 (0.01, 0.20) |
| 15–26 weeks | 0.16 (−6.97, 7.28) | 0.04 (−0.01, 0.09) | 0.06 (−0.02, 0.14) |
| 23–31 weeks | 0.74 (−7.22, 8.70) | 0.05 (−0.01, 0.11) | 0.07 (−0.03, 0.17) |
| 33–39 weeks | 4.01 (−2.35, 10.37) | 0.07 (0.03, 0.12) | 0.11 (0.02, 0.20) |
| Free leptin indexb | |||
| 10–14 weeks | −0.54 (−69.01, 67.92) | 0.00 (−0.42, 0.42) | −0.31 (−1.61, 0.99) |
| 15–26 weeks | −22.19 (−94.12, 49.73) | −0.30 (−0.72, 0.11) | −0.96 (−1.92, −0.01) |
| 23–31 weeks | −56.41 (−121.76, 8.94) | −0.26 (−0.75, 0.23) | −0.34 (−1.76, 1.07) |
| 33–39 weeks | −72.63 (−123.81, −21.44) | −0.30 (−0.60, 0.01) | −0.46 (−1.41, 0.50) |
| HMW-adiponectin, μg/ml | |||
| 10–14 weeks | 3.81 (−30.25, 37.87) | 0.01 (−0.15, 0.16) | 0.08 (−0.16, 0.32) |
| 15–26 weeks | −3.24 (−37.79, 31.31) | −0.03 (−0.21, 0.15) | −0.01 (−0.28, 0.26) |
| 23–31 weeks | −53.48 (−89.46, −17.49) | −0.26 (−0.51, 0.00) | 0.06 (−0.52, 0.64) |
| 33–39 weeks | −39.86 (−67.69, −12.03) | −0.18 (−0.40, 0.05) | 0.18 (−0.39, 0.75) |
| Total Adiponectin, μg/ml | |||
| 10–14 weeks | 1.96 (−23.15, 27.07) | 0.00 (−0.13, 0.12) | 0.02 (−0.13, 0.17) |
| 15–26 weeks | 4.79 (−19.30, 28.89) | −0.01 (−0.14, 0.13) | −0.01 (−0.18, 0.16) |
| 23–31 weeks | −38.14 (−63.84, −12.44) | −0.19 (−0.37, 0.00) | −0.05 (−0.42, 0.33) |
| 33–39 weeks | −25.96 (−50.13, −1.79) | −0.11 (−0.27, 0.06) | 0.07 (−0.35, 0.49) |
Abbreviations: CI, confidence interval; HMW, high molecular weight; sOB-R, soluble leptin receptor.
Adjusted for maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian), education (High school or less, some college/associate degree, 4-year college degree or higher), nulliparity (yes/no), pre-pregnancy body mass index (continuous), gestational weight gain up to the respective visit (continuous), gestational week at blood collection (continuous), and postnatal days at neonatal assessment (continuous; length and skinfolds model only).
Free leptin index calculated as Leptin (ng/ml)/sOB-R (ng/ml).
Results stratified by pre-pregnancy obesity status are shown in Table 4. Once stratified, free leptin was inversely associated with birthweight among women without obesity only. Adiponectin late in pregnancy was inversely associated with birthweight among women with and without obesity, but stronger in women with obesity. Throughout pregnancy free leptin tended to be associated with neonatal length; however, associations were differential by obesity status such that free leptin was inversely associated among women without obesity and positively among women with obesity. Adiponectin was inversely associated with neonatal length among women with obesity only. Free leptin across pregnancy tended to be inversely associated with neonatal skinfold thickness among women without obesity, while free leptin in early pregnancy was positively associated with neonatal skinfold thickness. Adiponectin was inversely associated with neonatal skinfold thickness among women with obesity only.
Table 4.
Longitudinal maternal plasma leptin, sOB-R, and HMW-adiponectin concentrations and the association with neonatal birthweight, length, and sum of skinfolds among women with pre-pregnancy normal or overweight or obesity.
| Neonatal Outcomes | ||||||
|---|---|---|---|---|---|---|
| Birthweight, g | Length, cm | Sum of Skinfolds, mm | ||||
| Pre-Pregnancy BMI | Pre-Pregnancy BMI | Pre-Pregnancy BMI | ||||
| <30.0 kg/m2 | ≥30.0 kg/m2 | <30.0 kg/m2 | ≥30.0 kg/m2 | <30.0 kg/m2 | ≥30.0 kg/m2 | |
| Maternal Biomarker | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) |
| Leptin, ng/ml | ||||||
| 10–14 weeks | −2.34 (−8.76, 4.08) | −3.17 (−13.84, 7.50) | −0.01 (−0.05, 0.03) | 0.00 (−0.06, 0.06) | −0.02 (−0.08, 0.03) | 0.10 (0.02, 0.17) |
| 15–26 weeks | −3.70 (−9.55, 2.15) | 3.04 (−5.66, 11.75) | −0.02 (−0.05, 0.01) | 0.00 (−0.05, 0.05) | −0.03 (−0.09, 0.02) | 0.11 (0.06, 0.16) |
| 23–31 weeks | −3.68 (−8.74, 1.38) | 0.56 (−4.61, 5.74) | −0.02 (−0.05, 0.00) | 0.09 (0.05, 0.13) | −0.02 (−0.08, 0.05) | 0.03 (−0.03, 0.08) |
| 33–39 weeks | −4.05 (−8.19, 0.10) | 2.83 (−4.46, 10.13) | −0.01 (−0.03, 0.02) | 0.06 (0.03, 0.09) | 0.00 (−0.05, 0.04) | 0.02 (−0.05, 0.10) |
| sOB-R, ng/ml | ||||||
| 10–14 weeks | 0.49 (−8.35, 9.34) | −14.87 (−31.32, 1.57) | 0.04 (−0.03, 0.11) | −0.09 (−0.21, 0.03) | 0.15 (0.06, 0.24) | −0.34 (−0.48, −0.19) |
| 15–26 weeks | 1.78 (−5.74, 9.03) | −4.87 (−22.97, 13.23) | 0.04 (−0.01, 0.09) | 0.04 (−0.07, 0.16) | 0.07 (0.00, 0.14) | −0.15 (−0.32, 0.01) |
| 23–31 weeks | −0.18 (−8.27, 7.90) | −5.47 (−25.12, 14.18) | 0.05 (−0.01, 0.11) | −0.36 (−0.57, −0.16) | 0.08 (−0.01, 0.17) | −0.25 (−0.53, 0.03) |
| 33–39 weeks | 3.33 (−3.00, 9.67) | −5.50 (−20.67, 9.67) | 0.07 (0.02, 0.12) | −0.27 (−0.36, −0.18) | 0.10 (0.02, 0.19) | −0.09 (−0.36, 0.18) |
| Free leptin Index | ||||||
| 10–14 weeks | −32.77 (−133.10, 67.55) | 63.13 (−8.32, 134.58) | −0.34 (−0.88, 0.20) | 0.52 (0.18, 0.85) | −1.56 (−2.54, −0.58) | 1.26 (0.77, 1.75) |
| 15–26 weeks | −87.54 (−178.47, 3.38) | 60.26 (−49.94, 170.47) | −0.56 (−1.12, −0.01) | 0.10 (−0.51, 0.71) | −1.62 (−2.67, −0.57) | 1.13 (0.30, 1.97) |
| 23–31 weeks | −73.69 (−184.50, 37.12) | 7.49 (−52.04, 67.02) | −0.68 (−1.20, −0.15) | 0.89 (0.47, 1.31) | −1.28 (−2.61, 0.05) | 0.45 (−0.37, 1.27) |
| 33–39 weeks | −111.89 (−179.83, −43.95) | 21.61 (−7.80, 51.01) | −0.55 (−0.83, −0.28) | 0.49 (0.34, 0.65) | −1.05 (−1.68, −0.42) | 0.60 (−0.38, 1.57) |
| HMW-adiponectin, μg/ml | ||||||
| 10–14 weeks | 7.94 (−27.57, 43.44) | −52.38 (−127.59, 22.83) | 0.03 (−0.14, 0.19) | −0.46 (−0.94, 0.03) | 0.08 (−0.15, 0.31) | −0.36 (−1.47, 0.75) |
| 15–26 weeks | −0.49 (−36.34, 35.35) | 28.72 (−65.59, 123.03) | −0.01 (−0.20, 0.18) | −0.16 (−0.78, 0.46) | −0.01 (−0.27, 0.26) | 0.11 (−0.89, 1.10) |
| 23–31 weeks | −60.88 (−98.04, −23.72) | 37.11 (−57.12, 131.34) | −0.25 (−0.51, 0.01) | −0.92 (−1.92, 0.07) | 0.06 (−0.51, 0.64) | −0.83 (−2.01, 0.36) |
| 33–39 weeks | −49.15 (−79.40, −18.90) | −125.76 (−211.70, −39.81) | −0.19 (−0.42, 0.03) | −1.29 (−1.74, −0.85) | 0.14 (−0.40, 0.68) | −1.46 (−2.23, −0.68) |
| Total Adiponectin, μg/ml | ||||||
| 10–14 weeks | 5.45 (−20.57, 31.47) | −42.24 (−93.91, 9.44) | 0.01 (−0.12, 0.15) | −0.24 (−0.56, 0.07) | 0.03 (−0.11, 0.17) | −0.22 (−0.84, 0.40) |
| 15–26 weeks | 7.07 (−18.07, 32.22) | 25.17 (−32.69, 83.03) | 0.01 (−0.13, 0.15) | −0.06 (−0.47, 0.36) | 0.00 (−0.17, 0.16) | 0.23 (−0.31, 0.77) |
| 23–31 weeks | −45.71 (−72.69, −18.73) | 17.48 (−49.05, 84.01) | −0.19 (−0.38, 0.00) | −0.15 (−0.66, 0.37) | −0.03 (−0.44, 0.38) | −0.67 (−1.46, 0.11) |
| 33–39 weeks | −36.21 (−61.17, −11.24) | −106.52 (−168.81, −44.22) | −0.13 (−0.31, 0.04) | −0.89 (−1.26, −0.52) | 0.03 (−0.39, 0.46) | −1.07 (−1.58, −0.56) |
Abbreviations: BMI, body mass index; CI, confidence interval; HMW, high molecular weight; sOB-R, soluble leptin receptor.
Adjusted for maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian) education (high school or less, some college/associate degree, 4-year college degree or higher), nulliparity (yes/no), pre-pregnancy body mass index (continuous), gestational weight gain up to the respective visit (continuous), gestational week at blood collection (continuous), and postnatal days at neonatal assessment (continuous; length and skinfolds model only).
Free leptin index calculated as Leptin (ng/ml)/sOB-R (ng/ml).
Results were also in a similar direction and magnitude when multiple imputation was used to impute missing data (Supplemental Table 3). Skinfold thickness results were similar when additionally adjusted for neonatal weight at post-delivery exam (Supplemental Table 4). All results were similar after excluding women who smoked prior to pregnancy (data not shown). Furthermore, when excluding preterm deliveries overall patterns of results and magnitude of estimates were generally consistent with main findings (Supplemental Table 5). Results using birthweight z-score yielded similar conclusions as with birthweight (Supplemental Table 6). Lastly, when adjusting week 33–39 adipokines for GDM and preeclampsia, results were consistent with little attenuation of estimates (Supplemental Table 7). There were two additional findings such that after adjustment for GDM and preeclampsia, among women with normal weight or overweight leptin at weeks 33–39 was significantly associated with decreased birthweight and among women with obesity sOB-R at weeks 33–39 was significantly associated with decreased birthweight.
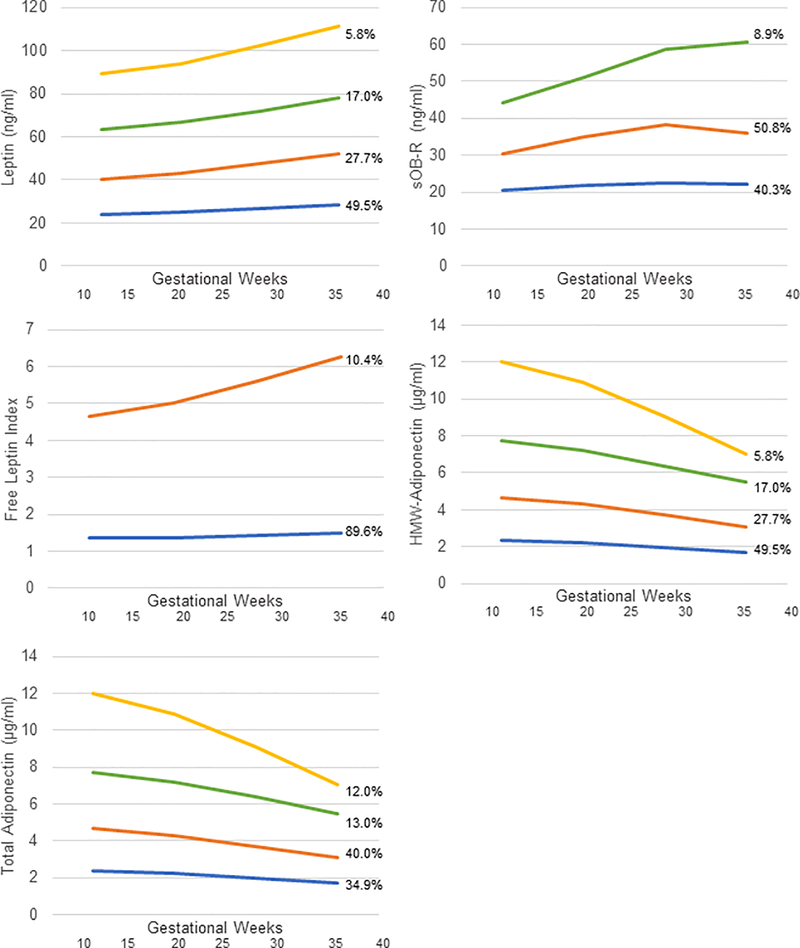
Figure 1 displays group-based trajectories for each biomarker across gestation. Four distinct groups were identified for leptin, HMW-adiponectin, and total adiponectin, three groups were identified for sOB-R, and two groups were identified for free leptin. Results based on maternal adipokine trajectories were in general similar to inferences from results on specific weeks of gestation with only a few notable differences (Table 5). For example, leptin was not significantly associated with birthweight using the original approach, but with trajectories, we observed that among women without pre-pregnancy obesity, higher baseline leptin that increased across pregnancy was inversely associated with birthweight compared to the lowest level of baseline leptin that remained relatively constant across pregnancy. Interestingly, however, the findings related to adiponectin and birthweight among women without obesity were null based on the trajectory approach.
Figure 1.
Maternal adipokine trajectories across gestation.
a Trajectories identified based on a weighted latent class model.
b Group 1 shown in orange. Group 2 shown in blue. Group 3 shown in green. Group 4 shown in yellow. Percentages represent the proportion of the data estimated to make up each group.
Table 5.
Maternal plasma leptin, sOB-R, and HMW-adiponectin concentration trajectories across gestation and the association with neonatal birthweight, length, and sum of skinfolds among women with pre-pregnancy normal or overweight or obesity.
| Neonatal Outcomes | ||||||
|---|---|---|---|---|---|---|
| Birthweight, g | Length, cm | Sum of Skinfolds, mm | ||||
| Pre-Pregnancy BMI | Pre-Pregnancy BMI | Pre-Pregnancy BMI | ||||
| <30.0 kg/m2 | ≥30.0 kg/m2 | <30.0 kg/m2 | ≥30.0 kg/m2 | <30.0 kg/m2 | ≥30.0 kg/m2 | |
| Maternal Biomarkerb | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) | Adjusteda β (95% CI) |
| Leptin, ng/ml | ||||||
| Group 4 | −568.1 (−1151.4, 15.2) | 234.8 (−472.7, 942.3) | −2.8 (−5.0, −0.7) | −2.5 (−6.4, 1.4) | −2.6 (−10.1, 5) | 6.4 (−1.5, 14.2) |
| Group 3 | −170.0 (−399.8, 59.8) | 232.2 (−279.5, 743.9) | −0.1 (−1.9, 1.8) | −1.6 (−4.2, 0.9) | −1.1 (−5.4, 3.3) | 10.5 (1.8, 19.2) |
| Group 2 | −198.5 (−347.8, −49.2) | 269.9 (−280.7, 820.5) | −1.3 (−2.3, −0.4) | −3.5 (−6.7, −0.4) | −1.6 (−3.6, 0.4) | 7.2 (−1.7, 16.1) |
| Group 1 | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| sOB-R, ng/ml | ||||||
| Group 3 | 88.6 (−172.1, 349.4) | --d | 2.1 (0.3, 3.9) | --d | 3.5 (0.7, 6.3) | --d |
| Group 2 | 116.8 (−64.6, 298.3) | −227.9 (−466.9, 11.1) | 0.4 (−0.8, 1.6) | 1.3 (−0.2, 2.8) | 2.7 (0.7, 4.7) | −10.1 (−16.4, −3.8) |
| Group 1 | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| Free leptin Indexc | ||||||
| Group 2 | −316.5 (−661.1, 28.1) | −307.3 (−667.4, 52.7) | −2.1 (−3.6, −0.7) | 0.0 (−2.1, 2.1) | −9.6 (−13.3, −5.9) | 4.3 (−1.0, 9.7) |
| Group 1 | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| HMW-adiponectin, μg/ml | ||||||
| Group 4 | 86.8 (−179.7, 353.3) | --d | 0.8 (n−1.1, 2.7) | --d | 0.0 (−4.4, 4.4) | --d |
| Group 3 | −145 (−367.6, 77.5) | −687.0 (−1294.7, −79.2) | −0.6 (−2.2, 1.0) | −5.5 (−8.7, −2.4) | −0.4 (−4.0, 3.3) | −2.8 (−10.7, 5.1) |
| Group 2 | 19.3 (−123.9, 162.4) | 121 (−83.9, 325.9) | 0.3 (−0.7, 1.3) | −0.2 (−2.6, 2.2) | −0.2 (−2.8, 2.3) | −4.1 (−8.0, −0.2) |
| Group 1 | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| Total Adiponectin, μg/ml | ||||||
| Group 4 | 67.6 (−198.6, 333.7) | --d | 0.5 (−1.3, 2.3) | --d | 0.2 (−3.1, 3.6) | --d |
| Group 3 | −164.3 (−386.7, 58.1) | −808.0 (−1429.9, −186) | −0.8 (−2.3, 0.6) | −5.3 (−9.4, −1.2) | −0.1 (−3.1, 2.8) | 1.3 (−8.2, 10.7) |
| Group 2 | −19.3 (−162.4, 123.9) | −121.0 (−325.9, 83.9) | −0.3 (−1.3, 0.7) | 0.2 (−2.2, 2.6) | 0.2 (−2.3, 2.8) | 4.1 (0.2, 8.0) |
| Group 1 | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
Abbreviations: BMI, body mass index; CI, confidence interval; HMW, high molecular weight; sOB-R, soluble leptin receptor.
Adjusted for maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian) education (high school or less, some college/associate degree, 4-year college degree or higher), nulliparity (yes/no), pre-pregnancy body mass index (continuous), gestational weight gain up to the last visit at 33–39 weeks (continuous), and postnatal days at neonatal assessment (continuous; length and skinfolds model only).
Groups were classified based on trajectories identified based on a weighted latent class model (see Figure 1).
Free leptin index calculated as Leptin (ng/ml)/sOB-R (ng/ml).
The sample size within the group of women with obesity was too small to estimate associations.
Discussion
Several key insights into fetal growth were gained from this analysis of longitudinal associations between maternal adipokine levels across pregnancy and neonatal size. First, in women with pre-pregnancy obesity we observed that leptin, sOB-R, and free leptin were associated with neonatal length and skinfold thickness (i.e., adiposity). Conversely among women without obesity, only free leptin was associated with neonatal length, while sOB-R and free leptin were associated with skinfold thickness. Interestingly, the direction of the associations was different in women with than without obesity. For example, free leptin was positively associated with neonatal length and skinfolds in women with obesity but negatively in women without. Furthermore, leptin and sOB-R were not associated with birthweight when stratified by obesity status, highlighting the importance of using more specific measures of neonatal size. Lastly, adiponectin levels (HMW and total), in the latter half of pregnancy were associated with birthweight in women with and without obesity, but only neonatal length and skinfolds were associated with adiponectin in women with obesity. These data suggest that maternal adipokines are associated with multiple pathways that influence fetal growth, and the associations may be differential between women who start pregnancy with obesity and those who do not.
Recent findings from our diverse cohort demonstrated that fetal long bones (femur length and humerus length) were longer in women with pre-pregnancy obesity, compared to without, starting from the second trimester.(2) The differences were not attributed to gravid complications and the underlying mechanism remained unclear. Our current in-depth analysis builds on this prior finding by demonstrating that maternal adipokine levels may be an important player in the differences in fetal bone growth between women with and without obesity. We observed that free leptin, potentially starting from as early as 10 weeks of gestation was positively associated with neonatal length among women with pre-pregnancy obesity, while it was inversely associated with neonatal length among women without obesity. Notably, women with obesity tend to have higher leptin and lower sOB-R levels, suggestive of leptin resistance, and thus we hypothesize that leptin resistance may play a role in women with pre-pregnancy obesity having longer neonates. We also observed that adiponectin was inversely associated with neonatal length, but only in women with obesity. These findings are novel as there has been little epidemiological research examining maternal adipokine levels and neonatal length or bone mass. Much of the prior literature is focused on cord blood or neonatal blood leptin levels.(35) While there is evidence in non-pregnant individuals for a direct and indirect function of leptin on bone health,(36) the exact mechanisms for an association between maternal leptin and fetal bone growth remain unknown and are further complicated by the fact that maternal leptin does not cross the placenta.(37) Furthermore it is interesting that free leptin was negatively associated with neonatal length in women without obesity, but positively in women with obesity. Yet the long-term clinical implications of these findings require additional research. In general, longer bone lengths at birth are associated taller adult height,(38) but also with an increased risk for wrist fractures in childhood.(39) Thus it is unclear if longer bone lengths at birth to women with obesity translates to healthier bones into adulthood and consequently the area of fetal programming of osteoporosis is an important emerging area of research.(40)
In line with traditional roles of adipokines in energy regulation, we observed that higher leptin was positively associated with neonatal skinfold thickness (i.e., adiposity), but only in women with pre-pregnancy obesity. This was consistent with previous studies.(41–43) While our study had leptin measured multiple times across pregnancy, we observed the association between leptin and neonatal adiposity in early pregnancy only. Given that fetal adipose does not tend to accumulate until late in pregnancy,(44) we hypothesize that maternal leptin may be more likely to have indirect influences on fetal adiposity potentially through regulation of maternal glucose levels or deposition of maternal subcutaneous fat mass early in pregnancy.(3) Also, free leptin was negatively associated with neonatal adiposity in women without obesity. We hypothesize that this may suggest that even in women without obesity increased leptin sensitivity may be associated with lower neonatal adiposity, but this requires additional investigation.(5) We also observed that adiponectin was inversely associated with neonatal adiposity, but only late in pregnancy among women with obesity. This corresponds to the catabolic state of late pregnancy when nutrients are shifted to the fetus. It has been hypothesized by others that maternal adiponectin has an indirect negative impact on fetal growth through improved insulin sensitivity and via placental signaling and nutrient transport to the fetus.(45) Notably, the findings we observed in this analysis are independent of neonatal weight and thus do not just reflect a larger neonate with greater fat mass. While the long-term implications of these findings require more research, studies have shown that neonatal fat mass is positively associated with obesity in childhood.(19)
There are a few potential limitations of this work. First, it is important to emphasize that these findings are observational and do not represent causal effects, but rather observed associations. The enrollment criteria for women with obesity was slightly less restrictive than for women without.(20) In sensitivity analyses we excluded women with obesity who smoked (n=5) and observed similar findings, but cannot rule out residual confounding due to other factors such as chronic hypertension which has been associated with lower birthweight.(46) Also, pre-pregnancy weight was self-reported at enrollment and there could be error in this measure, however, self-reported pre-pregnancy weight is typically highly correlated (r=0.90) with measured weight.(47) Lastly, we did not have biospecimens on the neonates, so the current work is limited solely to maternal adipokine levels.
Notable strengths lie in the robust longitudinal measurement of maternal adipokines. Studies with a single measurement in pregnancy may miss these dynamic associations. In addition to assessing the associations between the adipokines at various timepoints in gestation and birth outcomes, we also examined the associations using trajectory models based on the pattern of change in maternal adipokines over pregnancy. In general, the results were consistent between approaches with a few discrepancies likely due to our small sample. We also had maternal weight gain at each time point of biospecimen collection, which is important as many studies only have total gestational weight gain, and as such we adjusted for maternal weight gain up to each visit in our analyses. We also examined both total and HMW-adiponectin; notably the results were similar. Neonatal measurements were taken according to a strict protocol. Lastly, this was a diverse cohort of women from multiple race-ethnic groups from across the U.S. increasing generalizability.
Conclusions
We observed that leptin, sOB-R, and adiponectin are associated with multiple pathways that influence fetal growth differentially, depending on the timing in pregnancy and maternal pre-pregnancy obesity status. We observed novel findings associating maternal adipokines with neonatal length. In addition, maternal adipokines were associated with offspring adiposity with notable findings suggesting that even in women without pre-pregnancy obesity, leptin sensitivity may be associated with lower neonatal adiposity. Future research is needed to examine long-term intergenerational impact of maternal adipokines on offspring bone health and adiposity into adolescence and adulthood. This work provides new potential insights into the timing and mechanisms for which maternal obesity may impact fetal growth and may inform future work on developing a model for predicting fetal growth in women with obesity.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING: This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding as well as the American Recovery and Reinvestment Act funding (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z).
Footnotes
CONFLICT OF INTEREST DISCLOSURES: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors have no disclosures of interest.
REFERENCES
- 1.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, et al. Association of Maternal Obesity With Longitudinal Ultrasonographic Measures of Fetal Growth: Findings From the NICHD Fetal Growth Studies–Singletons. JAMA pediatrics. 2018;172(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King JC. Physiology of pregnancy and nutrient metabolism. The American journal of clinical nutrition. 2000;71(5):1218s–25s. [DOI] [PubMed] [Google Scholar]
- 4.Allison MB, Myers MG, Jr. 20 YEARS OF LEPTIN: Connecting leptin signaling to biological function. J Endocrinol. 223: (c) 2014 Society for Endocrinology.; 2014. p. T25–t35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaab M, Kratzsch J. The soluble leptin receptor. Best Pract Res Clin Endocrinol Metab. 2015;29(5):661–70. [DOI] [PubMed] [Google Scholar]
- 6.Misra VK, Straughen JK, Trudeau S. Maternal serum leptin during pregnancy and infant birth weight: the influence of maternal overweight and obesity. Obesity (Silver Spring). 2013;21(5):1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194(6):1537–45. [DOI] [PubMed] [Google Scholar]
- 8.Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauguel-de Mouzon S, Catalano P. Adiponectin: are measurements clinically useful in pregnancy? Diabetes Care. 2013;36(6):1434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, Roberts JM. Maternal leptin across pregnancy in women with small-for-gestational-age infants. Am J Obstet Gynecol. 2007;196(6):558 e1–8. [DOI] [PubMed] [Google Scholar]
- 11.Papastefanou I, Samolis S, Panagopoulos P, Tagia M, Bale C, Kouskoukis A, et al. Correlation between maternal first trimester plasma leptin levels and birth weight among normotensive and preeclamptic women. J Matern Fetal Neonatal Med. 2010;23(12):1435–43. [DOI] [PubMed] [Google Scholar]
- 12.Tamura T, Goldenberg RL, Johnston KE, Cliver SP. Serum leptin concentrations during pregnancy and their relationship to fetal growth. Obstet Gynecol. 1998;91(3):389–95. [DOI] [PubMed] [Google Scholar]
- 13.Nanda S, Akolekar R, Sarquis R, Mosconi AP, Nicolaides KH. Maternal serum adiponectin at 11 to 13 weeks of gestation in the prediction of macrosomia. Prenat Diagn. 2011;31(5):479–83. [DOI] [PubMed] [Google Scholar]
- 14.Valdes ER, Lattes KA, Munoz HS, Barja PY, Papapietro KV. First-trimester adiponectin and subsequent development of preeclampsia or fetal growth restriction. Gynecol Obstet Invest. 2011;72(3):152–6. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Shang LX, Dong X, Wang X, Wu N, Wang SH, et al. Relationship of adiponectin and resistin levels in umbilical serum, maternal serum and placenta with neonatal birth weight. Aust N Z J Obstet Gynaecol. 2010;50(5):432–8. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey K, Walker‐Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16(9):1694–703. [DOI] [PubMed] [Google Scholar]
- 17.Fewtrell MS, Cole TJ, Bishop NJ, Lucas A. Neonatal factors predicting childhood height in preterm infants: evidence for a persisting effect of early metabolic bone disease? The Journal of pediatrics. 2000;137(5):668–73. [DOI] [PubMed] [Google Scholar]
- 18.Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small-or large-for-gestational-age. Pediatrics. 1998;102(5):e60–e. [DOI] [PubMed] [Google Scholar]
- 19.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, De Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation–. The American journal of clinical nutrition. 2009;90(5):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA, et al. Cohort Profile: NICHD Fetal Growth Studies–Singletons and Twins. Int J Epidemiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213(4):449 e1–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Mendola P, Albert PS, Bao W, Hinkle SN, Tsai MY, et al. Insulin-Like Growth Factor Axis and Gestational Diabetes Mellitus: A Longitudinal Study in a Multiracial Cohort. Diabetes. 2016;65(11):3495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Training course on child growth assessment. Geneva: WHO; 2008:p17–25. [Google Scholar]
- 24.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82(3):165–77. [DOI] [PubMed] [Google Scholar]
- 25.Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997;24(5):497–505. [DOI] [PubMed] [Google Scholar]
- 26.de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1 Suppl):S27–36. [DOI] [PubMed] [Google Scholar]
- 27.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. The American journal of clinical nutrition. 2002;76(5):1096–100. [DOI] [PubMed] [Google Scholar]
- 28.Weile B Caliper skinfold measurements in newborns: analysis of a method. Biol Neonate. 1986;50(4):192–9. [DOI] [PubMed] [Google Scholar]
- 29.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics and gynecology (New York 1953). 2018;131(2):e49–e64. [DOI] [PubMed] [Google Scholar]
- 30.Samuelsen SO. A psudolikelihood approach to analysis of nested case-control studies. Biometrika. 1997;84(2):379–94. [Google Scholar]
- 31.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–9. [DOI] [PubMed] [Google Scholar]
- 32.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139. [DOI] [PubMed] [Google Scholar]
- 35.Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta‐analysis. Paediatr Perinat Epidemiol. 2011;25(2):150–63. [DOI] [PubMed] [Google Scholar]
- 36.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism-Clinical and Experimental. 2015;64(1):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geary M, Pringle PJ, Persaud M, Wilshin J, Hindmarsh PC, Rodeck CH, et al. Leptin concentrations in maternal serum and cord blood: relationship to maternal anthropometry and fetal growth. BJOG. 1999;106(10):1054–60. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen HT, Sabroe S, Rothman KJ, Gillman M, Steffensen FH, Fischer P, et al. Birth weight and length as predictors for adult height. Am J Epidemiol. 1999;149(8):726–9. [DOI] [PubMed] [Google Scholar]
- 39.Jones IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: evidence from a birth cohort study. Am J Epidemiol. 2004;159(4):343–50. [DOI] [PubMed] [Google Scholar]
- 40.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int. 2006;17(3):337–47. [DOI] [PubMed] [Google Scholar]
- 41.Josefson JL, Zeiss DM, Rademaker AW, Metzger BE. Maternal leptin predicts adiposity of the neonate. Horm Res Paediatr. 2014;81(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patenaude J, Lacerte G, Lacroix M, Guillemette L, Allard C, Doyon M, et al. Associations of Maternal Leptin with Neonatal Adiposity Differ according to Pregravid Weight. Neonatology. 2017;111(4):344–52. [DOI] [PubMed] [Google Scholar]
- 43.Valsamakis G, Papatheodorou DC, Naoum A, Margeli A, Papassotiriou I, Kapantais E, et al. Neonatal birth waist is positively predicted by second trimester maternal active ghrelin, a pro-appetite hormone, and negatively associated with third trimester maternal leptin, a pro-satiety hormone. Early Hum Dev. 2014;90(9):487–92. [DOI] [PubMed] [Google Scholar]
- 44.Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol. 2003;179(3):293–9. [DOI] [PubMed] [Google Scholar]
- 45.Aye IL, Powell TL, Jansson T. Review: Adiponectin--the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34 Suppl:S40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population-based study. The Journal of reproductive medicine. 2007;52(11):1046–51. [PubMed] [Google Scholar]
- 47.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18(3):350–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.